Published online Sep 8, 2017. doi: 10.4254/wjh.v9.i25.1064
Peer-review started: March 17, 2017
First decision: May 3, 2017
Revised: May 17, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: September 8, 2017
Processing time: 174 Days and 5.4 Hours
To investigate daclatasvir (DCV) and asunaprevir (ASV) efficacy in hepatitis C (HCV) patients, with respect to resistance-associated substitutions (RASs).
A total of 392 HCV-infected patients from multiple centers were included in this study. We evaluated their clinical courses and sustained virologic responses (SVR) according to pretreatment factors (gender, age, history of interferon-based regimens, platelet counts, level of viremia, pretreatment NA5A:L31, and Y93 substitutions). We also analyzed the pretreatment and post-treatment major RASs of NS3:D168, NS5A:L31 and Y93 substitutions using a direct-sequencing method in 17 patients who were unable to achieve SVR at 12 wk after treatment completion (SVR12).
The overall SVR12 rate was 88.3%. Thirty-one patients discontinued treatment before 24 wk because of adverse events, 23 of whom achieved SVR12. There were no significant differences in SVR12 rates with respect to gender, age, history of interferon-based regimens, and platelet counts. The SVR12 rate in patients with viral loads of ≥ 6.0 log IU/mL was significantly lower than those with viral loads of < 6.0 log IU/mL (P < 0.001). The SVR12 rate in patients with Y93 substitution-positive was significantly lower than those with Y93 substitution-negative (P < 0.001). The L31 substitution-positive group showed a lower SVR12 rate than the L31 substitution-negative group, but the difference was not statistically significant. Seventeen patients who did not achieve SVR12 and had available pretreatment and post-treatment sera had additional RASs in NS3:D168, NS5:L31, and Y93 substitution at treatment failure.
Combination of DCV and ASV is associated with a high SVR rate. Baseline RASs should be thoroughly assessed to avoid additional RASs after treatment failure.
Core tip: Hepatitis C - infected patients treated with daclatasvir and asunaprevir were evaluated for sustained virological response (SVR) according to pretreatment factors. The overall rate of SVR12 was 88.3%. The SVR12 rate in the ≥ 6.0 log IU/mL group was significantly lower than in the < 6.0 log IU/mL group. The SVR12 rate in Y93 substitution-positive patients was significantly lower than that in non-Y93 substitution patients. The L31 substitution-positive group had a lower SVR 12 rate than the L31 substitution-negative group. Seventeen patients who did not achieve SVR 12 had additional RASs in NS3:D168, NS5:L31, and Y93 post-treatment. Baseline RASs should be thoroughly assessed to avoid additional RASs after treatment failure.
- Citation: Fujii H, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Nakamura H, Yasui K, Minami M, Tanaka S, Ishikawa H, Kimura H, Takami S, Nagao Y, Shima T, Itoh Y. Real-world efficacy of daclatasvir and asunaprevir with respect to resistance-associated substitutions. World J Hepatol 2017; 9(25): 1064-1072
- URL: https://www.wjgnet.com/1948-5182/full/v9/i25/1064.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i25.1064
Hepatitis C virus (HCV) is one of the most important chronic infections worldwide. An estimated 170-200 million people are infected with HCV worldwide[1], with approximately 1.0-1.5 million infected people in Japan[2]. HCV treatments have dramatically changed recently. Pegylated interferon (PEG-IFN) and ribavirin (RBV) dual therapy has long been the standard treatment for genotype 1 chronic hepatitis C (CHC). Recently, however, newer anti-HCV drugs, termed direct-acting antiviral agents (DAAs), have become available[3].
Telaprevir (TVR) was the first nonstructural protein 3 (NS3) protease inhibitor (PI)[4] approved in Japan, followed by the second generation NS3 PIs, simeprevir (SMV) and vaniprevir[5-8]. These drugs were scheduled to be administered in combination with PEG-IFN and RBV, and could enhance treatment efficacy. However, both PEG-IFN and RBV can cause various side effects, and they are contraindicated in elderly patients and/or patients with certain comorbid conditions.
