Published online Jun 16, 2021. doi: 10.4253/wjge.v13.i6.170
Peer-review started: January 25, 2021
First decision: March 1, 2021
Revised: March 15, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 16, 2021
Processing time: 136 Days and 4 Hours
In the classic descriptions of the human liver, the common hepatic duct forms at the confluence of left and right hepatic ducts. Many authors have documented variations in the intra-hepatic ductal system, but to the best of our knowledge there has been no report on bile duct variations in Caribbean populations.
To evaluate the variations in bile duct anatomy using magnetic resonance cholangiography (MRC) in unselected patients at a major hepatobiliary referral centre in the Eastern Caribbean. Knowledge of the intra-hepatic biliary anatomy is important to optimize service delivery for any physician treating liver and biliary disorders.
This study was carried out at a tertiary referral hospital for hepatobiliary diseases in the Eastern Caribbean. We retrospectively evaluated magnetic resonance cholangiograms in 152 consecutive patients at this facility over a two-year period from April 1, 2017 to March 31, 2019. Two consultant radiologists experienced in MRC interpretation reviewed all scans and described biliary anatomy according to the Huang’s classification. A systematic review of published studies was performed and relevant data were extracted in order to calculate the global prevalence of each biliary variant. The variants in our population were compared to the global population.
There were 152 MRCs evaluated in this study in 86 males and 66 females. There were 109 (71.7%) persons with “classic” biliary anatomy (type A1) and variants were present in 43 (28.3%) persons. There was no statistical relationship between the presence of anatomic variants and gender or ethnicity. We encountered the following variants: 29 (19.1%) type A2, 7 (4.6%) type A3, 6 (3.95%) type A4, 0 type A5 and a single variant (quadrification) that did not fit the classification system. Compared to the global prevalence, our population had a significantly greater occurrence of A1 anatomy (71.7% vs 62.6%; P = 0.0227) and A2 trifurcations (19.1% vs 11.5%; P = 0.0069), but a significantly lower incidence of A3 variants (4.61% vs 11.5%; P = 0.0047).
There are significant differences in intra-hepatic biliary anatomy in this unselected Eastern Caribbean population compared to global statistics. Specifically, persons of Caribbean descent have a greater incidence of Huang A2 trifurcations and a lower incidence of Huang A3 variants.
Core Tip: Many authors have documented variations in the intra-hepatic ductal system, but to the best of our knowledge there has been no report on bile duct variations in Caribbean populations. In the unselected Eastern Caribbean population, 71.7% of persons have normal intra-hepatic biliary anatomy. Variant anatomy in this population occurs with the following frequencies: A2 (19.1%), A3 (4.6%) and A4 (3.95%).
- Citation: Cawich SO, Sinanan A, Deshpande RR, Gardner MT, Pearce NW, Naraynsingh V. Anatomic variations of the intra-hepatic biliary tree in the Caribbean: A systematic review. World J Gastrointest Endosc 2021; 13(6): 170-183
- URL: https://www.wjgnet.com/1948-5190/full/v13/i6/170.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i6.170
There have been prior reports of variant surface anatomy[1] and vascular supply[2] of the hepatobiliary tree in Caribbean populations. However, to the best of our knowledge there has been no report on bile duct variations in Caribbean populations. This study sought to evaluate the variations in bile duct anatomy using magnetic resonance cholangiography (MRC) at a hepatobiliary referral centre in the Eastern Caribbean.
This study was carried out at the Port-of-Spain General Hospital in Trinidad and Tobago. This 750-bed hospital was a major tertiary referral centre for hepatobiliary diseases serving patients in the Eastern Caribbean. At this centre, a dedicated multidisciplinary team met on a weekly basis to plan the management of patients with hepatobiliary diseases. Permission was granted to examine consecutive MRCs in all patients evaluated at multidisciplinary team meetings between April 1, 2017 to March 31, 2019.
All MRCs were performed using a 1.5 T Magnet with a phased array body coil. Our MRC protocols did not include the use of gadolinium compounds or morphine augmentation. The biliary anatomy on each scan was reported from these studies. The following scans were excluded: duplicate scans, scans with incomplete demographic data and scans with inadequate coverage of the biliary tree.
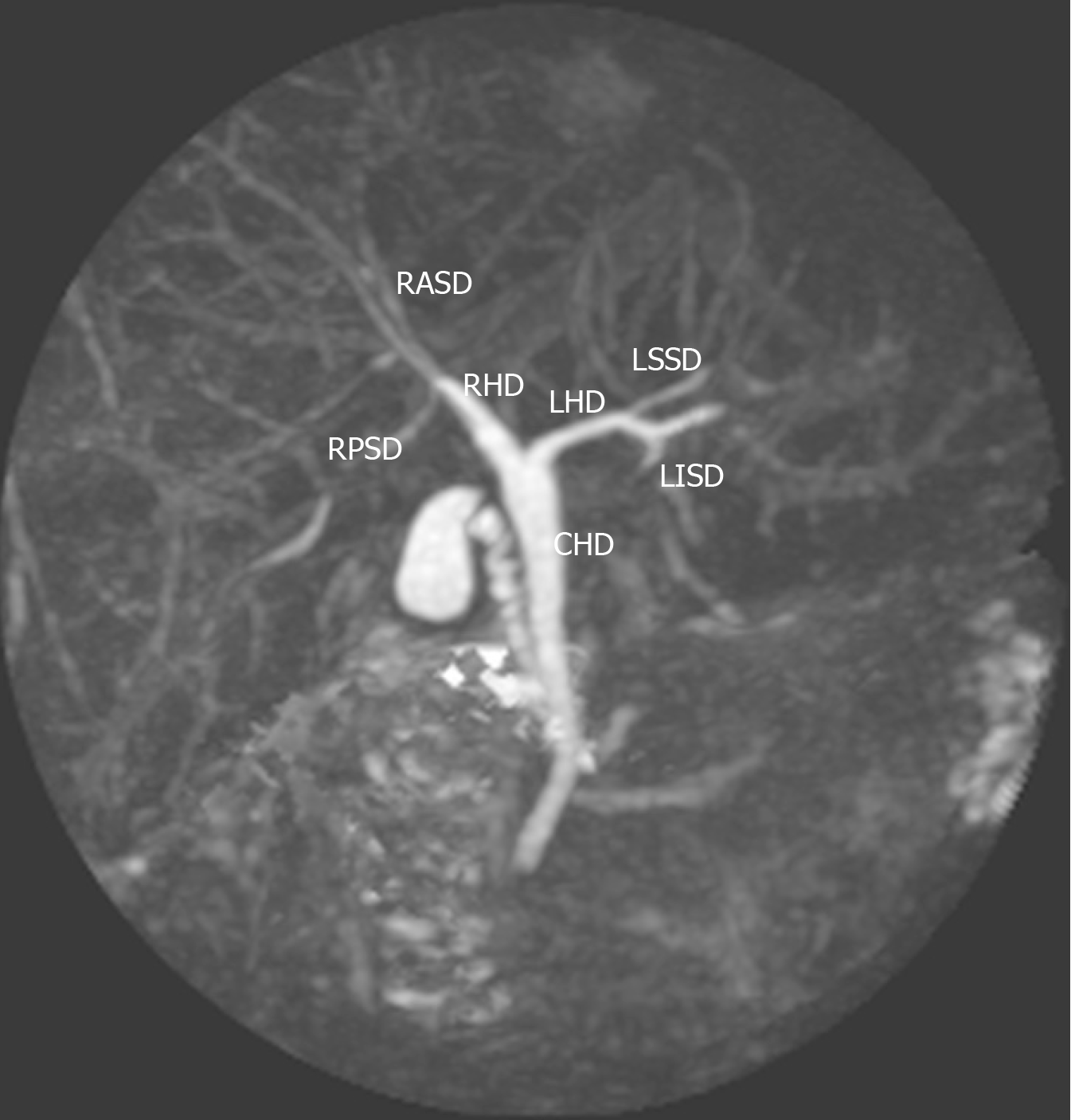
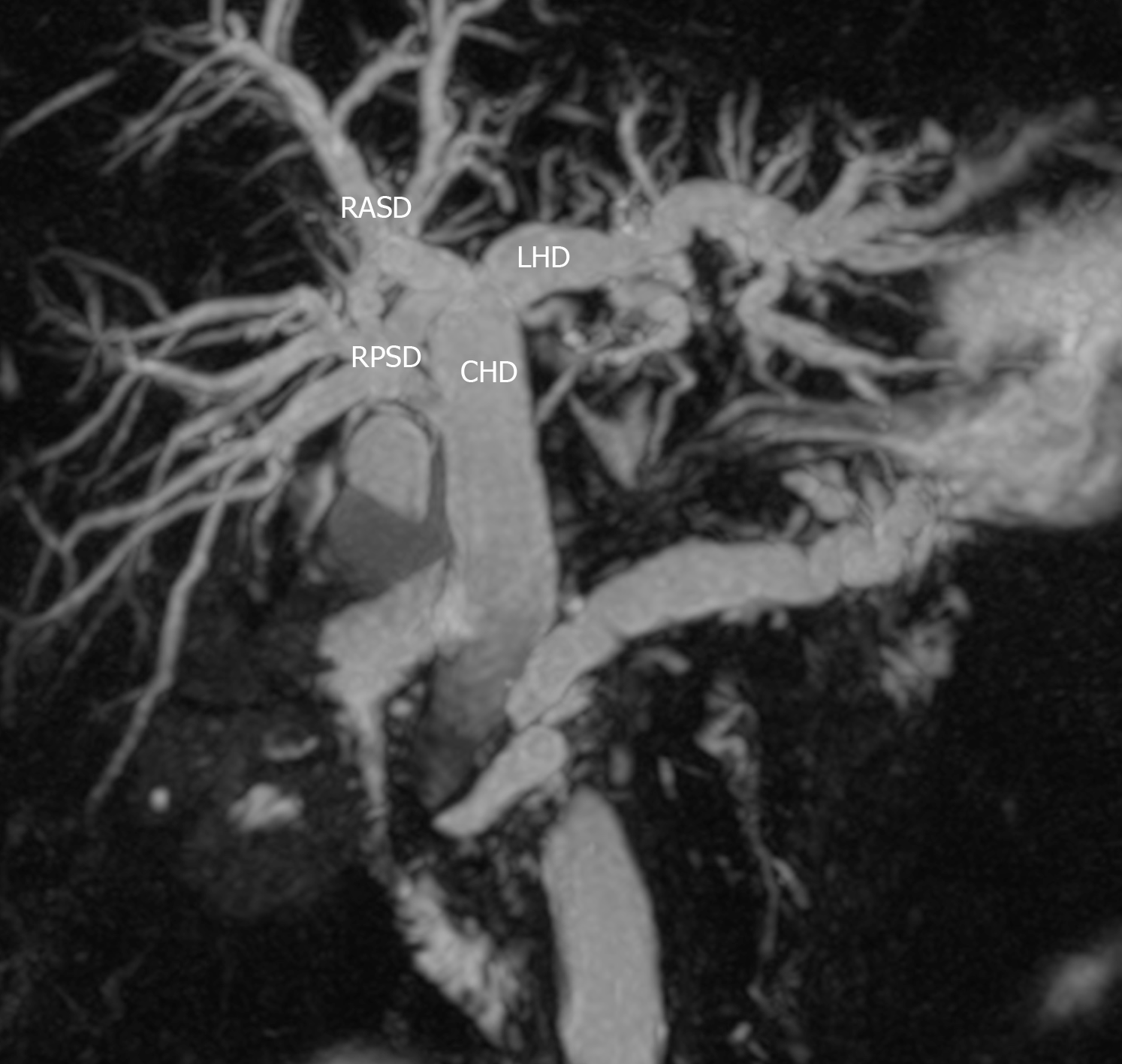
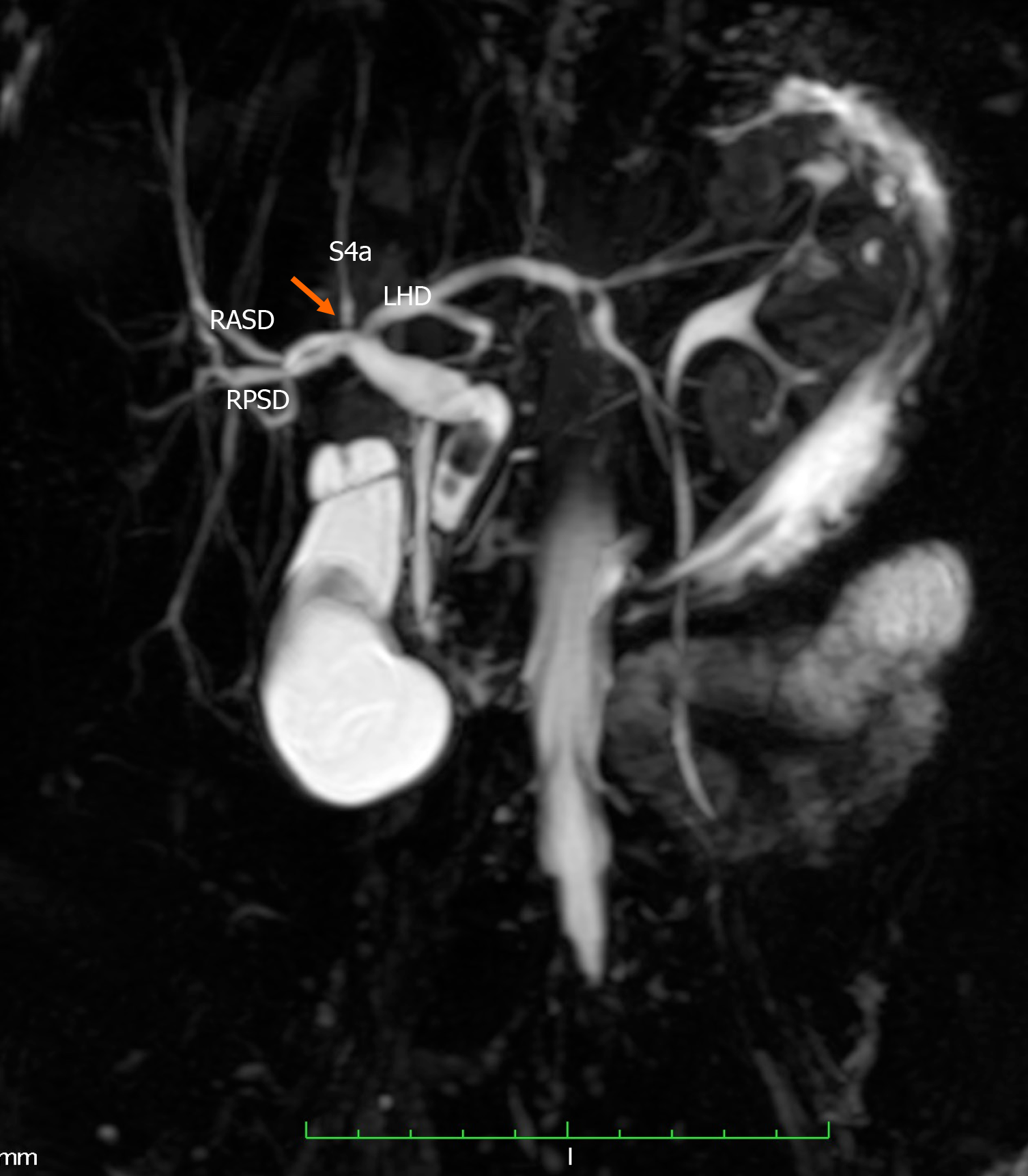
We described the biliary anatomy on MRC according to the classification proposed in 1996 by Huang et al[3]. This classification system was the one most commonly used in the medical literature. In this system, the “classic arrangement” of the intra-hepatic biliary tree is for the left hepatic duct (LHD) and right hepatic duct (RHD) to join, forming the common hepatic duct (CHD). The RHD has two tributaries: the right posterior sectoral duct (RPSD) that drains hepatic segments VI and VII coursing in a horizontal plane and the right anterior sectoral duct (RASD) that drains hepatic segments V and VIII, coursing in a vertical plane. In the left hemi-liver, the left superior sectional duct that drains segment IVa joins the left inferior sectional duct that drains segment II, III and IVb. Both tributaries form the LHD that drains the left hemi-liver. Biliary drainage from the caudate lobe is variable and may join either the LHD or RHD at its origin. The normal anatomy and described variants are illustrated in Table 1 and Supplementary Figure 1.
| Description | Taiwan[3], n = 958 (%) | Caribbean, n = 152 (%) |
| Type A1: The RHD and LHD join to form the CHD. The intra-hepatic RHD is formed by the union of RASD and RPSD. The LHD is formed by the union of LSSD and LISD | 600 (62.6) | 109 (71.7) |
| Type A2: A trifurcation is formed by the union of RASD, RPSD and LHD | 182 (19) | 29 (19.1) |
| Type A3: The RPSD or RASD drains directly into the LHD | 105 (11) | 7 (4.61) |
| Type A4: The RPSD drains directly into the CHD | 56 (5.8) | 6 (3.95) |
| Type A5: The RPSD drains into the cystic duct | 15 (1.6) | 0 |
In this study, two consultant radiologists experienced in MRC interpretation reviewed all scans and independently interpreted the images. In cases where there was disagreement in interpretation, the images were re-examined to achieve consensus. Data from the MRC scans were recorded in a Microsoft Excel® table and descriptive analyses were performed using SPSS version 20 statistical software.
We then conducted a systematic literature search using medical archiving platforms, including PubMed, Medline, Google Scholar and the Cochrane database of Systematic Reviews. We used the following search terms: “intra-hepatic duct”, “bile duct variant”, “biliary variant”, “ductal anatomy”, “hepatic duct variant” and “aberrant bile duct”. All relevant studies were retrieved and the data and images reviewed in detail. Inclusion criteria were: case series reporting > 15 cases, reports with detailed descriptions of variants, studies in adults > 18 years of age and those using magnetic resonance cholangiopancreatography imaging to detect ductal anatomy. We excluded data from duplicated publications, individual case reports and small series with less than 15 cases. In instances where other classifications were used, we studied the written descriptions and published images within the articles of the variants in order to re-classify them in keeping with Huang’s classification[3]. When the variant was not reported or the data could not be reliably extrapolated from published descriptions, data and/or images, the study data were excluded from the global prevalence statistics.
Raw data extracted from the published studies were used to calculate the global prevalence of anatomic variants[4]. The global prevalence was defined as the total number of individuals with a defined anatomic variant divided by the sum of the total number of individuals in each study. The global prevalence was then compared with the prevalence of each variant in our population using Chi square tests to compare contingency tables in SPSS version 20 (IBM Corp, Armonk, NY, United States). Fisher exact tests were used for values < 5. A P value < 0.05 was considered significant.
There were 159 MRCs performed during the study period. Seven scans on six patients were excluded from the final analysis because: one patient was scanned twice, three images were of insufficient quality for analysis and three were not retrievable from the digital archiving system. Therefore, a total of 152 MRCs were evaluated in this study.
There were 86 males and 66 females included in the final analysis with a mean age of 62.6 years (SD ± 10.8; median 65; range 34-80 years). These patients were of Indio-Caribbean (74), Afro-Caribbean (55), Asian (10), Caucasian (9) and Latin (4) descent.
Of 152 examinations analyzed, 109 (71.7%) had the “classic” type 1 biliary anatomy (Figure 1). There were 63 men and 46 women with “classic” anatomy. These persons were of Afro-Caribbean (41), Indio-Caribbean (57), Asian (7), Caucasian (3) and Latin (1) descent. In one patient with classic intra-hepatic biliary anatomy, a solitary Type 1c choledochal cyst was noted at the common bile duct in the extra-hepatic biliary tree.
There were variations from the “classic” biliary anatomy in 23 (15.1%) men and 20 (13.2%) women. There was no statistical relationship between the presence of anatomic variants and gender (26.7% vs 30.3%; P = 0.717), Afro-Caribbean (25.5% vs 29.9%; P = 0.581), Indio-Caribbean (22.97% vs 33.3%; P = 0.207), Asian (30% vs 28.2%; P = 1.000) or Latin ethnicity (75% vs 27%; P = 0.0687). Bile duct variants were commoner in persons of Caucasian ethnicity (66.7% vs 25.9%; P = 0.0158), although the statistical power of this association was reduced since there were only 9 (5.9%) Caucasians in the study population.
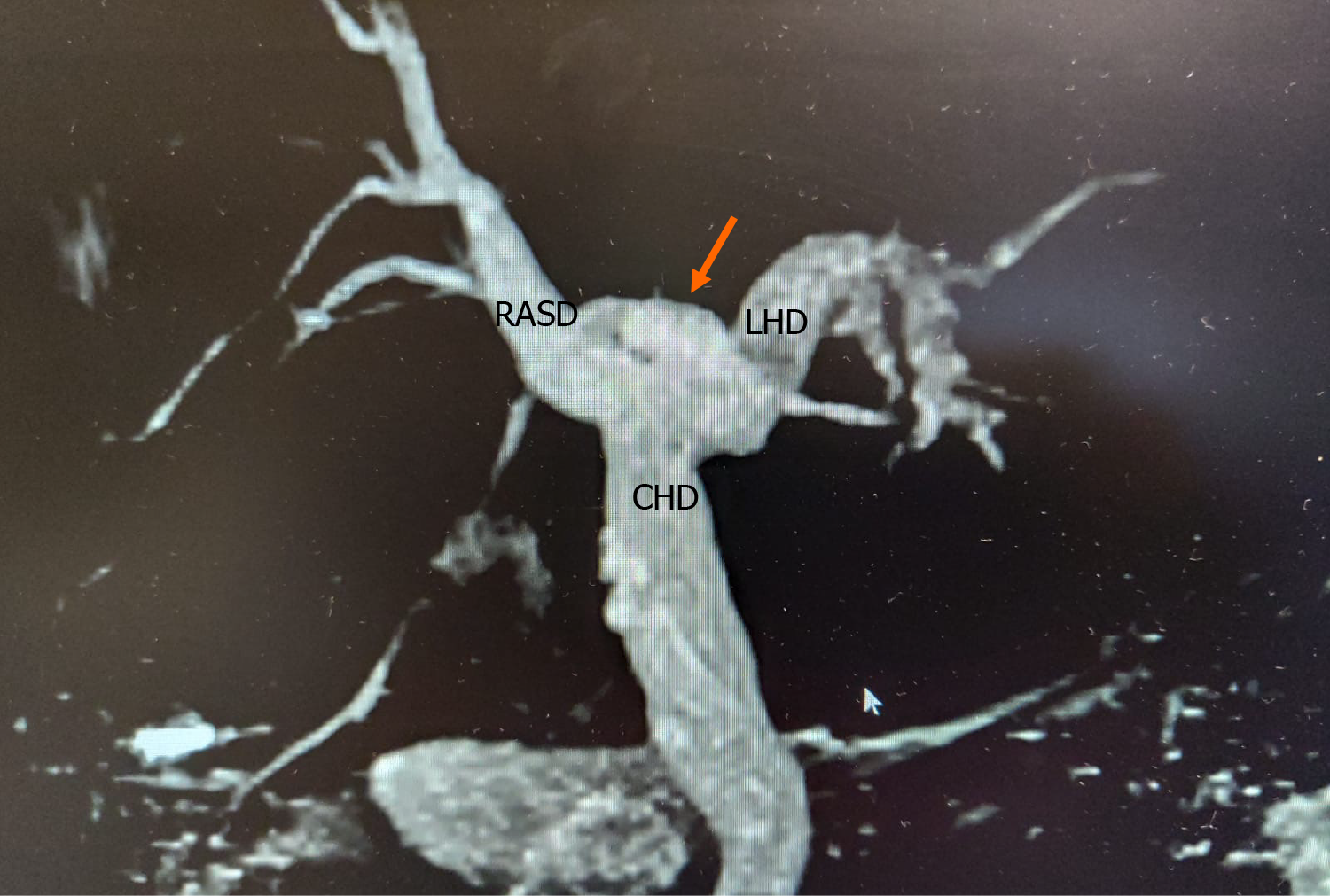
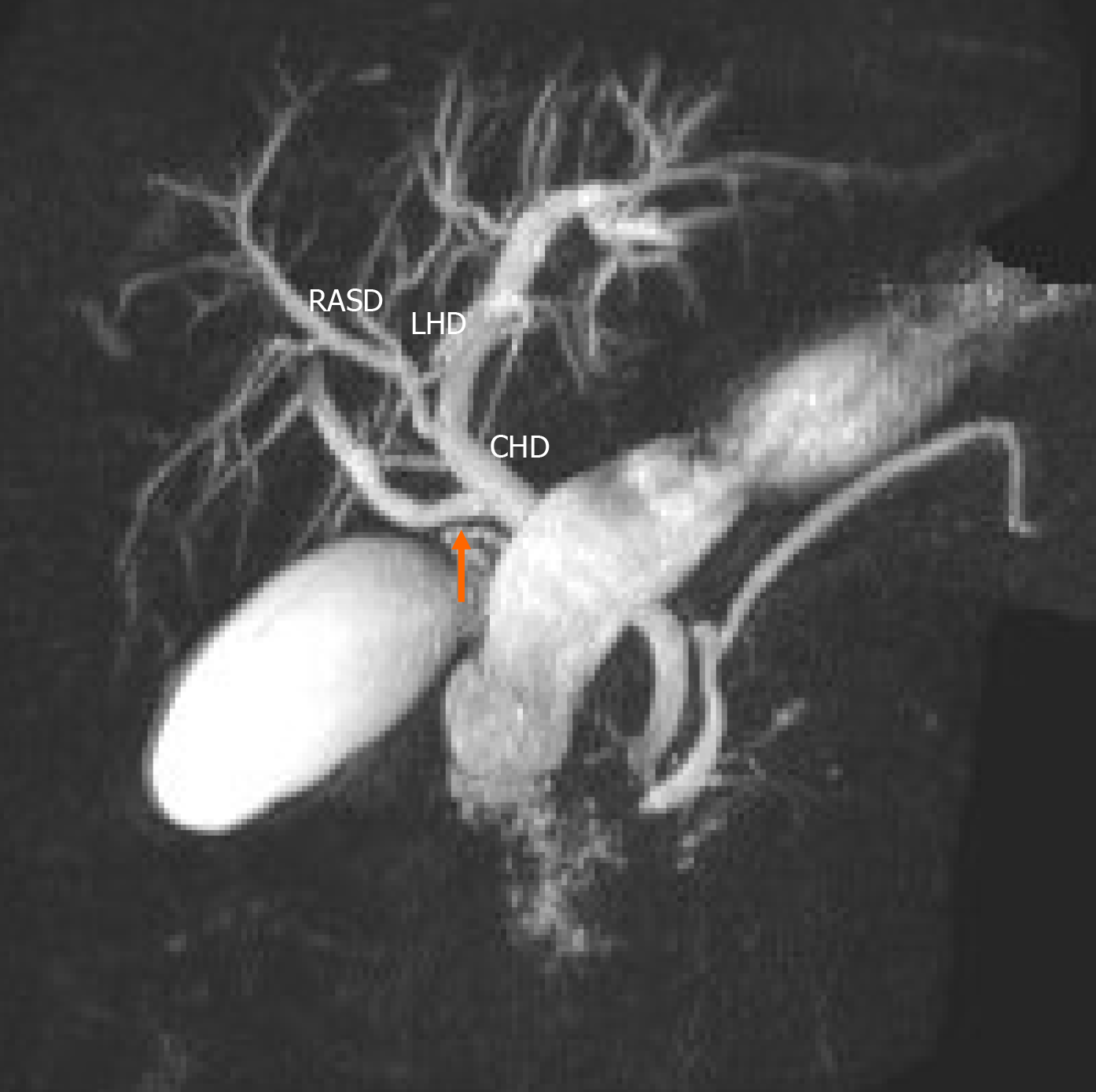
Type A2 anatomy was present in 29 (19.1%) individuals (Figure 2), type A3 variants in 7 (4.6%) individuals (Figure 3) and type A4 variants in 6 (3.95%) individuals (Figure 4). In this study population, we did not encounter any type A5 variants. One person had a variant that did not fit into the Huang classification. This individual had a quadrification where RASD, RPSD, LHD and segment IVa ducts met at the hilum to form the CHD (Figure 5).
In order to calculate the global prevalence of each variation, we conducted a systematic literature search using medical archiving platforms. We retrieved 47 articles that reported on variations in intra-hepatic biliary ductal anatomy in a total of 17045 persons[3,5-50]. Table 2 summarizes the data extracted from published reports of intra-hepatic bile duct variations across the globe. There were 10668 type A1 variants reported in 17045 persons. The global prevalence of type A1 variants (62.6%) was significantly lower than seen in our population (71.7%; P = 0.0227).
| Ref. | Country | Classification | Study population | Huang classification of biliary variants | |||||
| A1 (%) | A2 (%) | A3 (%) | A4 (%) | A5 (%) | Other | ||||
| Couinaud[5], 1957 | France | Couinaud1 | 298 | 1732 (58) | 332 (11.1) | 532 (30.6) | 172 (5.7) | 0 | 22 |
| Puente and Bannura[6], 1983 | Chile | Descriptive1 | 3845 | 2217 (57.6) | 426 (11.1) | 498 (13.0) | 249 (6.5) | NS | 455 |
| Huang et al[3], 1996 | Taiwan | Huang | 958 | 600 (62.6) | 182 (19) | 105 (11) | 56 (5.8) | 15 (1.6) | 0 |
| Yoshida et al[7], 1996 | Japan | Yoshida1 | 1094 | 741 (67.7) | 193 (17.7) | 66 (6.0) | 88 (8.0) | 0 | 0 |
| Cheng et al[8], 1997 | Taiwan | Huang | 958 | 624 (65.1) | NS | 105 (11) | NS | 0 | 200 (21) |
| Nakamura et al[9], 2002 | Japan | Couinaud1 | 120 | 78 (65) | 11 (9.2) | 10 (8.3) | 19 (15.8) | 2 (1.7) | 0 |
| Kitagawa et al[10], 2003 | Taiwan | Huang | 180 | 113 (62.7) | 36 (20.0) | 26 (14.4) | 5 (2.8) | 0 | 0 |
| Choi et al[11], 2003 | South Korea2 | Choi1 | 300 | 188 (63) | 292 (10) | 342 (11) | 192 (6) | 62 (2) | 282 |
| Ayuso et al[12], 2004 | Spain | Couinaud1 | 25 | 102 (40) | 12 (4) | 22 (8) | 102 (40) | 22 (8) | 0 |
| Ohkubo et al[13], 2004 | Japan | Ohkubo1 | 110 | 722 (65) | 62 (5) | 132 (12) | 52 (4.6) | 12 (0.9) | 13 |
| Düşünceli et al[14], 2004 | Turkey | Descriptive1 | 475 | 3602 (75.8) | 42 (0.8) | NS | 272 (5.7) | 0 | 84 |
| Lee et al[15], 2004 | United States | Couinaud1 | 108 | 782 (72.2) | 62 (5.6) | 42 (3.7) | 32 (2.8) | 12 (9.3) | 16 |
| Limanond et al[16], 2004 | United States | Huang | 27 | 19 (70.4) | 5 (18.5) | 2 (7.4) | 1 (3.7) | 0 | 0 |
| Wang et al[17], 2005 | United States | Yoshida1 | 62 | 352 (56.0) | 72 (11) | 112 (18) | 82 (13) | 0 | 1 |
| Chen et al[18], 2005 | United States | Couinaud1 | 56 | 332 (58.9) | 72 (12.5) | 102 (17.9) | 52 (1.8) | 0 | 1 |
| MacDonald et al[19], 2005 | United States | Choi1 | 39 | 242 (61.5) | 32 (7.7) | 72 (17.9) | 12 (2.6) | 0 | 4 |
| Kim et al[20], 2005 | Canada | Champetier1 | 30 | 172 (56.7) | 12 (3.3) | 92 (30) | 22 (6.7) | 12 (3.3) | 0 |
| Wietzke-Braun et al[21], 2006 | Germany | Ohkubo1 | 18 | 22 (11) | 22 (11) | 42 (22) | 12 (6) | 0 | 9 |
| Kitami et al[22], 2006 | Japan | Ohkubo1 | 158 | 116 (73) | 8 (5.1) | 19 (12) | 5 (3) | NS | 10 |
| Vidal et al[23], 2007 | France | Descriptive1 | 45 | 36 (80) | 2 (4.4) | 1 (2.25) | 3 (6.6) | 0 | 3 |
| Cho et al[24], 2007 | Japan | Cho1 | 60 | 382 (63.3) | 142 (23.3) | 72 (12) | 12 (2) | 02 | 0 |
| Sirvanci et al[25], 2007 | Turkey | Modified Huang1 | 62 | 43 (69.3) | 6 (9.7) | 9 | 3 | 0 | 1 |
| Song et al[26], 2007 | South Korea | Modified Huang1 | 111 | 67 (60.4) | 9 (8.1) | 22 (19.8) | 8 (7.2) | 2 (1.8) | 3 |
| Karakas et al[27], 2008 | Turkey | Karakas1 | 112 | 612 (55) | 162 (14) | 242 (21) | 112 (10) | 0 | 0 |
| De Filippo et al[28], 2008 | Italy | Descriptive1 | 350 | 2022 (57.7) | 282 (7.9) | 112 (3.1) | NS | NS | 109 |
| Kim et al[29], 2008 | South Korea | Modified Yoshida1 | 33 | 25 (75.8) | 1 (3) | 3 (9.1) | 0 | 1 (3) | |
| Sharma et al[30], 2008 | India | Couinaud1 | 253 | 134 (52.9) | 29 (11.5) | 46 (18.2) | 18 (7.1) | 1 (0.4) | 25 |
| Kashyap et al[31], 2008 | United States | Couinaud1 | 36 | 22 (61.1) | 4 (11.1) | 4 (11.1) | 3 (8.3) | 1 (2.8) | 2 |
| Cucchetti et al[32], 2011 | Italy | Choi1 | 200 | 129 (64.5) | 28 (14) | 24 (12) | 16 (8) | NS | 3 |
| Lyu et al[33], 2012 | Taiwan | Yoshida1 | 462 | 307 (65.8) | 42 (9.1) | 60 (13) | 41 (8.9) | 15 (3.2) | 0 |
| Tawab et al[34], 2012 | Egypt | Huang1 | 106 | 672 (63.2) | 112 (10.4) | 182 (17) | 82 (7.5) | 2 (1.9) | 0 |
| Thungsuppawattanakit and Arjhansiri[35], 2012 | Thailand | Couinaud1 | 163 | 106 (65) | 28 (17.2) | 15 (9.2) | 9 (5.5) | 0 | 5 (3.1) |
| Barsoum et al[36], 2013 | Egypt | Hakki1 | 50 | 282 (56) | 32 (6) | 152 (30) | 22 (4) | 02 | 0 |
| Mariolis-Sapsakos et al[37], 2012 | Greece | Couinaud1 | 73 | 482 (65.7) | 72 (9.59) | 112 (15.1) | 22 (2.74) | 12 (1.37) | 4 E |
| Uysal et al[38], 2014 | Turkey | Choi1 | 1011 | 803 (79.4) | 81 (8.01) | 42 (4.15) | 73 (7.23) | NS | 12 |
| Deka et al[39], 2014 | North India | Choi1 | 299 | 173 (57.8) | 242 (8) | 522 (17.4) | 202 (6.6) | 72 (2.3) | 23 |
| Al-Jiffry[40], 2015 | Saudi Arabia | Couinaud1 | 177 | 1042 (58.8) | 192 (10.7) | 72 (3.9) | 122 (6.8) | 22 (1.1) | 332 |
| Khanduja et al[41], 2016 | North India | Huang | 100 | 63 (63) | 18 (18) | 9 (9) | 8 (8) | 0 | 23 |
| Nayman et al[42], 2016 | Turkey | Yoshida1 | 2143 | 1329 (62) | 202 (95) | 245 (11) | 149 (7) | 1 (0.05) | 9 |
| Sarawagi et al[43], 2016 | North India | Karakas1 | 224 | 124 (55.3) | 26 (9.3) | 62 (27.6) | 9 (4) | 2 (0.8) | 0 |
| Adwan et al[44], 2016 | Jordan | Yoshida1 | 120 | 822 (68.4) | 102 (8.3) | 152 (12) | NS | NS | |
| Taghavi et al[45], 2017 | Iran | Huang | 362 | 163 (45) | 78 (21.5) | 48 (13.3) | 13 (3.6) | 0 | 60 (16.6) |
| Mazroa et al[46], 2017 | Egypt | Hakki1 | 50 | 242(48) | 42(8) | 152(30) | 72(14) | 02 | 0 |
| Adatepe et al[47], 2016 | Turkey | Choi1 | 1041 | 6162 (40.7) | 1332 (12.8) | 1262 (12.1) | 522 (4.99) | 02 | 114 |
| Abdelkareem et al[48], 2019 | Palestine | Modified Huang1 | 342 | 266 (77.8) | 29 (8.5) | 2 (0.6) | 1 (0.3) | 1 (0.3) | 43 |
| El Hariri et al[49], 2019 | Egypt | Modified Huang1 | 120 | 79 (65.8) | 14 (11.7) | 16 (13.3) | 9 (7.5) | 2 (1.67) | 0 |
| Medişoğlu et al[50], 2020 | Turkey | Huang | 79 | 29 (36.7) | 27 (34.2) | 16 (20.3) | 7 (8.9) | 02 | 0 |
| Global prevalence | Global | - | 17045 | 10668/17045 (62.6) | 1853/16087 (11.5) | 1903/16570 (11.5) | 1006/15617 (6.4) | 66/11361 (0.58) | |
| Present study | Caribbean | Huang | 152 | 109 (71.7) | 29 (19.1) | 7 (4.61) | 6 (3.95) | 0 | 1 |
| P value | 0.0227 | 0.0069 | 0.0047 | 0.2466 | 1.0 | - | |||
One published study did not report the number of A2 variants[8]. Therefore, data from this study were not included in the calculation of global prevalence of type A2 variants. In the remaining studies there were 1853 A2 variants in 16087 persons. There was a significantly greater prevalence of Huang A2 variants in our population (19.1% vs 11.5%; P = 0.0069).
After excluding one published study that did not specify the number of A3 variants[14], there were 1903 type A3 variants in 16570 persons. There were significantly less type A3 variants in our population (4.61% vs 11.5%; P = 0.0047).
The number of A4 variants were not reported and could not be reliably extrapolated from published descriptions and/or images in three publications[8,28,44]. Therefore, these studies were not included in the calculation of A3 global prevalence. The remaining studies documented 1006 type A4 variants in a total of 15617 persons. There was no statistical difference between the prevalence of type A4 variants in our population and the global prevalence (3.95% vs 6.4%; P = 0.2466).
The number of A4 variants were not reported and could not be reliably extrapolated from published descriptions and/or images in six publications[6,22,28,32,38,44]. In the remaining publications, there were 66 (0.58%) type A5 variants in a total of 11,361 persons. We did not encounter A5 variants in our population.
Although there are many techniques used to evaluate biliary anatomy, we agree that MRC is ideal[39,51,52] because it is non-invasive, does not require the administration of iodine-based contrast media and is associated with minimal patient-associated risk. Conventional T2-weighted MRC works on the concept that T2-weighted images demonstrate high signal intensity from fluid-containing structures, but it is limited in its ability to demonstrate small ducts and those not distended with bile[33,52].
Unfortunately, there is no standardized classification system to describe biliary anomalies. Numerous classification systems have been proposed and all are used in medical literature. These include classification systems described by Yoshida et al[7], Couinaud[5], Huang et al[3], Choi et al[11], Ohkubo et al[13], Karakas et al[27], Barsoum et al[36] and Champetier[53]. Each system has its individual merits. For example, some classifications[11] document the presence of accessory ducts, reportedly found in 2%[11,52] to 14%[39] of persons, while other systems do not include these data. As another example, many systems focus on biliary anatomy in the right-hemi liver[5,11,39,53] while others[3,7,13] also include detailed information on left-sided biliary anatomy.
Each system also has individual drawbacks. For example, the detailed classification proposed by Ohkubo et al[13]does not describe separate drainage from multiple segment IV ducts into LHD. Evaluating this from another perspective, most authors who proposed a classification system found anomalies that did not fit into their classifications: 1% by Choi et al[11], 2% by Khanduja et al[41], 3.3% by Couinaud[5], 11.1% by Karakas et al[27], 34% by Champetier[53]and 9.4% by Ohkubo et al[13]. In the general medical literature, the classification proposed by Huang et al[3] was the most commonly utilized system[3,8,10,16,25,26,34,41,45,48-50]. Therefore, we used the Huang classification to characterize variations encountered in our population.
All classification systems in use describe the “classic” anatomic pattern. This information is important when performing any operative or interventional radiologic procedures on the liver. This pattern is considered ideal for harvesting liver where a right or left lobe is required for living donor liver transplant[11]. This “classic” anatomic pattern was present in 71.7% of unselected persons in our population. In the general medical literature, the prevalence of the “classic” anatomic pattern ranged from 36.7%[50] to 80%[23]. Wietzke-Braun et al[21] reported type A1 variants in only 11% of their population. However, this was a small series of only 18 highly-selected individuals undergoing transplant evaluation. Therefore, we did not consider this outlier to be representative of A1 variants in the general population. The global prevalence of A1 anatomy was lower than encountered in our population (62.6% vs 71.7%; P = 0.0227).
In our population, 28.3% of unselected persons had variant intra-hepatic biliary anatomy. This compared well with published global data in which the prevalence of bile duct variants ranges from 20% in France[23] up to 60% in Spain[12]. Cucchetti et al[32] suggested that there was a relationship between gender and biliary anatomy, with significantly more women having biliary anomalies (45% vs 26%; P = 0.005), but we found no statistically significant relationship between the presence of anatomic variants and gender (27.6% vs 29.2%; P = 1.000) in our study. This was consistent with most other reports in the literature[3,5,26,33,39,52].
In our population, there are equal proportions of persons from the West African (40%) and North Indian (40%) diaspora as a result of the trans-Atlantic slave trade and indentured labour systems. Therefore, we sought to compare the prevalence of variants to studies from these geographic locations. There were no published studies reporting biliary variants in West African populations but there were four studies reporting 494 variants in 876 individuals from North Indian populations[30,39,41,43]. In our population there was a significantly lower incidence of all bile duct variants than that seen in Indian populations (28.3% vs 56.4%, P < 0.001), probably due to decades of population mixing in our setting.
The most common variant we encountered was a triple confluence (A2), occurring in 19.5% of unselected individuals. In the general medical literature, the prevalence of a trifurcation ranges from 0.8%[14] to 34.2%[50] and the calculated global prevalence was 11.5%. Therefore, in our population there was a significantly greater prevalence of A2 trifurcations (19.1% vs 11.5%; P = 0.0069). Interestingly, it was closest to the 18% prevalence reported by Khanduja et al[41] in a North Indian population and we previously noted that 40% of our population is from the North Indian diaspora. It is important for transplant surgeons to be aware that trifurcations are more common in persons of Caribbean extract. This has important implications for partial liver transplantation. For both, a formal right-left lobe split and a right lobe living donation, it would need a bi-ductal anastomosis in a recipient with higher chances of post-operative biliary complications. It is sometimes considered to be a relative contraindication for right lobe living donation[52].
The second most prevalent anomaly was a type-A3 variant that was present in 4.6% of our population. In the medical literature the prevalence of type 3 variations ranges from 0.6%[48] to 30%[5,36,46] and the global prevalence was calculated to be 11.5%. Type A3 variants are clinically significant for many reasons. The presence of this variant predisposes patients to inadvertent biliary tract injury in the donor[13]. However, this anomaly can be identified either during a pre-operative donor or intra-operative cholangiography during donor right hepatectomy. Patients with an unrecognized type-A3 variant who undergo a left hepatectomy may be at risk for significant post-operative bile leak from the transected RPSD if not properly secured in the liver remnant. This type of bile leak would remain unresolved despite an ERCP. Alternatively, the RPSD is at risk for ligation leading to biliary stasis, repeated infections and finally cirrhosis in the right posterior section.
In patients with type-3 anomaly, a Bismuth 3b hilar choangiocarcinoma is often misinterpreted as a type 4 Lesion since the right anterior and posterior sectoral ducts are deemed not to join. Such patients can then be incorrectly labelled as inoperable.
Finally, there may also be a theoretic relationship between a type-3 variant and hepatolithiasis[11]. Consider the prevailing theory that biliary stasis and secondary cholangitis may contribute to intra-hepatic lithiasis[11,54]. This is supported by the fact that intra-hepatic lithiasis is more common in the left liver because the LHD joins at a more acute angle than the RHD[54]. But the most acute angulation would be present in a person with a type-3 variation, where the RPSD joins the LHD[11]. Therefore, these patients are theoretically more likely to experience stasis and a greater incidence of intra-hepatic lithiasis[11].
The type A4 variant was present in 3.95% of our population. The prevalence of this variant in the general medical literature ranges from 0.3%[48] to 15.8%[9] and the calculated global prevalence was 6.4%. General surgeons should make an effort to identify A4 variants before performing laparoscopic cholecystectomy because the abnormal RPSD might be mistaken as the cystic duct[33], putting it at risk for inadvertent bile duct injury. Interventional radiologists should also attempt to identify A4 variants before percutaneous drainage procedures, because drain placement in the left duct system would not effectively drain the right posterior segment when this anatomy is present[11]. The issues with the right-left split and right lobe living donation are somewhat similar to the ones discussed for the type A3 variant. However, a type A4 is probably more favourable since the RASD is essentially inserted low into the CHD and can often be dissected out extra-hepatically and for a longer length, thereby making a bi-ductal recipient anastomosis comparatively easier.
We did not encounter any persons with type A5 variants in our population. This was not surprising as many authors published series without identifying A5 variants[3,5,8,10,14,16-19,21,23-25,27,35,36,41,45,46,50]. In the general medical literature, the frequency of A5 variants ranged from 0.05[42] to 9.3%[15] and we calculated the global prevalence of A5 variants to be 0.58%.
There was one person in our study with a quadrification that did not fit the Huang classification. Although uncommon, this variation has been reported before. Adatepe et al[47] reported a quadrification in 0.38% of 1,041 persons, which was similar to the prevalence in our population (0.65%). The clinical significance of this variation is probably similar to that of a Huang A2 variant. Most of the existing classification systems downplay the significance of the segment IV ducts due to the vagrant nature of its insertion, and in fact many of the classification systems do not mention the variable segment IV duct. Additionally, we believe that in many instances the segment IV duct is too small to be meaningfully represented on MRCs. Moreover, it has little bearing on resectability of a hilar cholangiocarcinoma, right lobe or a left lateral section living donor.
There were no accessory ducts in our study. The term accessory duct refers to extra bile ducts draining a single liver segment in addition to its normal drainage[33,55]. Accessory bile ducts are reported to occur in 2%[55] to 6%[11] of persons in the medical literature. Accessory ducts are important to transplant surgeons who would tailor harvesting techniques in the donor. They may also be inadvertently ligated at operation leading to the formation of biliary fistulae, biliary sepsis and biliary cirrhosis.
A limitation of this study was that the MRCs were done on a scanner with a 1.5 T magnet without the administration of gadolinium compounds. Although we did not identify aberrant or accessory ducts, we do appreciate that small accessory ducts and aberrant ducts may not have been detected because they were below the resolution of the protocol scan/equipment.
In this Eastern Caribbean population, MRC identified variant anatomy in 28.3% of unselected persons. There are significant differences in intra-hepatic biliary anatomy in this unselected Eastern Caribbean population compared to global statistics. Specifically, Caribbean persons have a greater incidence of Huang A2 trifurcations and a lower incidence of Huang A3 variants.
There have been many documented variations of the anatomy of the intra-hepatic bile ducts, but to the best of our knowledge there has been no report on bile duct variations in Caribbean populations. This information is important to optimize healthcare services for providers with interests in treating liver disorders.
This research sought to determine the bile duct variations in a Caribbean population. This will help to optimize hepatobiliary services in the region. We have also defined the global prevalence which will serve as a basis for further research in this field.
We sought to document the variations in bile duct anatomy using magnetic resonance cholangiography (MRC) at a major hepatobiliary referral centre in the Eastern Caribbean.
We evaluated MRC images from 152 consecutive patients over a two-year period and described biliary anatomy according to the Huang’s classification. A systematic review of all available published studies was performed. Raw data were extracted and used to calculate the global prevalence of each variant for comparisons to the variants in our population.
Classic anatomy was present in 71.7% of persons and 28.3% of persons had variant anatomy. The most common variant was Huang type 2 (19%), followed by type 3 (4.6%), type 4 (3.95%) and type 5 (0). One variant did not fit the Huang classification system. This Caribbean population had a significantly greater number of type 2 variants (19.1% vs 11.5%; P = 0.0069), but a significantly lower incidence of type 3 variants (4.61% vs 11.5%; P = 0.0047).
There are significant differences in biliary anatomy in this Caribbean population compared to global statistics. The new method this study proposes is to use the definition of global prevalence to compare anatomic variations.
Future research can focus on variations of extra-hepatic biliary anomalies using the global prevalence template.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Caribbean Chapter American Hepato-Pancreato-Biliary Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Trinidad and Tobago
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen W, Peng XC S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Cawich SO, Gardner MT, Barrow M, Barrow S, Thomas D, Ragoonanan V, Mahabir A, Ali R, Naraynsingh V. Inferior Hepatic Fissures: Anatomic Variants in Trinidad and Tobago. Cureus. 2020;12:e8369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Johnson PB, Cawich SO, Roberts P, Shah S, Gardner MT, Gordon-Strachan G, Pearce NW. Variants of hepatic arterial supply in a Caribbean population: a computed tomography based study. Clin Radiol. 2013;68:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc. 1996;28:1669-1670. [PubMed] |
| 4. | Cawich SO, Gardner MT, Shetty R, Pearce NW, Deshpande R, Naraynsingh V, Armstrong T. Human liver umbilical fissure variants: pons hepatis (ligamentum teres tunnel). Surg Radiol Anat 2021; 43: 795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Couinaud C. Le foie: etudes anatomiques et chirurgicales. Paris Masson Cie. 1957;13:530-532. |
| 6. | Puente SG, Bannura GC. Radiological anatomy of the biliary tract: variations and congenital abnormalities. World J Surg. 1983;7:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Yoshida J, Chijiiwa K, Yamaguchi K, Yokohata K, Tanaka M. Practical classification of the branching types of the biliary tree: an analysis of 1,094 consecutive direct cholangiograms. J Am Coll Surg. 1996;182:37-40. [PubMed] |
| 8. | Cheng YF, Huang TL, Chen CL, Chen YS, Lee TY. Variations of the intrahepatic bile ducts: application in living related liver transplantation and splitting liver transplantation. Clin Transplant. 1997;11:337-340. [PubMed] |
| 9. | Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, Ueda M, Kaihara S, Egawa H, Ozden I, Kobayashi N, Uemoto S. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002;73:1896-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Kitagawa Y, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K, Oda K, Ohta A, Jan YY, Cheng LP, Hwang TL, Chen MF. Intrahepatic segmental bile duct patterns in hepatolithiasis: a comparative cholangiographic study between Taiwan and Japan. J Hepatobiliary Pancreat Surg. 2003;10:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Choi JW, Kim TK, Kim KW, Kim AY, Kim PN, Ha HK, Lee MG. Anatomic variation in intrahepatic bile ducts: an analysis of intraoperative cholangiograms in 300 consecutive donors for living donor liver transplantation. Korean J Radiol. 2003;4:85-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Ayuso JR, Ayuso C, Bombuy E, De Juan C, Llovet JM, De Caralt TM, Sánchez M, Pagés M, Bruix J, García-Valdecasas JC. Preoperative evaluation of biliary anatomy in adult live liver donors with volumetric mangafodipir trisodium enhanced magnetic resonance cholangiography. Liver Transpl. 2004;10:1391-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ohkubo M, Nagino M, Kamiya J, Yuasa N, Oda K, Arai T, Nishio H, Nimura Y. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Düşünceli E, Erden A, Erden I. [Anatomic variations of the bile ducts: MRCP findings]. Tani Girisim Radyol. 2004;10:296-303. [PubMed] |
| 15. | Lee VS, Morgan GR, Lin JC, Nazzaro CA, Chang JS, Teperman LW, Krinsky GA. Liver transplant donor candidates: associations between vascular and biliary anatomic variants. Liver Transpl. 2004;10:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Limanond P, Raman SS, Ghobrial RM, Busuttil RW, Lu DS. The utility of MRCP in preoperative mapping of biliary anatomy in adult-to-adult living related liver transplant donors. J Magn Reson Imaging. 2004;19:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Wang ZJ, Yeh BM, Roberts JP, Breiman RS, Qayyum A, Coakley FV. Living donor candidates for right hepatic lobe transplantation: evaluation at CT cholangiography--initial experience. Radiology. 2005;235:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Chen JS, Yeh BM, Wang ZJ, Roberts JP, Breiman RS, Qayyum A, Coakley FV. Concordance of second-order portal venous and biliary tract anatomies on MDCT angiography and MDCT cholangiography. AJR Am J Roentgenol. 2005;184:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Macdonald DB, Haider MA, Khalili K, Kim TK, O'Malley M, Greig PD, Grant DR, Lockwood G, Cattral MS. Relationship between vascular and biliary anatomy in living liver donors. AJR Am J Roentgenol. 2005;185:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kim RD, Sakamoto S, Haider MA, Molinari M, Gallinger S, McGilvray ID, Greig PD, Grant DR, Cattral MS. Role of magnetic resonance cholangiography in assessing biliary anatomy in right lobe living donors. Transplantation. 2005;79:1417-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Wietzke-Braun P, Braun F, Muller D, Lorf T, Ringe B, Ramadori G. Adult-to-adult right lobe living donor liver transplantation: comparison of endoscopic retrograde cholangiography with standard T2-weighted magnetic resonance cholangiography for evaluation of donor biliary anatomy. World J Gastroenterol. 2006;12:5820-5825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kitami M, Takase K, Murakami G, Ko S, Tsuboi M, Saito H, Higano S, Nakajima Y, Takahashi S. Types and frequencies of biliary tract variations associated with a major portal venous anomaly: analysis with multi-detector row CT cholangiography. Radiology. 2006;238:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Vidal V, Hardwigsen J, Jacquier A, Le Corroller T, Gaubert JY, Moulin G, Bartoli JM, Petit P, Champsaur P. [Anatomic variants of the biliary tree with MR Cholangiography: feasibility and surgical applications]. J Chir (Paris). 2007;144:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Cho A, Asano T, Yamamoto H, Nagata M, Takiguchi N, Kainuma O, Soda H, Mori M, Narumoto S, Okazumi S, Makino H, Ochiai T, Ryu M. Relationship between right portal and biliary systems based on reclassification of the liver. Am J Surg. 2007;193:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Sirvanci M, Duran C, Ozturk E, Balci D, Dayangaç M, Onat L, Yüzer Y, Tokat Y, Killi R. The value of magnetic resonance cholangiography in the preoperative assessment of living liver donors. Clin Imaging. 2007;31:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Song GW, Lee SG, Hwang S, Sung GB, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY, Kim BS, Moon KM, Jung DH. Preoperative evaluation of biliary anatomy of donor in living donor liver transplantation by conventional nonenhanced magnetic resonance cholangiography. Transpl Int. 2007;20:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Karakas HM, Celik T, Alicioglu B. Bile duct anatomy of the Anatolian Caucasian population: Huang classification revisited. Surg Radiol Anat. 2008;30:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | De Filippo M, Calabrese M, Quinto S, Rastelli A, Bertellini A, Martora R, Sverzellati N, Corradi D, Vitale M, Crialesi G, Sarli L, Roncoroni L, Garlaschi G, Zompatori M. Congenital anomalies and variations of the bile and pancreatic ducts: magnetic resonance cholangiopancreatography findings, epidemiology and clinical significance. Radiol Med. 2008;113:841-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Kim SY, Byun JH, Hong HS, Choi EK, Lee SS, Park SH, Lee MG. Biliary tract depiction in living potential liver donors at 3.0-T magnetic resonance cholangiography. Invest Radiol. 2008;43:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Sharma V, Saraswat VA, Baijal SS, Choudhuri G. Anatomic variations in intrahepatic bile ducts in a north Indian population. J Gastroenterol Hepatol. 2008;23:e58-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Kashyap R, Bozorgzadeh A, Abt P, Tsoulfas G, Maloo M, Sharma R, Patel S, Dombroski D, Mantry P, Safadjou S, Jain A, Orloff M. Stratifying risk of biliary complications in adult living donor liver transplantation by magnetic resonance cholangiography. Transplantation. 2008;85:1569-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Cucchetti A, Peri E, Cescon M, Zanello M, Ercolani G, Zanfi C, Bertuzzo V, Di Gioia P, Pinna AD. Anatomic variations of intrahepatic bile ducts in a European series and meta-analysis of the literature. J Gastrointest Surg. 2011;15:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Lyu SY, Pan KT, Chu SY, hSu MY, Chen CM, Hung CF, Tseng JH. Common and Rare Variants of the Biliary Tree: Magnetic Resonance Cholangiographic Findings and Clinical Implications. J Radiol Sci. 2012;37:59-67. |
| 34. | Tawab MA, Tamer F, Ali T. Anatomic variations of intrahepatic bile ducts in the general adult Egyptian population: 3.0-T MR cholangiography and clinical importance. Egyptian J Radiol Nucl Med. 2012;43:111-117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Thungsuppawattanakit P, Arjhansiri K. Anatomic variants of intrahepatic bile ducts in Thais. Asian Biomed. 2012;6:51-57. |
| 36. | Barsoum NR, Samie AA, Adel L, Asaad RE. Role of MRCP in assessment of biliary variants in living donor liver transplantation. Egyptian J Radiol Nuclear Med. 2013;44:131-136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Mariolis-Sapsakos T, Kalles V, Papatheodorou K, Goutas N, Papapanagiotou I, Flessas I, Kaklamanos I, Arvanitis DL, Konstantinou E, Sgantzos MN. Anatomic variations of the right hepatic duct: results and surgical implications from a cadaveric study. Anat Res Int. 2012;2012:838179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Uysal F, Obuz F, Uçar A, Seçil M, Igci E, Dicle O. Anatomic variations of the intrahepatic bile ducts: analysis of magnetic resonance cholangiopancreatography in 1011 consecutive patients. Digestion. 2014;89:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
| 39. | Deka P, Islam M, Jindal D, Kumar N, Arora A, Negi SS. Analysis of biliary anatomy according to different classification systems. Indian J Gastroenterol. 2014;33:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Al-Jiffry B. Anatomic variations of intra- and extra- hepatic biliary system in the Kingdom of Saudi Arabia. Saudi J Health Sci. 2015;4:147-150. |
| 41. | Khanduja N, Chauhan RS, N, Jobta A, Sood RG, Kaundal AP, Chawla K, YogeSh Diwan Y. Anatomical Variations of Intrahepatic Bile Ducts on MRC in Himachal Pradesh, North India. Int J Anato Radiol Surg. 2016;5:21-24. |
| 42. | Nayman A, Özbek O, Erol S, Karakuş H, Kaya HE. Magnetic resonance cholangiopancreatography evaluation of intrahepatic bile duct variations with updated classification. Diagn Interv Radiol. 2016;22:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Sarawagi R, Sundar S, Raghuvanshi S, Gupta SK, Jayaraman G. Common and Uncommon Anatomical Variants of Intrahepatic Bile Ducts in Magnetic Resonance Cholangiopancreatography and its Clinical Implication. Pol J Radiol. 2016;81:250-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Adwan A, Shawaqfeh J, Banihani M. Biliary Tree Variants among Potential Living Liver Donors: Experience at King Hussein Medical Center. J Royal Med Service. 2016;23:64-67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Taghavi SA, Niknam R, Alavi SE, Ejtehadi F, Sivandzadeh GR, Eshraghian A. Anatomical Variations of the Biliary Tree Found with Endoscopic Retrograde Cholagiopancreatography in a Referral Center in Southern Iran. Middle East J Dig Dis. 2017;9:201-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Mazroa JA, Gaballa GM, SHEHA OA, Ouda MA. Preoperative MRCP Compared with Intraoperative Cholangiography in Assessment of Potential Right Lobe Living Liver Transplantation Donors. Semant Schola. 2017;199554626. |
| 47. | Adatepe M, Adibelli ZH, Esen OS, Imamogul C, Yildrim M, Erkan N. Anatomic Variations of Biliary Ducts: Magnetic Resonance Cholangiopancreatography Findings of 1041 Consecutive Patients. Eur Surg. 2016;48:296-303. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Abdelkareem H, Ali R, Jibrini M, Nazzal Z, Maree M, Hamaida J, Demyati K. A study of the anatomic variations of the pancreatico-biliary system in Palestine: a national study. Int Surg. 2019;6:1020-1028. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | El Hariri M, Riad MM. Intrahepatic Bile Duct Variation: MR cholangiography and implication in hepatobiliary surgery. Egyptian J Radiol Nuclear Med. 2019;50:362-363. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Medişoğlu MS, Tuncay C, Ahmet Y, Çam I, Mesut S, Baltrak Y. Investigation of Biliary Canal Variations as a Cause of Stone Formation in the Choledochal Canal. Trop Heal Med Res. 2020;2:77-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Hyodo T, Kumano S, Kushihata F, Okada M, Hirata M, Tsuda T, Takada Y, Mochizuki T, Murakami T. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Catalano OA, Singh AH, Uppot RN, Hahn PF, Ferrone CR, Sahani DV. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | Champetier J. Les voies biliaires. In: Chevrel JP. Anatomie Clinique, Le tronc. Paris: Springer, 1994: 416-417. |
| 54. | Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am J Gastroenterol. 1995;90:540-548. [PubMed] |
| 55. | Mortelé KJ, Ros PR. Anatomic variants of the biliary tree: MR cholangiographic findings and clinical applications. AJR Am J Roentgenol. 2001;177:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |