Published online Mar 16, 2019. doi: 10.4253/wjge.v11.i3.231
Peer-review started: January 27, 2019
First decision: February 20, 2019
Revised: February 28, 2019
Accepted: March 11, 2019
Article in press: March 11, 2019
Published online: March 16, 2019
Processing time: 49 Days and 16.6 Hours
Biliary ductal cancer (BDC) is a lethal disease; however, diagnosing BDC is challenging. Biliary biopsies are performed to pathologically diagnose BDC, but the appropriate parameters for biliary biopsy [number of biliary biopsies, number of endoscopic retrograde cholangiopancreatography (ERCP) sessions, etc.] are unknown.
To clarify what constitutes an adequate method for biliary biopsy.
In total, 95 patients who underwent endoscopic biliary biopsy without choledochoscopy and who were pathologically diagnosed with BDC were enrolled in this study. The patients were divided into two groups. Seventy-six patients who were diagnosed by biliary biopsy were defined as the positive group (P group), and nineteen patients who were not diagnosed by biliary biopsy were defined as the negative group (N group). The patient characteristics and ERCP-related procedures were compared between the P and N groups.
The numbers of ERCP sessions and biliary biopsies were significantly different between the two groups [ERCP sessions (one/two), P group 72/4 vs N group 15/4, P value = 0.048; number of biliary biopsies, P group 2 (1-6) vs N group 2 (1-7), P value = 0.039]. In a multivariate analysis, fewer than 2 ERCP sessions was an independent factor influencing the positivity of the biliary biopsies.
This study clarified that ERCP and biliary ductal biopsy should only be performed once. If biliary cancer is not pathologically diagnosed after the first ERCP session, other methods (Endoscopic ultrasonography-guided fine needle aspiration or choledochoscopy-guided biliary ductal biopsy) should be employed.
Core tip: The appropriate parameters for biliary biopsy [number of biliary biopsies, number of endoscopic retrograde cholangiopancreatography (ERCP) sessions, etc.) are unknown. In this report, fewer than 2 ERCP sessions was an independent factor influencing the positivity of the biliary biopsies. If biliary cancer is not pathologically diagnosed after the first ERCP session, other methods (Endoscopic ultrasonography-guided fine needle aspiration or choledochoscopy-guided biliary ductal biopsy) should be employed.
- Citation: Takagi T, Sugimoto M, Suzuki R, Konno N, Asama H, Sato Y, Irie H, Watanabe K, Nakamura J, Kikuchi H, Takasumi M, Hashimoto M, Hikichi T, Ohira H. Appropriate number of biliary biopsies and endoscopic retrograde cholangiopancreatography sessions for diagnosing biliary tract cancer. World J Gastrointest Endosc 2019; 11(3): 231-238
- URL: https://www.wjgnet.com/1948-5190/full/v11/i3/231.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i3.231
Biliary ductal cancer (BDC) is a lethal disease; however, diagnosing BDC is challenging. The pathological diagnostic methods for BDC are biliary cytology, biliary brush cytology, and biliary biopsy by endoscopic retrograde cholangiopan-creatography (ERCP).
The sensitivity of biliary cytology for diagnosing malignant biliary strictures is reported to be 32%-57%[1-9], and the sensitivity of biliary brush cytology is 33%-58%[3,4,10,11]. The sensitivity of biliary biopsy for diagnosing malignant biliary strictures is reported to be 36%-81%[3,4,7,9,11-13]. All reports except two indicate that the sensitivity of biliary biopsy is less than 65%. The sensitivities of biliary brush cytology and biliary biopsy are 61%-70.4%[4,11]. In addition, the sensitivity of repeated biliary cytology by endoscopic nasobiliary drainage tube is reported to be 72.4%[14].
Of these procedures for diagnosing BDC, the appropriate method of biliary biopsy is not clearly defined. In particular, the correct number of biliary biopsies is unknown, as is whether additional ERCP sessions are appropriate if the first session does not result in the pathological diagnosis of BDC. Therefore, the aim of this study was to clarify the appropriate parameters for biliary biopsy for the diagnosis of BDC.
This study was a retrospective study conducted to determine the adequate parameters for biliary biopsy used to diagnose BDC. Informed consent was not required for this study because the analysis utilized anonymous clinical data that were obtained after each patient agreed to treatment by providing written informed consent. This study was approved by the Institutional Review Board of Fukushima Medical University.