The combination of oral daclatasvir (DCV), a NS5A inhibitor, and asunaprevir (ASV), a second generation NS3 PI, has been the first drug combination approved in Japan for the treatment of HCV genotype 1-infected patients. This drug combination showed high rates of sustained virologic response (SVR) and better tolerability[9,10]. Thus, many patients for whom conventional IFN-based treatment was intolerable or incurable have been medicated. We performed a retrospective cohort study to evaluate the safety, tolerability, and effectiveness of DCV and ASV combination therapy in real-world clinical practice. Moreover, we evaluated the presence of pretreatment and post-treatment major resistance-associated substitutions (RASs) (NS5A:L31 and Y93 substitution, and NS3:D168 substitution) using a direct-sequencing method in 17 patients who did not achieve SVR12.
Patients were enrolled at Kyoto Prefectural University of Medicine and seven affiliated hospitals in the Kinki area of Japan (Kyoto, Osaka, Nara, Shiga Prefecture) from 2014 to 2015. Study protocols were approved by the ethics committee of each institution and conformed to the provisions of the Declaration of Helsinki. Eligible patients were those aged at least 20 years with HCV genotype 1 infection diagnosed by board-certified hepatologists. Patients with decompensated liver cirrhosis, chronic hepatitis B, HIV, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, or Wilson’s disease, were excluded. Patients with uncontrollable hypertension, those with a history of alcohol abuse or clinically significant medical conditions (severe renal disease, severe heart disease, active drug users, pregnancy, and those receiving drugs which interact with DCV or ASV) were also excluded. Patients were followed up monthly or every 2 wk to assess liver function and virological markers during treatment and until 12 wk after the completion of DCV and ASV therapy. All patients gave informed consent to participate in this study. Five patients were lost to follow-up or underwent extreme protocol deviation (e.g., death by accident). Those lost to follow-up or had extreme protocol deviation were excluded from the analysis.
Patients received oral daclatasvir (Daklinza; Bristol-Myers Squibb Company) 60 mg once daily with oral asunaprevir (Sunvepra; Bristol-Myers Squibb Company) 200 mg twice daily, in accordance with prescribing information, for 24 wk. Patients were followed up until at least 12 wk after final treatment administration to assess SVR12.
HCV RNA responses during therapy were classified into the following groups: The non-response group (NR), patients whose HCV RNA remained detectable during treatment, resulting in treatment discontinuation; the breakthrough group (BT), patients whose HCV RNA was once undetectable but reappeared during treatment; and the relapse group (REL), patients whose HCV RNA was undetectable at the end of the 24-wk treatment but became detectable again during follow-up. SVR12 was defined as undetectable serum HCV RNA levels at 12 wk after the end of treatment (EOT). Therapeutic effects were evaluated using per-protocol analysis and included patients who received at least 2 wk of this therapy.
Continuation of treatment was decided by board-certified hepatologists. In general, patients whose serum HCV RNA was positive at 8 or 12 wk were judged as NR, and the treatment was terminated at that time. Patients whose serum HCV RNA reappeared were diagnosed as belonging to the BT group, and the treatment was stopped at the time. Dose reduction or discontinuation of DCV or ASV was determined by board-certified hepatologists. Discontinuation of the treatment was generally considered when grade 3-4 adverse events according to Common Terminology Criteria for Adverse Events v4.0 occurred.
Blood samples were obtained for routine biochemical and hematological assessments at treatment initiation, on treatment weeks 4, 8, 12, 16, 20, 24, at EOT, and at 12 wk after EOT. Antiviral effects were mostly assessed by measuring serum HCV RNA levels using the COBAS TaqMan HCV test (Roche Molecular Diagnostics, Tokyo, Japan) with a lower limit of quantitation (LLOQ) of 15 IU/mL (with a quantitation range of 1.2-7.8 log10 IU/mL). Missing data points were deemed a success if the immediately preceding and subsequent time points were successful; otherwise, data points were termed as failures. Patients who had missing data because of premature discontinuations were considered failures from the point of discontinuation.
Pretreatment major RASs of NS5A, L31 or Y93 substitutions were assessed using commercially available assays of direct-sequencing method, the cycleave probe method, or invader assays. Furthermore, the pretreatment and post-treatment major RASs of NS3:D168, NS5A:L31, and Y93 substitution were investigated in 21 patients with virological failure by using a direct-sequencing method. In brief, HCV RNA was extracted from blood serum using a commercially available kit (QIAamp viral RNA kit; QIAGEN, Valencia, CA, United States). This sample was used for reverse transcription with random hexamer primers (SuperScript III First-Strand Synthesis System for RT-PCR cDNA synthesis kit; Invitrogen, Carlsbad, CA, United States). The NS3 and NS5A regions were amplified by nested PCR using Takara Ex Taq HS (Takara Ex Taq, Otsu, Japan). The PCR primer sequences were NS3 forward primer: gccgcgatgccatcatcctcc, gtccaaatggccttcatgaagctgg, caatgtagaccaggacctcgtcgg and reverse primer: tggtgaaggtgggatccaagctgaa; or NS5A forward primer: atcctctccagccttaccatcact and reverse primer: ccatgaccaactcgggctggacctt. The PCR products were separated by electrophoresis on 1% agarose gels. These were purified using the QIAquick gel extraction kit (QIAGEN, Hilden, Germany) and sequenced with second-round PCR primers using a dye terminator sequencing kit (BigDye Terminator v 1.1 cycle sequencing kit; Applied Biosystems, Foster City, CA, USA) and ABI PRISM 310 genetic analyzer (Applied Biosystems).
Baseline continuous data were expressed as median with interquartile ranges in parentheses, and categorical variables were expressed as numbers. Some baseline data were categorized, and univariate analyses were performed using the χ2 or Mann-Whitney U-tests as appropriate. All P-values of < 0.05 of two-tailed tests were considered significant. All statistical analyses were performed using the SPSS 22.0 statistical package (SPSS Incorporated, Chicago, IL, United States).
The baseline patient characteristics are shown in Table 1. In total, 392 patients were included in this study. Female patients were predominant. Enrolled patients were generally older (median, 71.0 years) and had lower platelet counts. As for prior treatments, 70 patients had received IFN, 147 patients had received PEG-IFN or IFN plus RBV, 13 patients had a history of PEG-IFN plus RBV plus TVR, and 9 patients had a history of PEG-IFN plus RBV plus SMV. Concerning RASs, L31, and Y93 substitutions at pretreatment were seen in 3.5% (10/288) and 8.4% (27/321) of patients, respectively (Table 1). Two patients had both L31M and Y93H RASs.
| No. of patients | n = 392 | |
| Gender (male/female) | 159/233 | |
| Age, yr | 71.0 | (64.0-77.0) |
| < 65 yr vs ≥ 65 yr | 99/293 | |
| Laboratory data | ||
| Level of viremia (log IU/mL) | 6.2 | (5.8-6.5) |
| < 6.0 vs ≥ 6.0 | 137 vs 255 | (35.1% vs 64.9%) |
| Platelet count (× 104/mm3) | 12.6 | (9.2-16.7) |
| 10 < vs ≥ 10 | 114/278 | (29.0% vs 70.4%) |
| ALT (IU/L) | 41 | (29-65) |
| γ-GTP(IU/L) | 34 | (22-57) |
| Other data | ||
| Prior treatment | ||
| IFN vs PEG plus RBV vs TVR vs SMV | 70/147/13/9 | |
| NS5A polymorphisms, n (%) | ||
| L31 substitution, n = 288 | 10 (3.5) | |
| Y93 substitution, n = 321 | 27 (8.4) | |
| L31 and/or Y93, n = 321 | 35 (10.9) |
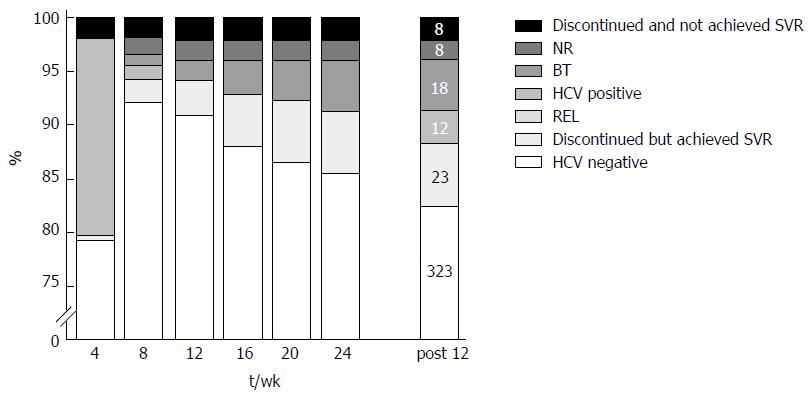
Undetectable HCV RNA levels were achieved in 79.7% (299 of 375), 94.1% (367 of 390), 94.1% (369 of 392), 92.8% (363 of 391), 92.2% (355 of 385), 91.3% (358 of 392), and 88.3% (346 of 392) of patients at treatment weeks 4, 8, 12, 16, 20, 24, and at 12 weeks’ post-treatment, respectively (Figure 1). Because two of the SVR12 patients experienced late relapse of chronic hepatitis C and two additional patients were lost to follow-up, the final SVR24 resulted in 87.2%. Treatment was discontinued in 8 NR patients (2.0%) because the therapeutic effect was hardly expected, and in 18 BT patients (4.6%). Twelve patients (3.0%) received 24 wk of treatment but ended in REL. Thirty-one patients discontinued treatment before 24 wk because of adverse events. Reasons for discontinuation included liver dysfunction (15 patients), fever increase (6), detection of HCC (2), edema or ascites (2), and other reasons (6). Of these, 23 patients (15 liver dysfunction, 2 fever increase, 2 HCC, 1 edema and ascites, and 3 with other reasons) eventually achieved SVR12. Eight patients who received treatment less than 8 wk due to adverse events achieved SVR12. There were no treatment-related deaths.
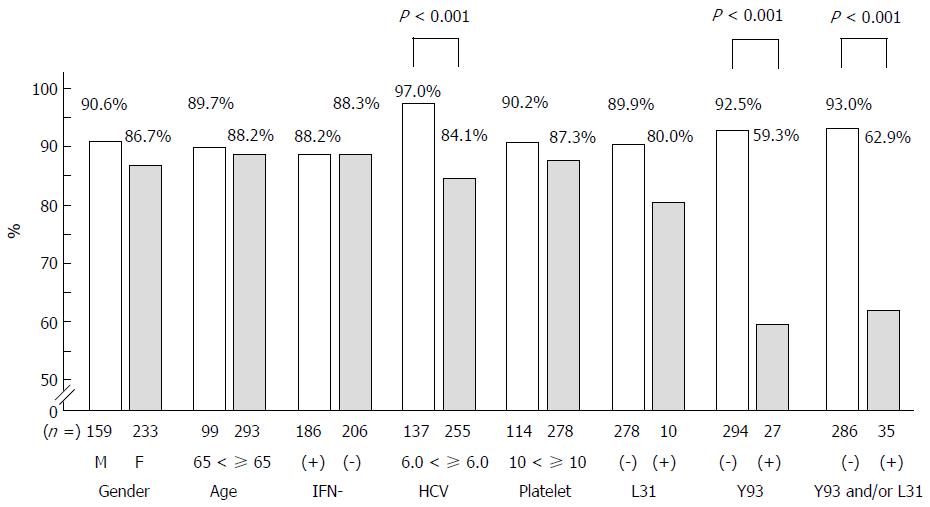
We assessed the SVR12 rate according to gender, age (< 65 vs ≥ 65), history of IFN-based treatment, platelet counts (< 10 × 104/mm3vs ≥ 10 × 104/mm3), level of viremia (< 6.0 log IU/mL vs ≥ 6.0 log IU/mL), and pretreatment L31 and Y93 substitution (negative or positive). The SVR12 rate in the ≥ 6.0 log IU/mL group was significantly lower than in the < 6.0 logIU/mL group (P < 0.001). As for Y93 substitutions, the SVR12 in the Y93 substitution-positive group was significantly lower than that in the Y93 substitution-negative group (P < 0.001). As for L31 substitutions, the L31 substitution-positive group showed a lower SVR 12 rate than their negative counterparts, but without statistical significance (P = 0.28). Other parameters were similar between the two groups (Figure 2).
We investigated the pretreatment and post-treatment major RASs (NS5A:L31 and Y93 substitutions and NS3:D168 substitution) using a direct-sequencing method in 21 patients who did not achieve SVR12. The results of direct-sequencing were of poor quality in four patients, leaving 17 patients who could be investigated completely. Five patients had NS3:D168 substitution, three had NS5A:L31 substitution, and six had NS5A:Y93 substitution before treatment. Five patients had neither NS3:D168 nor NS5A:L31 or Y93 substitutions before treatment. Analysis at the time of virological failure revealed that 14, 14 and 13 patients had NS3:D168, NS5A:L31, and NS5A:Y93 substitutions, respectively. All 17 patients whose pretreatment and post-treatment sera were available had one of the major RASs at the time of virological failure. Moreover, many patients had additional amino acid substitutions like NS5A Q54H (Table 2).
| No. | Pretreatment | Post-treatment | |||||||||
| C.C. | D168 | L31 | Y93 | Other NS3 | Other NS5A | D168 | L31 | Y93 | Other NS3 | Other NS5A | |
| 1 | BT | E | - | - | - | - | E | - | - | - | R30H |
| 2 | REL | - | L/I | - | - | A92T | - | M | - | - | A92K |
| 3 | NR | - | - | H | V170I | Q54Y | - | I | H | V170I | Q54Y |
| 4 | BT | Y | - | - | - | Q54H | Y | F | H | - | Q54H |
| 5 | NR | E | - | - | Q80R, V170I | Q54H | E | V | H | Q80R, V170I | Q54H |
| 6 | BT | E | - | - | - | Q54H | E | V/M | H | V170I | Q54H |
| 7 | BT | - | M/L | - | - | Q54V | V | M/V | H | - | Q54V |
| 8 | BT | - | - | H | - | Q54H | V | M | H | - | Q54H |
| 9 | REL | - | - | H | - | Q62E | V | I | H | - | Q62E |
| 10 | BT | - | - | H/Y | - | - | T | M | H | - | - |
| 11 | BT | - | - | H/Y | - | - | V | V/F | H | V170I | - |
| 12 | BT | Y | F | H | - | - | D | F | H | - | Q54H |
| 13 | BT | - | - | - | - | Q54H, A92T | V | - | - | - | Q54H, A92K |
| 14 | REL | - | - | - | - | Q54H | E | - | - | - | P32L, Q54H, A92K |
| 15 | BT | - | - | - | - | Q62N | V | V | H | - | Q62N |
| 16 | BT | - | - | - | - | - | - | V | H | - | - |
| 17 | REL | - | - | - | - | - | E | M | H | - | - |
We compared the Fibrosis-4 (FIB-4) index[11] at baseline and at 12 wk after the end of treatment. Baseline FIB-4 index was 4.14 and it remarkably decreased to 3.78 at 12 wk after the end of treatment in the SVR12 group (P < 0.001). Meanwhile, bseline FIB-4 index was 3.84 and it slightly decreased to 3.57 in the non-SVR12 group (P = 0.03) FIB-4 index was more markedly reduced in the SVR12 group.
In the present study, we presented the efficacy of DCV and ASV in real-world clinical practice. Most patients were rapidly cleared of HCV and achieved SVR12 with this combination of DAAs. Interestingly, some patients who discontinued treatment after a short duration because of side effects also achieved SVR. Resistance analyses revealed that patients who did not carry both Y93H and L31 mutations before treatment achieved 93.0% SVR12. All 17 patients who could not achieve SVR 12 and who were investigated for major RASs had either NS3-D168 substitution, or NS5A L31 and/or Y93 substitutions, including five patients who did not carry any RASs before treatment.
The high SVR rate of 88.3% in our present study is consistent with previous reports. Serum HCV RNA levels decreased rapidly, and was undetectable by 12 wk in the majority of patients[9,10]. In general, the therapeutic efficacy of a novel IFN-based HCV therapy in real-world clinical practice demonstrates lower SVR rates and higher rates of adverse events than observed in clinical trials. The stable therapeutic effect in a real-world setting is one of the notable benefits of the DCV and ASV combination therapy.
Baseline characteristics of gender, advanced age, history of IFN-based regimens, and low platelet counts (suggestive of advanced fibrosis or cirrhosis) did not influence SVR12 rates. An important finding was that patients with pretreatment viral loads of ≥ 6.0 log IU/mL showed a significantly lower rate of SVR12 than patients with pretreatment viral loads of < 6.0 log IU/mL. As for DCV and ASV treatment, Wang et al[12] showed that patients with lower viral loads (< 8 × 105 IU/mL: 8.0 × 105 IU/mL = 5.9 log IU/mL) seemed to have higher SVR rates than those with higher viral loads (≥ 8 × 105IU/mL) in their meta-analysis[12]. Comparing the background characteristics of patients with viral loads of ≥ 6.0 logIU/mL and < 6.0 logIU/mL in this study showed that they were not significantly different with respect to gender, age, platelet counts, number of treatment discontinuations, and preexisting major RASs (L31 substitution or Y93 substitution). The number of patients with a history of IFN-based treatment was significantly greater in patients with viral loads of ≥ 6.0 log IU/mL than those with < 6.0 log IU/mL (P = 0.04). PEG-IFN and RBV-based treatment was covered by public health insurance only for high viral loads (≥ 5.0 log IU/mL) in Japan. This might have caused the background difference in our study. The HCV RNA loads alone may have influenced treatment efficacy.
Twenty-three patients who experienced adverse events discontinued the treatment but nevertheless achieved SVR12. It is notable that eight patients who were treated for < 8 wk still achieved SVR12. The shortest treatment duration was only 2 wk. The factors that contributed to HCV clearance in such a short treatment duration are unknown. Because alanine aminotransferase (ALT) elevation was the main cause (15 in 23 patients) of stopping treatment early, elevated blood levels of ASV may be both a cause of ALT elevation and an enhanced treatment efficacy[13]. Interestingly, in our study, patients who discontinued treatment because of ALT elevation tended to have lower viral loads. This background factor of low viral load might influence treatment effectiveness. Patients with adverse events such as ALT elevation can still achieve SVR even with short treatment periods.
Resistance analyses before treatment revealed that patients who did not carry both Y93 and L31 substitutions using commercially available tests achieved 93.0% SVR12 (Figure 2). The SVR ratio of the Y93 substitution-positive group was significantly lower than in the Y93 non-substitution group. However, the SVR ratio of the L31 substitution-positive group was not significantly different from that of the L31 non-substitution group. The baseline prevalence of L31 substitution in our study was lower than that in other studies and this might have affected our statistical analyses.
We investigated RASs before treatment and after failure in 17 patients who failed to achieve SVR12. All 17 patients had major well-known substitutions (either NS5A L31 substitution and/or Y93 substitution, or NS3 D168 substitution) at the time of failure. The appearance pattern of these RASs was different in each patient, but can be classified into one of two patterns. The first pattern (Cases 13-17) had no major substitutions before treatment, but carried more than one major variant after treatment. The other (Cases 4-11) had one major substitution before treatment but carried three major substitutions after treatment. The mechanism of occurrence for the first pattern is obscure, but it might have been influenced by the detection sensitivity of direct sequence methods. The mechanism of the second pattern is also obscure, but it revealed an important problem of this therapy for the next generation of DAA treatments from the viewpoint of drug resistance. At any rate, there are still many problems to be solved concerning RASs.
The first problem of pretreatment RAS analysis is that there are no available commercial assays for NS3 mutations in the real world. We did not check the RASs in NS3 for two reasons. One reason is that naturally occurring NS3 RASs are reported to be rare[14,15]. Another reason is that the guideline for the treatment of hepatitis C edited by the Japan Society of Hepatology do not recommend to check NS3 RASs, but recommend to check NS5A RASs before starting DCV/ASV treatment. The second problem is the difference in sensitivities of available assays[16,17]. We could measure NS5A variants using three different methods: Direct-sequencing, the cycleave probe method, and invader assay. Although ultra-deep sequencing is the most sensitive method and can detect minor variants with frequencies of < 1% (Miura et al[18]), this method is too expensive and complicated. The appropriate proportion of RASs to predict this treatment’s efficacy has been reported in several studies. Ide et al[19] reported that SVR rates were clearly altered by the proportion of Y93 substitutions. In our unpublished data, patients who had > 10% pretreatment Y93 substitutions by the cycleave probe method tended to experience virological failure. Thus, this may be the appropriate cutoff value in our study (data not shown). Except for L31 and Y93 substitutions which can be checked commercially, other rare NS5A RASs were reported to have a rather small influence on therapeutic effect[20]. After all, although RASs have a great influence on the therapeutic efficacy of DCV and ASV combination treatment, we cannot determine the best method at present. Further larger-scale studies are needed to clarify this point.
The eradication of HCV can ameliorate liver inflammation as well as liver fibrosis[21]. We calculated the values of FIB4 index both at baseline and after SVR12. We found that FIB-4 index was more markedly reduced in the SVR12 group. This data indicate that liver fibrosis is improved in SVR12 group in the future
A major limitation of the present study was the inability to evaluate several factors known to influence HCV treatment efficacy. We did not examine IL28B[22], amino acid substitutions of the HCV core region 70 and 91[23], NS5A interferon sensitivity determining region[24], interferon/ribavirin resistance determining region[25]. These factors were mainly related to the efficacy of IFN based therapy and were not easily available in real-world.
DCV and ASV combination therapy is associated with a high SVR rate in real-world clinical practice. The SVR12 rate in patients with viral loads of HCV RNA ≥ 6.0 log IU/mL was significantly lower than that in patients with HCV RNA < 6.0 log IU/mL. The ratio of SVR12 in the Y93 substitution-positive group was significantly lower than that in the Y93 substitution-negative group. The pretreatment and post-treatment NS3:D168 substitution, and NS5A:L31 and Y93 substitutions were evaluated in 17 patients who did not achieve SVR 12 using direct-sequencing method. All 17 patients had increased RASs after treatment failure. Baseline RASs should be thoroughly assessed to avoid additional RASs after treatment failure.
The modality for treating hepatitis C has rapidly progressed in a recent years. The usage of directly acting antiviral (DAA) has changed the treatment dramatically. The combination of oral daclatasvir (DCV), a NS5A inhibitor, and asunaprevir (ASV), a second generation NS3 PI, is the first drug combination approved in Japan for the treatment of hepatitis C (HCV) genotype 1-infected patients. This drug combination showed high rates of sustained virologic response (SVR) and better tolerability. They performed a retrospective cohort study to evaluate the effectiveness of DCV and ASV combination therapy in real-world clinical practice. Moreover, they evaluated the presence of pretreatment and post-treatment major resistance-associated substitutions (RASs) (NS5A:L31 and Y93 substitution, and NS3:D168 substitution) using a direct-sequencing method in 17 patients who did not achieve SVR12.
In the era of DAA treatment for hepatitis C, drugs are designed targeting NA3, NA5A, and NS5B of HCV. Evaluation of the efficacy/tolerability of the DAAs regimens as well as the acquisition of RASs in DAAs treatment are major interest in the field of hepatology. Above all, attention is paid for RASs (NS5A: L31 and Y93 substitution, and NS3:D168 substitution) in DAC/ASV treatment.
All 17 patients who failed to achieve SVR12 in DAC/ASV treatment had major well-known RASs (either NS5A L31 RAS and/or Y93 RAS, or NS3 D168 RAS) after treatment failure. The appearance pattern of these RASs was different, but can be classified into two patterns. The first pattern: No major RASs before treatment, but more than one major RASs after treatment failure. The second pattern: One major RAS before treatment but three major RASs after treatment failure.
DCV/ASV combination therapy is associated with a high SVR rate in real-world clinical practice, but appearance of RASs were seen in patients with treatment failure. Baseline critical RASs should be checked to avoid additional RASs after treatment failure.
Very interesting paper on DCV and ASV efficacy in HCV patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bock CT, Luo HS, Romanelli RG S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1846] [Article Influence: 153.8] [Reference Citation Analysis (3)] |
| 2. | Namiki I, Nishiguchi S, Hino K, Suzuki F, Kumada H, Itoh Y, Asahina Y, Tamori A, Hiramatsu N, Hayashi N. Management of hepatitis C; Report of the Consensus Meeting at the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:347-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Bartenschlager R, Lohmann V, Penin F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat Rev Microbiol. 2013;11:482-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 292] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 5. | Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Kumada H, Hayashi N, Izumi N, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Rito K, Komada Y, Seto C. Simeprevir (TMC435) once daily with peginterferon-α-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: The CONCERTO-4 study. Hepatol Res. 2015;45:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Hayashi N, Nakamuta M, Takehara T, Kumada H, Takase A, Howe AY, Ludmerer SW, Mobashery N. Vaniprevir plus peginterferon alfa-2b and ribavirin in treatment-naive Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase III study. J Gastroenterol. 2016;51:390-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 10. | Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1606] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 12. | Wang HL, Lu X, Yang X, Xu N. Effectiveness and safety of daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: Systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Akuta N, Sezaki H, Suzuki F, Kawamura Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y, Arase Y. Relationships between serum asunaprevir concentration and alanine aminotransferase elevation during daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. J Med Virol. 2016;88:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Bartels DJ, Sullivan JC, Zhang EZ, Tigges AM, Dorrian JL, De Meyer S, Takemoto D, Dondero E, Kwong AD, Picchio G, Kieffer TL. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment. J Virol. 2013;87:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Suzuki F, Sezaki H, Akuta N, Suzuki Y, Seko Y, Kawamura Y, Hosaka T, Kobayashi M, Saito S, Arase Y, Ikeda K, Kobayashi M, Mineta R, Watahiki S, Miyakawa Y, Kumada H. Prevalence of hepatitis C virus variants resistant to NS3 protease inhibitors or the NS5A inhibitor (BMS-790052) in hepatitis patients with genotype 1b. J Clin Virol. 2012;54:352-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Tadokoro K, Kobayashi M, Suzuki F, Tanaka C, Yamaguchi T, Nagano M, Egashira T, Kumada H. Comparative quantitative analysis of hepatitis C mutations at amino acids 70 and 91 in the core region by the Q-Invader assay. J Virol Methods. 2013;189:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Uchida Y, Kouyama J, Naiki K, Mochida S. A novel simple assay system to quantify the percent HCV-RNA levels of NS5A Y93H mutant strains and Y93 wild-type strains relative to the total HCV-RNA levels to determine the indication for antiviral therapy with NS5A inhibitors. PLoS One. 2014;9:e112647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Miura M, Maekawa S, Sato M, Komatsu N, Tatsumi A, Takano S, Amemiya F, Nakayama Y, Inoue T, Sakamoto M. Deep sequencing analysis of variants resistant to the non-structural 5A inhibitor daclatasvir in patients with genotype 1b hepatitis C virus infection. Hepatol Res. 2014;44:E360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Ide T, Eguchi Y, Harada M, Ishii K, Morita M, Morita Y, Sugiyama G, Fukushima H, Yano Y, Noguchi K. Evaluation of Resistance-Associated Substitutions in NS5A Using Direct Sequence and Cycleave Method and Treatment Outcome with Daclatasvir and Asunaprevir for Chronic Hepatitis C Genotype 1. PLoS One. 2016;11:e0163884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Uchida Y, Kouyama JI, Naiki K, Sugawara K, Inao M, Imai Y, Nakayama N, Mochida S. Development of rare resistance-associated variants that are extremely tolerant against NS5A inhibitors during daclatasvir/asunaprevir therapy by a two-hit mechanism. Hepatol Res. 2016;46:1234-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Tada T, Kumada T, Toyoda H, Mizuno K, Sone Y, Kataoka S, Hashinokuchi S. Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J Gastroenterol Hepatol. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [PubMed] |
| 23. | Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Someya T, Kobayashi M, Saitoh S, Watahiki S, Sato J. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non-virological response to interferon-ribavirin combination therapy. Intervirology. 2005;48:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Ogura Y, Izumi N, Marumo F, Sato C. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 734] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 25. | El-Shamy A, Nagano-Fujii M, Sasase N, Imoto S, Kim SR, Hotta H. Sequence variation in hepatitis C virus nonstructural protein 5A predicts clinical outcome of pegylated interferon/ribavirin combination therapy. Hepatology. 2008;48:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |