Copyright
©2013 Baishideng Publishing Group Co.
World J Gastrointest Endosc. May 16, 2013; 5(5): 265-269
Published online May 16, 2013. doi: 10.4253/wjge.v5.i5.265
Published online May 16, 2013. doi: 10.4253/wjge.v5.i5.265
Figure 1 SX-ELLA biodegradable biodegradable esophageal stent.
The stent is flared at both ends to reduce the risk of migration and is fitted with radiopaque markers at the midpoint and at the ends to enable precise stent positioning under fluoroscopic control. Because of reduced long-term elasticity, the stent is supplied separate from the delivery system and needs to be manually loaded just before the implantation procedure (see manufacturer’s “Instructions for Use”).
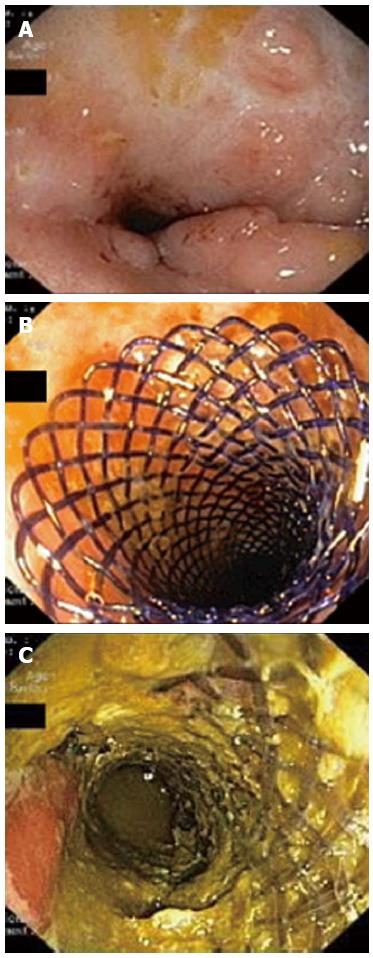
Figure 2 Endoscopic images.
A: Stricture of the distal sigmoid colon before stent insertion. The surrounding mucosa show no signs of active inflammation and few inflammatory polyps; B: Biodegradable stent deployed in the stricture at the end of the procedure; C: Endoscopic view 1 mo after the stent placement. The stent fibers present a translucent appearance and partial fragmentation.
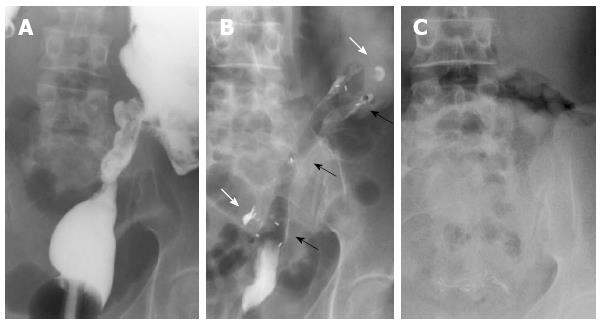
Figure 3 Radiographic images.
A: Stricture of the distal sigmoid colon outlined by luminal filling with barium using a rectal catheter (supine position); B: Biodegradable stent in situ immediately after insertion (supine position). The stent is radiolucent, but the radiopaque markers at the midpoint and at the ends are visible (black arrows). The margins of the stricture are marked with lipiodol (white arrows); C: Disappearance of radiopaque markers at 4 mo confirming complete stent degradation (erect position).
- Citation: Rodrigues C, Oliveira A, Santos L, Pires E, Deus J. Biodegradable stent for the treatment of a colonic stricture in Crohn’s disease. World J Gastrointest Endosc 2013; 5(5): 265-269
- URL: https://www.wjgnet.com/1948-5190/full/v5/i5/265.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i5.265