We enrolled 95 patients who underwent endoscopic biliary biopsy without choledochoscopy and who were pathologically diagnosed with BDC between February 2007 and March 2018. These patients underwent ERCP and biliary cytology or brush cytology and biliary biopsy. If they were not diagnosed by ERCP-related procedures, they were diagnosed by endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA), biopsy from duodenal invasion, or biopsy using choledochoscopy. The patients were divided into two groups. Seventy-six patients who were diagnosed by biliary biopsy were defined as the positive group (P group). Nineteen patients who were not diagnosed by biliary biopsy were defined as the negative group (N group).
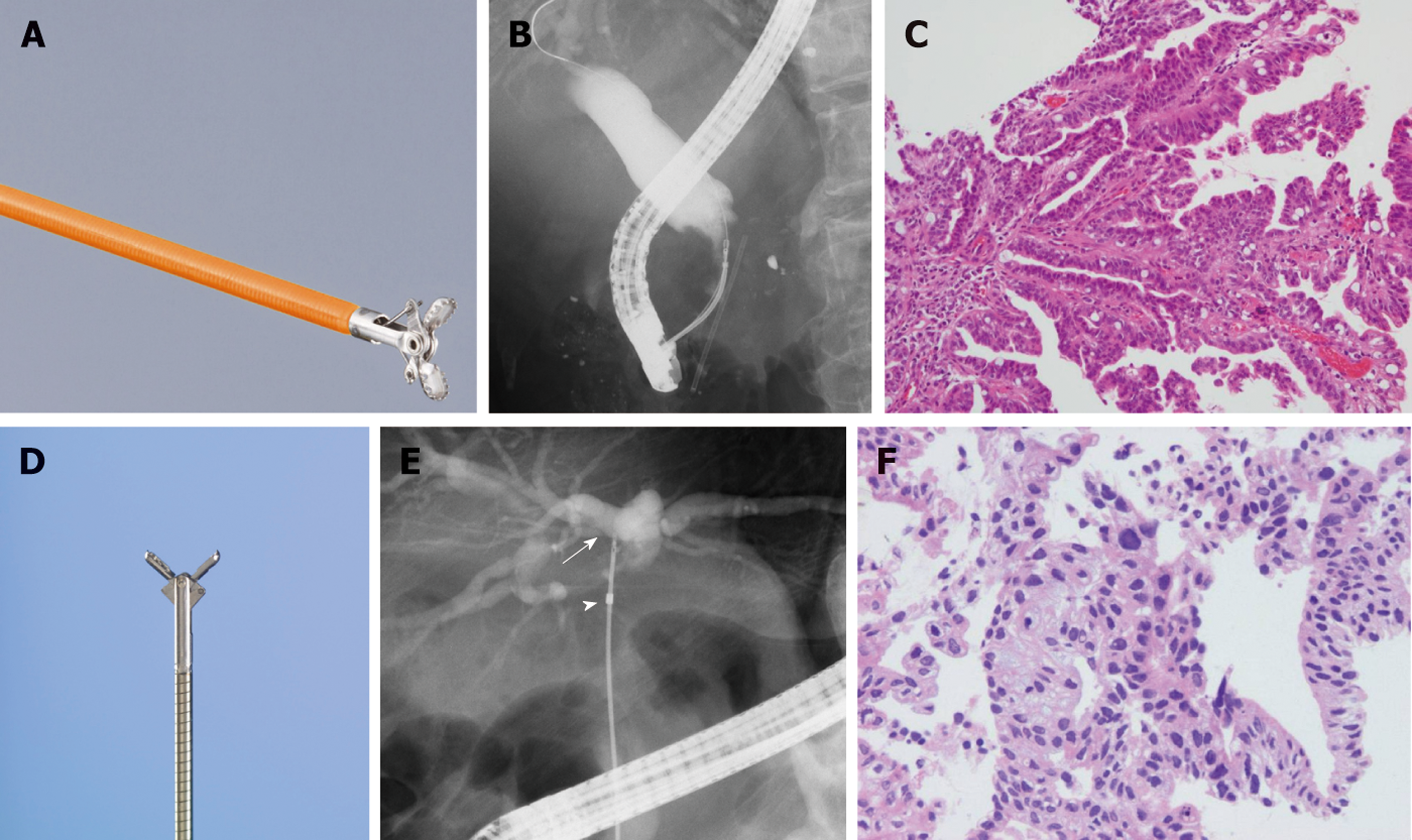
In all patients, an endoscope was inserted after they were sufficiently sedated with midazolam. After the endoscope reached the descending part of the duodenum, the biliary cannulation was started. If the biliary cannulation was successful, bile was collected for biliary cytology. After a range of malignant biliary strictures were confirmed by cholangiography, endoscopic sphincterotomy (EST) or intraductal ultrasonography, biliary brush cytology was performed if deemed appropriate. At this stage, biliary biopsy was performed to diagnose the malignancy or the status of BDC progression. The number of biliary biopsies was determined randomly by each endoscopist. The collection of a sufficient specimen was visually confirmed. If a patient had already received a biliary stent, the stent was removed before biliary cannulation. In five patients, endoscopic nasobiliary drainage (ENBD) was performed. The bile that was used for cytology was turned in the pathological department twice a day for three days. JF260V, JF240, and TJF240 ERCP endoscopes (Olympus, Tokyo, Japan) were used. An MTW ERCP catheter taper (MTW Endoskopie, Wesel, Germany), Tandem XL (Boston Scientific Japan, Tokyo, Japan) or PR-233Q (Olympus) was used as the ERCP catheter. A Clever Cut 3V or an RX Needle Knife (Boston Scientific Japan, Tokyo, Japan) were used for endoscopic sphincterotomy (EST). An Endo Jaw FB231K (Olympus) or a Radial JawTM 4 Biopsy Forceps (Boston Scientific Japan) was used for the biliary biopsy (Figure 1). If the biliary stricture was too tight to allow the insertion of the Radial Jaw, a SpyBite (Boston Scientific Japan, Tokyo, Japan) was used for the biliary biopsy. Reverse α-type or α-type ENBD catheters (Gadelius Medical, Tokyo, Japan) or a FleximaTM Nasobiliary Catheter single pigtail (Olympus) was used for the ENBD catheter. The choledochoscope used in this study was a SpyGlass DSTM (Boston Scientific Japan).
Patient characteristics (age, gender, receipt of antithrombotic drugs, location of tumor, Union for International Cancer Control (UICC) stage, cholangitis within the last month) and ERCP-related procedures (number of ERCP sessions; diagnosability of BDC from bile, brush cytology or ENBD cytology; EST; cup diameter of biopsy forceps (1 mm or 2 mm); total biopsy number; biopsy number before biliary stenting; biopsy number after biliary stenting; adverse events; post-ERCP pancreatitis (PEP)) were compared between the P group and the N group. Cholangitis was diagnosed according to the presence of an elevated white blood cell (WBC) count or C-reactive protein (CRP) level (WBC ≥ 10000/μL or CRP ≥ 5 mg/dL). The biopsy number was defined as the number of biopsies taken from the main stricture of the biliary cancer minus the number of screening and mapping biopsies. PEP was diagnosed by the presence of hyperamylasemia more than three times the normal level more than 24 hours after ERCP and abdominal pain[15]. In addition, we confirmed peripancreatic inflammation by contrast CT imaging in all PEP patients. The seriousness of PEP was determined according to the consensus guidelines proposed by Cotton et al[15] (mild: planned hospitalization was prolonged by 2-3 d, moderate: planned hospitalization was prolonged by 4-10 d, severe: planned hospitalization was prolonged by more than 10 d, a pseudocyst was present, intervention (percutaneous drainage or surgery) was necessary, or hemorrhagic pancreatitis developed).
The Mann-Whitney U test was used for the comparisons of continuous and ordinal variables. Fisher’s exact test was used for the comparisons of nominal variables. Multivariate logistic regression analysis was used. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using the EZR platform (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R commander that was designed to perform functions that are frequently used in biostatistics[16].
Regarding patient characteristics, no items except age were significantly different between the P group and N group (Table 1). Age was significantly higher in the P group than in the N group [P group 75 (29 - 90) years vs N group 68 (43-82) years, P value = 0.012; median (range)].
| P group (n = 76) | N group (n = 19) | P value | |
| Age (yr) | 75 (29-90) | 68 (43-82) | 0.012 |
| Males | 60 (78.9) | 12 (63.2) | 0.229 |
| Received antithrombotic drugs | 14 (18.4) | 0 (0) | 0.064 |
| Location of tumor (distal/hilar) | 45/31 | 8/11 | 0.205 |
| UICC stage (1/2/3/4) | 27/29/10/10 | 7/7/3/2 | 0.91 |
| Cholangitis within the last month | 10 (13.2) | 3 (15.8) | 0.719 |
Regarding ERCP-related procedures, the number of ERCP sessions and the total number of biopsies were significantly different between the two groups (ERCP session (one/two), P group 72/4 vs N group 15/4, P value = 0.048; total number of biopsies, P group 2 (1-6) vs N group 2 (1-7), P value = 0.039) (Table 2).
| P group (n = 76) | N group (n = 19) | P value | |
| Number of ERCP sessions (1/2) | 72/4 | 15/4 | 0.048 |
| EST | 74 (97.4) | 17 (89.5) | 0.177 |
| Diagnosability of bile or brush or ENBD cytology | 16/681 (23.5) | 5/19 (26.3) | 0.77 |
| Cup diameter of biopsy forceps (1 mm/2 mm) | 8/68 | 2/17 | 1.0 |
| Total number of biopsies | 2 (1 - 6) | 2 (1 - 7) | 0.039 |
| Number of biopsies before biliary stenting | 2 (1 - 4) | 2 (1 - 3) | 0.119 |
| Number of biopsies after biliary stenting | 2 (1 - 4) | 1 (1 - 6) | 0.065 |
| PEP | 4 (5.3) | 0 (0) | 0.58 |
| Moderate | 2 | ||
| Severe | 2 |
In univariate analysis, only fewer than two ERCP sessions significantly influenced the positivity of biliary biopsies (Table 3). In multivariate analysis including two factors (total number of biopsies ≤ 1, number of ERCP sessions < 2; the P values of these two factors were lower than the others in univariate analysis), fewer than two ERCP sessions was the independent factor influencing the positivity of biliary biopsies (Table 4).
| P group (n = 76) | N group (n = 19) | P value | |
| Total number of biopsies ≤ 1 | 32 (42.1) | 4 (21.1) | 0.116 |
| Total number of biopsies ≤ 2 | 62 (81.6) | 12 (63.1) | 0.120 |
| Total number of biopsies ≤ 3 | 69 (90.8) | 15 (78.9) | 0.222 |
| Number of ERCP sessions < 2 | 72 (94.7) | 15 (78.9) | 0.048 |
| OR | 95%CI | P value | |
| Number of ERCP sessions < 2 | 4.8 | 1.08-21.4 | 0.04 |
In this study, we verified an adequate method of biliary biopsy for the diagnosis of BDC. Although the number of biliary biopsies did not affect the positivity of the biliary biopsies, it was revealed that multiple ERCP sessions for the diagnosis of BDC were not useful. If the result of the biliary biopsy is negative after the first ERCP session, other methods should be subsequently employed.
In past reports, EUS-FNA and choledochoscopy were introduced as additional methods. The efficacy of EUS-FNA for diagnosing malignant biliary strictures was reported in previous studies. The sensitivity of EUS-FNA for the diagnosis of malignant biliary strictures is 45%-94.0% with a specificity of 77%-100% and an accuracy of 68%-94.0%[17-23]. Ohshima et al[24] reported that 10 bile duct cancer cases not diagnosed by ERCP (brush cytology and biopsy) were successfully diagnosed by EUS-FNA. Nayar et al[25] and DeWitt et al[23] reported that EUS-FNA was successful after poor results were obtained with ERCP-related diagnostic methods. In addition, malignant lymph node swelling in pancreaticobiliary tract cancers were successfully diagnosed by EUS-FNA[26,27].
Starting approximately ten years ago, SpyGlass® (Boston Scientific Japan, Tokyo, Japan) has been increasingly used as the preferred choledochoscope. SpyGlass® was introduced in 2006 and is a very thin reusable fiber that is used with a disposable delivery catheter (SpyScope®, Boston Scientific Japan, Tokyo, Japan), which can be moved in four directions. The SpyGlass® system can be controlled by a single operator. In a systematic review by Navaneethan et al[28], the sensitivity and specificity of biliary biopsy with the SpyGlass® system were 74.7% and 93.3%, respectively, for the diagnosis of malignant biliary strictures that had previously failed to be diagnosed by brushings or biopsy[28-32]. In addition, a patient with an indeterminate biliary stricture who was not diagnosed by ERCP (brush cytology, intraductal biopsy) or EUS-FNA was diagnosed with cholangiocarcinoma by SpyGlass®-guided biopsy[33]. Recently, an advanced version of SpyGlass®, SpyGlass® DS (Boston Scientific Japan, Tokyo, Japan), was released. The image transmitted by SpyGlass® DS is clearer than the image transmitted by the original SpyGlass®, and the delivery system for SpyGlass® DS is easier to operate than that of SpyGlass®. The efficacy of SpyGlass® DS-guided biliary biopsy for the diagnosis of malignant biliary strictures that remained undiagnosed by previous brush cytology, biliary biopsy or EUS-FNA has been reported[34,35].
Then, the diagnostic methods used in the 19 patients in the N group were considered; 10 patients were diagnosed via surgery, 4 were diagnosed after bile cytology, 2 were diagnosed via biliary brush cytology, 1 was diagnosed through choledochoscopy-guided biopsy, 1 was diagnosed via EUS-FNA of metastatic lymph nodes, and 1 was diagnosed via a biopsy from duodenal invasion. Bile cytology and biliary brush cytology were performed with biliary biopsy. In addition, 3 of the 4 patients who underwent 2 sessions of ERCP remained undiagnosed before surgery. Therefore, other methods, such as EUS-FNA or choledochoscopy-guided biliary biopsy, should be performed if biliary cancer is not diagnosed in the first ERCP session.
This study had some limitations. First, this study was performed with a small sample size and at a single institution. Thus, a statistical bias might exist. Second, this study was retrospective. Therefore, the indications regarding the volumes of the specimens sampled by biliary biopsies were absent except for visually confirming the presence of a sufficient specimen. In the future, a larger sample size and prospective multicenter study are needed. Third, the volumes of the specimens sampled by biliary biopsy were not assessed. The correlation between the pathological diagnosis and the volume of biliary cancer specimens should be verified.
In conclusion, this study clarified that ERCP for biliary ductal biopsy should only be performed once. If biliary cancer is not pathologically diagnosed after the first session of ERCP, other methods (EUS-FNA or choledochoscopy-guided biliary ductal biopsy) should be employed.
Biliary ductal cancer (BDC) is a lethal disease; however, the histological diagnosis of BDC is difficult.
Histological diagnosis of BDC is achieved by endoscopic biliary biopsy except for surgery. However, the appropriate method (i.e., the number of times, the number of ERCP sessions) for biliary biopsy is unknown.
This study aims to clarify the appropriate method of endoscopic biliary biopsy.
The subjects of this study were patients who were histologically diagnosed with BDC. The patients who could be diagnosed by biliary biopsy were determined as the positive group (P group), and the patients who could not be diagnosed by biliary biopsy were determined as the negative group (N group). The methods for ERCP procedures were compared between the P group and the N group.
Multiple ERCP sessions did not contribute to the improvement of the diagnosability of biliary biopsy.
If biliary cancer is not pathologically diagnosed after the first session of ERCP, other methods should be employed.
From the results of this study, several methods will be developed and tested for diagnosing BDC.
We are grateful to the staff at the Department of Gastroenterology, Fukushima Medical University, School of Medicine; the medical staff at the Department of Endoscopy, Fukushima Medical University Hospital; and the medical staff at the Gastroenterology Ward at Fukushima Medical University Hospital.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fogli L, Lee CL S- Editor: Gong ZM L- Editor: A E- Editor: Zhang YL
| 1. | Foutch PG, Kerr DM, Harlan JR, Kummet TD. A prospective, controlled analysis of endoscopic cytotechniques for diagnosis of malignant biliary strictures. Am J Gastroenterol. 1991;86:577-580. [PubMed] |
| 2. | Lee JG, Leung JW, Baillie J, Layfield LJ, Cotton PB. Benign, dysplastic, or malignant--making sense of endoscopic bile duct brush cytology: results in 149 consecutive patients. Am J Gastroenterol. 1995;90:722-726. [PubMed] |
| 3. | Ponchon T, Gagnon P, Berger F, Labadie M, Liaras A, Chavaillon A, Bory R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 264] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Pugliese V, Conio M, Nicolò G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 190] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Glasbrenner B, Ardan M, Boeck W, Preclik G, Möller P, Adler G. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999;31:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Jailwala J, Fogel EL, Sherman S, Gottlieb K, Flueckiger J, Bucksot LG, Lehman GA. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Macken E, Drijkoningen M, Van Aken E, Van Steenbergen W. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254-259. [PubMed] |
| 9. | Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465-467. [PubMed] |
| 10. | Howell DA, Parsons WG, Jones MA, Bosco JJ, Hanson BL. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointest Endosc. 1996;43:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Schoefl R, Haefner M, Wrba F, Pfeffel F, Stain C, Poetzi R, Gangl A. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol. 1997;32:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Kubota Y, Takaoka M, Tani K, Ogura M, Kin H, Fujimura K, Mizuno T, Inoue K. Endoscopic transpapillary biopsy for diagnosis of patients with pancreaticobiliary ductal strictures. Am J Gastroenterol. 1993;88:1700-1704. [PubMed] |
| 13. | Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U, Werner M. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Uchida N, Kamada H, Ono M, Aritomo Y, Masaki T, Nakatsu T, Kuriyama S. How many cytological examinations should be performed for the diagnosis of malignant biliary stricture via an endoscopic nasobiliary drainage tube? J Gastroenterol Hepatol. 2008;23:1501-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2037] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 16. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13334] [Article Influence: 1111.2] [Reference Citation Analysis (0)] |
| 17. | Matsubayashi H, Matsui T, Yabuuchi Y, Imai K, Tanaka M, Kakushima N, Sasaki K, Ono H. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22:628-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 18. | Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Byrne MF, Gerke H, Mitchell RM, Stiffler HL, McGrath K, Branch MS, Baillie J, Jowell PS. Yield of endoscopic ultrasound-guided fine-needle aspiration of bile duct lesions. Endoscopy. 2004;36:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Meara RS, Jhala D, Eloubeidi MA, Eltoum I, Chhieng DC, Crowe DR, Varadarajulu S, Jhala N. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology. 2006;17:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Ohshima Y, Yasuda I, Kawakami H, Kuwatani M, Mukai T, Iwashita T, Doi S, Nakashima M, Hirose Y, Asaka M, Moriwaki H. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Nayar MK, Manas DM, Wadehra V, Oppong KE. Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology. 2011;58:1862-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Gleeson FC, Rajan E, Levy MJ, Clain JE, Topazian MD, Harewood GC, Papachristou GI, Takahashi N, Rosen CB, Gores GJ. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Kurita A, Kodama Y, Nakamoto Y, Isoda H, Minamiguchi S, Yoshimura K, Kuriyama K, Sawai Y, Uza N, Hatano E, Uemoto S, Togashi K, Haga H, Chiba T. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest Endosc. 2016;84:467-475.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608-14.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Manta R, Frazzoni M, Conigliaro R, Maccio L, Melotti G, Dabizzi E, Bertani H, Manno M, Castellani D, Villanacci V, Bassotti G. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013;27:1569-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Siddiqui AA, Mehendiratta V, Jackson W, Loren DE, Kowalski TE, Eloubeidi MA. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol. 2012;10:466-71; quiz e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Nishikawa T, Tsuyuguchi T, Sakai Y, Sugiyama H, Miyazaki M, Yokosuka O. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: a prospective study. Gastrointest Endosc. 2013;77:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Figueroa Marrero A, Chavarría-Herbozo CM, de la Serna Higuera C, Pérez-Miranda M. Long-standing indeterminate biliary stricture with iterative negative tissue sampling revealed as cholangiocarcinoma under SpyGlassTM cholangiocoscopy. Rev Esp Enferm Dig. 2017;109:220-221. [PubMed] |
| 34. | Tanaka R, Itoi T, Honjo M, Tsuchiya T, Kurihara T, Tsuji S, Tonozuka R, Kamada K, Sofuni A, Mukai S. New digital cholangiopancreatoscopy for diagnosis and therapy of pancreaticobiliary diseases (with videos). J Hepatobiliary Pancreat Sci. 2016;23:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Varadarajulu S, Bang JY, Hasan MK, Navaneethan U, Hawes R, Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? (with video). Gastrointest Endosc. 2016;84:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |