Published online Dec 16, 2019. doi: 10.4253/wjge.v11.i12.589
Peer-review started: June 10, 2019
First decision: August 2, 2019
Revised: October 17, 2019
Accepted: November 4, 2019
Article in press: November 5, 2019
Published online: December 16, 2019
Processing time: 168 Days and 3.4 Hours
Evaluation of biliary strictures primarily focuses on ruling out malignancy in older age groups. With endoscopic tools such as endoscopic ultrasound (EUS) and cholangioscopy, improved biliary visualization has enhanced the investigation of intraluminal biliary lesions and provided modalities for targeted biopsies. Benign biliary strictures, however, may pose a diagnostic dilemma.
A 71-year-old female with past medial history of hypothyroidism presenting for abnormal biliary imaging. Patient’s previous evaluation was concerning for common bile duct dilation with cholelithiasis, for which she underwent a cholecystectomy. Due to persistent symptoms and worsening liver function tests, she presented to our institution for further workup. Subsequently, the patient underwent an EUS and multiple ERCP’s with cholangioscopy; biliary biopsies revealed no evidence of malignancy but concerning for prominent eosinophilic infiltration. After further review of multiple pathology specimens and the benign clinical course, we diagnosed the patient with eosinophilic cholangitis.
Eosinophilic cholangitis is a rare disease and can present as a challenging case diagnostically. This case raises the potential utility of quantitative eosinophilic infiltration reporting in creating an objective diagnostic metric for eosinophilic cholangitis.
Core tip: Eosinophilic cholangitis is a rare cause of benign biliary strictures. The diagnosis is based predominantly on histopathologic findings in the setting of excluding malignancy, particularly in older age group. Previously, no quantitative eosinophilic threshold has been discussed in establishing the diagnosis of eosinophilic cholangitis, and hence the diagnosis is based on exclusion and suggestive findings. This case report provides the first look at eosinophils per high power field to help define eosinophilic cholangitis.
- Citation: Dodda A, Matsukuma K, Urayama S. Eosinophilic cholangitis: A case report of diagnostically challenging eosinophilic infiltrative biliary obstruction. World J Gastrointest Endosc 2019; 11(12): 589-595
- URL: https://www.wjgnet.com/1948-5190/full/v11/i12/589.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i12.589
Biliary strictures can be a challenging condition to determine an etiology. Previously, due to the limitations of endoscopic and radiographic modalities, when the etiology of strictures was not identified, they were labeling as “indeterminant strictures”[1,2]. With the advent of endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, and cholangioscopy, accuracy in diagnosing the cause of strictures have improved, with evaluation primarily centered on evaluating for the presence or absence of malignancy in older age population[3]. Previous studies have shown majority of strictures being secondary to malignancy (pancreatic adenocarcinoma and cholangiocarcinoma), with up to 30% due to benign pathologies[4].
However, establishing a benign biliary disease may be difficult in certain cases as they mimic the findings of malignant disease. With endoscopic tools such as endoscopic ultrasound (EUS) and cholangioscopy[5], improved biliary visualization has enhanced the investigation of intraluminal biliary lesions and provided modalities for targeted biopsies[6]. Along with these improvements, tumor markers have also been implemented (serum CA 19-9) to help with diagnosis[7]. The importance of this workup is imperative as timely diagnosis may affect a patient’s survival and candidacy for therapeutic interventions (surgery, chemotherapy, radiation, en-doscopic decompression)[8].
A 71-year-old Caucasian female was referred to us with findings of abnormal biliary imaging.
Seventeen months ago, she presented to another hospital with two weeks of fatigue and abdominal pain. She had mild transaminitis with normal bilirubin levels (Table 1). An ultrasound showed abnormal thickening of the common bile duct (CBD), pericholecystic fluid, and gallbladder sludge without wall thickening. HIDA scan demonstrated delayed gallbladder filling, with magnetic resonance imaging (MRI) significant for mid common hepatic duct (CHD) narrowing, distal CBD tapering with no filling defect, and CBD dilation to 8mm. Esophagogastroduodenoscopy showed mild chronic gastritis. With persistent symptoms and worsening liver function tests (LFTs) (Table 1) over the next two weeks, patient underwent a cholecystectomy. Surgical pathology was significant for acalculous chronic cholecystitis, with the presence of prominent eosinophilic infiltration.
| Pre-Cholecystectomy | Post-Cholecystectomy | ERCP 1 | ERCP 2 | Most Recent | |
| AST (U/L) | 134 | 193 | 151 | 82 | 27 |
| ALT (U/L) | 207 | 302 | 222 | 38 | 27 |
| Alkaline Phosphatase (U/L) | 461 | 558 | 765 | 61 | 65 |
| Bilirubin (mg/dL) | 0.3 | 0.3 | 0.3 | 0.1 | 0.5 |
| % Eosinophils (ULN < 5%) | 13.6 | 27.2 | 11.4 | 7.5 | 5.9 |
| CA 19-9 (U/mL) | < 3 |
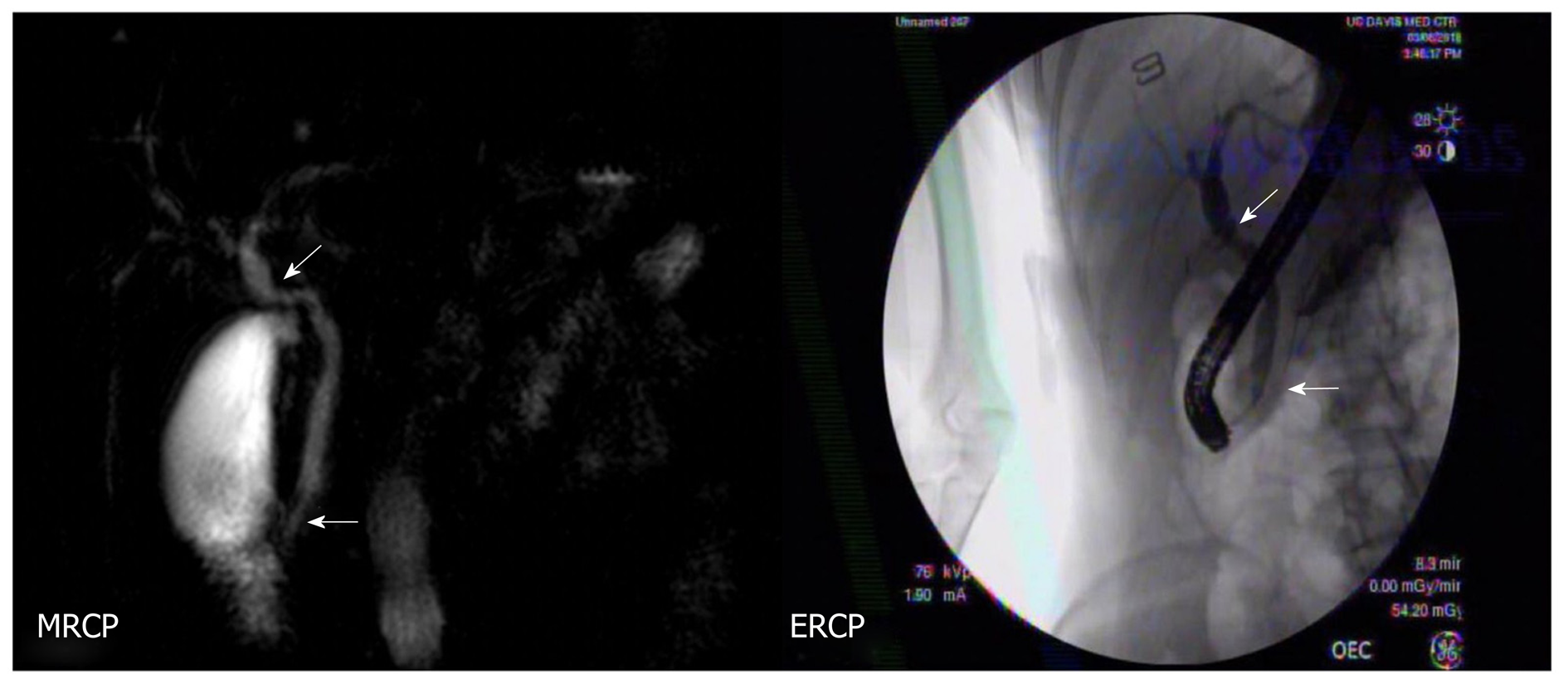
Post-cholecystectomy, the patient’s LFTs remained elevated. Repeat MRCP was concerning for biliary dilation, focal narrowing and tapering at the ampulla, and no filling defects (Figure 1). Due to abnormal imaging, patient presented to our institution for an EUS.
The patient was previously diagnosed with hypothyroidism.
The patient’s temperature was 36.7°C, heart rate was 75, blood pressure was 119/73. The clinical abdominal examination revealed non-tender, soft, with evidence of laparoscopy incision (healed with no tenderness or drainage).
Blood analysis revealed transaminitis with aspartate aminotransferase 151 U/L and alanine aminotransferase 222 U/L, with elevated alkaline phosphatase 765 U/L and normal bilirubin 0.3 mg/dL. Patient also had mild peripheral eosinophilia with 11.4 % eosinophils.
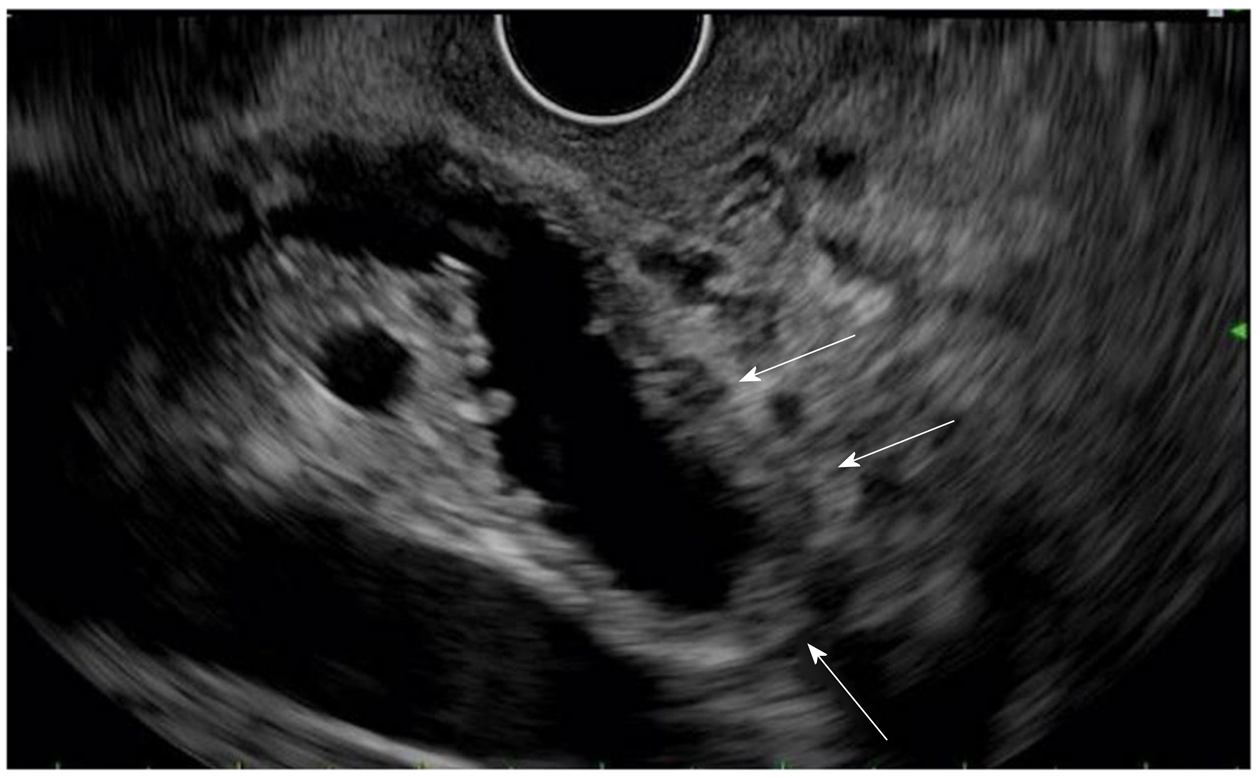
EUS showed extrahepatic biliary dilation, irregular and diffusely thickened bile duct walls, and no focal mass (Figure 2).
Therefore, benign pathologies such as primary sclerosing cholangitis and IgG4-associated cholangitis, were also pursued. However, laboratory results showed normal IgG4 levels and autoimmune markers (myeloperoxidase antibody, anti-smooth muscle antibody, HIV antibody and anti-mitochondrial antibody).
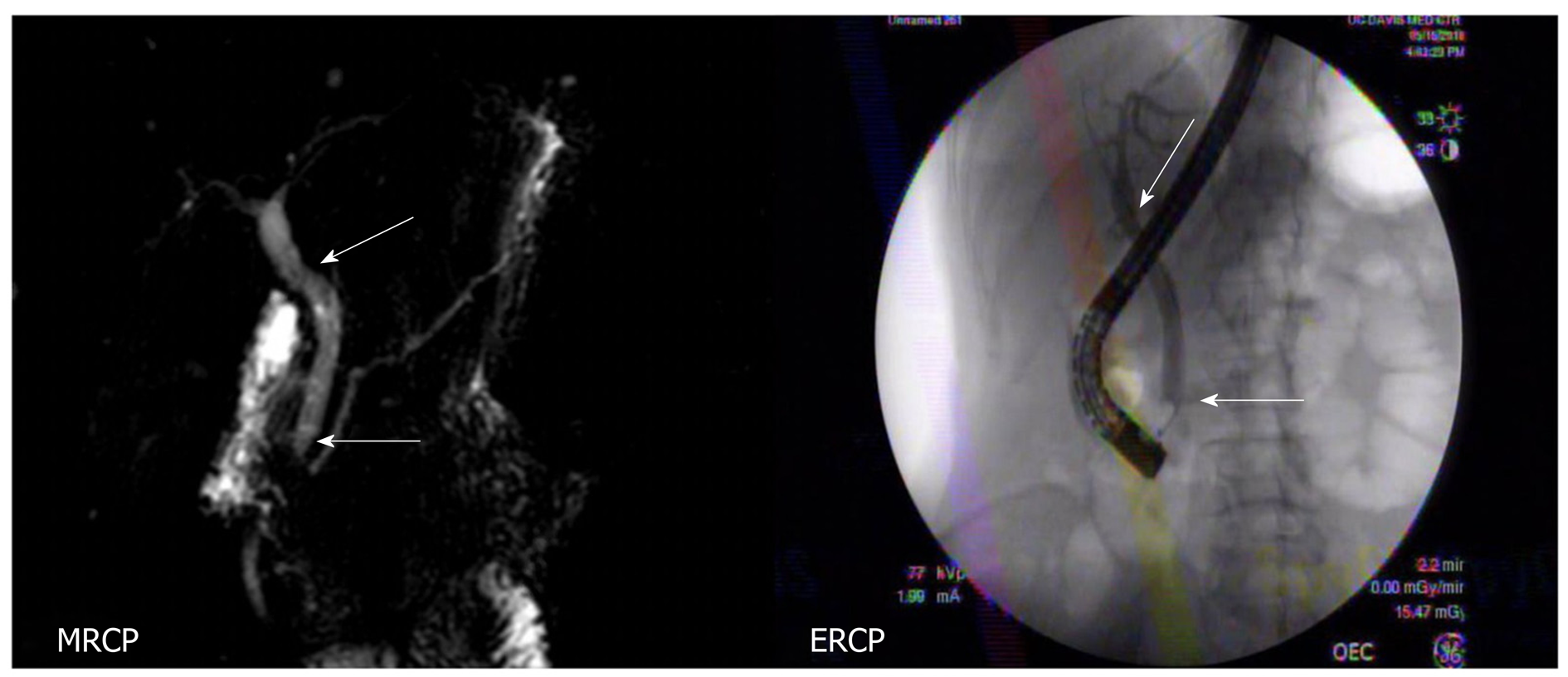
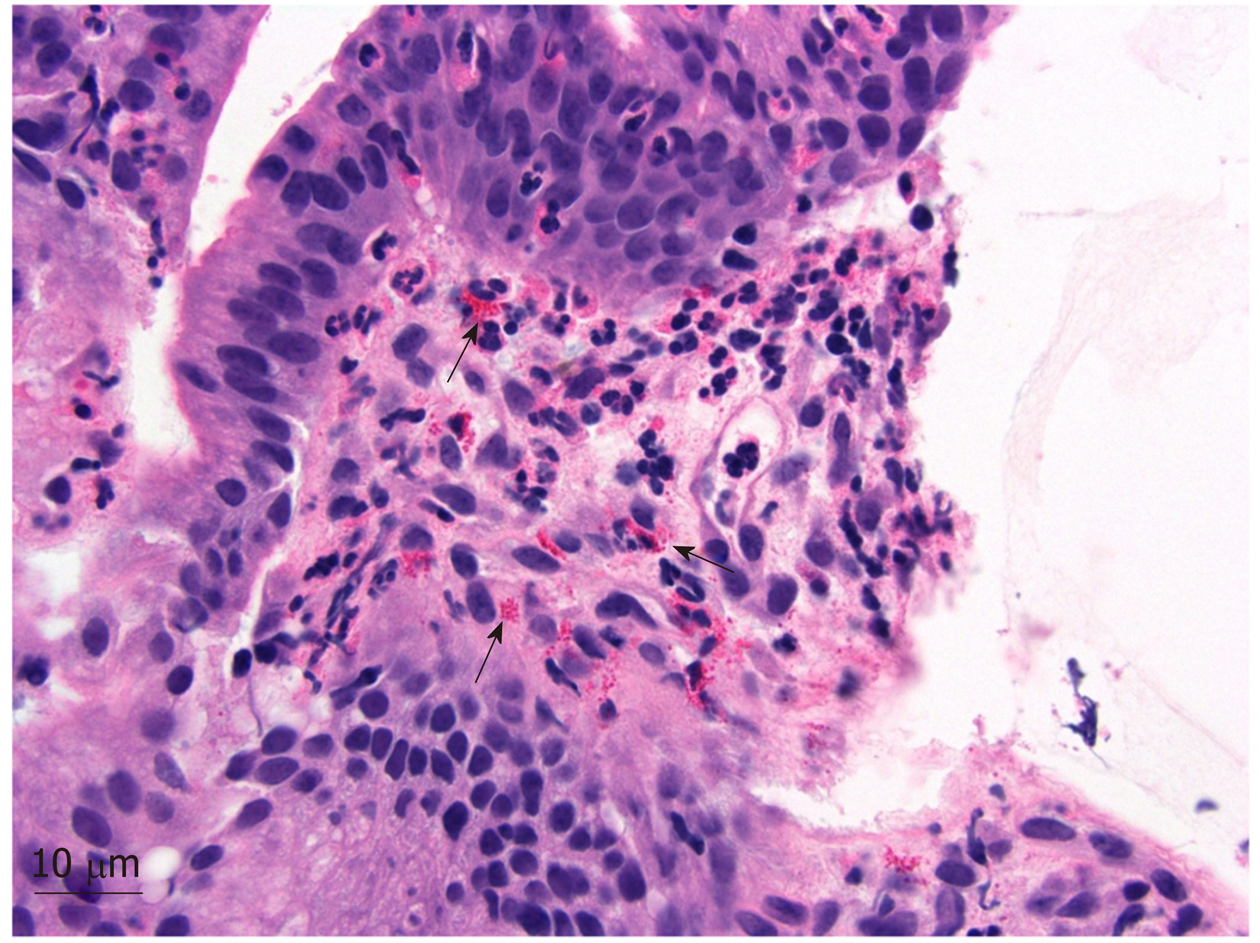
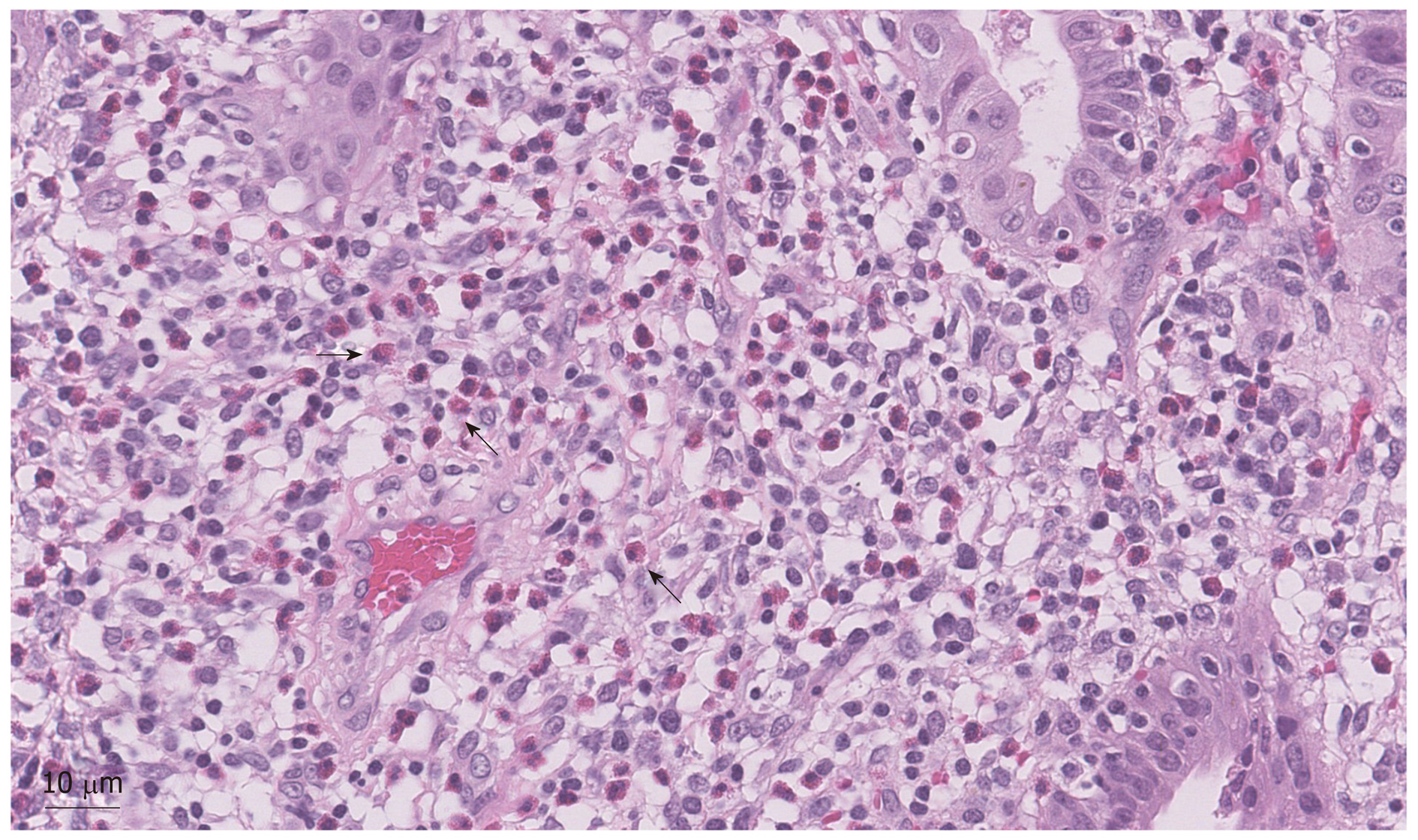
To address the biliary stricture and rule out malignancy, the patient underwent an ERCP, demonstrating CHD narrowing and a distal CBD stricture with extra and intrahepatic dilation (Figure 1). A plastic biliary stent was placed, and biliary brushings/biopsies were obtained. Pathology showed scant stroma with mild eosinophilic infiltrate of 4 eosinophils per high power field (eos/hpf) without malignancy. On repeat ERCP, cholangiogram demonstrated no distal bile duct stricture (Figure 3). Cholangioscopy identified frond-like ductal lesions in the mid-CBD, and multiple biopsies were obtained. Pathology demonstrated reactive biliary epithelium associated with a mixed infiltrate of neutrophils and eosinophils (up to 9 eos/hpf) (Figure 4).
Due to the unusual clinical features of this case and mild prominence of eosinophils in the biliary specimens, a second review of the previous pathology specimens was performed. The patient’s gallbladder was notable for a prominent diffuse, eosinophilic infiltrate (211 eos/hpf) (Figure 5). Gastric biopsies also showed a focal prominence of eosinophils. Further retrospective review showed peripheral eosinophilia (Table 1).
Karen Matsukuma, MD, PhD, Assistant Professor, Department of Pathology and Laboratory Medicine, University of California–Davis.
The histopathologic evidence of eosinophilic infiltrate in the gallbladder and bile duct specimens in the setting of known peripheral eosinophilia is suggestive of eosinophilic cholangitis.
The final diagnosis of the presented case is eosinophilic cholangitis (EC).
Steroids were initially considered, but with spontaneous improvement in the patient’s biliary narrowing and resolving LFT abnormalities without a biliary stent, no treatment was administered.
On the most recent follow-up (approximately 1 year since patient’s initial presentation to us), patient remained asymptomatic, and the repeat MRI showed no extra or intrahepatic dilation (Figure 3).
EC is a rare cause of benign biliary obstruction, and can closely resemble the clinical presentation of cholangiocarcinoma[9]. EC was first reported in 1980 and represents a broader category of gastrointestinal disease, characterized by eosinophilic infiltration of the gastrointestinal tract[10]. EC encompasses eosinophilic cholecystitis, reported initially in 1949, and eosinophilic cholangiopathy, described in 1990 as having biliary involvement without cholecystitis[11,12]. With eosinophilic infiltration in multiple organs, we feel that our patient exhibited EC.
Diagnosing EC may be clinically challenging. Studies have described a correlation with hypereosinophilic syndrome, but no clear association has been established[13]. Prior to making the diagnosis of EC, other benign etiologies of biliary strictures should be ruled out, including immune-mediated inflammation (primary sclerosing cholangitis, sarcoidosis, IgG4-related sclerosing cholangitis, mast cell cholangitis), choledocholithiasis, ischemic cholangiopathy, infection (parasitic, recurrent pyogenic cholangitis, tuberculosis), radiation therapy, cystic fibrosis and follicular cholangitis[14]. And EC should be considered in the setting of cho-lecystitis without the presence of cholelithiasis.
Furthermore, there are no established concrete criteria for the diagnosis of EC, but a combination of histopathologic, radiographic, endoscopic, and hematologic findings is used in evaluation. In 2007, the following findings were suggested to establish a diagnosis of EC: (1) Wall thickening or stenosis of the biliary system; (2) Histopathological findings of eosinophilic infiltration; and (3) Reversibility of biliary abnormalities without treatment or following steroid treatment[13]. Previous studies discussed a correlation with peripheral eosinophilia in about 70% of patients, but its presence is neither specific nor sensitive[15]. Imaging may show biliary wall thickening, with cholangiography identifying stricturing and biliary mucosal irregularities. Cholangioscopy provides direct visualization and targeted biopsies, aiding in establishing the diagnosis[16]. EUS is also becoming increasingly important in detailed evaluation of biliary tree, including a specific finding of a parenchymal echo in the bile duct wall using contrast-enhanced method[17].
In other gastrointestinal eosinophilic diseases, studies have focused on identifying numerical thresholds for eosinophilic counts that distinguish eosinophil-driven disease from non-specific inflammation. One summary proposed the following (average eos/hpf in 5 hpf) to establish the diagnosis: Stomach (> 30), duodenum (> 52), ileum (> 56), right colon (> 100), transverse and descending colon (> 84) and rectosigmoid colon (> 64)[18]. For eosinophilic esophagitis (EoE), the diagnosis is suspected when there are symptoms of esophageal dysfunction and at least 15 eos/hpf. EoE is confirmed when there is esophageal dysfunction and at least 15 eos/hpf after evaluation for other causes of esophageal eosinophilia[19].
To our knowledge, we are the first to report the eosinophil counts in the setting of EC. While the number of eosinophils was relatively mild in the bile duct biopsies, it is notable that the eosinophils comprised the predominant inflammatory component in the bile duct biopsies and gallbladder, a less common finding in most other inflammatory conditions in which eosinophils are found. We recorded a histological eosinophilic concentration in our patient, showing 211 eos/hpf in the gallbladder and 9 eos/hpf in the bile duct. As eosinophilic infiltration of the bile duct in various disease state may be variable, we propose that future reporting should record the degree of eosinophilic infiltration in order to establish a quantifiable value of biliary eosinophilia for developing potentially more definitive diagnostic criteria. Also, consideration can be made to retrospectively evaluate quantitative eosinophilic counts in previously diagnosed patients.
Treatment of EC consists of conservative management or steroid therapy. Most improve with monitoring, but symptomatic patients (pain, transaminitis, hy-perbilirubinemia), may respond to steroids. In one case, a patient responded to long-term hydroxyurea treatment[20]. Recurrence has been documented in only a few cases, presenting with focal eosinophilic inflammation in multiple organs or abdominal pain. Surgical resection of the refractory biliary stricture has also been reported[20].
EC remains a rare diagnosis of benign biliary obstruction. Evaluation requires carefully excluding malignancy and various other potential benign disease processes. Many patients, including ours, improve with conservative management after placement of temporary biliary stent, although steroid therapy and surgical resection have been used in other cases. In the future, accumulation of the biliary eosinophilia metrics from biopsy specimens may help establish the diagnostic criteria for this disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jonaitis LV, Sugimoto M S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL
| 1. | Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf). 2015;3:22-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Naveen BK, Pavan T, Jennifer LL, Banke A. EUS guided fine needle aspiration is useful in diagnostic evaluation of indeterminate proximal biliary strictures. Open J Gastroenterology. 2012;2:33–9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Wakai T, Shirai Y, Sakata J, Maruyama T, Ohashi T, Korira PV, Ajioka Y, Hatakeyama K. Clinicopathological features of benign biliary strictures masquerading as biliary malignancy. Am Surg. 2012;78:1388-1391. [PubMed] |
| 4. | Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Rana SS, Bhasin DK, Sharma V, Rao C, Gupta R, Singh K. Role of endoscopic ultrasound in evaluation of unexplained common bile duct dilatation on magnetic resonance cholangiopancreatography. Ann Gastroenterol. 2013;26:66-70. [PubMed] |
| 7. | Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, Pinto E, Roviello F. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Nesbit GM, Johnson CD, James EM, MacCarty RL, Nagorney DM, Bender CE. Cholangiocarcinoma: diagnosis and evaluation of resectability by CT and sonography as procedures complementary to cholangiography. AJR Am J Roentgenol. 1988;151:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Goode EC, Simpson BW, Rushbrook SM. A rare cause of cholangiopathy. Gastroenterology. 2013;144:e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Leegaard M. Eosinophilic cholecystitis. Acta Chir Scand. 1980;146:295-296. [PubMed] |
| 11. | ALBOT G, POILLEUX F. [Not Available]. Presse Med. 1949;57:558. [PubMed] |
| 12. | Tenner S, Roston A, Lichtenstein D, Brooks D, Herlihy E, Carr-Locke D. Eosinophilic cholangiopathy. Gastrointest Endosc. 1997;45:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Matsumoto N, Yokoyama K, Nakai K, Yamamoto T, Otani T, Ogawa M, Tanaka N, Iwasaki A, Arakawa Y, Sugitani M. A case of eosinophilic cholangitis: imaging findings of contrast-enhanced ultrasonography, cholangioscopy, and intraductal ultrasonography. World J Gastroenterol. 2007;13:1995-1997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Nguyen Canh H, Harada K. Adult bile duct strictures: differentiating benign biliary stenosis from cholangiocarcinoma. Med Mol Morphol. 2016;49:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Hoilat JN, Hoilat GJ, AlQahtani S, Alhussaini HF, Alabbad SI. Atypical Presentation of a Rare Disease: Eosinophilic Cholangitis Posing as a Cancer. Am J Case Rep. 2018;19:76-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Grauer L, Padilla VM, Bouza L, Barkin JS. Eosinophilic sclerosing cholangitis associated with hypereosinophilic syndrome. Am J Gastroenterol. 1993;88:1764-1769. [PubMed] |
| 17. | Walter D, Hartmann S, Albert JG. Indeterminate biliary stricture with suspicion for malignancy unmasked as eosinophilic cholangitis by cholangioscopy. Gastrointest Endosc. 2017;85:265-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, Spechler SJ, Attwood SE, Straumann A, Aceves SS, Alexander JA, Atkins D, Arva NC, Blanchard C, Bonis PA, Book WM, Capocelli KE, Chehade M, Cheng E, Collins MH, Davis CM, Dias JA, Di Lorenzo C, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox A, Gonsalves NP, Gupta SK, Katzka DA, Kinoshita Y, Menard-Katcher C, Kodroff E, Metz DC, Miehlke S, Muir AB, Mukkada VA, Murch S, Nurko S, Ohtsuka Y, Orel R, Papadopoulou A, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Rothenberg ME, Schoepfer A, Scott MM, Shah N, Sheikh J, Souza RF, Strobel MJ, Talley NJ, Vaezi MF, Vandenplas Y, Vieira MC, Walker MM, Wechsler JB, Wershil BK, Wen T, Yang GY, Hirano I, Bredenoord AJ. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022-1033.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 815] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 20. | Abdalla EK, Vauthey JN. Eosinophilic cholangiopathy. Surgery. 2002;131:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |