Published online Feb 28, 2017. doi: 10.4254/wjh.v9.i6.318
Peer-review started: July 29, 2016
First decision: September 28, 2016
Revised: December 23, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 28, 2017
Processing time: 214 Days and 14.2 Hours
To investigate the relationship between baseline platelet count, clauss fibrinogen, maximum amplitude (MA) on thromboelastography, and blood loss in orthotopic liver transplantation (OLT).
A retrospective analysis of our OLT Database (2006-2015) was performed. Baseline haematological indices and intraoperative blood transfusion requirements, as a combination of cell salvage return and estimation of 300 mls/unit of allogenic blood, was noted as a surrogate for intraoperative bleeding. Two groups: Excessive transfusion (> 1200 mL returned) and No excessive transfusion (< 1200 mL returned) were analysed. All data analyses were conducted using IBM SPSS Statistics version 23.
Of 322 OLT patients, 77 were excluded due to fulminant disease; redo transplant or baseline haemoglobin (Hb) of < 80 g/L. One hundred and fourteen (46.3%) were classified into the excessive transfusion group, 132 (53.7%) in the no excessive transfusion group. Mean age and gender distribution were similar in both groups. Baseline Hb (P ≤ 0.001), platelet count (P = 0.005), clauss fibrinogen (P = 0.004) and heparinase MA (P = 0.001) were all statistically significantly different. Univariate logistic regression with a cut-off of platelets < 50 × 109/L as the predictor and Haemorrhage as the outcome showed an odds ratio of 1.393 (95%CI: 0.758-2.563; P = 0.286). Review of receiver operating characteristic curves showed an area under the curve (AUC) for platelet count of 0.604 (95%CI: 0.534-0.675; P = 0.005) as compared with AUC for fibrinogen level, 0.678 (95%CI: 0.612-0.744; P ≤ 0.001). A multivariate logistic regression shows United Kingdom model for End Stage Liver Disease (P = 0.006), Hb (P = 0.022) and Fibrinogen (P = 0.026) to be statistically significant, whereas Platelet count was not statistically significant.
Platelet count alone does not predict excessive transfusion. Additional investigations, e.g., clauss fibrinogen and viscoelastic tests, provide more robust assessment of bleeding-risk in thrombocytopenia and cirrhosis.
Core tip: Current literature describing bleeding risk in thrombocytopenia and cirrhosis does not take into account the impact of fibrinogen. The minimal platelet count to form a clot with normal strength is unknown, and would be influenced by fibrinogen. Viscoelastic testing, particularly maximum amplitude (MA, thromboelastography) or maximum-clot-firmness (MCF, thromboelastometry), reflects platelet-fibrinogen interaction and allows assessment of clot strength. Low platelet count and low fibrinogen levels lead to low MA/MCF and correlate strongly with increased bleeding tendency. Assessment of platelet count alone does not accurately predict bleeding, but is useful in conjunction with other indices such as clauss fibrinogen and MA/MCF.
- Citation: Thakrar SV, Mallett SV. Thrombocytopenia in cirrhosis: Impact of fibrinogen on bleeding risk. World J Hepatol 2017; 9(6): 318-325
- URL: https://www.wjgnet.com/1948-5182/full/v9/i6/318.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i6.318
Thrombocytopenia is a common finding in patients with advanced liver disease. In most instances it is well tolerated but it is traditionally thought to increase the likelihood of surgical or traumatic bleeding. Moderate thrombocytopenia (defined as platelet count < 50 × 109/L) occurs in approximately 13% of those with liver disease and is associated with significant morbidity[1]. A number of factors contribute to thrombocytopenia in liver disease, including low thrombopoietin levels, and sequestration of platelets in hypersplenism as a result of portal hypertension[2].
Derangements of other haematological indices in cirrhosis include prolongation of prothrombin time (PT), prolongation of activated thromboplastin time (APTT) and dysfibrinogenaemia. Conventionally, these changes were thought to lead to an increased bleeding risk. Over the last 10 years, however, a new paradigm of haemostasis in liver disease has been described. There is now considered to be a “rebalancing” with a reduction in pro-coagulant molecules being accompanied by a reduction in anticoagulant molecules. Thrombin generation is normal, or even increased and patients with cirrhosis are now considered to have an elevated risk of thrombosis rather than have complications of bleeding[3].
Standard tests of coagulation such as PT and APTT do not accurately reflect coagulation status in vivo, as they cannot assess cellular contributions or the effects of anticoagulant molecules. In-vitro studies in cirrhosis have shown a compensatory increase in levels of Von Willebrand Factor (vWF) - a platelet adhesion protein and reductions in ADAMTS-13, the cleavage enzyme responsible for the breakdown of vWF[4]. Additionally, platelet hyperactivity has been reported in cholestatic liver disease[5]. A systematic review evaluating platelet function concluded that in patients with cirrhosis, primary haemostasis is not defective[6].
Whole blood viscoelastic testing provides valuable information about dynamic clot formation. It measures changes in clot tensile strength with time and is used in goal-orientated algorithms to target transfusion. Clot strength is assessed by maximum amplitude (MA) or maximum clot firmness (MCF) and is influenced by both platelet count and by fibrinogen level. MA or MCF can be maintained in the face of low platelet counts by normal or increased levels of fibrinogen[7]. Whole blood global viscoelastic tests such as thromboelastography (TEG®) or thromboelastometry (ROTEM®) may provide more clinically relevant information about coagulation profiles in liver disease. Increasingly observed blood transfusion free orthotopic liver transplantation (OLT) suggests that conventional tests of coagulation are inadequate in predicting bleeding.
Studies of low platelet count in cirrhosis suggest that thrombin generation may be reduced in cases of severe thrombocytopenia[8]. In-vitro studies, however, have shown that a platelet count of 20-30 × 109/L is likely to be adequate to initiate haemostasis and generate enough thrombin to allow normal MA on TEG[9]. Despite a reduction in thrombin production, clot strength is likely to be adequate if the appropriate substrates for clot formation are present. Moderate reductions in platelet count, therefore, do not necessarily indicate an increased risk of bleeding in liver disease.
British Haematology Society guidelines for the use of platelet transfusions and consensus guidelines for percutaneous image guided interventions[10,11] recommend the prophylactic transfusion of platelets to a count of > 50 × 109/L prior to liver biopsy to prevent complications of bleeding. In view of current knowledge of coagulation and haemostasis in cirrhosis, the objectives of this study were to investigate the relationship between baseline platelet count, clauss fibrinogen, MA on TEG and the volume of blood transfused in patients undergoing orthotopic liver transplantation.
A retrospective study of patients who had undergone OLT at the Centre for Hepatobiliary Surgery, Royal Free London between 2006 and 2015 was conducted. The cohort of patients reviewed had transplantation for chronic end stage liver disease, with or without hepatocellular carcinoma. Data from their intraoperative course was retrieved from a database formed as part of standard care. Those with acute fulminant liver failure, paracetamol overdose or redo transplantation were excluded. Patients with starting haemoglobin of less than 80 g/L were also excluded in view of an increased risk of intraoperative blood transfusion. Data was anonymised and institutional research and development departmental approval was obtained for its use.
Patient demographic data, baseline haematological results, number of packed red cell units transfused intra-operatively and volume of cell salvaged blood returned to patients was retrieved electronically.
Baseline variables were retrieved from the OLT database and included patient characteristics such as age, gender, diagnosis and severity scoring with United Kingdom model for End Stage Liver Disease (UKELD score). Baseline clinical measurements were point-of-care (i.e., Medical diagnostic testing at the point of care) samples taken at the time of anaesthesia for liver transplantation from arterial catheters and measurements included haemoglobin concentration (Hb) and platelet count by pocH-100i full blood count analyser (Sysmex Europe GmbH). TEG variables were from TEG® 5000 (Haemonetics, Braintree, MA, United States. United Kingdom TD in particular MA on heparinase TEG was assessed. Heparinase TEGs were used for analysis to remove any influence that may have been exerted by endogenous heparinoids and to standardize results. Laboratory Clauss fibrinogen levels using ACL-TOP 700 (Werfen, United Kingdom) were obtained prior to transplantation and did not exceed 24 h prior to anaesthetic start time. All assays are controlled and monitored using laboratory quality assurance processes.
As a surrogate for intraoperative blood loss, an estimation of 300 mL of blood in a packed red cell unit given to patients was made, and the volume summated with cell salvage return volume to give a total volume of blood returned. Patients were divided into 2 groups according to total volume of blood returned: ≤ 1200 mL (no excessive transfusion) and > 1200 mL (excessive transfusion).
Descriptive statistics were performed on baseline variables and comparisons made between excessive transfusion (ET) and no excessive transfusion (NET) groups. Univariate logistic regression was performed for each variable independently as the predictor, and ET as the binary outcome. Receiver operating characteristic (ROC) curves for baseline platelet count and for baseline clauss fibrinogen were also constructed and area under the curves calculated. Binomial logistic regression with each variable as the predictor and ET as the outcome was also performed. Predicted probabilities from the binomial logistic regression were used for further ROC analysis. The relationship between platelet count and blood volume returned as well as fibrinogen and blood volume returned was further investigated by linear regression modeling. All data analyses were conducted using IBM SPSS Statistics version 23. All statistical analyses were reviewed by Ms Fatima Jichi, a trained biostatistician with the department of Biostatistics, University College London.
Results for 323 patients were reviewed and of these 37 patients had either acute, fulminant liver failure or a redo-liver transplant and were excluded. A further 40 patients had a baseline Hb less than 80 g/L and were also excluded. Of the remaining 246 patients, 114 (46.3%) had excessive transfusion and 132 (53.7%) had no excessive transfusion.
Mean patient ages were 53 years (± 1 SD 10.04 years) and were similar in ET and NET groups. The gender distribution of patients was 72.8% male and 27.2% female with a similar divide in both groups. Mean UKELD was 56 (± 10.55) in the ET group and 51 (± 5.08) in the NET group (P ≤ 0.001). Liver disease due to Infection (33.3%) or Alcohol (29.6%) was the commonest aetiology. Interestingly, primary sclerosing cholangitis (PSC) was more common in the NET group (18.9%) vs the ET group (7.9%). χ2 analysis of aetiologies in both groups revealed ×χ2 = 18.81, P = 0.016. Baseline Hb, platelet count, clauss fibrinogen and hep MA were all statistically significantly different between the two groups (Table 1).
| Excessive transfusion | No excessive transfusion | Total | P value | |
| n | 114 (46.3%) | 132 (53.7%) | 246 | |
| Age (yr) | ||||
| Mean | 53.12 | 52.72 | 52.91 | 0.757 |
| SD | 9.68 | 10.38 | 10.04 | |
| Gender | ||||
| Female | 28 (24.6%) | 39 (29.5%) | 67 (27.2%) | 0.381 |
| Male | 86 (75.4%) | 93 (70.5%) | 179 (72.8%) | |
| UKELD | ||||
| Mean | 56.11 | 51.11 | 53.02 | < 0.001 |
| SD | 10.55 | 5.08 | 6.22 | |
| Diagnosis | ||||
| ALD | 44 (38.6%) | 29 (21.9%) | 73 (29.6%) | |
| Infectious | 40 (35.1%) | 42 (31.8%) | 82 (33.3%) | |
| NASH | 4 (3.5%) | 6 (4.5%) | 10 (4.1%) | |
| PSC | 9 (7.9%) | 25 (18.9%) | 34 (13.8%) | |
| PBC | 3 (2.6%) | 6 (4.5%) | 9 (3.7%) | |
| AIH | 4 (3.5%) | 3 (2.3%) | 7 (2.8%) | |
| Wilsons | 1 (0.8%) | 0 (0%) | 1 (0.4%) | |
| Haemochromatosis | 1 (0.8%) | 0 (0%) | 1 (0.4%) | |
| Misc | 8 (7.0%) | 21 (15.9%) | 29 (11.8%) | |
| Hb (g/L) | ||||
| Mean | 101.90 | 109.71 | 106.09 | < 0.001 |
| SD | 16.89 | 15.38 | 16.53 | |
| Platelets (× 109/L) | ||||
| Mean | 83.18 | 107.29 | 96.12 | 0.005 |
| SD | 55.03 | 75.14 | 67.53 | |
| Median | 67 | 84.5 | 76.0 | |
| IQR | 42.5 | 77.5 | 61.25 | |
| Fibrinogen (g/L) | ||||
| Mean | 1.96 | 2.60 | 2.3 | 0.004 |
| SD | 0.91 | 1.15 | 1.09 | |
| Hep MA (mm) | ||||
| Mean | 42.15 | 47.71 | 45.14 | 0.001 |
| SD | 12.68 | 11.87 | 12.54 | |
| Total blood returned (mL) | ||||
| Mean | 3323 | 487 | 1802 | |
| SD | 2536 | 419 | 2251 |
A comparison of patient demographics and baseline measurements between those with platelet count < 50 and those ≥ 50 × 109/L is described in Table 2. Baseline fibrinogen was statistically significantly different between those with low platelet count (mean = 1.78 ± 0.62) and those without (2.45 ± 1.15, P ≤ 0.001). Baseline hep MA was also significantly different in the 2 groups (35.28 ± 9.49 vs 47.85 ± 11.93, P ≤ 0.001). The total volume of blood returned was not significantly different between the 2 groups (P = 0.69).
| Platelet count < 50 | Platelet count ≥ 50 | P value | |
| n | 53 (21.5%) | 193 (78.5%) | |
| Age (yr) | |||
| Mean | 51.23 | 53.37 | 0.13 |
| SD | 10 | 9.04 | |
| Median | 52.38 | 55.26 | |
| Range | 52 | 50 | |
| Gender | |||
| Female | 40 (75.5%) | 139 (72%) | 0.62 |
| Male | 13 (24.5%) | 54 (28%) | |
| UKELD | |||
| Mean | 54.16 | 52.72 | 0.09 |
| SD | 5.06 | 6.47 | |
| Diagnosis | |||
| ALD | 10 (19%) | 63 (33%) | |
| Infectious | 30 (56%) | 52 (27%) | |
| NASH | 3 (6%) | 7 (4%) | |
| PSC | 5 (9%) | 29 (15%) | |
| PBC | 1 (2%) | 8 (4%) | |
| AIH | 2 (4%) | 5 (2%) | |
| Wilsons | 0 | 1 (0.5%) | |
| Haemochromatosis | 0 | 1 (0.5%) | |
| Misc | 2 (4%) | 27 (14%) | |
| Hb (g/L) | |||
| Mean | 106.34 | 106.03 | 0.89 |
| SD | 15.08 | 16.94 | |
| Fibrinogen (g/L) | |||
| Mean | 1.78 | 2.45 | < 0.001 |
| SD | 0.62 | 1.15 | |
| Hep MA (mm) | |||
| Mean | 35.28 | 47.85 | < 0.001 |
| SD | 9.49 | 11.93 | |
| Total blood returned (mL) | |||
| Mean | 1692.13 | 1831.6 | 0.69 |
| SD | 1426.72 | 2431.54 | |
| Median | 1500 | 1110 | |
| Range | 6305 | 21237 |
A logistic regression analysis (Table 3) was performed to ascertain the independent effects of age, gender, UKELD, baseline Hb, baseline platelet count, baseline platelet count < 50 × 109/L or ≥ 50 × 109/L as a binary value, baseline clauss fibrinogen level and baseline heparinise MA on likelihood of excessive transfusion. UKELD (P ≤ 0.001), HB (P ≤ 0.001), platelet count (P = 0.007), clauss fibrinogen (P ≤ 0.001) and Hep MA (P = 0.001) were all statistically significant. A cut off value of platelet count less than 50 was not a good predictor of excessive transfusion (P = 0.286).
| Odds ratio | 95%CI | P value | |
| Age | 1.004 | 0.979-1.029 | 0.76 |
| Gender | 1.288 | 0.730-2.271 | 0.382 |
| UKELD | 1.130 | 1.076-1.188 | < 0.001 |
| Hb | 0.97 | 0.954-0.986 | < 0.001 |
| Platelet count | 0.994 | 0.990-0.998 | 0.007 |
| Platelets (Binary cut off < 50 and ≥ 50) | 1.393 | 0.758-2.563 | 0.286 |
| Fibrinogen | 0.523 | 0.388-0.703 | < 0.001 |
| Hep MA | 0.963 | 0.942-0.984 | 0.001 |
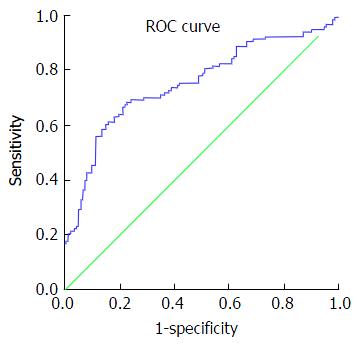
Review of ROC curves showed an area under the curve (AUC) for platelet count of 0.604 (standard error: 0.036; 95%CI: 0.534-0.675; P = 0.005). AUC for fibrinogen level was 0.678 (standard error: 0.034; 95%CI: 0.612-0.744; P ≤ 0.001) (Figure 1).
A multivariate logistic regression with all covariates with P ≤ 0.1 from univariate logistic regression added to a model, shows UKELD (P = 0.006), Hb (P = 0.022) and Fibrinogen (P = 0.026) to be statistically significant. Platelet count was not statistically significant (Table 4).
| OR | 95%CI | P value | |
| UKELD | 1.081 | 1.023-1.143 | 0.006 |
| Hb | 0.977 | 0.958-0.997 | 0.022 |
| Platelets | 0.999 | 0.994-1.004 | 0.700 |
| Fibrinogen | 0.682 | 0.487-0.955 | 0.026 |
| Hep MA | 0.986 | 0.957-1.015 | 0.338 |
The AUC from ROC curve analysis of predicted probabilities from multivariate logistic regression was 0.749 (standard error: 0.032; 95%CI: 0.686-0.812; P ≤ 0.001), suggesting that variables need to be considered together to better predict excessive transfusion (Figure 2).
Further investigation of the relationship between baseline platelet count and total volume of blood returned to patients showed that for every 1 point increase in platelet count, a 4.9 mL (P = 0.19) reduction in total blood volume returned was achieved. For every 1-point increase in fibrinogen level, however, a reduction of 525.95 mL (P ≤ 0.001) of blood returned to the patient was achieved (Table 5).
| Model | Regression coefficient B | 95%CI | P value |
| Platelet count | 2280.72-4.99 | -9.142-0.829 | < 0.001 |
| Fibrinogen | 3014.63-525.95 | -776.88-275.02 | < 0.001 |
Guidelines recommend the prophylactic transfusion of platelets to achieve a count of 50 × 109/L prior to invasive procedures such as liver biopsy. Understanding of haemostasis in the cirrhotic population has altered with the concept of a “rebalanced” haemostatic profile in liver disease. This study evaluated differences in baseline platelet count, fibrinogen levels, viscoelastic tests and blood transfusion requirements in those undergoing OLT for chronic liver disease. Patients in our study were divided according to whether they received excessive blood transfusion or not. On comparison, the 2 groups were well matched for gender and age, although UKELD was found to be significantly different. Severity of liver disease, in the form of childs-pugh score and Model for end stage liver disease (MELD) scoring, is associated with a prediction of increased intraoperative transfusion requirement[12]. This may explain the difference in UKELD between the 2 groups.
It was interesting to note a higher preponderance of PSC as the aetiology of liver disease in those not requiring excessive transfusion. Hypercoagulable haemostatic profiles have been described for those with biliary cirrhotic disease and in general this population does not have thrombocytopenia or low fibrinogen[5].
A statistically significant difference in baseline Hb, platelet count, Clauss fibrinogen and MA on heparinase TEG was observed between those who received excessive transfusion vs those who did not. This highlights an association with bleeding risk and indicates that possibly all of these measurements would be useful in predicting increased blood transfusion requirements.
Logistic regression performed to evaluate the probability of excessive transfusion with each variable shows clearly that a platelet threshold value of 50 × 109/L is not a good predictor of blood transfusion in this population. Although there has been a previously described association between a reduction in thrombin generation with a reduction in platelet count[8], the cut off value of platelets requiring transfusion in cirrhosis is likely to lie significantly below 50 x 109/L described in guidelines. One small prospective study of liver biopsy in severe thrombocytopenia associated with haematological malignancy suggests the likely cut off value lies below 30 × 109/L[13].
Much of the literature describing bleeding risk in cirrhosis and thombocytopenia does not take into account fibrinogen level. The minimal platelet count required for normal clot strength is unknown and is markedly affected by fibrinogen. MA on TEG is a composite reflection of platelet-fibrinogen interaction and can be used to assess clot strength. Assessment of MA shows that even in the face of a low platelet count, adequate clot strength may still be achieved if fibrinogen is normal or raised. A combination of low platelet count and low fibrinogen level always results in low MA and is strongly associated with an increased bleeding tendency[14,15]. Platelet count alone is not a true indicator of clot strength; therefore, if baseline platelet count is low, assessment of MA is useful in guiding whether to replace fibrinogen or to transfuse platelets. Thrombocytopenia predominately leads to a reduction in blood clot strength displayed as MA on TEG[16], but fibrinogen also contributes to clot firmness. The effect of the administration of fibrinogen concentrates in thrombocytopenia, at a count of 30 × 109/L, in the pig model has been studied. Velik-Salchner et al[17] showed an improvement in impaired clot formation and a reduction in blood loss in thrombocytopenia with the addition of fibrinogen.
The impact of fibrinogen on bleeding risk can be observed in the results of this study. Baseline clauss fibrinogen level is likely to have a greater protective effect than the other baseline haematological variables (OR: 0.52; 95%CI: 0.388-0.705; P ≤ 0.001). Similarly, Odds ratios for fibrinogen on multivariate analysis are the lowest when compared with other variables (0.682, 95%CI: 0.487-0.955) (Table 4). On comparison of AUCs on ROC curve analysis, baseline fibrinogen level is a better predictor of excessive transfusion than platelet count. Interestingly, linear regression analysis shows a 525.95 mL reduction in blood returned to patients with each 1-unit increase in baseline fibrinogen level (i.e., 1 g of fibrinogen factor concentrate) (Figure 1). In comparison, 1 pool of platelets (one adult therapeutic dose) increases platelet count by 20 × 109/L[11], equating to a 99.8 mL reduction in blood transfusion if the linear model is used. ROC analysis of predicted probabilities on multivariate analysis show an AUC greater than that of platelet count alone, indicating a better predictive value on assessing all the demographic and haematological variables simultaneously (Figure 2).
Although there are a number of in-vitro investigations into the associations between thrombocytopenia and fibrinogen concentration and clot strength, there is a lack of evidence relating to the influence of the two in vivo. There is also a lack of substantial evidence to validate a cut off value for prophylactic platelet transfusion in the cirrhotic population. This study highlights the contribution of fibrinogen in reducing the risk of excessive blood transfusion, and therefore bleeding risk.
Excessive transfusion was used as a surrogate for intraoperative bleeding in this study. Measurement of blood loss in suction and weight of swabs would provide more accurate information with regard to blood loss, but this information was unavailable retrospectively. Furthermore, OLT is complex surgery with other influences on haemorrhage apart from the haemostatic picture. These include presence of portal hypertension and varices, difficult operative dissection with multiple adhesions, surgical technique (i.e., Caval replacement surgery or “piggy back” technique for reperfusion) and the volume of fluid given to the patient[12,18]. Additionally, baseline low haemoglobin values increase the likelihood of requiring intraoperative blood transfusion. Low haematocrit also has an impact on laminar flow in blood vessels and therefore a disturbance in primary haemostasis may occur in anaemia[19]. We excluded those with baseline haemoglobin of < 80 g/L for this reason.
Although the results of our study point to the usefulness of measuring baseline clauss fibrinogen in conjunction with platelet count and assessment of TEG, we are unable to assess for specific cut off values for baseline platelet count and fibrinogen level. Results would require validation against external data sets to allow for cut off values, requiring further prospective research.
The transfusion of platelets is not without risk. Complications of platelet transfusion include allergic or anaphylactic reactions, haemolytic and non-haemolitic transfusion reactions, transfusion related acute lung injury and septic transfusion reactions of bacterial origin[20]. Furthermore, in liver transplantation, platelets have been shown to be involved in ischaemic reperfusion injury by interactions with activated sinusoidal endothelium and induction of apoptosis. Perioperative platelet transfusion has been identified as an independent risk factor for adverse post-operative outcomes[21]. In a large retrospective analysis of patients undergoing cardiac surgery, those receiving platelets were at an increased risk of postoperative infection, stroke and multiorgan failure[22].
The availability of platelets, largely due to short storage life, can also be limited. By demonstrating a lack of association between excessive blood transfusion and a threshold platelet count of 50 × 109/L, the question of unnecessary prophylactic platelet transfusion arises. If an increase in fibrinogen levels by 1 g reduces the volume of blood transfused significantly, the usefulness of fibrinogen concentrate rather than platelet transfusion should be considered. Fibrinogen concentrate appears to have a better safety profile than cryoprecipitate and fresh frozen plasma, particularly when considering the risk of blood borne infection. Other advantages of its use include the accuracy and rapidity of its administration[23].
In conclusion, further prospective evaluation to assess for the true baseline platelet count at which bleeding risk is increased needs to be performed. Studies have described an increase likelihood of bleeding associated with invasive procedure with low platelet counts (< 75 × 109/L), but it is important to note that these studies failed to assess the contribution of fibrinogen to clot strength in cases of thrombocytopenia[24]. Additional haematological indices such as Clauss fibrinogen and the use of viscoelastic testing may provide a more robust assessment of bleeding risk in thrombocytopenia associated with cirrhosis.
We would like to appreciate Dr. Eleanor Galtrey and Dr. Simon Goddard, Anaesthetic fellows, for their contribution in obtaining and processing data for this study. We also appreciate Felicity Blake, Point of Care laboratory manager for her continuing support in facilitating point of care assessment in patients undergoing OLT.
Thrombocytopenia is a common finding in patients with advanced liver disease. Concomitantly an increase in value of conventional tests of coagulation including prothombin time and activated partial thromboplastin time occurs. Anew paradigm of haemostasis in liver disease has been described and there is now considered to be a “rebalancing” of haemostasis in cirrhosis. As a result, the authors investigated the relationship between platelet count, clauss fibrinogen, thromboelastography, and blood loss in orthotopic liver transplantation (OLT).
Much of the literature describing bleeding risk in cirrhosis and thombocytopenia does not take into account fibrinogen level. There have been recent studies that show the effect of the administration of fibrinogen concentrates in thrombocytopenia, at a count of 30 × 109/L, in the pig model. Velik-salchner et al showed an improvement in impaired clot formation and a reduction in blood loss in thrombocytopenia with the addition of fibrinogen. Further studies evaluating the contribution of fibrinogen to clot strength in thrombocytopenia in humans are required.
Currently, there are limited studies evaluating the contribution of fibrinogen to bleeding risk. The increased use of thromboelastography and in particular the evaluation of clot strength with other haematological indices could provide a more robust method of evaluating bleeding risk and requires further investigation.
The transfusion of platelets does not come without risks to patients. The availability of this valuable resource is also limited. The study highlights that platelet count alone is not a true indicator of clot strength and the threshold at which bleeding occurs in the face of a normal fibrinogen level needs further evaluation. Many current guidelines suggest prophylactic transfusion of platelets up to a count of 50 × 109/L, when in fact the threshold for bleeding risk is likely to be lower.
Interesting study with respectable sample size. Τhis article evaluated retrospectively the association between coagulation parameters and need for transfusion in patients undergoing OLT. The study is well written with a clear message.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chuang WL, Manolakopoulos S, Procopet B, Wong GLH S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 2. | Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 555] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost. 2010;8:1994-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 420] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Pihusch R, Rank A, Göhring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55:1415-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Gunduz E, Akay OM, Bal C, Gulbas Z. Can thrombelastography be a new tool to assess bleeding risk in patients with idiopathic thrombocytopenic purpura? Platelets. 2011;22:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell’Era A, Fabris F, Salerno F, Mannucci PM. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Kawaguchi C, Takahashi Y, Hanesaka Y, Yoshioka A. The in vitro analysis of the coagulation mechanism of activated factor VII using thrombelastogram. Thromb Haemost. 2002;88:768-772. [PubMed] |
| 10. | Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WA. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 438] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 11. | Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 385] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Feltracco P, Brezzi M, Barbieri S, Galligioni H, Milevoj M, Carollo C, Ori C. Blood loss, predictors of bleeding, transfusion practice and strategies of blood cell salvaging during liver transplantation. World J Hepatol. 2013;5:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Wallace MJ, Narvios A, Lichtiger B, Ahrar K, Morello FA, Gupta S, Madoff DC, Hicks ME. Transjugular liver biopsy in patients with hematologic malignancy and severe thrombocytopenia. J Vasc Interv Radiol. 2003;14:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R, Gill R, McSporran W, Moor P, Rao Baikady R. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia. 2016;71:829-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Lang T, Johanning K, Metzler H, Piepenbrock S, Solomon C, Rahe-Meyer N, Tanaka KA. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Oshita K, Az-ma T, Osawa Y, Yuge O. Quantitative measurement of thromboelastography as a function of platelet count. Anesth Analg. 1999;89:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Velik-Salchner C, Haas T, Innerhofer P, Streif W, Nussbaumer W, Klingler A, Klima G, Martinowitz U, Fries D. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Donohue CI, Mallett SV. Reducing transfusion requirements in liver transplantation. World J Transplant. 2015;5:165-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | AlMomani T, Udaykumar HS, Marshall JS, Chandran KB. Micro-scale dynamic simulation of erythrocyte-platelet interaction in blood flow. Ann Biomed Eng. 2008;36:905-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Kiefel V. Reactions Induced by Platelet Transfusions. Transfus Med Hemother. 2008;35:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Pereboom IT, Lisman T, Porte RJ. Platelets in liver transplantation: friend or foe? Liver Transpl. 2008;14:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, Murkin J, Nadel A. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus. 2012;10:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 24. | Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8:899-902; quiz e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |