Published online Apr 16, 2021. doi: 10.4253/wjge.v13.i4.97
Peer-review started: January 6, 2021
First decision: February 11, 2021
Revised: February 19, 2021
Accepted: March 11, 2021
Article in press: March 11, 2021
Published online: April 16, 2021
Processing time: 95 Days and 23.3 Hours
With increasing volume and cost of gastrointestinal endoscopic procedures, the proper selection of patients for moderate sedation becomes increasingly relevant. The current literature lacks consistent findings that allow for appropriate selection of patients for moderate sedation.
To analyze a nationwide registry of patients to identify patient and procedural factors associated with lower sedation requirements for endoscopy.
The Clinical Outcomes Research Initiative National Endoscopic Database was queried to assess adult patients undergoing moderate sedation for esophagogastroduodenoscopy (EGD) and colonoscopy from 2008 to 2014. Patients were stratified into two groups [low dose (LD) and high dose sedation] based on sedation requirements. Anthropometric, procedural, and anesthesia data were compared, and multivariable analysis was performed to identify factors associated with LD sedation.
Of the 371102 patients included in the study, 63137 where stratified into the LD sedation group and 307965 were in the high dose group. Moderate sedation was managed primarily by endoscopists (50%) and anesthesia providers (47%). Patients undergoing EGDs and procedures performed in the inpatient setting, in ambulatory surgery centers, intensive care units or hospital wards, required less sedation than colonoscopies, outpatient procedures and procedures done in endoscopy suites, respectively (P < 0.0001 for all). On multivariable analysis, factors predictive of tolerance with lower sedation requirements for EGDs and colonoscopies were female gender, age ≥ 50, non-White race, Hispanic descent, body mass index ≤ 25 kg/m2, and higher American Society of Anesthesia Class (P < 0.0001 for all).
Clinicians should consider these patient profiles in determining which patients will better tolerate moderate sedation vs those better suited for alternative sedation methods.
Core Tip: Limited society guidelines currently exist to aid endoscopists in the selection of the most appropriate sedation method. Rather, it is at the discretion of the endoscopist on a case-by-case basis, with many decisions made based on gut feeling and previous personal experience. With the growing focus on patient satisfaction as a metric for reimbursement and an increased focus on healthcare cost containment initiatives, identifying which patients can safely and effectively undergo endoscopy without anesthesia-administered sedation is becoming exceedingly important. Existing studies on this topic to date have been small scale, single-center data with inconsistent findings. Robust data to drive practice patterns have been lacking. As such, we have capitalized upon nationwide data found in the Clinical Outcomes Research Initiative National Endoscopic Database to clarify these discrepancies and to identify patient and procedure characteristics that may predict better patient tolerance to endoscopy with moderate sedation.
- Citation: Passi M, Rahman F, Gurram S, Kumar S, Koh C. Identifying who best tolerates moderate sedation: Results from a national database of gastrointestinal endoscopic outcomes. World J Gastrointest Endosc 2021; 13(4): 97-110
- URL: https://www.wjgnet.com/1948-5190/full/v13/i4/97.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i4.97
Adequate sedation and analgesia are considered integral components of a good quality, endoscopic exam[1]. With the adoption of the patient-centered care model, there has been a rise in the use of procedural sedation where 98% of endoscopists in the United States routinely administer sedation during endoscopies[1,2]. The use of procedural sedation is primarily intended to reduce patient anxiety and discomfort, thereby improving tolerability and satisfaction for the procedure[1]. Sedation also provides the endoscopist with an ideal environment for a thorough exam allowing for improved outcomes. The importance of high-quality procedures, and the increasing patient awareness and expectation of a painless examination highlight the need for effective procedural sedation[3].
The use of moderate (conscious) sedation provides adequate control of pain and anxiety, a safety margin when compared with deep sedation and general anesthesia, and provides adequate anesthesia for the majority of routine endoscopies[4]. In the United States, more than 75% of endoscopists use a benzodiazepine plus narcotic regimen, with the combination of midazolam and either fentanyl or meperidine being the most common[2]. These drugs have a predictable pharmacokinetic profile, a rapid onset of action, analgesic and anxiolytic effects, a short recovery time, and minimal associated risks making them ideal for administration by a non-anesthesia provider[2]. While certain patient characteristics may help predict the dosage needed for adequate sedation, patients differ in their response to sedation and for any given sedative or analgesic, the range of individuals response to a specific drug can be up to 3-5 fold[4,5]. Thus, the ability to seek a balance between patient comfort and drug-related side effects is an art that comes with experience and requires careful consideration of the patient, the endoscopic facility, and the variabilities of the procedure itself[6].
The desire to identify the difficult-to-sedate patient both in terms of safety and patient satisfaction has been the subject of previous research efforts[7]. Certain characteristics that have been associated with higher levels of sedation include younger age, female gender, lower body mass index (BMI), chronic benzodiazepine or opioid use, higher income, higher education, and psychologic distress[5,7-9]. In 2018, the American Society of Gastrointestinal Endoscopy published updated guidelines for sedation in gastrointestinal (GI) endoscopy with acknowledgement that further investigation is needed for the selection of appropriate candidates for various types of sedation[5]. Clearly, certain patient populations require specific sedation strategies based on comorbid factors. Nonetheless, consensus is lacking, and thus the validity of existing studies is limited by inconsistent findings, small-scale, single-institution data, and use of non-standardized, post-procedure patient-administered surveys, introducing potential bias[10-12].
The National Endoscopic Database (NED) contains procedural data collected by the Clinical Outcomes Research Initiative (CORI) from 1995 to 2014. Using this nationwide database, we aimed to evaluate patient tolerance of endoscopy using current sedation practices with the goal of identifying patient and procedure characteristics that may predict better tolerance with moderate sedation.
We utilized the CORI database – a large national multi-center consortium of 108 sites from 87 practices, created for the means of studying outcomes and utilization of endoscopy in a variety of practice settings. The practice sites consist of 74% community practices, health maintenance organizations and private practices, 15% government agencies (e.g., military and Veterans Affairs Health Services), and 12% academic medical centers. Participating sites use a structured, computerized, report generator to process all endoscopic reports and comply with quality control requirements. Data are subsequently transmitted electronically to a central data repository – the National Endoscopic Database –which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
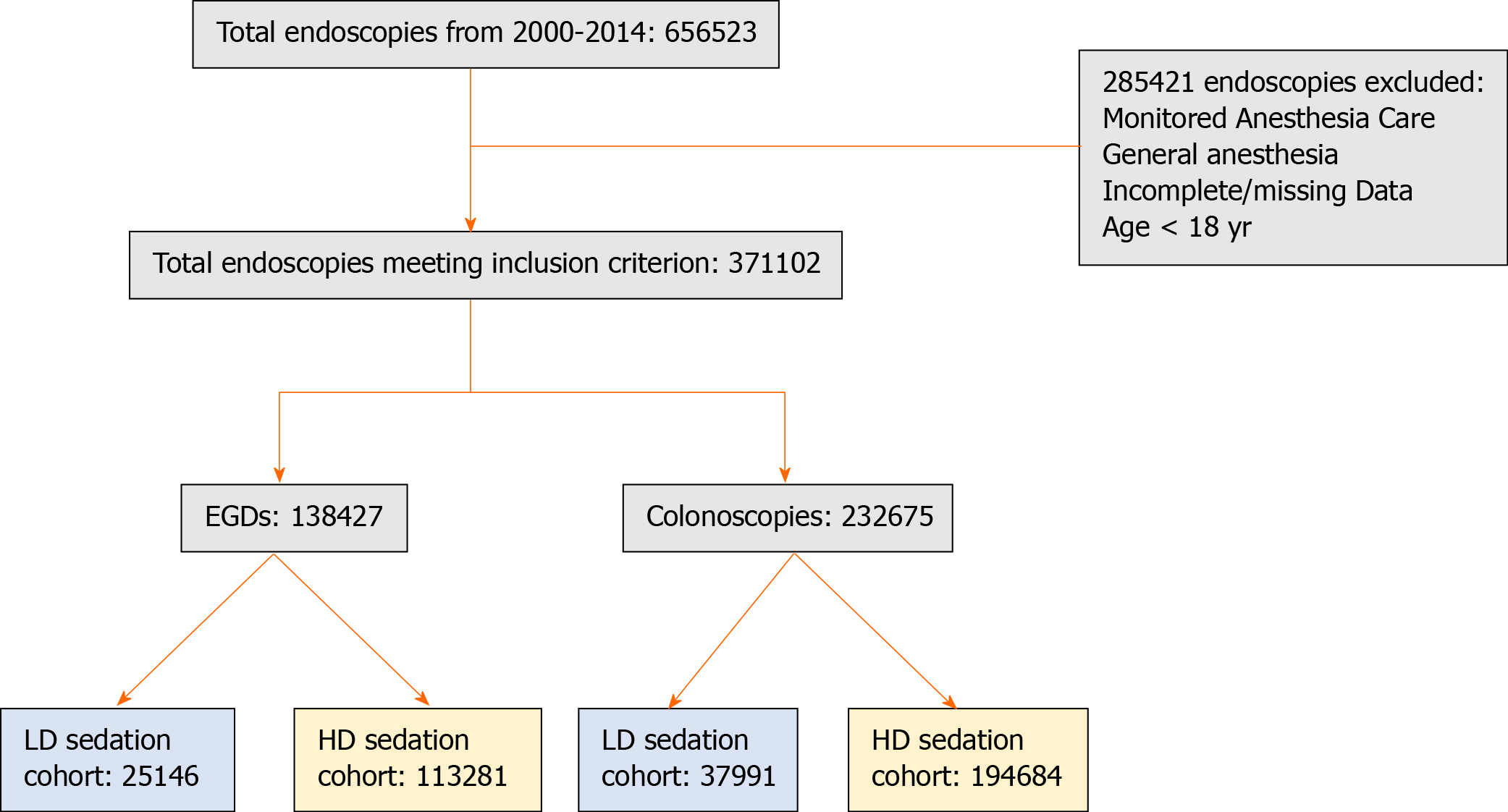
The CORI version 4 database was queried from 2008 to 2014 to identify all adult patients (≥ 18 years) undergoing moderate sedation for esophagogastroduodenoscopy (EGD) and colonoscopy. After separation into procedure type, patients were stratified into two groups based on sedation requirements: (1) Low dose sedation (LD) (fentanyl ≤ 50 µg or meperidine ≤ 50 mg and/or midazolam ≤ 2 mg), and (2) High dose sedation (HD) (fentanyl ≥ 200 µg or meperidine ≥ 150 mg and/or midazolam ≥ 6 mg and/or the requirement of diphenhydramine at any dose) (Figure 1). These sedation parameters where chosen because the recommended initial dose in the United States for endoscopic sedation for fentanyl is 50 µg, for meperidine is 50 mg and for midazolam is < 2 mg, and the maximum recommended dose for fentanyl is 200 µg, for meperidine is 150 mg and for midazolam is 6 mg. All patients who received any quantity of sedation outside the specified LD and HD sedation ranges (fentanyl > 50 µg to < 200 mg, meperidine > 50 mg to < 150 mg and midazolam > 2 mg to < 6 mg) were excluded from the study. Diphenhydramine is a well-established potentiator of benzodiazepine-narcotic regimens, leading to deeper levels of sedation and decreased pain with minimal hemodynamic side effects in patients undergoing GI endoscopy[2,13-15]. Current American Society of Gastrointestinal Endoscopy guidelines provide a strong recommendation for the use of diphenhydramine as an option in patients who are not adequately sedated with a benzodiazepine and opioid combination for GI endoscopy[16]. As such, patients who received diphenhydramine were considered to fall in the HD sedation group. Patients who received deep sedation or general anesthesia, as recorded in the CORI database, were excluded. In addition, patients < 18 years old and those with incomplete demographic and procedure related data were excluded.
Following stratification of patients into groups based on sedation requirements and procedure type, anthropometric, procedural and anesthesia data were compared utilizing a unique procedure identification. Specific data collected on these patients were: Age, sex, type of procedure, American Society of Anesthesia Class (ASA) class, BMI, race, admission status, endoscopy facility type, procedure duration, personnel administering sedation and type/does of conscious sedation administered. Further data on number of aborted procedures and unexpected intubations were recorded. Finally, patient tolerance during endoscopy as perceived by the endoscopist was captured and recorded as one of four categories: “excellent,” “good,” “fair,” and “poor”. These demographic and procedure-related variables were selected based on the findings from prior studies suggesting these factors may influence sedation requirements.
Although some of the patients included had more than one procedure performed during the study period, quantities observed in different procedures were assumed to constitute statistically independent observations for the purposes of data analysis. Summary statistics of baseline data are presented as either frequencies for categorical data or as means and standard deviations for continuous data, unless otherwise specified. The Student’s t-test or the chi-squared test, employing Yates’ correction for continuity where appropriate, were performed to understand differences in baseline labs between the LD and HD sedation groups. Univariate logistic regression analysis was performed to calculate an unadjusted odds ratio for factors related to lower sedation requirements. Adjusted odds ratios (aOR) were calculated using multivariate logistic regression. Additional multivariate analyses were done by procedure type (EGD vs colonoscopy) to understand factors related to tolerability by procedure. Demographic and procedure related variables that were statistically significant on univariate logistic regression were selected for multivariable analysis. All analysis was done in SAS 9.4. Statistical significance was set at P < 0.05. Only complete-case analysis was performed to account for missing values in CORI, as missing values were assumed to be missing at random. Additionally, it is recognized that there was multiple testing of outcome data arising from individual procedures. The multivariable linear regression analyses of factors associated with lower sedation (by type of procedure and overall) are offered as the main, definitive results and for which it is noted that correction for multiple testing by Bonferroni's method would not have removed statistical significance from any finding. The P values for all other statistical tests relating to outcomes should be considered preliminary and exploratory or else secondary; those P values are not corrected for multiple testing and are to be taken as descriptive only.
Clinical characteristics: During the study period, 656523 procedures were recorded and 371102 (56.5%) met criteria for inclusion. Upon further stratification by procedure type, colonoscopies comprised the majority of cases (63%, n = 232675) as compared to EGDs (37%, n = 138427) (Figure 1). Amongst the entire group, patients were mostly male (52%), non-Hispanic Whites (84%) with a mean age of 55 ± 18 years and ASA class I or II (88%). The mean BMI amongst the entire group was 28.2 ± 6.3 kg/m2. Demographic and clinical characteristics of these patients are shown in Table 1. In the LD group, the majority of patients were female (50.1%) whereas in the HD group, the majority were male (52.7%). Among both groups, patients were predominantly non-Hispanic Whites (64.1% in the LD group, 74.8% in the HD group), ≥ 50 years old (85.1% in the LD group, 79.2% in the HD group), and of ASA class I or II (82.4% in the LD group, 88.6% in the HD group).
| Variable | LD EGD (n = 25146), n (%) | HD EGD (n = 113281), n (%) | P value | LD colonoscopy (n = 37991), n (%) | HD colonoscopy (n = 194684), n (%) | P value |
| Age, mean ± SD | 51.9 ± 21.9 | 58.2 ± 19.2 | < 0.0001 | 56.8 ± 14.5 | 59.7 ± 22.6 | < 0.0001 |
| Female gender | 13095 (52.1) | 55617 (49.1) | < 0.0001 | 18548 (48.8) | 89973 (46.2) | < 0.0001 |
| Hispanic | 6091 (24.2) | 20479 (18.1) | < 0.0001 | 9052 (23.8) | 22533 (11.6) | < 0.0001 |
| White | 19704 (78.4) | 91529 (80.8) | < 0.0001 | 30643 (80.7) | 168539 (86.6) | < 0.0001 |
| Black | 2084 (8.3) | 6427 (5.7) | < 0.0001 | 2262 (6.0) | 8605 (4.4) | < 0.0001 |
| Asian | 1284 (5.1) | 3985 (3.5) | < 0.0001 | 2213 (5.8) | 5484 (2.8) | < 0.0001 |
| Other race | 2025 (8.1) | 9697 (8.6) | < 0.0001 | 3220 (8.5) | 10100 (5.2) | < 0.0001 |
| BMI (kg/m2), mean ± SD | 27.8 ± 15.2 | 28.2 ± 19.8 | < 0.0001 | 28.3 ± 19.9 | 28.2 ± 17 | < 0.0001 |
| Exam duration (min), mean ± SD | 9 ± 12 | 10 ± 8 | < 0.0001 | 20 ± 24 | 23 ± 23 | < 0.0001 |
| Medication dosage, mean ± IQR | ||||||
| Fentanyl (mcg) | 45 ± 8.3 | 205.6 ± 11.1 | 47.6 ± 9 | 255.9 ± 15.3 | ||
| Meperidine (µg) | 33.9 ± 7.2 | 170.3 ± 10.4 | 45.7 ± 12.3 | 152.8 ± 11.6 | ||
| Midazolam (mg) | 1.3 ± 9.4 | 6.3 ± 19.7 | 1.8 ± 17.4 | 8.8 ± 21.2 | ||
| Diphenhydramine (mg) | N/A | 25.2 ± 13.5 | N/A | 50.3 ± 11.5 | ||
| Personnel managing sedation, n (%) | ||||||
| Endoscopist | 11522 (45.8) | 63187 (55.8) | < 0.0001 | 15608 (41.1) | 96376 (49.5) | < 0.0001 |
| Anesthesiologist | 13572 (54.0) | 49656 (43.8) | < 0.0001 | 21288 (56.0) | 89120 (45.8) | < 0.0001 |
| Other | 9042 (36.0) | 216 (0.19) | < 0.0001 | 1095 (2.9) | 46 (0.02) | < 0.0001 |
| Unplanned intubations, n (%) | 132 (0.5) | 239 (0.2) | < 0.0001 | 5 (0.01) | 19 (0.01) | < 0.0001 |
| Aborted procedures, n (%) | 5791 (23.0) | 1371 (1.2) | < 0.0001 | 1602 (4.2) | 341 (0.2) | < 0.0001 |
| Admission status, n (%) | ||||||
| Inpatient | 4936 (19.6) | 12300 (10.9) | < 0.0001 | 1642 (4.3) | 4894 (2.5) | < 0.0001 |
| Outpatient | 20204 (80.3) | 100756 (88.9) | < 0.0001 | 36349 (95.7) | 189631 (97.4) | < 0.0001 |
| Location of procedure, n (%) | ||||||
| Ambulatory surgical center | 14469 (57.5) | 70643 (62.4) | < 0.0001 | 24967 (65.7%) | 137399 (70.6) | < 0.0001 |
| Endoscopy suite | 9790 (38.9) | 40263 (35.5) | < 0.0001 | 12735 (33.5) | 56352 (28.9) | < 0.0001 |
| Hospital ward | 150 (0.6) | 205 (0.2) | < 0.0001 | 24 (0.06) | 72 (0.04) | < 0.0001 |
| ICU | 668 (2.7) | 1678 (1.5) | < 0.0001 | 114 (0.3) | 205 (0.1) | < 0.0001 |
| Operating room | 16 (0.1) | 66 (0.06) | < 0.0001 | 4 (0.01) | 18 (0.01) | < 0.0001 |
| Other1 | 479 (1.9) | 201 (0.2) | < 0.0001 | 147 (0.4) | 47 (0.02) | < 0.0001 |
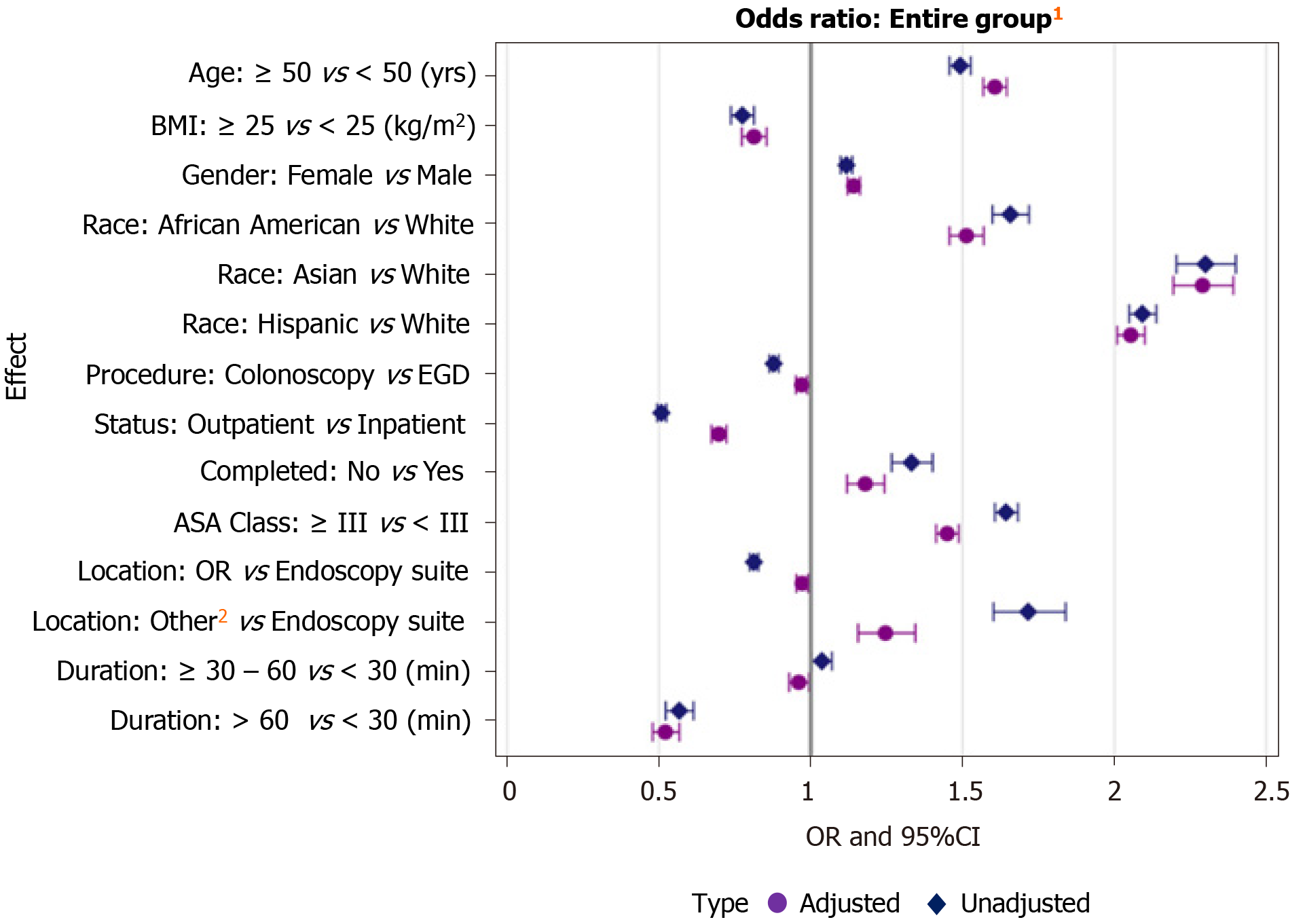
Among patient characteristics, female gender was a significant predictor of lower sedation requirements [aOR: 1.14, 95% confidence interval (CI): 1.12-1.16, P < 0.0001]. Additionally, older age was a predictor of lower sedation requirements when stratifying patients into ages ≥ 50 and < 50 (aOR: 1.61, 95%CI: 1.57-1.65, P < 0.0001). The adjusted odds ratios for low dose vs high dose sedation by decade of age for the entire study population can be found in Supplementary Table 1. Compared to Whites, African American patients had lower sedation requirements (aOR: 1.51, 95%CI: 1.46-1.57) as did Asians (aOR: 2.29, 95%CI: 2.19-2.39) and Hispanics (aOR: 2.06, 95%CI: 2.01-2.10) (P < 0.0001 for all). ASA class was also evaluated as a potential predictor of sedation requirements by comparing patients with an ASA class < III and ≥ III. Higher ASA class (≥ III) was predictive of less sedation requirements for both EGDs and colonoscopies as compared to lower ASA class (< III) (aOR: 1.45, 95%CI: 1.41-1.49, P < 0.0001). The adjusted odds ratios for low dose vs high dose sedation among each ASA class of patients can be found in Supplementary Table 2. Finally, BMI was evaluated in comparing overweight (BMI ≥ 25 kg/m2) vs normal/underweight (BMI < 25 kg/m2) patients; normal/underweight BMI was a significant predictor of lower sedation requirements (aOR: 0.81, 95%CI: 0.77-0.86, P < 0.0001) (Table 2, Figure 2).
| Characteristic | Adjusted OR1 (95%CI) | P value |
| Age ≥ 50 vs < 50 (yr) | 1.61 (1.57-1.65) | < 0.0001 |
| BMI ≥ 25 vs < 25 (kg/m2) | 0.81 (0.77-0.86) | < 0.0001 |
| Females (vs males) | 1.14 (1.12-1.16) | < 0.0001 |
| African Americans vs Whites | 1.51 (1.46-1.57) | < 0.0001 |
| Asians vs Whites | 2.29 (2.19-2.39) | < 0.0001 |
| Hispanics vs Whites | 2.06 (2.01-2.10) | < 0.0001 |
| Colonoscopy vs EGD | 0.97 (0.95-0.99) | 0.001 |
| Outpatient vs inpatient | 0.70 (0.67-0.72) | < 0.0001 |
| Completed: No vs yes | 1.18 (1.12-1.24) | < 0.0001 |
| ASA ≥ III vs ASA < III | 1.45 (1.41-1.49) | < 0.0001 |
| Location: OR vs endoscopy suite | 0.97 (0.95-0.99) | 0.005 |
| Location: Other site2 vs endoscopy suite | 1.25 (1.16-1.34) | < 0.0001 |
| Duration: ≥ 30-60 vs < 30 (min) | 0.96 (0.93-0.99) | 0.02 |
| Duration: > 60 vs < 30 (min) | 0.52 (0.48-0.57) | < 0.0001 |
Procedure related outcomes: For all procedures, moderate sedation was managed predominantly by endoscopists (50%) and anesthesia providers (47%). Within the LD group, sedation was primarily performed by anesthesia providers (48.3% vs 37.6% by endoscopists) compared to the HD sedation group in which sedation was more often managed by endoscopists than by anesthesia providers (53.3% vs 46.4%). The average sedation medication doses for EGDs and colonoscopies among patients in the LD and HD sedation groups are listed in Table 1.
The majority of patients in both the LD and HD groups had endoscopies performed as an outpatient (89.5% and 94.3%, respectively). Similarly, among both groups, cases were more commonly performed in ambulatory surgery centers (62.4% in the LD group, 67.6% in the HD group) followed by the endoscopy suite (35.7% in LD group, 31.4% in HD group). Average procedure duration for patients in the HD group was 2.1 min longer (18.6 ± 20.3 min) as compared to the LD group (mean of 16.5 ± 21.6 min). Unplanned intubations were uncommon among both groups (0.2% incidence in the LD group, 0.08% in the HD groups). Similarly, while the rate of aborted procedures was quite low among both groups, procedures were unexpectedly terminated about four times more often in the LD group (7393 cases) as compared to the HD group (1712 cases) (Table 1).
Admission status was assessed by comparing endoscopies performed as an inpatient vs those done as an elective, outpatient procedure. Inpatient procedures required significantly less sedation for both EGDs and colonoscopies as compared to those patients who had endoscopies performed on an outpatient basis (aOR: 0.70, 95%CI: 0.67-0.72, P < 0.0001). Additionally, location of procedure was evaluated by comparing cases performed in endoscopy suites vs those performed in ambulatory surgery centers, intensive care units (ICUs) and hospital wards. Procedures performed at sites other than the endoscopy suite required significantly less sedation as compared to those performed in endoscopy suites (aOR: 1.25, 95%CI: 1.16-1.34, P < 0.0001). Conversely, procedures performed in the endoscopy suite required less sedation as compared to those performed in the operating room (OR) (aOR: 0.97, 95%CI: 0.95-0.99, P = 0.005). Procedures that were aborted before completion required significantly less sedation as compared to those that were completed (aOR: 1.18, 95%CI: 1.12- 1.24, P < 0.0001). Procedure duration was assessed by comparing procedures less than 30 min (< 30 min) long, procedures 30 to 60 min (≥ 30–60 min) long, and procedure longer than 60 min (> 60 min). Significantly less sedation was required for all procedures < 30 min long as compared to both those ≥ 30-60 min and those > 60 min long (aOR: 0.96, 95%CI: 0.93-0.99, P = 0.02 and aOR: 0.52, 95%CI: 0.48-0.57, P < 0.0001, respectively) (Table 2, Figure 2).
Regarding patient tolerance as perceived by the endoscopist, patients were deemed to have “good” tolerance the majority of the time (65.9% in the LD sedation group, 60.9% in the HD group). On the other hand, patients in the HD group were 12.6% more likely to be “poorly tolerant” per endoscopist report compared to patients in the LD group.
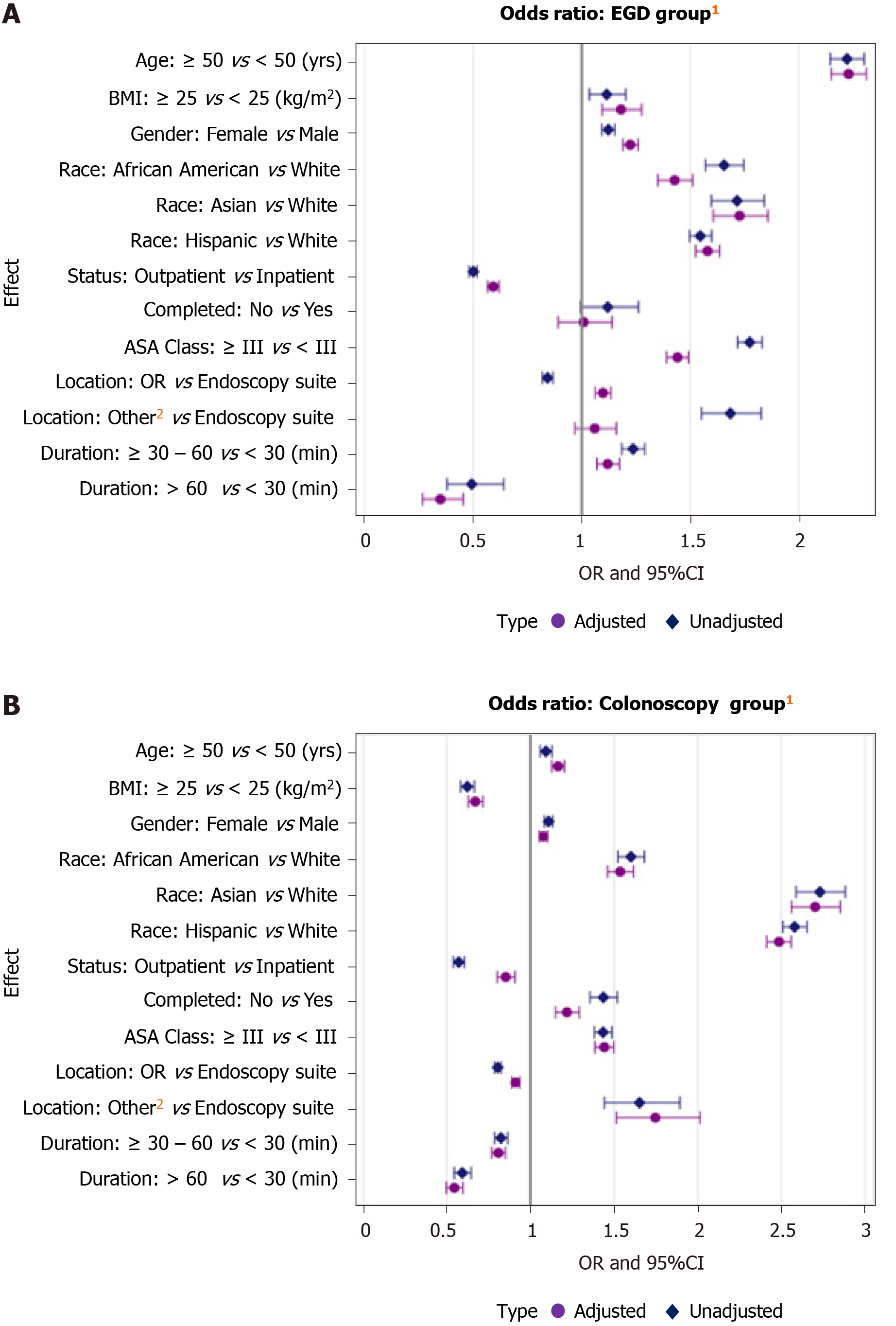
Clinical characteristics: Analyzing the data by procedure type provided additional insight into specific factors that affect tolerance for different procedures as shown in Table 3. When stratifying by procedure type, older patients (≥ 50 years old) were more likely to require less sedation compared to younger patients (< 50) for both EGDs (aOR: 2.23, 95%CI: 2.15-2.31) and colonoscopies (aOR: 1.16, 95%CI: 1.13-1.20) (P < 0.0001 for both). Female gender was also predictive of lower sedation requirements as compared to males (EGD: aOR: 1.23, 95%CI: 1.19-1.26; colonoscopy: aOR: 1.08, 95%CI: 1.05-1.10; P < 0.0001 for both). African American, Asian, and Hispanic races all had higher odds of requiring less sedation as compared to Whites for both EGDs and colonoscopies (P < 0.0001 for all). While a higher BMI (≥ 25 kg/m2) was predictive of lower sedation requirement for EGDs (aOR: 1.18, 95%CI: 1.09-1.28), a lower BMI (BMI < 25 kg/m2) was a significant predictor of lower sedation requirements for colonoscopies (aOR: 0.67, 95%CI: 0.63-0.72) (P < 0.0001 for both). Interestingly, a higher ASA class (≥ III) was predictive of requiring less sedation for both EGDs (aOR: 1.44, 95%CI: 1.39-1.49) and colonoscopies (aOR: 1.44, 95%CI: 1.39-1.50) (P < 0.0001 for both) as compared to a lower ASA class (ASA I and II) (Table 3, Figure 3).
| Characteristic | EGD group adjusted OR1 (95%CI) | P value | Colonoscopy group adjusted OR1 (95%CI) | P value |
| Age ≥ 50 vs < 50 (yr) | 2.23 (2.15-2.31) | < 0.0001 | 1.16 (1.13-1.20) | < 0.0001 |
| BMI ≥ 25 vs < 25 (kg/m2) | 1.18(1.09-1.28) | < 0.0001 | 0.67 (0.63-0.72) | < 0.0001 |
| Females (vs males) | 1.23 (1.19-1.26) | < 0.0001 | 1.08 (1.05-1.10) | < 0.0001 |
| African Americans vs Whites | 1.43 (1.35-1.51) | < 0.0001 | 1.54 (1.46-1.62) | < 0.0001 |
| Asians vs Whites | 1.73 (1.61-1.86) | < 0.0001 | 2.70 (2.56-2.85) | < 0.0001 |
| Hispanics vs Whites | 1.58 (1.53-1.64) | < 0.0001 | 2.49 (2.42-2.56) | < 0.0001 |
| Outpatient vs inpatient | 0.59 (0.57-0.62) | < 0.0001 | 0.85 (0.80-0.91) | < 0.0001 |
| Completed: No vs yes | 1.01 (0.89-1.14) | 0.89 | 1.22 (1.15-1.29) | < 0.0001 |
| ASA ≥ III vs ASA < III | 1.44 (1.39-1.49) | < 0.0001 | 1.44 (1.39-1.50) | < 0.0001 |
| Location: OR vs endoscopy suite | 1.10 (1.06-1.14) | < 0.0001 | 0.91 (0.89-0.94) | < 0.0001 |
| Location: Other site2 vs endoscopy suite | 1.06 (0.97-1.16) | 0.20 | 1.75 (1.51-2.01) | < 0.0001 |
| Duration: ≥ 30-60 vs < 30 (min) | 1.12 (1.07-1.17) | < 0.0001 | 0.81 (0.77-0.85) | < 0.0001 |
| Duration: > 60 vs < 30 (min) | 0.35 (0.27-0.45) | < 0.0001 | 0.55 (0.50-0.60) | < 0.0001 |
Procedure related outcomes: Inpatients status was more predictive of lower sedation requirements among patients undergoing EGDs (aOR: 0.59, 95%CI: 0.57-0.62) and colonoscopies (aOR: 0.85, 95%CI: 0.80-0.91) (P < 0.0001 for both). Colonoscopies performed at sites outside the endoscopy suite (i.e. in ambulatory surgery center, ICUs, and hospital wards) had a significantly higher odds of requiring less sedation as compared to procedures done in the endoscopy suite (aOR: 1.75, 95%CI: 1.51-2.01, P < 0.0001). On the other hand, for EGDs, there was no significant difference in sedation requirements for procedures performed at sites outside the endoscopy suite as compared to those performed in the endoscopy suite (P = 0.20). On the contrary, while colonoscopies performed in the endoscopy suite was predictive of lower sedation requirements as compared to those performed in the OR (aOR: 0.91, 95%CI: 0.89-0.94), the inverse was true for EGDs (i.e. those performed in the OR were predictive of lower sedation requirements compared to the endoscopy suite) (aOR: 1.10, 95%CI: 1.06-1.14), (P < 0.0001 for both). While for colonoscopies, procedures that were aborted prior to completion required significantly less sedation as compared to those that were completed (aOR: 1.01, 95%CI: 0.89-1.14, P < 0.0001), there was no significant difference in sedation requirements for EGDs that were terminated early vs completed (P = 0.89). For colonoscopies, significantly less sedation was required for all procedures < 30 min long as compared to both those ≥ 30-60 min and those > 60 min long (aOR: 0.81, 95%CI: 0.77-0.85 and aOR: 0.55, 95%CI: 0.50-0.60, P < 0.0001 for both). On the other hand, for EGDs, while procedures < 30 min long were predictive of lower sedation requirements as compared to those > 60 min long (aOR: 0.35, 95%CI: 0.27-0.45, P < 0.0001), EGDs that were ≥ 30-60 min long were predictive of lower sedation requirements as compared to those < 30 min (aOR: 1.12, 95%CI: 1.07-1.17, P < 0.0001) (Table 3; Figure 3).
In this large, multi-center study evaluating a nationwide group spanning academic, government-based, and community practice experiences, we compared two groups of patients stratified by sedation needs, to discern factors associated with lower sedation requirements using moderate sedation. We found that female gender, older age, non-White race, Hispanic descent, higher ASA class, procedures performed as inpatient status and those done at locations other than the endoscopy suites (i.e., ICUs, ambulatory surgery centers, hospital wards), were identified as factors associated with lower sedation requirements for completion. These factors were predictive on entire group analysis and remained predictive upon subgroup analysis when assessing EGD and colonoscopy groups separately, with the exception of BMI. Not only does this study add to the current body of literature, but it also provides definitive evidence informed by nationwide, multi-institutional data to illustrate the profile of the prototypical patient most likely to tolerate endoscopy under moderate sedation vs those better suited for an alternative sedation method. Our results should serve as a clinical guide to better inform the appropriate sedation practice utilized during GI endoscopy.
ASA class I and II patients undergoing routine endoscopy are generally deemed suitable for moderate sedation[5]. In low to average risk patients undergoing standard endoscopy, sedation administered by an endoscopist has previously been shown to be safe and offers patient satisfaction comparable with sedation administered by an anesthesia provider[17]. Alternatively, we found that higher ASA class and older age patients have lower sedation requirements. The pharmacokinetics of midazolam, the most widely used sedative in the United States, are influenced by patient age and renal and hepatic clearance, which affect the availability and functioning of cytochrome enzymes responsible for its metabolism[2,18,19]. This may explain the lower sedation requirements among patients of older age and higher ASA class, a surrogate for the presence of comorbidities, as compared to their younger, healthier counterparts. Moreover, with regards to colonoscopy, younger patients often have tighter mesentery tissues, as opposed to elderly patients whose mesenteries are more elastic and therefore, easier to navigate for the endoscopist. As such, older patients are likely more tolerable of colonoscopy with less sedation requirements. Another explanation could be that extra caution was exercised and less sedation was administered to patients of higher ASA class due to concern for the risk of sedation-related adverse events.
In our study, African Americans, Asians, and patients of Hispanic ethnicity uniformly had lower sedation requirements as compared to Whites. Previous studies have demonstrated conflicting data with regards to the role of race on pain perception[20,21]. This is largely attributable to the complex interplay among various factors including social and cultural beliefs, expressiveness towards pain, psychological factors, as well as biological factors such as genetics and alterations in the endogenous pain control systems, implicated in pain and tolerance to discomfort[22]. This remains an interesting area for further study on how race affect a patient’s perception of the endoscopic experience.
Contrary to other studies, our findings suggest that females have lower sedation requirements as compared to males[7,11,23]. Alternatively, one prospective cohort study found that gender has no impact on sedation requirements during endoscopy[12]. Younger females tend to have longer colons with decreased mobility due to higher organ burden in the abdominopelvic cavity and acute bends in the sigmoid colon[24,25]. This can make colonoscopies in this patient demographic challenging for the endoscopist, creating potential patient discomfort and translating into higher sedation requirements. In our study, however, younger females (i.e., females < 40 years old) were grossly under-represented, comprising only 10% of all female patients included in our study as compared to females between the ages of 50 and 69, which comprised > 50% of our entire female study population (Supplementary Table 1). As such, our findings may be more reflective of the older, female population. Nonetheless, it is worth noting that compared to prior studies, our study had significantly more patients across all age subgroups, and thus, our findings are likely more generalizable[7,11]. Additionally, the inconsistent findings in sedation requirements with regards to BMI among patients undergoing EGD and colonoscopy in our study is unclear. Recent data on midazolam implies that while the peripheral volume of distribution increases with higher BMI, the clearance of the drug with CYP3A is unaffected with higher BMI, challenging the notion that midazolam clearance is influenced by weight[24]. This may help to explain the variable findings regarding BMI in our colonoscopy and EGD groups. Nonetheless, while the effects of benzodiazepine agents are better studied, there remains a paucity of data with regards to the effects of patient demographics on opioid response in the procedure setting, which could be an interesting avenue for further research.
Our study is not without limitations. The CORI database is a clinical database, not an analytical data set, and is subject to human error and misclassification biases. In addition, the database has missing information, thus possibly introducing an inadvertent selection bias. We stratified our study population into two groups with opposite experiences in regard to sedation requirements to help emphasize demographic and procedural factors predictive of procedural sedation needs; however, we acknowledge that there are some patients who may fall into a “gray zone” with moderate sedation requirements. Furthermore, in this study, we assumed that amount of sedation administered was titrated to patient comfort; however, we recognize that practices may differ in their determination of what constitutes a suitable sedation level. This may help to explain our finding of higher ASA class patients “requiring” less sedation; in reality, less sedation may have been given as a result of the comfort level of the personnel administering the sedation. We would also like to recognize the subjective nature of “patient tolerance” during endoscopy as perceived by the endoscopist; since this study includes multi-center data input from different endoscopists, without a means for standardizing this data point, the patient “tolerance” parameter is subject to induce significant heterogeneity. Additionally, due to incomplete data in the CORI database, we could not account for procedural indications; had we done so, we likely would have identified a difference in tolerance of moderate sedation between procedures performed for screening or surveillance purposes and those performed for diagnostic and therapeutic purposes. Finally, due to limitations with the available data in the CORI repository, this study did not reflect upon the endoscopist’s experience and its effect on sedation tolerance. It is conceivable that an experienced endoscopist may have a significant effect on patient comfort and tolerance, and this is a potential area for future investigation.
In conclusion, younger age, low/normal BMI, female sex, African American and Asian race, Hispanic ethnicity, and higher ASA class were shown to be significant predictors of lower sedation requirements and, thereby, improved tolerance to moderate sedation. This is substantive data to guide sedation practices during GI endoscopy, a source of debate in recent years. The utilization of monitored anesthesia care for endoscopy has been steadily rising. Given the high volume of GI endoscopies, payment for anesthesia services which accounts for 40% of the total overhead cost of an endoscopic exam, could be substantial. The use of anesthesiologist administered sedation for otherwise healthy, low risk patients undergoing routine endoscopy, has no proven benefit with respect to patient safety, satisfaction, and procedure efficacy. Thus, identifying those patients suitable for moderate sedation for GI endoscopy becomes even more critical to decrease discretionary spending and overutilization of anesthesia resources. Mitigation strategies to reduce aerosolized airborne pathogen exposure in the endoscopy suite has come to the forefront of endoscopic practice over the recent months; therefore, it has become increasingly important to identify those patients who would benefit from conscious sedation vs those requiring higher levels of sedation and possible intubation. This study utilizes a nationwide registry, and to our knowledge it is the largest study examining the potential factors predictive of lower sedation requirements for endoscopy with moderate sedation. These findings are novel and increase our understanding of how patients should be assessed prior to undergoing sedation for routine endoscopic procedures.
Moderate (conscious) sedation administered by endoscopists provides adequate sedation and analgesia for the majority of American Society of Anesthesia (ASA) class I and II patients undergoing routine gastrointestinal (GI) endoscopy. Deep sedation and general anesthesia are traditionally reserved for patients at higher risk for sedation-related adverse events.
Currently, there are limited society guidelines and insufficient data to aid endoscopists in the selection of the most appropriate sedation method. Rather, this decision is often based on the endoscopist's personal discretion and prior experience.
The study’s main objective was to identify patient and procedure characteristics that may predict better tolerance with moderate sedation for routine GI endoscopy.
This was a retrospective cohort study utilizing a nationwide, multi-center repository of endoscopic outcomes. Sedation dose requirements for all adult patients undergoing moderate sedation for esophagogastroduodenoscopy (EGD) and colonoscopy were identified from which patients were stratified into one of two groups based on sedation dose needs (low vs high dose). Anthropometric, procedural, and anesthesia-related data were compared between the two sedation groups, and logistic regression analysis was used to identify factors associated with lower sedation requirements.
Among 371102 patients included, 63137 patients were stratified into the low dose sedation group and 307965 patients were stratified into the high dose sedation group. Patients undergoing EGDs vs colonoscopies, procedure performed in the inpatient vs outpatient setting, and those performed in ambulatory surgery centers vs endoscopy suites were associated with lower moderate sedation requirements. On further multivariable analysis, factors predictive of tolerance with lower sedation requirements for both EGDs and colonoscopies included female gender, older age (≥ 50 years old), non-White race, Hispanic descent, lower BMI (≤ 25 kg/m2) and higher ASA class.
We have provided substantive data identifying key demographic and procedure related variables associated with lower sedation requirements during routine GI endoscopy and thereby, improved tolerance with moderate sedation.
While our findings can help to guide appropriate sedation practices during GI endoscopy, future prospective studies are needed to clarify the effects of patient demographic and procedure related variables on opioid and benzodiazepine response in the procedure setting.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; and American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe J S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD Jr; AGA Institute. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 2. | Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19:463-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 172] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (3)] |
| 3. | Ferreira AO, Cravo M. Sedation in gastrointestinal endoscopy: Where are we at in 2014? World J Gastrointest Endosc. 2015;7:102-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Rex DK. Review article: moderate sedation for endoscopy: sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther. 2006;24:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | ASGE Standards of Practice Committee; Early DS, Lightdale JR, Vargo JJ 2nd, Acosta RD, Chandrasekhara V, Chathadi KV, Evans JA, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Shergill AK, Cash BD, DeWitt JM. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2018;87:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 6. | American Association for Study of Liver Diseases. American College of Gastroenterology; American Gastroenterological Association Institute; American Society for Gastrointestinal Endoscopy; Society for Gastroenterology Nurses and Associates, Vargo JJ, DeLegge MH, Feld AD, Gerstenberger PD, Kwo PY, Lightdale JR, Nuccio S, Rex DK, Schiller LR. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc. 2012;76:e1-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | McCain JD, Stancampiano FF, Bouras EP, DeVault KR, Gilbert EL, Ryan T, Maillis A, Heckman MG, Diehl NN, Palmer WC. Creation of a score to predict risk of high conscious sedation requirements in patients undergoing endoscopy. Gastrointest Endosc 2020; 91: 595-605. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Mahajan RJ, Johnson JC, Marshall JB. Predictors of patient cooperation during gastrointestinal endoscopy. J Clin Gastroenterol. 1997;24:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Schutz SM, Lee JG, Schmitt CM, Almon M, Baillie J. Clues to patient dissatisfaction with conscious sedation for colonoscopy. Am J Gastroenterol. 1994;89:1476-1479. [PubMed] |
| 10. | Hazeldine S, Fritschi L, Forbes G. Predicting patient tolerance of endoscopy with conscious sedation. Scand J Gastroenterol. 2010;45:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Braunstein ED, Rosenberg R, Gress F, Green PH, Lebwohl B. Development and validation of a clinical prediction score (the SCOPE score) to predict sedation outcomes in patients undergoing endoscopic procedures. Aliment Pharmacol Ther. 2014;40:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Bal BS, Crowell MD, Kohli DR, Menendez J, Rashti F, Kumar AS, Olden KW. What factors are associated with the difficult-to-sedate endoscopy patient? Dig Dis Sci. 2012;57:2527-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Sachar H, Pichetshote N, Nandigam K, Vaidya K, Laine L. Continued midazolam versus diphenhydramine in difficult-to-sedate patients: a randomized double-blind trial. Gastrointest Endosc. 2018;87:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Tu RH, Grewall P, Leung JW, Suryaprasad AG, Sheykhzadeh PI, Doan C, Garcia JC, Zhang N, Prindiville T, Mann S, Trudeau W. Diphenhydramine as an adjunct to sedation for colonoscopy: a double-blind randomized, placebo-controlled study. Gastrointest Endosc. 2006;63:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | El Shahawy MS, El-Fayoumy M. The Influence of Adding Diphenhydramine Before Initiation of Moderate Sedation with Midazolam and Pethidine for Improving Quality of Colonoscopy. J Natl Med Assoc. 2019;111:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy; Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO, Shen B, Stewart L, Khan K, Vargo JJ. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Poincloux L, Laquière A, Bazin JE, Monzy F, Artigues F, Bonny C, Abergel A, Dapoigny M, Bommelaer G. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Dig Liver Dis. 2011;43:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Cinar K, Yakut M, Ozden A. Sedation with midazolam versus midazolam plus meperidine for routine colonoscopy: a prospective, randomized, controlled study. Turk J Gastroenterol. 2009;20:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kinirons MT, O'Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012;2:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Wyatt R. Pain and ethnicity. Virtual Mentor. 2013;15:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Belfer I. Nature and nurture of human pain. Scientifica (Cairo). 2013;2013:415279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Shingina A, Ou G, Takach O, Svarta S, Kwok R, Tong J, Donaldson K, Lam E, Enns R. Identification of factors associated with sedation tolerance in 5000 patients undergoing outpatient colonoscopy: Canadian tertiary center experience. World J Gastrointest Endosc. 2016;8:770-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;335-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 25. | Tarway NK, Jain M, Rajavel VP, Melpakkam S, Srinivasan V, Ravi R, Varghese J, Michael T, Venkataraman J. Patient satisfaction and safety profile with sedation during gastrointestinal endoscopy. Indian J Gastroenterol. 2017;36:330-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |