Published online Oct 18, 2017. doi: 10.4254/wjh.v9.i29.1141
Peer-review started: May 27, 2017
First decision: July 11, 2017
Revised: July 26, 2017
Accepted: August 16, 2017
Article in press: August 17, 2017
Published online: October 18, 2017
Processing time: 144 Days and 7.7 Hours
To analyze liver tests before and following treatment with herbal Traditional Chinese Medicine (TCM) in order to evaluate the frequency of newly detected liver injury.
Patients with normal values of alanine aminotransferase (ALT) as a diagnostic marker for ruling out pre-existing liver disease were enrolled in a prospective study of a safety program carried out at the First German Hospital of TCM from 1994 to 2015. All patients received herbal products, and their ALT values were reassessed 1-3 d prior to discharge. To verify or exclude causality for suspected TCM herbs, the Roussel Uclaf Causality Assessment Method (RUCAM) was used.
This report presents for the first time liver injury data derived from a prospective, hospital-based and large-scale study of 21470 patients who had no liver disease prior to treatment with herbal TCM. Among these, ALT ranged from 1 × to < 5 × upper limit normal (ULN) in 844 patients (3.93%) and suggested mild or moderate liver adaptive abnormalities. However, 26 patients (0.12%) experienced higher ALT values of ≥ 5 × ULN (300.0 ± 172.9 U/L, mean ± SD). Causality for TCM herbs was RUCAM-based probable in 8/26 patients, possible in 16/26, and excluded in 2/26 cases. Bupleuri radix and Scutellariae radix were the two TCM herbs most commonly implicated.
In 26 (0.12%) of 21470 patients treated with herbal TCM, liver injury with ALT values of ≥ 5 × ULN was found, which normalized shortly following treatment cessation, also substantiating causality.
Core tip: Worldwide research on herbal medicine safety is still limited. Adverse effects are range from clinically not relevant to more severe ones including suspected liver injury. We conducted a prospective hospital-based study to report the number of new liver injury in patients with no liver disease prior to treatment with herbal Traditional Chinese Medicine. Liver injury was detected in 26/21470 patients (0.12%) with alanine aminotransferase values of ≥ 5 × upper limit normal. The Roussel Uclaf Causality Assessment Method assessed the causality of suspected cases and showed a causality level of “possible” for the majority of the liver injury cases.
- Citation: Melchart D, Hager S, Albrecht S, Dai J, Weidenhammer W, Teschke R. Herbal Traditional Chinese Medicine and suspected liver injury: A prospective study. World J Hepatol 2017; 9(29): 1141-1157
- URL: https://www.wjgnet.com/1948-5182/full/v9/i29/1141.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i29.1141
Traditional Chinese Medicine (TCM) with the focus on its herbal constituents is an individual treatment option with growing worldwide popularity[1,2], despite still insufficiently documented efficacy[3] and known adverse reactions[4,5]. In particular, the risk of liver injury in patients under therapy using TCM herbs has appeared as a major problem for many decades[6]. This issue has been known at least since 1983[7] and is in line with many subsequent case reports and case series[8-15]. However, there have been attempts to downgrade the hepatotoxic risk of herbal TCM but such proposals were vague and rejected since no proof for this claim was provided[16]. Other problems were recognized as variables, which confounded establishing valid causality[17-19]. Among these variables were co-medication with other herbal products or synthetic, potentially hepatotoxic Western drugs, low case data quality, incomplete consideration of alternative causes, and questionable quality of herbal TCM products. Indeed, some herbal TCM products are confronted with problems of misidentified herbs, impurities, pesticides, heavy metals, or adulteration by Western drugs to enhance or provide efficacy[20-24].
Other challenges included the fact that not all publications used a sophisticated, robust causality assessment method. Nevertheless, Roussel Uclaf Causality Assessment Method (RUCAM)[25] was successfully applied in many cases of suspected liver injury by TCM herbs[25,26], including as examples some more recent reports[9,27-31]. Further, there was also uncertainty as to whether the observed liver disease could have been present prior to the initiation of the TCM use rather than caused by the herbal TCM therapy itself. Meeting the objections regarding pre-existing liver disease would have required an analytical approach whereby a study protocol is prospectively applied to patients without any liver disease, in whom therapy with herbal TCM is intended and liver tests can be analyzed under such treatment conditions. In patients with new abnormal liver tests under the therapy, causality for the suspected herbal TCM product can easily be assessed using RUCAM. So far, such a systematic prospective, large-scale investigation has not been published on liver-healthy individuals, at least not in the scientific literature available in the English language.
In this report, we present for the first time liver injury data derived from a prospective, hospital-based and large-scale study of 21470 patients, who had no liver disease prior to treatment by herbal TCM. This study focused on the effect of herbal TCM use on the liver integrity of patients with normal liver test results of alanine aminotransferase (ALT), used as a diagnostic biomarker to exclude liver disease. Since this study followed a strict protocol and was conducted under clinical conditions, the risk of confounding variables appeared low and should provide valid data.
TCM study cohort: To assess to what extent herbal TCM treatment leads to liver injury, we designed a protocol for a prospective study in consecutive patients, who were admitted to the Hospital for TCM in Bad Kötzting, Germany. Hospital admission was commonly arranged by patients’ general practitioners or medical specialists with the intention of a TCM-based therapy. No restrictions on admission exist for patients residing in Germany, as hospital costs are covered by most German statutory sickness funds. Treatment modalities including indications, choice of specific herbal TCM products, daily dosage, and duration of therapy are based on the recommendations of the Beijing University of Chinese Medicine (BUCM), China[32,33].
Included in the TCM study cohort were all in-patients with normal ALT values on admission or the following day, who had received treatment with TCM herbs during their hospital stay, and were discharged between January 1, 1994, and December 31, 2015. Initial ALT results were obtained along with a routine blood sampling analysis. The inclusion criteria of normal serum ALT activities on admission ensured a lack of a preexisting liver disease that could later confound the potential diagnosis of liver injury along with herbal TCM treatment. For reasons of transparency, these patients represent the TCM study cohort. ALT was chosen as a specific diagnostic biomarker to clearly exclude or establish a liver disease[25,34]. Patients with increased ALT values on admission were excluded from the study.
To ensure the good medical care of the patients, six German hospital physicians and eight Chinese physicians who trained at the University of Chinese Medicine in Beijing were in charge of the patients at the 80-bed TCM hospital[35]. Also included in the team was also a pharmacist. On admission, hospital physicians provided a complete physical examination for all patients and recorded their past medical history. They also assessed all normal and elevated laboratory values and documented these together with any adverse or medical event during hospitalization in a standardized adverse event record as part of a hospital-based safety and quality assurance program. During the last three days before discharge, the occurrence of liver injury was tested using serum ALT as the appropriate diagnostic tool.
Treatment with TCM was carried out with TCM herbs, given as decoctions from raw materials[36,37]. Overall TCM treatment may also include acupuncture, Chinese manual therapy, and relaxation therapy, as outlined previously[19,35]. Western therapies were continued or prescribed if necessary. Details of prescriptions, each single Western drug, all specific TCM treatment modalities, and the duration of treatment were documented systematically in the hospital files.
Herbal TCM products were obtained from China[38,39]. Prior to use in patients, all herbal TCM products delivered to the hospital underwent a comprehensive preclinical drug control program under the guidance of the Center for Drug Research of the Ludwig-Maximilian University Munich and other drug control centers in China. For herbal TCM product quality and safety assessment, established methods were used that included HPLC, colored TLC photographs, and botanical authenticity proof[40]. This approach aimed to reduce the risk of possible falsification of the herbal products and to ensure concentrations of heavy metals, aflatoxins, and microbial contamination were within the allowed limits. Some of the herbal products were thus rejected for human use before being prescribed to any patient, mostly due to a lack of pharmaceutical quality criteria or detection of contaminants outside the regulatory requirements. All herbal TCM products were also analyzed for microbial contamination[41].
Liver injury study cohort: The liver injury cohort consists of and is limited to those patients of the TCM study cohort who experienced liver injury in connection with treatment with TCM herbs. Liver injury is defined by an elevated serum ALT activity of at least 5 × upper limit normal (ULN) in patients with normal ALT values on admission[25]. Case data of this liver injury cohort were recruited by further scrutinizing the files and adverse event reports supplied by the hospital physicians. Case identification covers age, sex, diagnosis, past medical history, treatment with herbal TCM drugs and conventional drugs, a course of laboratory data, and any adverse or medical event during hospitalization. The case details were recorded and summarized in individual narratives as part of the patients’ hospital documents.
For patients identified with newly emerging liver injury, the suspected herbal TCM products were analyzed and closely reviewed. The aim was to highlight TCM medications that might be associated with an increased risk of liver injury. As this safety analysis of herbal TCM drugs was an outcome study within a routine quality assurance program, approval by an ethical review board was not requested. All patients on admission provided informed written consent prior to study enrollment.
In line with a previous report[19] and the recommendations of the Chinese Society of Hepatology (GSH)[42], a causality assessment of herb induced liver injury (HILI) for individual cases and herbal TCM products was achieved using RUCAM[25]. This is the most commonly used liver-specific, and validated tool for liver injury cases, and a standard form was used to extract core elements of RUCAM[25]. This assessment requires the initial evaluation of liver injury criteria and its pattern in each suspected case. The core elements of RUCAM include: The time period from the beginning until the cessation of herb intake in relation to disease onset or from the cessation of herb use to the onset of the liver injury; de-challenge characteristics with a course of ALT values after cessation or continuation of the herb use; risk factors such as alcohol abuse, age and pregnancy; co-medication with synthetic drugs or other herbs; search for alternative causes with special care for all hepatitis types; available information on previous herbal hepatotoxicity; and response to unintentional re-exposure. RUCAM was performed for the hepatocellular type of injury, and scoring was independently conducted by three hospital physicians (Stefan Hager, Sabine Albrecht, Dieter Melchart). Final RUCAM scores commonly range from -5 to + 14 points and the resulting causality levels are defined as follows: ≤ 0 points, excluded causality; 1-2, unlikely; 3-5, possible; 6-8, probable; and ≥ 9, highly probable[25].
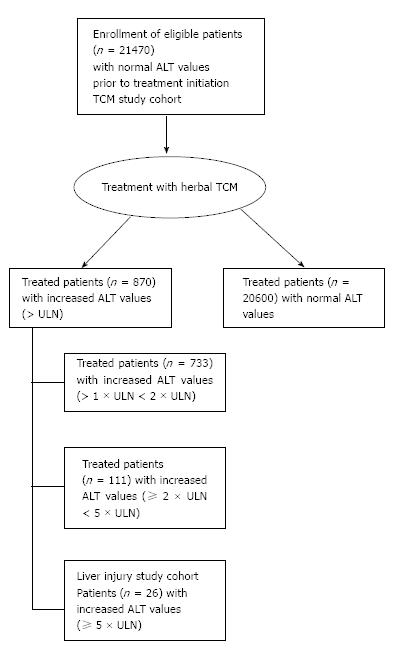
The inclusion criteria of the TCM study cohort were strict, especially the criterion of normal ALT values on admission and before the initiation of the treatment with herbal TCM. During the study period from 1994 to 2015, overall, 21896 patients were admitted to the hospital, but 426 patients of these had increased ALT values and were not eligible for inclusion in the study, which corresponds to 1.91%. Consequently, 21470 patients fulfilled the inclusion criteria of the TCM study cohort and were treated with herbal TCM (Figure 1). Among these patients of the TCM study cohort, ALT values remained in the normal range in 20600 patients (95.94%) under the TCM treatment. However, treatment led in 733 patients (3.41%) to abnormal ALT values (> 1 × ULN < 2 × ULN), in 111 patients (0.51%) to ALT values of ≥ 2 × ULN < 5 × ULN, and 26 patients (0.12%) showed ALT ≥ 5 × ULN, representing the liver injury study group (Figure 1).
ALT abnormalities with values in a range from 2 × up to 5 × ULN observed in 111 patients in the TCM study cohort are clearly caused by the herbal TCM treatment (Figure 1 and Table 1), with a preference of the ALT range between 2 × and 3 × ULN (Table 1). These small increases are commonly without clinical relevance and likely due to metabolic adaptation caused by events associated with the metabolism of TCM plant chemicals.
| ALT as multiples of the ULN | Patients (n) with ALT elevation |
| Total > 1 × ULN < 2 × ULN | 733 |
| ≥ 2 × ULN < 3 × ULN | 71 |
| ≥ 3 × ULN < 4 × ULN | 32 |
| ≥ 4 × ULN < 5 × ULN | 8 |
| Total ≥ 2 × ULN < 5 × ULN | 111 |
| ≥ 5 × ULN < 6 × ULN | 6 |
| ≥ 6 × ULN < 7 × ULN | 2 |
| ≥ 7 × ULN < 8 × ULN | 4 |
| ≥ 8 × ULN < 9 × ULN | 3 |
| ≥ 9 × ULN < 10 × ULN | 2 |
| ≥ 10 × ULN | 9 |
| Total ≥ 5 × ULN | 26 |
| Total number of elevated values of ALT | 870 |
Analysis of the TCM study cohort showed that the age was 52.7 ± 14.0 years (mean ± SD), and females accounted for 71.9% (Table 2). All patients in this cohort suffered for about 7.8 years (median) from chronic disorders that led to hospital admission (Table 2).
| Parameter | TCM study cohort | Liver injury study cohort | P (difference between both cohorts) |
| Patients (n) | 21470 | 26 | |
| ALT (U/L, mean ± SD) | NA | 300.0 ± 172.9 | |
| Females (%) | 71.9 | 84.6 | NS |
| Age (yr, mean ± SD) | 52.7 ± 14.0 | 57.6 ± 10.5 | NS |
| Chronic diseases (%) | 58.9 | 66.6 | NS |
| Duration of complaints (yr, median) | 7.8 | 8.5 | NS |
| Duration of herbal TCM treatment (d, median, range) | 20 (8-77) | 19.5 (7-28) | NS |
| Total dosage of herbal TCM (g, mean, range) | 88 ± 18 (18-208) | 95 ± 30 (43-155) | < 0.05 |
| Hospital stay (d, mean ± SD) | 26.2 ± 5.2 | 26.1 ± 4.0 | NS |
Chronic diseases or health conditions prevailed in the patients of the TCM study cohort (Table 2), whereby the majority experienced psychosomatic diseases as well as chronic pain syndromes. Additional diagnoses were, for example, hypertension and sleep disturbance. Herbal TCM decoctions were provided with four to five prescriptions of about 11 TCM herbs (ranging from a minimum of 6 to a maximum of 19 different herbs) per prescription during the hospital stay. The dosage of each herb was 6-15 g/d. The total daily dosage per prescription was mean 88 ± 18 g (range 18-208), provided by two dosages a day.
The liver injury study cohort consisting of 26 patients with serum ALT ≥ 5 × ULN (Table 1) merits further consideration (Table 2). Compared with the large TCM study cohort, patients in the liver injury study cohort were older (52.7 ± 14.0 years vs 57.6 ± 10.5 years) and contained a higher percentage of women (71.9% vs 84.6%), whereas the duration of the hospital stay was similar in both cohorts (Table 2). There is a long list of indications for herbal TCM treatment in the patients in the liver injury study group, along with individual TCM herbs that were used as medication (Table 3). For these patients with confirmed liver injury, details are given for maximum ALT values, which range from 140 U/L to 1052 U/L (Table 3).
| Cases | Indication for TCM treatment | Maxi-mum ALT (U/L) | Suspected TCM herbs | RUCAM-based causality |
| Patient 1 | Asthma Depression Lower back pain syndrome | 341 | Bupleuri radix Glycyrrhizae radix Scutellariae radix | Possible (score +4) |
| Patient 2 | Posttraumatic paralysis of both legs | 140 | Bupleuri radix Glycyrrhizae radix Scutellariae radix | Possible (score +3) |
| Patient 3 | Chronic bronchitis Emphysema Sleeping disorder | 234 | Bupleuri radix Ephedrae herba Glycyrrhizae radix Scutellariae radix | Probable (score +7) |
| Patient 4 | Chronic migraine | 168 | Bupleuri radix Glycyrrhizae radix | Probable (score +6) |
| Patient 5 | Post herpes zoster state Hypertension Diabetes mellitus | 330 | Bupleuri radix Dictamni radicis cortex Scutellariae radix | Excluded (score -1) |
| Patient 6 | Chronic migraine Cervico-brachial pain syndrome Low back pain syndrome Diarrhoea | 530 | Bombyx batryticatus (t) Psoraleae fructus (semen) Scutellariae radix | Possible (score +3) |
| Patient 7 | Lumbosacral plexus syndrome Cervicobrachial pain syndrome | 132 | Bupleuri radix Dictamni radices cortex Ephedrae herba Scutellariae radix | Possible (score +5) |
| Patient 8 | Polyneuropathy | 193 | Decoction; none identified suspected herb | Possible (score +3) |
| Patient 9 | Polymyalgia rheumatica Fibromyalgia | 162 | Bupleuri radix Scutellariae radix | Possible (score +4) |
| Patient 10 | Chronic migraine Tension headache | 195 | Bombyx batryticatus (t) Bupleuri radix Meliae toosendan fructus Scutellariae radix | Possible (score +3) |
| Patient 11 | Difficulty of walking Polyneuropathy Low back pain syndrome | 325 | Glycyrrhizae radix | Excluded (score -1) |
| Patient 12 | Chronic fatigue Depressive episodes Gastrointestinal symptoms | 751 | Meliae toosendan fructus | Probable (score +7) |
| Patient 13 | Low back pain syndrome Sleeping disorder | 389 | Cassiae semen | Possible (score +5) |
| Patient 14 | Chronic osteomyelitis | 1052 | Meliae toosendan fructus Scutellariae radix | Probable (score +7) |
| Patient 15 | Chronic fatigue Chronic cephalgia | 290 | Bupleuri radix Meliae toosendan fructus Scutellariae radix | Possible (score +4) |
| Patient 16 | Lichen sclerosus Cervical spondylosis | 715 | Bombyx batryticatus (t) Bupleuri radix Scutellariae radix | Possible (score +5) |
| Patient 17 | Chronic migraine Depression | 252 | Bombyx batryticatus (t) Bupleuri radix Cassiae semen Scutellariae radix | Probable (score +6) |
| Patient 18 | Spondylosis cervicalis Depression Migraine | 233 | Bombyx batryticatus (t) Bupleuri radix Scutellariae radix | Probable (score +6) |
| Patient 19 | Carcinophobia | 249 | Bombyx batryticatus (t) | Probable (score +6, 2011) |
| (2011) | Tinnitus | Bupleuri radix | ||
| (2014) | Allergic sensitivity syndrome | 295 | Ephedrae herba Puerariae radix Polygoni multiflora caulis Scutellariae radix | Probable (score +7, 2014) |
| Patient 20 | Migraine Lower back pain syndrome Depressive episodes | 207 | Bupleuri radix Ephedrae herba Glycyrrhizae radix Polygoni cuspidate rhizoma Scutellariae radix | Possible (score +4) |
| Patient 21 | Neurasthenia Fibromyalgia | 221 | Bupleuri radix Glycyrrhizae radix Rhei radix et rhizoma Scutellariae radix | Possible (score+5) |
| Patient 22 | Tension headache Somatoform pain disorder Polyarthritis | 361 | Glycyrrhizae radix Scutellariae radix | Possible (score +3) |
| Patient 23 | Alopecia cranialis totalis Hashimoto-Thyroiditis | 268 | Bupleuri radix Polygoni multiflori radix Psoraleae fructus (semen) | Possible (score +4) |
| Patient 24 | Chronic migraine Depression | 210 | Bombyx batryticatus (t) Bupleuri radix Polygoni multiflori caulis Scutellariae radix | Probable (score +6) |
| Patient 25 | Chronic pain syndrome | 359 | Bupleuri radix Scutellariae radix | Possible (score +5) |
| Patient 26 | Somatization Gastrointestinal symptoms | 182 | Bupleuri radix Glycyrrhizae radix Meliae toosendan fructus Scutellariae radix | Possible (score +3) |
TCM herbs were rarely applied as a mono-preparation, but mostly as mixtures consisting of several herbs adding up to 35 different drugs during the patients’ four-week stay. The daily dosage was 95 ± 30 g and thus slightly higher than in the TCM study cohort (Table 2). Among the many herbal TCM used by the 26 patients in the liver injury cohort, Bupleuri radix and Scuterllariae radix were the two TCM herbs most frequently implicated in liver injury, with variable RUCAM-based causality gradings. Most of the patients received one to six TCM drugs that were associated with potential liver injury as evidenced from the scientific literature, e.g., one patient (case 8) received six potentially hepatotoxic herbal TCM drugs during their hospital stay (Table 4).
| Potentially hepatotoxic TCM herbs | Total herbs (n) | RUCAM-based causality grading: Probable Cases | RUCAM-based causality grading: Possible Cases | RUCAM-based causality grading: Excluded Cases |
| Bombyx batryticatus(t) | 7 | 17, 18, 19, 24 | 6, 10, 16 | |
| Bupleuri radix | 19 | 3, 4, 17, 18, 19, 24 | 1, 2, 7, 9, 15, 16, 17, 20 21, 23, 25, 26 | 5 |
| Cassiae semen | 2 | 17 | 13 | |
| Dictamni radicis cortex | 2 | 7 | 5 | |
| Ephedrae herba | 4 | 3, 19 | 7, 20 | |
| Glycyrrhizae radix | 9 | 3, 4 | 1, 2, 20, 21, 22, 26 | 11 |
| Meliae toosendan | 5 | 12, 14 | 10, 15, 26 | |
| Polygoni cuspidate rhizoma | 1 | 23 | ||
| Polygoni multiflori caulis | 2 | 19, 24 | ||
| Polygoni multiflori radix | 1 | 23 | ||
| Psoraleae fructus (semen) | 1 | 23 | ||
| Puerariae radix | 1 | 19 | ||
| Rhei radix et rhizoma | 1 | 21 | ||
| Scutellariae radix | 20 | 3, 14, 17, 18, 19, 24 | 1, 2, 6, 7, 9, 10, 15, 16, 20, 21, 22, 25, 26 | 5 |
Narratives are essential for case details including treatment conditions and are presented for reasons of transparency and possible re-evaluation by peers or regulatory agencies. The narratives were documented in the hospital case records and are provided for all 26 patients in the liver injury study cohort (Table 5). In only one patient (case 8), none of the potential hepatotoxic TCM herbs was prescribed. Half of the patients were also under co-medication with synthetic drugs, initiated prior to admission, and only a few of these drugs are known for their hepatotoxic potential. The RUCAM analysis excluded all co-medicated drugs as the cause of liver injury in the cases under consideration (Table 5).
| Patients | Narratives |
| Case 1 male 51 yr (1994) | Patient with asthma (ICD-9 493.9), chronic low back pain (ICD-9 724.2), and reactive depression (ICD-9 300.4) treated with TCM decoctions with 8 drugs for 23 d: Angelicae sinensis radix, Asari herba, Astragali radix, Atractylodis macrocephalae rhizoma, Bupleuri radix, Glycyrrhizae radix, Paeoniae rubrae radix, Poria (parts), Scutellariae radix. Total daily dose: 80 g. Co-medication theophylline, fluocortolon. No alcohol abuse. Adverse events: nausea after drinking the decoction. ALT 293 U/L. First control after 5 d: ALT 341 U/L. Second control 14 d after discharge: ALT 17 U/L. No hepatitis serology RUCAM-based causality for Bupleuri radix, Glycyrrhizae radix and Scutellariae radix: Possible (score +4) |
| Case 2 male 73 yr (1994) | Patient suffered from unclear paralytic symptoms in both legs after trauma (ICD-9 344). Herbal TCM treatment with 9 drugs: Angelicae sinensis radix, Asari herba, Astragali radix, Atractylodis macrocephalae rhizoma, Bupleuri radix, Glycyrrhizae radix, Paeoniae rubrae radix, Poria (Stücke), Scutellariae radix for 22 d. Total daily dose: 60 g. Co-medication: digoxine, carbochol, nitrofurantoin, and sulfadiazine. No alcohol abuse. No adverse event symptoms. At discharge: ALT 140 U/L. First control 3 d later: ALT 100 U/L. Second control 3 wk later: ALT 22 U/L. No hepatitis serology RUCAM-based causality for Bupleuri radix, Glycyrrhizae radix, and Scutellariae radix: Possible (score +3) |
| Case 3 female 68 yr 1995 | Patient with chronic bronchitis (ICD-9 491), emphysema (ICD-9 492), and sleeping disorder (ICD-9 780.50) was treated with herbal TCM decoctions (10 drugs) for 26 d: Angelicae sinensis radix, Asari herba, Astragali radix, Atractylodis macrocephalae rhizoma, Bupleuri radix, Ephedrae herba, Glycyrrhizae radix, Paeoniae rubrae radix, Poria (Stücke), Scutellariae radix. Total daily dose: 80 g. No co-medication. No alcohol abuse. No adverse event symptoms. ALT at discharge: 234 U/L. First control at 4 wk after discharge: ALT 7 U/L. No hepatitis serology RUCAM-based causality for Bupleuri radix, Ephedrae herba, Glycyrrhizae radix, and Scutellariae radix: Probable (score +7) |
| Case 4 female 47 yr (1996) | Patient with migraine (ICD-9 346.0) was treated for 28 d with the following herbal TCM decoctions (15 drugs): Angelicae dahuricae radix, Angelicae sinensis radix, Armeniacae amarum semen, Asari herba, Bupleuri radix, Codonopsis pilosulae radix, Evodiae fructus, Forsythiae fructus, Glycyrrhizae radix, Isatidis radix, Ligustici chuanxiong rhizoma, Ligustici rhizoma, Lonicerae flos, Platycodi radix, Prunellae spica. Total daily dose: 130 g. No comedication. No alcohol abuse. No adverse event symptoms. ALT at discharge 168 U/L. At control 4 wk later: 18 U/L; Hepatitis serology post increased ALT detection: anti-HAV (IgM/IgG) negative; HBs-Ag negative; anti-HBs negative; anti-HBc (IgM/IgG) negative RUCAM-based causality for Bupleuri radix and Glycyrrhizae radix: Probable (score +6) |
| Case 5 male 77 yr (1998) | Patient with post herpes zoster state (ICD-9 053.13), hypertension (ICD-9 401), diabetes mellitus (ICD-9 250) was treated for 12 d with 11 herbal TCM decoctions: Bupleuri radix, Chebulae fructus, Dictamni radicis cortex, Gentianae macrophyllae rhizoma, Margaritifera usta concha (t), Moutan radicis cortex, Myristicae semen, Paeoniae rubrae radix, Rehmanniae radix, Scutellariae radix, Sophorae flavescentis radix. Total daily dose: 110 g. Co-medication with potentially hepatotoxic drugs: zolpidem, anti-factor 10 Xa-activity. No alcohol abuse. Adverse event symptoms: Fever 38.6 °C, erythema, and transient scleral jaundice. At discharge ALT of 330 U/L, at control 12 wk after discharge < 24 U/L. No hepatitis serology RUCAM-based causality for Bupleuri radix, Dictamni radicis cortex, and Scutellariae radix: Excluded (score -1) |
| Case 6 female 60 yr (1999) | Patient Suffered From Chronic Migraine (ICD-9 346), Cervico-Brachial Pain Syndrome Left Side (ICD-9 723.3), Lower Back Pain Syndrome (ICD-9 724.2), And Diarrhea (ICD-9 787.91) Without Clear Gastrointestinal Diagnosis Since 4 yr. Herbal TCM Medication (22 Drugs) As Decoction For 22 D: Angelicae Dahuricae Radix, Astragali Radix, Atractylodis Rhizoma, Bombyx Batryticatus (T), Chrysanthemi Flos, Cicadae Periostracum (T), Cinnamomi Ramulus, Citri Reticulatae Pericarpium, Codonopsis Pilosulae Radix, Coicis Semen, Euryales Semen, Evodiae Fructus, Ligustici Rhizome, Lycopi Herba, Moutan Radicis Cortex, Myristicae Semen, Pinelliae Praeparatae Rhizome, Poria (Parts), Psoraleae Fructus (Semen), Punicae Granati Pericarpium, Rehmanniae Praeparatae Rhizome, Scutellariae Radix, Uncariae Cum Uncis Ramulus, Viticis Fructus. Total Daily Dose: 150 G. Co-Medication With Potentially Hepatotoxic Drugs: Ibuprofen 800 And Piroxicam 10. Alcohol Consumption 1 Drink Beer Daily. After Treatment For 5 D, Improvement Of Diarrhea, And After 22 D Migraine Attack Treated With Ibuprofen. Directly After Intake Of Ibuprofen, She Noticed Symptoms With Stomach Pain, Nausea, Vomiting. ALT At Discharge 530 U/L, At Control 47 D Later: 14 U/L. Hepatitis Serology Post Increased ALT: Anti-HAV-Igg Positive; Anti-HAV-Igm Negative; Anti-Hbs Negative; Anti-Hbc Negative RUCAM-Based Causality For Bombyx Batryticatus (T), Psoraleae Fructus (Semen), And Scutellariae Radix: Possible (Score +3) |
| Case 7 female 58 yr (1999) | Patient with lumbosacral plexus syndrome (ICD-9 953.5) and cervico-brachial syndrome (ICD-9 723.3) was treated with 24 drugs for 26 d with decoctions: Angelicae sinensis radix, Asteris radix, Bupleuri radix, Cinnamomi ramulus, Coptidis rhizoma, Dictamni radicis cortex, Ephedrae herba, Farfarae flos, Forsythiae fructus, Ginkgo semen, Glehniae radix, Isatidis folium, Lonicerae flos, Lumbricus (t), Ophiopogonis radix, Paeoniae alba radix, Paeoniae rubra radix, Perillae fructus, Pinelliae praeparatae rhizoma, Platycodi radix, Rehmanniae praeparatae rhizome, Rehmanniae radix, Scutellariae radix. Total daily dose 110 g. Co-medication: theophylline, vitamin E. No alcohol abuse. Adverse event symptoms: Diarrhea, headache, nausea, and vomiting. ALT at discharge: 35 U/L; at first control 5 d later ALT 132 U/L, at second control 4 wk later 8 U/L. No hepatitis serology RUCAM-based causality for Bupleuri radix, Dictamni radices cortex, Ephedrae herba, and Scutellariae radix: Possible (score +5) |
| Case 8 male 65 yr (2000) | Patient with polyneuropathy (ICD-9 357.2), who was treated with 14 drugs for 22 d with Angelicae pubescentis radix, Astragali radix, Chaenomelis fructus, Cinnamomi ramulus, Coptidis rhizoma, Corydalis rhizoma, Lonicerae caulis, Lumbricus (t), Mori ramulus, Moutan radicis cortex, Paeoniae rubrae radix, Rehmanniae praeparatae rhizoma, Spatholobi caulis, and Trachelospermi caulis. Total daily dose 130 g. Co-medication with potential liver toxicity: allopurinol 300 mg, atorvastatin 10 mg. Alcohol consumption 3-4 drinks beer per day. No adverse events. Safety check after 10 d of treatment: ALT < 24 U/L; at discharge: 193 U/L; first control 16 d later: ALT 24 U/L RUCAM-based causality for all used TCM herbs: Possible (score +3) |
| Case 9 female 78 yr 2000 | Patient with polymyalgia rheumatica (ICD-9 725) and fibromyalgia (ICD-9 74.1), treated with 17 drugs for 23 d with: Astragali radix, Bupleuri radix, Carthami flos, Cinnamomi ramulus, Coptidis rhizoma, Curcumae longae rhizoma, Cyperi rhizoma, Glehniae radix, Ligustri lucidi fructus, Luffae fructus retinervus, Lycopi herba, Mori ramulus, Paeoniae rubrae radix, Rehmanniae praeparatae rhizoma, Scutellariae radix, Sparganii tuber (rhizoma), Trachelospermi caulis. Total daily dose: 84 g. Co-medication with potential liver toxicity: Triamterene, diclofenac. No alcohol abuse. No adverse event symptoms. ALT at discharge 162 U/L; first control 21 d later: 12 U/L. Hepatitis serology post increased ALT: anti-HAV IgG positive: Anti-HAV IgM negative; HBs-antigen negative; anti-HBs 250 U/L, anti-HBc positive RUCAM-based causality for Bupleuri radix and Scutellariae radix: Possible (score +4) |
| Case 10 female 35 yr (2002) | Patient suffered from migraine (ICD-9 346.0) and tension headache (ICD-9 307) since 20 yr. Treatment with 20 drugs: Albiziae cortex, Amomi cardamomi semen, Angelicae dahuricae radix, Angelicae sinensis radix, Artemisiae argyi folium, Bombyx batryticatus (t), Bupleuri radix, Codonopsis pilosulae radix, Dolichoris album semen, Evodiae fructus, Ligustici rhizoma, Margaritifera usta concha (t), Meliae toosendan fructus, Mori ramulus, Notopterygii rhizoma seu radix, Paeoniae albae radix, Prunellae spica, Puerariae radix, Scutellariae radix, Tribuli fructus as decoctions. Total daily dose: 95 g. Herbal TCM treatment for 28 d. Co-medication with potentially hepatotoxic drug: estradiol. Occasional alcohol use. Adverse event symptoms: abdominal pain and diarrhea. ALT at discharge 195 U/L; at first control 10 d later ALT 56 U/L. No hepatitis serology RUCAM-based causality for Bombyx batryticatus (t), Bupleuri radix, Meliae toosendan fructus, and Scutellariae radix: Possible (score +5) |
| Case 11 female 74 yr (2003) | Patient with difficulty of walking (ICD-9 719.7), polyneuropathy (ICD-9 357.2), and low back pain (ICD-9 724.2). Herbal TCM treatment with decoctions (25 drugs): Achyranthis bidentatae radix, Albiziae cortex, Amomi cardamomi semen, Angelicae pubescentis radix, Angelicae sinensis radix, Astragali radix, Atractylodis macrocephalae rhizoma, Chaenomelis fructus, Coicis semen, Coptidis rhizoma, Corydalis rhizoma, Glycyrrhizae radix, Margaritifera usta concha (t), Mori ramulus, Moutan radicis cortex, Paeoniae albae radix, Paeoniae rubrae radix, Phellodendri cortex, Rehmanniae praeparatae rhizoma, Salviae miltiorrhizae radix, Sappan lignum, Spatholobi caulis, Trachelospermi caulis, Tribuli fructus, Ziziphi spinosae semen.Total daily dose: 150 g. Co-medication with potentially hepatotoxic drug: candesartan 16 mg/die (regular), enalapril (rare). No alcohol abuse. No adverse event symptoms. Safety check after 14 d: ALT 165 U/L. At discharge: ALT 325 U/L. First control: ALT 61 U/L, Hepatitis serology post increased ALT: hepatitis B and C excluded; anti-HAV (IgG) positive RUCAM-based causality for Glycyrrhizae radix: Excluded (score -1) |
| Case 12 female 62 yr (2004) | Patient with chronic fatigue (ICD-10 L53), depressive episodes (ICD-10 F32.9), and gastrointestinal symptoms (ICD-10 K59.9) including abdominal pains and flatulence. Treatment with herbal TCM decoctions (18 drugs) for 26 d: Albiziae cortex, Amomi cardamomi semen, Angelicae sinensis radix, Astragali radix, Aurantii fructus, Citri sarcodactylis fructus, Codonopsis pilosulae radix, Coicis semen, Corydalis rhizoma, Curcumae radix, Eriocauli flos, Meliae toosendan fructus, Moutan radicis cortex, Notoginseng radix, Phragmitis rhizoma, Platycodi radix, Poria (parts), Schisandrae fructus. Total daily dose 96 g. Co-medication with clorazepate. No alcohol abuse. ALT at discharge 751 U/L. Seventeen days after cessation of herbal TCM products: ALT 148 U/L. No more subsequent ALT results available. Adverse event symptoms: Directly after discharge from the TCM-hospital, the patient was admitted at another hospital with a department of internal medicine due to deterioration of gastrointestinal symptoms. Serology: EBV-IgG 590 U/L, EBV-IgM negative RUCAM-based causality for Meliae toosendan fructus: probable (score +7) |
| Case 13 female 57 yr (2004) | Patient with arthralgia (ICD-10 M25.5), lower back pain syndrome (ICD-10 M54.4), and sleeping disorder (ICD-10 G 47.9) was treated with herbal TCM decoctions (14 drugs) for 25 d: Achyranthis bidentatae radix, Albiziae cortex, Angelicae sinensis radix, Astragali radix, Cassiae semen, Cinnamomi ramulus, Curcumae longae rhizoma, Gentianae macrophyllae rhizoma, Loranthi ramulus, Notoginseng radix, Notopterygii rhizoma seu radix, Periploca radicis cortex, Psoraleae fructus (semen), Spatholobi caulis. Total daily dose: 110 g. Co-medication: L-thyroxine, aminophylline. No alcohol abuse. Adverse event symptoms: abdominal pain, loss of appetite, and single vomiting. ALT at discharge 389 U/L; at first control 4 d later: ALT 191 U/L, and at second control 15 d later: ALT 22 U/L. Hepatitis serology post increased ALT: anti-HAV (IgG+IgM) positive; anti-HAV IgM negative; HBs-Antigen negative; anti-HBs positive; anti-HBc negative; HCV ab: negative RUCAM-based causality for Cassiae semen: possible (score +5) |
| Case 14 Female 52 yr (2006) | Patient with chronic osteomyelitis (ICD-10 M86.99) left leg and a six-year history after open fracture. PMH of hepatitis A 1968. Treatment with herbal TCM decoctions (12 drugs) for 24 d: Achyrantis bidentatae radix, Amomi cardamom semen, Chaenomelis fructus, Citri grandis exocarpium, Coicis semen, Corydalis rhizoma, Mangolie officinalis cortex, Meliae toosendan fructus, Paeoniae rubra radix, Poria (parts), Scutellariae radix, Zingiberis rhizoma. Total daily dose: 155 g. Co-medication with potential hepatotoxicity: use of not overdosed paracetamol (once) and ibuprofen (when needed, but presently no intake). No alcohol abuse. At day 20 after admission, patient showed adverse event symptoms like abdominal pain of the colic type with intestinal cramps, nausea, and mushier diarrhea. No ascites, no splenomegaly, no hyperbilirubinemia. ALT at discharge 1052 U/L. Three days later: 692 U/L and 35 d later: 33 U/L. Normal hepatitis serology with exclusion of hepatitis A, B, and C RUCAM-based causality for Meliae toosendan fructus and Scutellariae radix: Probable (score +7) |
| Case 15 female 43 yr (2007) | Patient suffered from unclear post-infectious chronic fatigue (ICD-10 G93; 10 F.43) and chronic cephalgia (ICD-10 R51). Known history of EBV infection. Treatment with herbal TCM decoctions containing the following 23 components for 19 d: Albiziae cortex, Anemarrhenae rhizoma, Astragali radix, Bambusae caulis in taeniam, Bupleuri radix, Chaenomelis fructus, Cinnamomi ramulus, Citri reticulatae pericarpium, Curcumae longae rhizoma, Epimedii herba, Leonuri herba, Ligustri lucidi fructus, Lycii fructus, Magnoliae officinalis cortex, Meliae toosendan fructus, Paeoniae rubra radix, Polygalae radix, Poria (parts), Pseudostellariae radix, Pyrrosiae folium, Salviae miltiorrhizae radix, Scutellariae radix, Tribuli fructus. Total daily dose: 69 g. Co-medication: L-thyroxine. No alcohol abuse. Adverse event symptom: abdominal pain. ALT at discharge 290 U/L; 12 d later at first control: ALT 181 U/L. Second control 24 d later: ALT 81 U/L; third control 28 d later: normal ALT values. No hepatitis serology RUCAM-based causality for Bupleuri radix, Meliae toosendan fructus, and Scutellariae radix: Possible (score +4) |
| Case 16 female 58 yr (2007) | Patient with lichen sclerosus (ICD-10 L90.0) and cervical spondylosis (ICD-10 M47.0) was treated with herbal TCM decoctions (11 drugs) for 25 d: Amomi cardamomi semen, Atractylodis rhizoma, Bambusae caulis in taeniam, Bombyx batryticatus (t), Bupleuri radix, Coicis semen, Kochiae fructus, Phellodendri cortex, Rehmanniae radix, Salviae miltiorrhizae radix, Scutellariae radix. Total daily dose 43 g. No co-medication. No alcohol abuse. No adverse event symptoms. ALT values at discharge normal. Continued use of herbal TCM at home, but at reduced daily dose of 26 g (corresponding to about 60% of the individual hospital dosage). Twenty-one days after hospital stay, safety check: ALT 715 U/L. Cessation of the herb use. Fifteen days after the first control: ALT 113 U/L. Again 14 d thereafter, at a second control: ALT 44 U/L; and at a third control, ALT 23 U/L. Hepatitis serology post first increased ALT values: Hepatitis B and C excluded. Anti-HAV-IgG positive; Anti-HAV-IgM negative RUCAM-based causality for Bombyx batryticatus (t), Bupleuri radix, and Scutellariae radix: Possible (score +5) |
| Case 17 female 48 yr (2010) | Patient with migraine (ICD-10 G 43.0) and depression (ICD-10 F 32.0) was treated with 11 herbal TCM components as decoctions for 20 d: Angelicae dahuricae radix, Bambusae caulis in taeniam, Bombyx batryticatus (t), Bupleuri radix, Cassiae semen, Curcumae longae rhizoma, Dipsaci radix, Gentianae macrophyllae rhizoma, Scutellariae radix, Siegesbeckiae herba, Trichosanthis fructus. Total daily dose: 60 g. No co-medication. No alcohol, Adverse event symptom: abdominal pain. Safety control: ALT 279 U/L. Cessation of the herb use. Five days after cessation and at discharge: ALT 252 U/L. First control 14 d after hospital discharge: ALT 12 U/L. Hepatitis serology post increased ALT: Hepatitis A, B, C excluded. Anti-EBV-VCA-IgG > 100; EBV-VCA-IgM < 0.9; HBsAb 474 Units; HBc ab negative; HCV ab not reactive; EBV-EBNA1-Ab (IgG) > 100 Units; Anti-HAV (IgM) negative; Anti-HBc (IgM) negative RUCAM-based causality for Bombyx baytryticuatus (t), Bupleuri radix, Cassiae semen and Scutellariae radix: Probable (score +6) |
| Case 18 female 52 yr (2010) | Patient with depression (ICD-10 F32.1), migraine (ICD-10 G43.0), spondylosis cervical (ICD-10 M47.8), and cephalgia (ICD-10 R51) treated with 16 drugs: Achyranthis bidentatae radix, Angelicae dahuricae radix, Bambusae caulis in taeniam, Bombyx batryticatus (t), Bupleuri radix, Citri reticulatae pericarpium, Coicis semen, Curcumae longae rhizoma, Magnoliae officinalis cortex, Paeoniae rubra radix, Polygalae radix, Poria (Stücke), Scutellariae radix, Tribuli fructus, Viticis fructus, Zingiberis rhizoma. Total daily dose: 96g. Duration of treatment for only 7 d because of adverse event symptoms of diarrhea ad deterioration of headache. Co-medication: L-thyroxine. Safety check 14 d after admission: ALT 76 U/L; At first control 20 d after admission: ALT 233 U/L. At discharge: ALT 198 U/L. Control 30 d after discharge: ALT 35 U/L. Serology post increased ALT: Anti-HAV (IgG, IgM) negative; anti-HAV (IgM) negative; HBs-antigen negative, Anti-HBs < 10 IU/L; Anti-HBc (IgG + IgM) negative; Anti-HCV negative RUCAM-based causality for Bombyx batryticatus (t), Bupleuri radix, and Scutellariae radix: Probable (score +6) |
| Case 19 female 60 yr (2011) | In 2011, patient with carcinophobia (ICD-10 F45.2), allergic sensitivity (ICD-10 T78.4) and tinnitus (ICD-10 H93.1) was treated with 28 drugs for 19 d with: Achyranthis bidentatae radix, Angelicae sinensis radix, Armeniacae amarum semen, Astragali radix, Atractylodis macrocephalae rhizoma, Aurantii immaturus fructus, Bambusae caulis in taeniam, Bombyx batryticatus (t), Bupleuri radix, Chrysanthemi flos, Cinnamomi ramulus, Curcumae longae rhizoma, Curcumae radix, Ephedrae herba, Edebouriellae radix, Ligustri lucidi fructus, Liquidambaris fructus, Luffae fructus retinervus, Lycii fructus, Menthae herba, Mori ramulus, Paeoniae albae radix, Paeoniae rubrae radix, Persicae semen, Peucedani radix, Polygoni multiflori caulis, Schisandrae fructus, Scutellariae radix. Daily drug dose 119 g. No co-medication. No alcohol. No adverse event symptoms. ALT at discharge 249 U/L; at first control after 3 d 123 U/L; at second control after 69 d 30 U/L. No hepatitis serology RUCAM-based causality in 2011 for Bombyx batryticatus (t), Bupleuri radix, Ephedrae herba, Polygoni multiflori caulis, and Scutellariae radix: Probable (score +6) |
| 63 yr (2014) | In 2014, the patient was treated again with some of the previous herbal components as in 2011, now with 13 drugs: Atractylodis rhizoma; Bupleuri radix; Carthami flos; Clematidis radix; Curcumae longae rhizoma; Curcumae radix; Lycopodii herba; Mori ramulus; Pinelliae praeparatae rhizoma; Poria (parts); Puerariae radix; Sappan lig; Scutellariae radix. Total daily dose: 104 g. No co-medication. No alcohol abuse known. No adverse event symptoms. Because of the previous experience, liver enzyme control already after 7 d: ALT 295 U/L; cessation of all herbal TCM products. Six days later ALT 182 U/L, and 3 d thereafter: ALT 86 U/L. Eleven days later: ALT 34 U/L. No hepatitis serology RUCAM-based causality in 2014 for Bupleuri radix, Puerariae radix, and Scutellariae radix: Probable (score +7) |
| Case 20 female 53 yr (2012) | Patient with depressive episode (ICD-10 F33.1), migraine (ICD-10 G43.1), and low back-pain (ICD-10 M54.1) was treated for 22 d the with following 12 components: Achyranthis bidentatae radix, Angelicae dahuricae radix, Bupleuri radix, Cuscutae semen, Ligustici chuanxiong rhizome, Liquidambaris fructus, Lycii fructus, Rehmanniae radix, Scutellariae radix, Trachelospermi caulis, Tribuli fructus, Uncariae cum uncis ramulus. Total daily dose: 88 g. Additional wind-heat-mixture: Polygoni cuspidate rhizoma, Glycyrrhizae radix for 3 d. Wind-cold-mixture: Ephedra herba and Polygoni cuspidate rhizoma. Total daily dose: No comedication. No alcohol. No adverse effect symptoms. ALT at discharge 207 U/L, 22 d later at first control 30 U/L. No hepatitis serology RUCAM-based causality for Bupleuri radix, Ephedra herba, Glycyrrhizae radix, Polygoni cuspidate rhizoma, and Scutellariae radix: Possible (score +4) |
| Case 21 female 53 yr (2013) | Patient with neurasthenia (ICD-10 F48.0) and fibromyalgia (ICD-10 M79.7) received herbal TCM decoction for 18 d with the following 24 herbs: Achyranthis bidentatae radix, Astragali radix; Atractylodis rhizoma; Aurantii immaturus fructus, Bambusae caulis in taeniam, Bupleuri radix, Carthami flos, Citri reticulatae pericarpium, Citri sarcodactylis fructus, Curcumae longae rhizoma, Dipsaci radix, Glycyrrhizae radix, Inulae flos, Loranthi ramulus, Ophiopogonis radix, Paeoniae albae radix, Paeoniae rubrae radix, Persicae semen, Pinelliae praeparatae rhizoma, Pseudostellariae radix, Rehmanniae radix, Rhei radix et rhizoma, Scutellariae radix, Siegesbeckiae herba. Total daily dose: 78 g. No potentially hepatotoxic co-medication (only L-thyroxine). No alcohol. No adverse event symptoms. ALT at discharge 221 U/L and 41 U/L at control 14 d later. No hepatitis serology RUCAM-based causality for Bupleuri radix, Glycyrrhizae radix, Rhei radix et rhizoma, and Scutellariae radix: Possible (score +5) |
| Case 22 female 52 yr (2013) | Patient with somatoform pain disorder (ICD-10F45.0), drug induced tension headache (ICD-10 G45.2), and polyarthritis (ICD-10 M05.9), who was treated with herbal TCM decoctions for 22 d. The following 35 herbs were applied: Achyranthis bidentatae radix, Anemarrhenae rhizoma, Angelicae pubescentis radix, Astragali radix, Atractylodis rhizoma, Bambusae caulis in taeniam, Chaenomelis fructus, Cinnamomi ramulus, Clematidis radix, Coicis semen, Coptidis rhizoma, Curcumae longae rhizoma, Cyperi rhizoma, Dipsaci radix, Glycyrrhizae radix, Homalomenae rhizoma, Ligustri lucidi fructus, Liquidambaris fructus, Lonicerae caulis, Loranthi ramulus, Luffae fructus retinervus, Magnoliae officinalis cortex, Mori ramulus, Notopterygii rhizoma seu radix, Paeoniae rubrae radix, Pinelliae praeparatae rhizoma, Polygalae radix, Scutellariae radix, Siegesbeckiae herba, Sinomenii caulis, Sparganii tuber (rhizoma), Trachelo-spermi caulis, Tribuli fructus, Trichosanthis fructus, Uncariae cum uncis ramulus. Total daily dose was 78 g. Co-medication with the potentially hepatotoxic drug: omega-3-acidethylester. No alcohol-abuse, Adverse event symptoms: diarrhea, abdominal pain, and flatulence. Hepatitis B serology negative; HBs-Ag negative, Anti-HCV negative. At discharge: ALT 361 U/L and 15 U/L at control 90 d later RUCAM-based causality for Glycyrrhizae radix and Scutellariae radix: Possible (score +3) |
| Case 23 female 46 yr (2014) | Patient with alopecia cranialis totalis (ICD-10 L63.0) and Hashimoto-thyroiditis (ICD-10 E06.3) was treated for 28 d with a decoction containing 15 TCM herbs: Achyranthis bidentatae radix, Angelicae sinensis radix, Atractylodis macrocephalae rhizoma, Bambusae caulis in taeniam, Bupleuri radix, Citri reticulatae pericarpium, Cuscutae semen, Ledebouriellae radix, Lycii fructus, Periploca radicis cortex, Polygonati rhizoma, Polygoni multiflori radix, Poria (parts), Psoraleae fructus (semen), Testudinis plastrum (t). Total daily dose: 72 g. Co-medication with L-thyroxine. No alcohol. Adverse event symptom: flatulence. At discharge ALT 268 U/L, with 210 U/L on day 20 and 62 U/L on day 30 RUCAM-based causality for Bupleuri radix, Polygoni multiflori radix, and Psoraleae fructus (semen): Possible (score +4) |
| Case 24 female 51 yr (2014) | Patient with depression (ICD-10 F33.1) and migraine (ICD-10 G43.0) took for 17 d the herbal TCM decoction with the following 18 herbs: Achyranthis bidentatae radix, Angelicae sinensis radix, Bombyx batryticatus (t), Bupleuri radix, Curcumae longae rhizoma, Curcumae radix, Kochiae fructus, Ligustri lucidi fructus, Lycii fructus, Mori radicis cortex, Mori ramulus, Paeoniae rubrae radix, Polygoni multiflori caulis, Salviae miltiorrhizae radix, Scutellariae radix, Sparganii tuber (rhizoma), Spatholobi caulis, Tribuli fructus. Total daily dose was 60 g. Comedication: cimicifuga, zolpidem. No alcohol. Lack of adverse event symptoms. At discharge and control: ALT 210 U/L and 191 U/L. At a subsequent control 19 d later, ALT 36 U/L RUCAM-based causality for Bombyx batryticatus (t), Bupleuri radix, Polygoni multiflori caulis, and Scutellariae radix: Probable (score +6) |
| Case 25 female 53 yr (2015) | Patient with chronic pain syndrome (ICD-10 F 45.41; G43.9) received for 3 wk (twice daily) herbal TCM decoctions containing 20 different herbs: Angelicae sinensis radix, Aurantii fructus, Bambusae caulis in taeniam, Bupleuri radix, Carthami flos, Citri sarcodactylis fructus, Curcumae longae rhizoma, Curcumae radix, Ligustici chuanxiong rhizoma, Loranthi ramulus, Lycopodii herba, Mori ramulus, Persicae semen, Poria (parts), Puerariae radix, Scutellariae radix, Sparganii tuber (rhizoma), Spatholobi caulis, Tribuli fructus, Viticis fructus. Total daily dose: 87 g. Co-medication: intermittent use of sumatriptane and the potentially hepatotoxic drug paracetamol. No alcohol. No adverse effect symptoms. ALT at discharge 359 U/L, at control 18 d later ALT 69 U/L. Hepatitis A and B were excluded serologically RUCAM-based causality for Bupleuri radix and Scutellariae radix: Possible (score +5) |
| Case 26 female 61 yr -2015 | Patient with unspecified somatization (ICD-10 F45.1), who suffered from abdominal symptoms of nausea, diarrhea, and loss of appetite, received herbal TCM decoction therapy for 23 d with 26 herbs: Amomi cardamomi semen, Amomi fructus, Armeniacae amarum semen, Atractylodis macrocephalae rhizoma, Bambusae caulis in taeniam, Bupleuri radix, Cinnamomi ramulus, Citri sarcodactylis fructus, Codonopsis pilosulae radix, Coicis semen, Corydalis rhizoma, Cyperi rhizoma, Forsythiae fructus, Glehniae radix, Glycyrrhizae radix, Ledebouriellae radix, Meliae toosendan fructus, Mori radicis cortex, Ophiopogonis radix, Paeoniae albae radix, Paeoniae albae radix, Peucedani radix, Pinelliae praeparatae rhizoma, Poria (parts), Scutellariae radix, Zingiberis rhizoma. Total daily dose: 87 g. Intermittent co-medication with the potentially hepatotoxic pantoprazole. Alcohol use with 2 drinks a day. During hospital stay, she experienced deterioration of her gastrointestinal symptoms. At discharge, ALT 182 U/L. Two weeks later, normalization of ALT (30 U/L). No hepatitis serology RUCAM-based causality for Bupleuri radix, Glycyrrhizae radix, Meliae toosendan fructus, and Scutellariae radix: Possible (score +3) |
Among the liver injury study cohort, 12/26 (46%) of the patients experienced one or more gastrointestinal symptoms such as abdominal pain, diarrhea (6/12), nausea (4/12), vomiting (3/12), and intestinal colicky cramps (3/12) (Table 2). These symptoms are most likely the result of incipient liver injury due to herbal TCM and may be interpreted as a clinical warning signal. Following the discontinuation of herbal TCM treatment, the symptoms rapidly vanished and ALT values normalized in virtually all patients in the liver injury study cohort.
For all 26 cases in the liver injury study cohort, causality for the used herbal TCM and co-medicated synthetic drugs used was assessed using RUCAM. RUCAM-based causality for TCM herbs was probable in 8/26 patients, possible in 16/26, and excluded in 2/26 cases. All details are presented to facilitate thorough information and reassessment by other groups or regulators (Table 6).
| RUCAM items with attribution of scores (SC) | CASES 1-26 | ||||||||||||||||||||||||||
| SC | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | |
| 1 Time to onset from the beginning of the herb | |||||||||||||||||||||||||||
| 5-90 d | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +1 | +2 | +2 | +2 | +2 | +2 | +2 | +2 |
| < 5 or > 90 d | +1 | ||||||||||||||||||||||||||
| Alternative: Time to onset from cessation of the herb | |||||||||||||||||||||||||||
| ≤ 15 d | +1 | ||||||||||||||||||||||||||
| 2 Course of ALT after cessation of the herb | |||||||||||||||||||||||||||
| Decrease ≥ 50% within 8 d | +3 | +3 | +3 | +3 | +1 | +3 | |||||||||||||||||||||
| Decrease ≥ 50% within 30 d | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | ||||||||
| No information of continued drug use | 0 | 0 | 0 | ||||||||||||||||||||||||
| Decrease ≥ 50% after the 30th day | 0 | 0 | |||||||||||||||||||||||||
| Decrease < 50% after the 30th day or recurrent increase | -2 | ||||||||||||||||||||||||||
| 3 Risk factors | |||||||||||||||||||||||||||
| Alcohol use (current drinks/d: > 2 for woman, > 3 for men) | +1 | ||||||||||||||||||||||||||
| Alcohol use (current drinks/d: ≤ 2 for woman, ≤ 3 for men) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age ≥ 55 yr | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | ||||||||||||
| Age < 55 yr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
| 4 Concomitant drug(s)/herb(s) | |||||||||||||||||||||||||||
| None or no information | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/0 | 0 | |||||||||||||||||
| Concomitant drug/herb with incompatible time to onset | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| Concomitant drug/herb with compatible or suggestive time to onset | -1 | -1 | -1 | -1 | |||||||||||||||||||||||
| Concomitant drug/herb known as hepatotoxin and with compatible or suggestive time to onset | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | ||||||||||||||||||
| Concomitant drug with evidence for its role in this case (positive rechallenge or validated test) | -3 | ||||||||||||||||||||||||||
| 5 Search for alternative causes Group I (7 causes) | |||||||||||||||||||||||||||
| HAV: Anti-HAV-IgM | Ø | Ø | N | N | Ø | N | Ø | Ø | Ø | Ø | N | N | Ø | Ø | N | N | Ø/Ø | Ø | Ø | Ø | Ø | Ø | N | Ø | |||
| HBV: Anti-HBC-IgM, HBV-DNA | Ø | Ø | N | N | Ø | N | Ø | Ø | Ø | Ø | N | N | N | N | Ø | Ø | N | N | Ø/Ø | Ø | Ø | N | Ø | Ø | N | Ø | |
| HCV: Anti-HCV, HCV-RNA | Ø | Ø | N | Ø | Ø | N | N | Ø | Ø | Ø | N | N | N | N | Ø | Ø | N | N | Ø/Ø | Ø | Ø | N | Ø | Ø | Ø | Ø | |
| HEV: Anti-HEV-IgM, anti-HEV-IgG, HEV-RNA | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø/Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | |
| Hepatobiliary sonography/colour Doppler sonography of liver vessels/endo-sonography/CT/MRC | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | N | Ø | Ø | Ø | Ø | Ø/Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | |
| Alcoholism | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N/N | N | N | N | N | N | N | N | |
| Acute recent hypotension history (particularly if underlying heart disease) | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N/N | N | N | N | N | N | N | N | ||
| Group II (5 causes) | |||||||||||||||||||||||||||
| Complications of underlying disease(s) such as sepsis, metastatic malignancy, autoimmune hepatitis, chronic hepatitis B or C, primary biliary cholangitis or sclerosing cholangitis, genetic liver diseases | |||||||||||||||||||||||||||
| Infection suggested by PCR and titre change for | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N/N | N | N | N | N | N | N | N | |
| CMV (anti-CMV-IgM, anti-CMV-IgG) | |||||||||||||||||||||||||||
| EBV (anti-EBV-IgM, anti-EBV-IgG) | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø/Ø | Ø | Ø | Ø | Ø Ø | Ø | Ø | Ø | |
| HSV (anti-HSV-IgM, anti-HSV-IgG) | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | + | Ø | Ø/Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | ||
| VZV (anti-VZV-IgM, anti-VZV- IgG) | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø/Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | |
| Evaluation of group I and II | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø/Ø | Ø | Ø | Ø | Ø | Ø | Ø | ||
| All causes - groups I and II - reasonably ruled out | +2 | ||||||||||||||||||||||||||
| The 7 causes of group I ruled out | +1 | ||||||||||||||||||||||||||
| 6 or 5 causes of group I ruled out | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Less than 5 causes of group I ruled out | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | -2 | 1 | -2 | -2 | -2 | -2 | -2 | -2 | ||||||||
| Alternative cause highly probable | -3 | ||||||||||||||||||||||||||
| 6 Previous information on hepatotoxicity of the herb | |||||||||||||||||||||||||||
| Reaction labelled in the product characteristics | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2/ +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | |||
| Reaction published but unlabelled | +1 | +1 | |||||||||||||||||||||||||
| Reaction unknown | 0 | 0 | 0 | ||||||||||||||||||||||||
| 7 Response to unintentional | |||||||||||||||||||||||||||
| reexposure | |||||||||||||||||||||||||||
| Doubling of ALT with the herb alone, provided ALT below 5 ULN before reexposure | +3 | ||||||||||||||||||||||||||
| Doubling of ALT with the herb already given at the time of first reaction | +1 | +1 | |||||||||||||||||||||||||
| Increase of ALT but less than ULN in the same conditions as for the first administration | -2 | ||||||||||||||||||||||||||
| Other situations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total score for case | 4 | 3 | 7 | 6 | -1 | 3 | 5 | 3 | 4 | 3 | -1 | 7 | 5 | 7 | 4 | 5 | 6 | 6 | 6/7 | 4 | 5 | 3 | 4 | 6 | 5 | 3 | |
Assessing causality in the 26 cases is indeed challenging, but RUCAM can handle this condition fairly well. All patients used a mixture of several TCM herbs (Table 5). The exposure conditions of the suspected herbs are identical, especially regarding start of use and discontinuation. Therefore, basic causality gradings should be identical, unless some herbs have a record of known previous liver injury, which gives two extra RUCAM points, as compared to other herbs without such records, which do not allow two extra points. Therefore, differences in causality grading for TCM herbs can be achieved considering the criteria of known hepatotoxicity. In the absence of such criteria, causality must be attributed to all the herbs together that were used, without the possibility of differentiating between the various herbs. Some patients in the liver injury study cohort also used conventional drugs, which were prescribed either before they were included in the study or during hospitalization. RUCAM was also applied to these co-medicated drugs, which may differ regarding their previous hepatotoxicity and their duration of use. However, it is unlikely that drugs may have caused the increases of ALT during the hospitalization since at the time of inclusion in the study, the ALT values were normal.
This report provides liver injury data derived from a prospective, hospital-based and large-scale study of 21470 patients, who had no liver disease prior to treatment with TCM herbs for the first time. Clinically relevant liver injury with ALT ≥ 5 × ULN developed in 26 patients (0.12%) (Figure 1 and Tables 1-5). These data suggest that TCM herbs carry a risk of liver injury in line with other reports[9,27,28,43] and concomitantly dismiss contrarian claims that TCM herbs lack hepatotoxic potency[16]. However, the surprisingly low frequency of liver injury caused by herbal TCM in this study (Figure 1) is at a variance with several reports implying that liver injury cases due to these herbs occur at a high frequency[2,9,11,17,19,27,28,43]. The rarity of liver injury cases found in the present investigation may be explained by the strict study protocol: (1) prospective rather than retrospective study approach; (2) valid exclusion of pre-existing liver disease prior to the start of the therapy with TCM herbs; (3) hospital-based treatment with specifically trained TCM physicians from Germany and China; (4) use of good quality TCM herbal products specifically ascertained by appropriate analyses; (5) therapy with a median of 19.5 d, avoiding prolonged treatment; (6) selective inclusion in the study only of those patients meeting the liver injury criteria ALT ≥ 5 × ULN; and (7) causality assessment using RUCAM and ascertaining ALT dechallenge following discontinuation of the herbal TCM therapy. Such excellent investigational conditions rarely exist under normal field conditions, where patients are evaluated in retrospective studies, and often provide cases of limited data quality[2,6,17], mostly with the lack of a robust causality assessment such as RUCAM[25]. Despite these encouraging data under hospital conditions, herbal TCM treatment outside a hospital setting may be associated with higher liver injury risks, requiring a cautionary statement. Consequently and to err on the side of caution, patients who opt for special therapy with herbal TCM should be informed about the low risk of liver injury and its clinical symptoms.
Supportive evidence of causality for TCM herbs in the injury cases was provided by the rapid decline of ALT to nearly normal values shortly following cessation of herbal use in 24/26 patients (Table 5), while two patients escaped final ALT analysis (Table 5). Causality is further supported by the lack of pre-existing liver diseases in the patients in the liver injury study cohort, ruling out that alternative liver diseases could compete with the newly emerging liver injury caused by TCM herbs. Finally, the causality of liver injury for various TCM herbs was established using the updated RUCAM (Table 6), as published in 2016[25]. Causality was excluded in two patients, whereas most cases achieved a possible or even a probable causality level. Using both, RUCAM-based causality assessment and positive tests of unintentional re-exposures, valid causality was provided for numerous TCM herbs by other published analyses of liver injury[9,27,28,43].
It appears that patients with acute liver injury due to TCM herbs commonly have a good prognosis and no transition to chronic liver injury, at least under the treatment conditions of a hospital, and is possibly attributed to the exclusion of prolonged treatment as described in the present study (Table 2). This favorable outcome is in line with a previous RUCAM-based study, which does not report on severe courses[27], but is in contrast to a retrospective study[9] and another analysis[28]: Both publications reported severe clinical courses with the risk of acute liver failure, requirement of liver transplant, and of death[9,28]. In more detail, acute liver failure was reported in 7.8%, a requirement for liver transplant in 0.6%, and a fatality rate in 3.2%, but associated RUCAM-based causality gradings were not published in the study[9]. This was done in another report of 54 patients with an RUCAM-based causality grading of probable for herbal TCM: One patient had used a herbal TCM product for 60 d and required a liver transplantation, while another one died after using TCM herb for 30 d[28]. The difference in outcome between the present study and previous publications[9,28] cannot validly be explained and is certainly open for discussion, especially regarding the duration of herbal TCM exposure, which was 19.5 d in this study (Table 2).
Of note, in addition to the 21470 patients, who were included in the TCM study cohort due to their normal ALT values, 472 patients corresponding to 2.3% had been admitted to the hospital for TCM treatment with increased ALT values on admission and were therefore not included in the TCM study cohort. If included, these patients may have alternative diagnoses as confounders, as initially increased ALT values may reflect already existing liver disease. Similar to the hospital conditions, alternative causes as confounding variables have been described in suspected cases of DILI[44,45] and HILI[46] including those caused by TCM herbs[9,46], and dietary supplements[46-50].
It is likely that in the real world, some patients seeking therapy with TCM herbs have a pre-existing, initially not recognized liver disease[9]. Uncertainty exists as to whether pre-existing liver disease is a risk factor for liver injury by TCM herbs, in analogy to some chronic liver diseases such as non-alcoholic fatty liver disease or alcoholic liver disease, which are risk factors of drug-induced liver injury (DILI) by some special drugs[51,52]. Our study clearly differentiated liver injury with ALT values of ≥ 5 × ULN from ALT abnormalities with ALT values of < 5 × ULN (Table 1). Those are considered as an adaptive phenomenon due to the metabolism of the chemicals provided from the herbs to the liver. These adaptive abnormalities are clinically not relevant. The present data do not allow the general clinical recommendations to analyze ALT before therapy with TCM herbs is considered, as liver injury is unpredictable. ALT assessment may be helpful in legal situations to prevent later patient claims.
The rarity of hepatotoxic reactions together with normal dosages of herbal TCM used in this study imply idiosyncrasy as a cause rather than an intrinsic mechanism, which is dose-dependent and can be elucidated by experimental studies. However, pathogenetic steps leading to the dose-independent idiosyncratic liver injury are largely unknown due to lack of appropriate animal models[17]. In analogy with other herbs, TCM herbs including those incriminated as causes of liver injury in the present study (Tables 3 and 5) contain dozens of known chemicals as ingredients but their specific hepatotoxic potency is difficult to assess and remains largely unknown[28]. Another problem of most TCM therapy regimens is the multiplicity of herbs included as ingredients in herbal mixtures[19,28], such as up to 35 in the present study (Table 5). Multiple plant chemicals of many herbs may lead to an increased risk of liver injury, which were described at least for DILI if several drugs were co-administered[53], and for herb-herb interactions or herb-drug interactions, if concomitantly used with Western drugs[54]. Used as co-medication to Western antipsychotic drugs such as quetiapine, clozapine, and olanzapine, in the case of Bupleuri radix it is known that this is associated with nearly 60% of the risk of adverse outcomes[4,12,55]. Other potential risk factors for liver injury by TCM herbs may include higher dosages and lipophilicity of chemicals and known conditions from DILI cases[56]. Publications on the quality problems of some herbal TCM products[20-24] called for providing excellent quality for our patients as high priority, which is a strength of this study and avoids discussions around product quality as causative for the observed liver injury.
In the present study, Bupleuri radix and Scutellariae radix are the two TCM herbs most implicated in liver injury (Tables 3-5). However, both herbs turned out to be the most frequently prescribed drugs in the TCM hospital in Bad Kötzting in general[57]. Even though liver injury from Polygonum multiflorum has increasingly been reported in recent years[9-11], but convincing evidence for causality is limited in the present analysis (Tables 3-5). Previous regulatory discussions focused on herbal products containing unsaturated pyrrolizidine alkaloids (PAs), and in 2012, EMA stated that herbal medicinal products containing herbal preparations with toxic, unsaturated PAs (even in very low amounts) should not be used orally[58]. Used in high amounts for a prolonged period, PAs can cause HSOS (hepatic sinusoidal obstruction syndrome)[19]. TCM herbs also include Jue Ming Zi (Cassia), but only its leaves and fruits contain PAs and may cause HSOS[19,58]. Cassiae semen lacks PAs that has been used by two patients, who as expected had no signs of HSOS (Table 5).
The use of herbal TCM is widely considered less risky as compared with synthetic drugs, although data on direct comparisons are not available in support of this view. Populations using herbal TCM, drugs, either alone, or combined experience more DILI than HILI, possibly due to a higher use of drugs[27]. Valid data of incidence and prevalence of HILI caused by TCM herbs are lacking[19], and respective data cannot be derived from the present study with a low frequency of liver injury of 0.12% among all 21470 patients treated with herbal TCM. Valid data were published for drugs, showing that idiosyncratic DILI is a rare event, in a population-based French study with an annual estimated incidence of 13.9 ± 2.4 cases per 100000 inhabitants[53]. A good overview of suspicious TCM herbs is provided in several reports[2,13-15,34], which were also used for comparison in our own survey (Table 5). Nevertheless, the list of suspected TCM herbs remains tentative (Tables 3-5).
Limitations of our study: The focus of our investigation was on ALT levels > 5 × ULN, considering thereby real HILI cases. Cases with ALT elevations between 2 and 5 × ULN are per definition not real but milder HILI due to treatment with TCM herbs, not requiring additional causality proof using RUCAM. As all patients with real HILI had a good outcome with ALT normalization during the relatively short follow-up periods, this favorable outcome can be expected also for patients with milder HILI. A single normal pre-treatment ALT value likely excludes pre-existing liver disease, though little uncertainty remains, which would decrease rather than increase the overall frequency of HILI by TCM. By study protocol, patients with increased ALT values were explicitly not included, although it would have been of interest how TCM treatment influences increased pre-treatment ALT values.
In this report, we present liver injury data for the first time derived from a prospective, hospital-based and large-scale study of 21470 patients, who received treatment with TCM herbs and had no liver disease before. ALT was used as a diagnostic biomarker to exclude liver disease prior to therapy initiation and to assess liver integrity during and after the therapy. This study of 21470 patients revealed that herbal TCM products cause rare liver injury in 26 patients corresponding to 0.12%. Liver injury rapidly improved in most patients following cessation of the therapy, also substantiating causality for the suspected TCM herbs. Under the present study conditions, a transition of acute liver injury to a chronic course was not observed. As these encouraging results are based on strict protocol in a hospital setting, it remains to be established whether these data can be transferred to normal field conditions. Indeed, in the real world confounding variables prevail, such as pre-existing chronic liver diseases, complex therapy conditions of co-medication with Western drugs, and possible problems of herbal TCM product quality regarding misidentification of herbs, impurities of heavy metals, pesticides and other toxins, and adulteration by Western drugs to enhance efficacy. To be on the side of caution and for risk minimizing physicians are well advised to inform patients about the low risk and symptoms of liver injury associated with the use of TCM herbs, if patients decide on this special therapy option.
Herbal Traditional Chinese Medicines (TCMs) are worldwide in common use, which is well documented in the literature. They are highly appreciated, as they are of natural origin, and mainly based the belief of their efficiency and lack of adverse events, and their preference as valuable alternatives over a conventional treatment with synthetic drugs. However, some criticism emerged regarding the issue of efficiency, and adverse reactions ranging from clinically not relevant events to more severe ones including suspected liver injury.
In a prospective, hospital-based study, patients with normal values of alanine aminotransferase (ALT) as a diagnostic marker for ruling out pre-existing liver disease were enrolled and reassessed on discharge by routine laboratory within a safety program carried out at the First German Hospital of TCM from 1994 to 2015. Liver injury was detected in 26/21470, patients (0.12%) with normal liver tests prior to treatment initiation. In most of the liver injury cases, the Roussel Uclaf Causality Assessment Method (RUCAM)-based causality for herbal TCM was graded as “possible”.
In this report, the authors present for the first time liver injury data derived from a prospective, hospital based and large-scale study of 21470 patients, who received treatment with TCM herbs and had no liver disease before.
The findings in this study may help to bring more objectification into discussion about TCM herbs and their risks of liver injury. As long as the therapeutic efficacy of TCM herbs is poorly documented in the scientific literature, even low risk of liver injury by TCM herbs has to be communicated to all potential consumers and to the academic public.
Liver injury was defined ALT ≥ 5 × ULN = upper limit of normal as clinically relevant.
This is an interesting and well-written study.
We thank the staff members at the TCM hospital in Bad Kötzting and all colleagues from the Beijing University of Chinese Medicine for their support and critical comments.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Borzio M, Carvalho-Filho RJ, Tziomalos K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Ge S, He TT, Hu H. Popularity and customer preferences for over-the-counter Chinese medicines perceived by community pharmacists in Shanghai and Guangzhou: a questionnaire survey study. Chin Med. 2014;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | National Institutes of Health (NIH) and LiverTox. Chinese and other Asian herbal medicines. 2016. Accessed on 23 April. 2017; Available from: http://livertox.nih.gov/ChineseAndOther AsianHerbalMedicines.htm. |
| 3. | Teschke R, Wolff A, Frenzel C, Eickhoff A, Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J Gastroenterol. 2015;21:4466-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (2)] |
| 4. | Teo DC, Ng PS, Tan SH, Lim AT, Toh DS, Chan SY, Cheong HH. Drug-induced liver injury associated with Complementary and Alternative Medicine: a review of adverse event reports in an Asian community from 2009 to 2014. BMC Complement Altern Med. 2016;16:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1344] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 6. | Teschke R. Traditional Chinese Medicine Induced Liver Injury. J Clin Transl Hepatol. 2014;2:80-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Kumana CR, Ng M, Lin HJ, Ko W, Wu PC, Todd D. Hepatic veno-occlusive disease due to toxic alkaloid herbal tea. Lancet. 1983;2:1360-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Teschke R, Wolff A, Frenzel C, Schulze J. Review article: Herbal hepatotoxicity--an update on traditional Chinese medicine preparations. Aliment Pharmacol Ther. 2014;40:32-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Zhu Y, Niu M, Chen J, Zou ZS, Ma ZJ, Liu SH, Wang RL, He TT, Song HB, Wang ZX. Hepatobiliary and pancreatic: Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol. 2016;31:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Yu J, Xie J, Mao XJ, Wang MJ, Li N, Wang J, Zhaori GT, Zhao RH. Hepatoxicity of major constituents and extractions of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata. J Ethnopharmacol. 2011;137:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | China Food and Drug Administration. Consideration of the risks of liver injury caused by Polygonum multiflorum. Accessed on May 05. 2017; Available from: http://www.sda.gov.cn/WS01/CL0051/102902.html. |
| 12. | Lee CH, Wang JD, Chen PC. Risk of liver injury associated with Chinese herbal products containing radix bupleuri in 639,779 patients with hepatitis B virus infection. PLoS One. 2011;6:e16064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (30)] |
| 13. | Woo HJ, Kim HY, Choi ES, Cho YH, Kim Y, Lee JH, Jang E. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine. 2015;22:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Dağ MS, Aydınlı M, Oztürk ZA, Türkbeyler IH, Koruk I, Savaş MC, Koruk M, Kadayıfçı A. Drug- and herb-induced liver injury: a case series from a single center. Turk J Gastroenterol. 2014;25:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Ou P, Chen Y, Li B, Zhang M, Liu X, Li F, Li Y, Chen C, Mao Y, Chen J. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. Springerplus. 2015;4:802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Teschke R, Wolff A, Frenzel C, Schulze J. Letter: Herbal hepaotoxicity--an update on traditional Chinese medicine preparations; authors‘ reply. Aliment Pharmacol Ther. 2014;40:738-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Teschke R, Eickhoff A. Herbal hepatotoxicity in traditional and modern medicine: actual key issues and new encouraging steps. Front Pharmacol. 2015;6:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Frenzel C, Teschke R. Herbal Hepatotoxicity: Clinical Characteristics and Listing Compilation. Int J Mol Sci. 2016;17:pii: E588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Teschke R, Larrey D, Melchart D, Danan G. Traditional Chinese Medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines. 2016;3:18. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Intern Med. 2002;252:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Shaw D. Toxicological risks of Chinese herbs. Planta Med. 2010;76:2012-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Zhang L, Yan J, Liu X, Ye Z, Yang X, Meyboom R, Chan K, Shaw D, Duez P. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012;140:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Posadzki P, Watson L, Ernst E. Contamination and adulteration of herbal medicinal products (HMPs): an overview of systematic reviews. Eur J Clin Pharmacol. 2013;69:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 26. | Teschke R, Danan G. Causality assessment methods in drug-induced liver injury. Editors: Minjun Chen and Yvonne Will. Series: Methods in Pharmacology and Toxicology/Y. James Kang David C. Casey. Berlin Germany: Springer Protocols, Springer 2017; . |
| 27. | Nin Chau T, Cheung WI, Ngan T, Lin J, Lee KW, Tat Poon W, Leung VK, Mak T, Tse ML; Hong Kong Herb-Induced Liver Injury Network (HK-HILIN). Causality assessment of herb-induced liver injury using multidisciplinary approach and Roussel Uclaf Causality Assessment Method (RUCAM). Clin Toxicol (Phila). 2011;49:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Zhang P, Ye Y, Yang X, Jiao Y. Systematic Review on Chinese Herbal Medicine Induced Liver Injury. Evid Based Complement Alternat Med. 2016;2016:3560812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Wang J, Ma Z, Niu M, Zhu Y, Liang Q, Zhao Y, Song J, Bai Z, Zhang Y, Zhang P. Evidence chain-based causality identification in herb-induced liver injury: exemplification of a well-known liver-restorative herb Polygonum multiflorum. Front Med. 2015;9:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Zhu Y, Liu SH, Wang JB, Song HB, Li YG, He TT, Ma X, Wang ZX, Wang-Li-ping , Zhou K. [Clinical Analysis of Drug-induced Liver Injury Caused by Polygonum multiflorum and its Preparations]. Zhongguo Zhongxiyi Jiehe Zazhi. 2015;35:1442-1447. [PubMed] |
| 31. | Papafragkakis C, Ona MA, Reddy M, Anand S. Acute Hepatitis after Ingestion of a Preparation of Chinese Skullcap and Black Catechu for Joint Pain. Case Reports Hepatol. 2016;2016:4356749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Hager S, Dai J, Fischer V, Lüthke F, Staudinger A. East Meets West: Synergy through Diversity. Forsch Komplementmed. 2016;23 Suppl 2:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Chinese Pharmacopoeia Commission (ed): Pharmacopoeia of the People’s Republic of China 2010, English Edition vol I, Appendix IID A-25. Beijing: China Medical Science Press 2010; . |
| 34. | Teschke R, Schulze J, Eickhoff A, Danan G. Drug Induced Liver Injury: Can Biomarkers Assist RUCAM in Causality Assessment? Int J Mol Sci. 2017;18:E803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Weidenhammer W, Melchart D. Quality Profiling at the TCM Hospital Bad Kötzting - Examples from an Ongoing Systematic Patient Documentation. Forsch Komplementmed. 2016;23 Suppl 2:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Wagner H, Bauer R, Melchart D. New Analytical Monographs on TCM Herbal Drugs for Quality Proof. Forsch Komplementmed. 2016;23 Suppl 2:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Feng DD, Tang T, Lin XP, Yang ZY, Yang S, Xia ZA, Wang Y, Zheng P, Wang Y, Zhang CH. Nine traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review. Neuropsychiatr Dis Treat. 2016;12:2387-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Melchart D, Linde K, Liao X, Hager S. Herbal treatment in a hospital for traditional Chinese medicine in Germany. Phytomedicine. 1996;3:SL-128. |
| 39. | Melchart D, Linde K, Weidenhammer W, Hager S, Liao J, Bauer R, Wagner H. Use of traditional drugs in a hospital of Chinese medicine in Germany. Pharmacoepidemiol Drug Saf. 1999;8:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Wagner H, Bauer R, Melchart D, Xiao PG, Staudinger A. Chromatographic fingerprint analysis of herbal medicines, vol. 1-4. Wien: Springer 2011; . |
| 41. | Bauer R, Tittel G. Quality assessment of herbal preparations as a precondition of pharmacological and clinical studies. Phytomedicine. 1996;2:193-198. [PubMed] |
| 42. | Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, Ding Y, Duan ZP, Fu QC, Guo XY, Hu P, Hu XQ, Jia JD, Lai RT, Li DL, Liu YX, Lu LG, Ma SW, Ma X, Nan YM, Ren H, Shen T, Wang H, Wang JY, Wang TL, Wang XJ, Wei L, Xie Q, Xie W, Yang CQ, Yang DL, Yu YY, Zeng MD, Zhang L, Zhao XY, Zhuang H; Drug-induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 43. | Teschke R, Zhang L, Long H, Schwarzenboeck A, Schmidt-Taenzer W, Genthner A, Wolff A, Frenzel C, Schulze J, Eickhoff A. Traditional Chinese Medicine and herbal hepatotoxicity: a tabular compilation of reported cases. Ann Hepatol. 2015;14:7-19. [PubMed] |
| 44. | Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J. Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol. 2014;13:248-255. [PubMed] |
| 45. | Shahbaz O, Mahajan S, Lewis JH. Highlights of Drug - and Herb- induced liver injury in the Literature from 2016: How Best to Translate New Information into Clinical Practice? Expert Opin Drug Metab Toxicol. 2017;13:935-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Teschke R, Schulze J, Schwarzenboeck A, Eickhoff A, Frenzel C. Herbal hepatotoxicity: suspected cases assessed for alternative causes. Eur J Gastroenterol Hepatol. 2013;25:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Teschke R, Schulze J, Eickhoff A, Wolff A, Frenzel C. Mysterious Hawaii liver disease case - Naproxen overdose as cause rather than OxyELITE Pro? J Liver Clin Res. 2015;2:1013. |
| 48. | Teschke R, Schwarzenboeck A, Frenzel C, Schulze J, Eickhoff A, Wolff A. The mystery of the Hawaii liver disease cluster in summer 2013: A pragmatic and clinical approach to solve the problem. Ann Hepatol. 2016;15:91-109. [PubMed] |
| 49. | Teschke R, Eickhoff A. The Honolulu Liver Disease Cluster at the Medical Center: Its Mysteries and Challenges. Int J Mol Sci. 2016;17:476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Teschke R, Eickhoff A. Suspected Liver Injury and the Dilemma of Causality. Dig Dis Sci. 2017;62:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Teschke R, Danan G. Diagnosis and Management of Drug-Induced Liver Injury (DILI) in Patients with Pre-Existing Liver Disease. Drug Saf. 2016;39:729-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Teschke R, Danan G. Drug-induced liver injury: Is chronic liver disease a risk factor and a clinical issue? Expert Opin Drug Metab Toxicol. 2017;13:425-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 536] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 54. | Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol. 2013;75:603-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 55. | Zhang ZJ, Tan QR, Tong Y, Wang XY, Wang HH, Ho LM, Wong HK, Feng YB, Wang D, Ng R. An epidemiological study of concomitant use of Chinese medicine and antipsychotics in schizophrenic patients: implication for herb-drug interaction. PLoS One. 2011;6:e17239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 57. | Melchart D, Hager S, Dai J, Weidenhammer W. Quality Control and Complication Screening Programme of Chinese Medicinal Drugs at the First German Hospital of Traditional Chinese Medicine - A Retrospective Analysis. Forsch Komplementmed. 2016;23 Suppl 2:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Yuen MF, Tam S, Fung J, Wong DK, Wong BC, Lai CL. Traditional Chinese medicine causing hepatotoxicity in patients with chronic hepatitis B infection: a 1-year prospective study. Aliment Pharmacol Ther. 2006;24:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |