Published online Sep 8, 2016. doi: 10.4254/wjh.v8.i25.1075
Peer-review started: May 30, 2016
First decision: July 6, 2016
Revised: July 17, 2016
Accepted: July 29, 2016
Article in press: August 1, 2016
Published online: September 8, 2016
Processing time: 109 Days and 3.2 Hours
To propose several alternatives treatment of type 1 hepatorenal syndrome (HRS-1) what is the most severe expression of circulatory dysfunction on patients with portal hypertension.
A group of eleven gastroenterologists and nephrologists performed a structured analysis of available literature. Each expert was designated to review and answer a question. They generated draft statements for evaluation by all the experts. Additional input was obtained from medical community. In order to reach consensus, a modified three-round Delphi technique method was used. According to United States Preventive Services Task Force criteria, the quality of the evidence and level of recommendation supporting each statement was graded.
Nine questions were formulated. The available evidence was evaluated considering its quality, number of patients included in the studies and the consistency of its results. The generated questions were answered by the expert panel with a high level of agreement. Thus, a therapeutic algorithm was generated. The role of terlipressin and norepinephrine was confirmed as the pharmacologic treatment of choice. On the other hand the use of the combination of octreotide, midodrine and albumin without vasoconstrictors was discouraged. The role of several other options was also evaluated and the available evidence was explored and discussed. Liver transplantation is considered the definitive treatment for HRS-1. The present consensus is an important effort that intends to organize the available strategies based on the available evidence in the literature, the quality of the evidence and the benefits, adverse effects and availability of the therapeutic tools described.
Based on the available evidence the expert panel was able to discriminate the most appropriate therapeutic alternatives for the treatment of HRS-1.
Core tip: The available evidence for the treatment of type 1 hepatorenal syndrome (HRS-1) was evaluated. The role of terlipressin and norepinephrine was confirmed as the pharmacologic treatment of choice. On the other hand the use of the combination of octreotide, midodrine and albumin without vasoconstrictors was discouraged. The role of several other options was also evaluated and the available evidence was explored and discussed. Liver transplantation is considered the definitive treatment for HRS-1 and the necessary conditions to optimize the recovery of renal function was also discussed.
- Citation: Arab JP, Claro JC, Arancibia JP, Contreras J, Gómez F, Muñoz C, Nazal L, Roessler E, Wolff R, Arrese M, Benítez C. Therapeutic alternatives for the treatment of type 1 hepatorenal syndrome: A Delphi technique-based consensus. World J Hepatol 2016; 8(25): 1075-1086
- URL: https://www.wjgnet.com/1948-5182/full/v8/i25/1075.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i25.1075
Hepatorenal syndrome (HRS) is a severe disease strictly related to the presence of portal hypertension (PHT). The cumulative probability of HRS in patients with cirrhosis and ascites was initially reported as 18% at one year and 39% at five years[1]. More recently and employing the Ascites Club criteria, the incidence of HRS was estimated as 7.1% in a cohort that was followed for 41 ± 3 mo[2]. Table 1 depicts the diagnostic criteria proposed by the Ascites Club for the diagnosis of HRS[3]. These criteria are the result of a consensus based on clinical knowledge of the condition, and at this time, there are no biochemical tests clinically available for the diagnosis of HRS. Two types of HRS have been defined by the Ascites Club. Type 2 (HRS-2) is a slowly progressive disease, clinically expressed as refractory ascites with an average median survival of approximately six months. In contrast, type 1 HRS (HRS-1) is a catastrophic disease characterized by a rapid decrease in glomerular filtration rate[3], and it is considered the most extreme expression of circulatory dysfunction secondary to PHT. Its diagnosis requires the exclusion of intrinsic renal disease, hypovolemia, shock and exposure to nephrotoxic drugs. It has a very poor prognosis with a mean survival of only two weeks without treatment[4]. Thus, HRS-1, is considered one of the most severe complications of cirrhosis and PHT, and it represents a therapeutic challenge for hepatologists.
| HRS |
| Presence of cirrhosis and ascites |
| Serum creatinine > 1.5 mg/dL (or 133 micromoles/L) |
| No improvement of serum creatinine (decrease equal to or less than 1.5 mg/dL) after at least 48 h of diuretic withdrawal and volume expansion with albumin (recommended dose: 1 g/kg per day up to a maximum of 100 g of albumin/day) |
| Absence of shock |
| No current or recent treatment with nephrotoxic drugs |
| Absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/d, microhematuria (> 50 RBCs/high power field, and/or abnormal renal ultrasound scanning |
| HRS-1 |
| Rapidly progressive renal failure defined by a doubling of the initial serum creatinine to a level greater than 2.5 mg/dL or 220 μmol/L in less than 2 wk |
| Although it may appear spontaneously, HRS-1 often develops with a precipitating event, particularly spontaneous bacterial peritonitis |
| HRS-1 occurs in the setting of an acute deterioration of circulatory function (arterial hypotension and activation of the endogenous vasoconstrictor systems) and is frequently associated to rapid impairment in liver function and encephalopathy |
| HRS-2 |
| Characterized by a moderate renal failure (serum creatinine greater than 1.5 mg/dL) which follows a steady or slowly progressive course. It appears spontaneously in most cases |
| HRS-2 is frequently associated with refractory ascites. Survival of patients with HRS-2 is shorter than that of patients with ascites but without renal failure |
Several alternatives have been proposed for the treatment of HRS-1. Although liver transplantation (LT) appears to be the logical definitive treatment for this condition, other interventions are needed while the patient is on the waitlist, sometimes for several weeks before LT. Moreover, for a myriad of reasons, some patients cannot be considered appropriate candidates for LT. Hence, LT it is not a proper alternative for every patient, and other therapeutic alternatives must be considered.
Recently, the Chilean Gastroenterology Society provided a consensus for the treatment of HRS-1. The aim of this article is to show the results of the consensus and to suggest a therapeutic approach based on the best available evidence.
The consensus organizing committee [under the sponsorship of the Chilean Society of Gastroenterology (http://sociedadgastro.cl)] assembled a group of adult gastroenterologists and nephrologists with expertise in the management of advanced liver disease patients and evidence-based medicine. The panel generated a list of questions relevant for the treatment of HRS-1. To address these questions, two members of the panel (JCC and JPA) performed separate searches in PubMed®, retrieving reports published in English or Spanish through June 2014. The search results were distributed. Simultaneously, each individual panelist contributed additional data and abstracts presented at meetings. Each expert was designated to review and answer a question. They generated draft statements for evaluation by all the experts; the answers were to be supported through a review of the literature. The quality of the evidence (Table 2) and the level of recommendation (Table 3) were graded following the United States Preventive Services Task Force criteria[5,6].
| Level of evidence | Description |
| Type I | Evidence obtained at least from one well-designed, randomized, controlled1 trial or from a systematic review of randomized clinical studies |
| Type II | II-1 evidence obtained from non-randomized, prospective, controlled1 studies |
| II-2 evidence obtained from cohort observational studies2 or case-control studies, preferably multi-centric | |
| II-3 evidence obtained from case series | |
| Type III | Opinion of authorities on the subject matter based on expertise, expert committees, case reports, pathophysiological studies or basic science studies |
| Recommendation | Description |
| A | The consensus strongly recommends the mentioned intervention or service. This recommendation is based on high quality evidence, with a benefit that significantly exceeds the risks |
| B | The consensus recommends the regular clinical use of the mentioned intervention or service. This recommendation is based on moderate quality evidence, with a benefit that exceeds the risks |
| C | The consensus does not make any positive or negative recommendation regarding the mentioned intervention or service. A categorical recommendation is not provided, because the evidence (of at least moderate quality) does not show a satisfactory risk/benefit relationship. The decision has to be made on a case-by-case basis |
| D | The consensus makes a negative recommendation against the mentioned intervention or service. The recommendation is based on at least moderate quality evidence, not showing any benefit or where the risk or damage exceeds the benefits of the intervention |
| I | The consensus concludes that the evidence is insufficient, due to low-quality studies, heterogeneous results or because the risk/benefit balance cannot be determined |
Initially, each expert wrote a draft recommendation statement and sent to organizing committee for evaluation and distribution among the entire panel. A 1 to 5 Likert scale (where 1 means “totally disagree” and 5 “totally agree”) was used to measure agreement. To reach a final consensus a modified three-round Delphi technique method was used as described by Arab et al[7].
The final statements and recommendations were exposed during the XLI Chilean Congress of Gastroenterology and the I Chilean Symposium on HRS-1 treatment in Coquimbo, Chile, in November 2014. The audience of approximately 450 physicians voted in real-time. The approved final recommendations (those with average scores ≥ 4 on the Likert scale) are presented below.
Statistical review of the study was performed by a biomedical statistician. Level of agreement from the Delphi panel was expressed in mean ± SD.
Terlipressin: Terlipressin is the vasoconstrictor of choice for the treatment of HRS-1, due to the large number of studies (and enrolled subjects) showing its effectiveness and its positive effects on survival. Terlipressin is a synthetic analogue of vasopressin acting through V1 receptors, increasing effective circulating volume, and by means of an increase in resistance in the splanchnic territory (which reduces portal pressure), it allows for the redistribution of the bloodstream, increasing renal perfusion[8]. Two important controlled, randomized and multicenter trials showed that terlipressin associated with albumin resulted in an improvement in renal function and could also reverse HRS-1[9,10]. A recently published meta-analysis, which included 320 subjects, proved 50% effectiveness with an OR of 7.5[11].
An Italian trial reported on the impact of terlipressin on the survival rate of HRS-1 patients. This randomized trial included 52 subjects and showed a higher and more significant probability of survival in the group treated with terlipressin[12]. These findings were confirmed in a recent meta-analysis published by Cochrane, including 6 trials and 309 subjects, in which a statistically significant reduction in the mortality rate was observed in HRS-1 patients treated with terlipressin (RR = 0.76, 95%CI: 0.61-0.95)[13].
For the treatment of HRS-1, it is recommended to start the administration of terlipressin at an initial dose of 0.5-1 mg every 4-6 h as an IV bolus, with the possibility of increasing the dose up to 2 mg every 4-6 h if there is no proper response after 3 d; a proper response is defined as a reduction > 25% from basal plasma creatinine. It is recommended to maintain the administration of terlipressin until creatinine levels decrease to less than 1.5 mg/dL or for a maximum of 14 d[14]. Recurrence can occur after discontinuation of the therapy (< 20%)[15], in which case, the recommendation is to repeat a new cycle. The most frequent side effects are abdominal pain, diarrhea, arrhythmia and ischemic complications. The incidence of serious effects requiring suspension of terlipressin is close to 7%[16]. The presence of coronary, vascular or peripheral arterial ischemic disease must be considered a contraindication for the use of terlipressin and other systemic vasoconstrictors.
Recommendation: Treatment with terlipressin associated with albumin represents the drug therapy of choice in HRS-1 patients, and it is capable of reversing this condition and reducing the associated mortality rate (Evidence Level I, grade of recommendation A, Agreement 5 ± 0).
Norepinephrine: Norepinephrine, an adrenergic agonist widely available in critical care units, is regarded as an alternative therapy in association with albumin, and it is effective and safe for the treatment of HRS-1[17]. Two randomized trials compared norepinephrine to terlipressin, reporting similar efficacy levels for reversal of HRS-1, as well as a comparable safety profile[18,19]. A recent study conducted in India enrolled 46 HRS-1 patients and reported results that confirmed previously published results[20], in addition to a recently published meta-analysis[21]. The effects of both drugs on mortality rates after 30 d and the probability of HRS-1 recurrence were also similar. In this meta-analysis, adverse effects were less common with the use of norepinephrine; however, only four studies were considered[21].
Norepinephrine is used as a continuous intravenous infusion at a 0.5 mg/h initial dose, with the purpose of achieving a > 10 mmHg increase in basal mean blood pressure (MBP). Accordingly, the dose can be adjusted by 0.5 mg/h every 4 h until a maximum dose of 3 mg/h is attained[20].
The significantly reduced cost and broad availability of norepinephrine are attractive[18,20]. It must be considered, however, that the number of cases treated with norepinephrine remains low, compared to the number of cases treated with terlipressin.
Recommendation: The use of norepinephrine associated with albumin represents an alternative to the use of terlipressin for the treatment of HRS-1; however, the currently available information is not as abundant in comparison to terlipressin (Evidence Level I, grade of recommendation B, Agreement 5 ± 0).
Octreotide plus midodrine: The most studied vasoconstrictor used for the treatment of HRS-1 is terlipressin, and it results in complete reversal of the disease and a reduction in the associated mortality rate. However, many studies have assessed the use of other vasoconstrictors, with or without volume expansion agents, with variable results[22]. The association of midodrine, a systemic vasoconstrictor acting on alpha-adrenergic receptors, and octeotride, a synthetic analogue of vasopressin that inhibits the release of endogenous vasodilators, has shown a benefit to mortality rates in some small studies[23,24]. Regarding reversal, some studies have shown complete response with reduction in creatinine to values less than 1.5 mg/dL; nevertheless, other studies have shown contradictory results[25]. These two points were assessed in a meta-analysis published in 2012[26] that included 256 subjects from 3 separate observational studies. This meta-analysis showed a reduction in mortality rates at 30 d (OR = 0.33; 95%CI: 0.18-0.60) and 90 d (OR = 0.17; 95%CI: 0.03-0.96) but no conclusive results in terms of HRS-1 reversal; however, a delay in progression was observed, based on a reduction in creatinine levels that was not statistically significant. It is important to note that, in the cases of the control groups in these three studies, two studies used dopamine, and one study used albumin. This reduction in the progression of renal function would be the mechanism believed to reduce the mortality rate, even without achieving HRS-1 reversal.
A randomized, monocentric trial including 23 subjects compared the association of midodrine and octeotride with noradrenaline[27], with complete response in 73% and 75% of subjects, respectively, without significant differences between the two therapeutic options. However, the small number of subjects included in this study limited the interpretation of the results. In contrast, a recent prospective, randomized trial compared the use of terlipressin plus albumin (27 subjects) with the combination of midodrine/octreotide plus albumin (22 subjects). This study showed a 70.4% response rate in the terlipressin branch vs a 28.6% response rate in the midodrine/octreotide branch. Moreover, the complete response rate was significantly higher in the terlipressin branch [55.5% vs 4.8% in the midodrine/octreotide group (P < 0.001)], showing low efficacy in terms of complete response in the midodrine/octreotide group[28]. These results were consistent with the low reversal rate described in previous studies.
Some studies have suggested that an increase in MBP is necessary for reverting alterations in renal hemodynamics specific to HRS-1; this increase is greater in patients responding to vasoconstrictor treatment compared with non-responding patients, regardless of the vasoconstrictor used. In a joint analysis of 501 patients from 21 studies, Velez et al[29] proved a significant correlation between an increase of 10 to 15 mm in MBP and the HRS-1 patient’s response to treatment, with improvement in renal function. Other studies have not been able to prove this association. The main limitation of the study by Velez et al[29] is that it gathered information from previous studies that were not designed to assess the measured result. Therefore, their study cannot be regarded as having sufficient evidence for issuing a recommendation.
With regard to the safety and efficacy of the use of midodrine and octreotide, these data were assessed in a retrospective study[30] including 60 HRS-1 patients, compared to 21 patients treated only with albumin. Midodrine treatment combined with octeotride was not associated with significant adverse effects.
Recommendation: Although the midodrine-octeotride combination is a safe treatment with easy administration, its beneficial effects on survival and improvement in renal function have not been consistent across trials. Therefore, we do not recommend its use for the treatment of HRS-1 (Evidence Level I, grade of recommendation B, Agreement 4.6 ± 0.5).
Vasopressin: Vasopressin has been proposed as a vasoconstrictor for the treatment of HRS-1 in some countries where no other therapies are available.
A retrospective study[31] compared the use of vasopressin alone and in combination with octeotride in HRS-1 patients vs the use of octeotride. This study showed a reduction in creatinine to values < 1.5 mg/dL with the use of vasopressin with or without octeotride vs octeotride alone (42% vs 38% vs 0%, respectively, P = 0.001), with an OR of 6.4 as well as an improvement in the survival rate and the possibility of being candidate for LT.
The dose required for achieving this objective has not yet been established. The aforementioned study required a dose of 0.23 + 0.19 U/min for a period of 5 to 9 d. In contrast, the use of low doses of vasopressin (1 U/h)[32] was effective for the restoration of urine volume in HRS-1 patients and patients with congestive heart failure, without improving the overall prognosis of the patients or their creatinine levels.
The use of vasopressin requires strict monitoring to avoid adverse effects associated with ischemic phenomena.
Recommendation: We do not recommend the use of vasopressin for the treatment of HRS-1, due to several adverse effects and the lack of randomized, clinical trials supporting its use (Evidence Level II-2, grade of recommendation I, Agreement 4.8 ± 0.3).
The use of human albumin in cirrhosis is based mainly on its hemodynamic properties, improving oncotic pressure in patients with circulatory disorders, and it is characterized by dilatation of the splanchnic territory, effective hypovolemia and activation of the renin-angiotensin-aldosterone system. In addition, albumin has antioxidant and immunomodulatory functions; albumin also has the capacity to transport and metabolize other substances and has hemostatic and endothelial stabilization effects. In cirrhotic patients, both plasma albumin concentration and its functional properties are diminished, becoming even more severe depending on the level of the patient’s renal failure[33].
The aforementioned situation has been the rationale for the use of albumin in decompensated cirrhosis. The main evidence in favor of its use is in the prevention of renal failure, both in the presence of spontaneous bacterial peritonitis and after a large-volume paracentesis.
In HRS-1, circulatory disorders are established at its highest levels, and by definition, these disorders are not reversed by the administration of albumin alone, the use of which is commonly suggested to expand intravascular volume and to improve cases of prerenal failure, thus disregarding the HRS-1 diagnosis in these patients[3].
Vasoconstrictors are the basis of HRS-1 treatment, and in the majority of well-designed, prospective and randomized studies, they have been associated with the use of albumin as a plasma expander and compared to the isolated use of albumin[9,12]. In these studies, there has been evidence of a significant difference in favor of combined therapy regarding HRS-1 reversal, improvement of renal function, MBP and diuresis.
The purpose of a meta-analysis performed by Dobre et al[11] was to prove the usefulness of terlipressin with or without albumin, compared with placebo with or without albumin. The study showed that HRS-1 reversal using the first alternative was significantly more common, with an OR of 7.47. Of the six studies included in the meta-analysis, only 1 did not use albumin, and it was the oldest (1998) and had the smallest number of subjects[11], proving that the majority of researchers have considered albumin to be a part of the basic treatment for HRS-1 patients.
Regarding the assumption that albumin provides an additional benefit to the use of vasoconstrictors alone, we only found evidence of an observational study designed to answer this question. Seventy-seven percent of the patients who used terlipressin and albumin experienced a resolution of HRS-1, compared to 25% in the group that used terlipressin alone. In addition, the group of patients treated with terlipressin plus albumin showed significant improvement in MBP and a reduction in the activation of the renin-aldosterone system[34].
Along these same lines, the retrospective study by Moreau et al[35] assessing the usefulness of terlipressin in HRS-1 showed that the respondent group used albumin in 79% of cases vs 68% in the non-respondent group, which was not a significant difference.
There is no evidence available that other fluids (such as crystalloids) can have a similar effect to albumin associated with vasoconstrictors. No studies have been designed for this purpose, and no such studies are likely to be performed because the use of crystalloids increases ascites and, therefore, intra-abdominal pressure, which in turn affects renal perfusion and reduces the likelihood of improvement of renal and circulatory failure. An observational study, in which a large-volume paracentesis with reposition of albumin was performed in HRS-1 patients, showed partial improvement of renal function, supporting the hypothesis that a reduction in intra-abdominal pressure could be useful for renal perfusion recovery[36].
Recommendation: The use of albumin with vasoconstrictors is the therapy of choice for treating HRS-1. The use of albumin without vasoconstrictors is only recommended in the stage prior to HRS-1 diagnosis to exclude patients with prerenal failure (Evidence Level II-2, grade of recommendation B, Agreement 4.8 ± 0.3).
There is scarce evidence for the use of trans-jugular intrahepatic portosystemic shunt (TIPS) in HRS-1. In a study including 16 patients, 6 with HRS-1 and 10 with HRS-2 and Child-Pugh scores of 7-9, a duplication of creatinine clearance in serum and increased sodium concentration excreted in the urine were observed two weeks after the TIPS procedure. Three of the HRS-1 patients required hemodialysis during the progression of the disease, and 12 and 18 d after the TIPS procedure, two patients were able to stop hemodialysis. There was an improvement in renal function, even after 6-8 wk. Three of the 16 HRS-1 and HRS-2 patients did not respond, and they died within a 6-wk period[37]. A prospective, non-randomized, phase II study included 41 patients with cirrhosis and HRS without indications for transplantation: 21 with HRS-1 and 20 with HRS-2. Thirty-one of these patients (14 type 1 and 17 type 2) received TIPS and were followed for a mean of 24 mo. The use of TIPS in HRS-1 and HRS-2 patients reduced significantly (P < 0.001) the hepatic venous pressure gradient and increased creatinine clearance and sodium excretion. Those patients who received TIPS showed higher survival rates than those who did not. There was only one death related to the procedure (3.2%). It is important to note that the HRS-2 patients had a significantly greater benefit and were identified as a variable independently correlated with survival[38]. Another uncontrolled study assessed 7 patients with cirrhosis and HRS-1. The TIPS procedure was associated with gradual improvements in the glomerular filtration rate (9 to 27 mL/min) and blood urea nitrogen and creatinine reduction. The majority of patients also showed a reduction in the activity of the renin-angiotensin system and in the sympathetic nervous system, suggesting an improvement in hemodynamic parameters. The average survival after the TIPS procedure was approximately 5 mo, which was longer than the survival rate expected for these patients[39].
Unfortunately, many HRS-1 patients are too sick to undergo a TIPS procedure, mainly because the procedure can present complications such as deterioration of hepatic encephalopathy and liver function (increased bilirubin levels), bleeding and intravenous contrast-induced nephropathy[40]. In a study that designed a predictive model for determining the survival rate after a TIPS procedure, patients with HRS-1 due to alcoholic cirrhosis or chronic cholestatic disease showed a 25% mortality rate 90 d after the procedure and a mortality rate of 80% in patients with cirrhosis due to other causes[41].
In general, these results suggest that TIPS could be considered for patients with relatively preserved liver function and as a bridge therapy to LT. However, due to the risks associated with the procedure and the lack of well-designed studies, this procedure should be considered only as a last resource.
Recommendation: Due to the lack of evidence, the consensus considers that the use of TIPS shall not be recommended in HRS-1 patients (Evidence Level II-2, grade of recommendation I, Agreement 4.7 ± 0.4).
Role of renal replacement therapies for the management of acute renal failure associated with HRS-1: If there is no response to proven pharmacological strategies, acute renal failure (ARF) in HRS-1 takes an irreversible course unless the patient undergoes LT. At this point, patients develop oliguria, hydrosaline balance disorders and severe metabolic disorders that can lead to the prescription of renal replacement therapies (RRTs).
For patients who will not undergo LT, the potential benefit of RRT is controversial due to the high morbi-mortality associated with RRT, basically determined by poor hemodynamic tolerance and/or hemorrhages associated with liver failure complications[42].
For patients who are non-respondent to pharmacological therapy or TIPS, who are waiting for a LT or who are under evaluation to undergo the surgery, the use of RRT is advised as a bridge to LT. In a retrospective study developed by Keller et al[43], the survival rate of patients with HRS-1 was 44% in the group that received RRT vs 10% in the group that did not receive the therapy. However, this higher survival rate could be related to reduced RRT tolerance, which could increase the number of hospitalizations. In a report of 4 patients who received hemodialysis while waiting for LT, Capling et al[44] observed an average survival of 236 d (31 to 460 d). All of the patients survived the initial event and were discharged, but 33% of the days gained were then spent in hospitalization due to intercurrent diseases. The most common cause of hospitalization was hepatic encephalopathy; the authors believe that avoiding lactulose during the days when the patient undergoes dialysis, to prevent diarrhea events, might have been a contributing factor[44].
Efficacy, safety and the best RRT modality in HRS-1 patients have not been systematically assessed. Potential advantages of continuous vs intermittent RRT include slower removal of fluids with higher hemodynamic stability and slower control of solute concentrations, which is why many clinicians prefer continuous RRT in patients with hemodynamic instability and in patients with evidence of cerebral edema[45]. In two studies, Davenport and Detry proved that continuous RRT was better tolerated than intermittent hemodialysis in patients with liver failure, evidenced by greater cardiovascular stability, gradual correction of hyponatremia and less variation in intracranial pressure[46,47].
Recommendation: We recommend the initiation of RRT in patients with HRS-1 refractory to pharmacological therapy who are candidates for LT. In patients with hemodynamic instability and/or evidence of cerebral edema, we recommend the use of other continuous RRTs; We recommend maintaining RRT in patients with HRS-1 who are candidates for LT and who must be temporarily removed from the waiting list because they have developed an intercurrent disease (Evidence Level II-3, grade of recommendation C, Agreement 4.6 ± 0.5).
Extracorporeal liver support with albumin dialysis (molecular adsorbent recirculating system): The molecular adsorbent recirculating system (MARS) is an extracorporeal liver support that, by means of recirculating albumin dialysis, helps to remove water-soluble substances, as well as protein-bound substances. The removal function has been shown to reduce bilirubin, ammonium, urea nitrogen, creatinine, fatty acids and bile salts, which are all substances that, in high concentrations, are related to liver and renal failure. As expected, the effect is temporary, and if there is no improvement in liver function, these parameters change again in the short term[48,49]. This system does not improve hepatic synthesis; it is used as a depuration system, comparable to renal hemodialysis. In contrast, some small studies have shown an improvement in the hemodynamics of these patients, with increased blood pressure that had been reduced due to the hyper-dynamic circulation characteristic of liver failure. The mechanism of the aforementioned benefit would be depuration and the reduction of substances such as renin, angiotensin and aldosterone, which are responsible for the hemodynamic disorders related to liver failure[48]. A recent retrospective, uncontrolled study showed that, of 32 HRS-1 patients receiving MARS therapy for an average of 3.5 ± 1.5 sessions, 13 (40%) experienced an improvement in renal function, but only 9 (28%) showed complete response in the form of renal function recovery. In contrast, of the 15 patients who survived > 28 d, only 9 achieved this stage without transplantation, and of these 9 patients, only 2 showed complete renal response using MARS therapy[50]. Therefore, it has been suggested that MARS therapy is capable of improving HRS-1 through the removal of vasodilators; however, this effect would more probably be caused by MARS’s hemofiltration function, which is an effect that is similar to conventional hemodiafiltration.
As mentioned, non-treated HRS-1 patients show high mortality rates in the short term. The effect of MARS therapy in this stage is controversial. There have been three studies that have not shown any benefit in terms of survival[51-53], and another two studies, both from the same author, did not show benefits[49,54].
Finally, a recent (2013) multi-center, randomized study[51] included patients with acute or chronic liver failure and randomized a total of 189 patients to a group with standard medical therapy plus MARS therapy (95 patients) vs another group using only standard medical therapy (94 patients); this study observed no benefit in survival at 28 d in the MARS group. A sub-analysis of the HRS-1 patients that were included (48 in the MARS group and 47 in the control group) also did not show differences in the survival rates.
Recommendation: We do not recommend the use of extracorporeal liver support with albumin dialysis (MARS) for the treatment of HRS-1 (Evidence Level I, grade of recommendation D, Agreement 4.8 ± 0.3).
LT is the therapy of choice for HRS-1 patients because it not only improves renal failure but also improves the underlying diseases, i.e., cirrhosis and PHT. Post-transplantation survival in HRS-1 patients seems to be lower than for transplanted patients without HRS-1; however, survival is considerably higher compared with that in HRS-1 patients without transplantation. In a retrospective study, the survival of HRS-1 transplanted patients after 1 and 3 years was 80.3% and 76.6%, respectively, and it was 90.7% and 85.3%, respectively, for recipients without HRS-1[55].
Although we can consider that treating HRS-1 with vasoconstrictor agents can improve post-transplant results by improving renal function before the procedure, there is no clear evidence of this effect. In a clinical study, 99 patients were randomized to receive terlipressin or placebo. Of these patients, 35% received LT. Subjects receiving albumin plus terlipressin showed a 100% survival rate among transplanted patients and a 34% survival in non-transplanted patients after 6 mo. In contrast, subjects receiving only albumin showed 94% survival in transplanted patients and only 17% survival in non-transplanted patients after 6 mo. The authors concluded that the use of terlipressin has no impact on post-transplant survival. The sole benefit of the use of terlipressin in patients who will undergo transplantation seems to be the facilitation of the use of calcineurin inhibitors post-transplant, reducing the need for anti-IL2 antibodies[56].
The majority of HRS-1 patients experience an improvement in renal function post-transplant; therefore, there seems to be no advantage in performing double liver and renal transplantation vs single LT. In a Chinese observational study, 32 HRS-1 patients received transplantation, and of these patients, 8 received dialysis, showing that 94% of the patients recovered renal function in an average of 24 d, with 65% survival after 1 year[57]. In another observational study with 28 patients, with an average MELD score of 30 ± 6, only 58% of patients recovered renal function. Four patients died, of whom 3 showed resolution of HRS-1[58]. However, patients that did not experience any improvement in renal function post-transplant showed poorer survival rates[59].
In a recent retrospective study, 62 HRS-1 patients received transplantation, with an average basal creatinine of 3.35 mg/dL and an average MELD score of 35 ± 1. The progression time of HRS-1 before transplantation was 18 d. Eleven patients continued dialysis after the surgery, and 5 patients died. Survival after 1 year in the patients who recovered renal function was 97% vs 60% in the group that did not show any improvement in renal function. After one year, the creatinine levels in the group with HRS-1 resolution were similar to the creatinine levels in the group of transplanted patients without HRS-1. The only factor associated with the non-resolution of HRS-1 after transplantation was the period of time on dialysis pre-transplantation. For each day of dialysis, the patient has a 6% increase in the risk of non-resolution of HRS-1. A patient who is on dialysis for more than 14 d has a 9.2 times greater relative risk of non-resolution of HRS-1[60].
Despite these findings, apart from the duration of dialysis time before transplantation, predictive factors for the improvement of renal function after transplantation have not been clearly established. Patients with ARF requiring dialysis more than two times per week for more than 4 wk must be assessed for double liver and renal transplantation, considering the risk factors at the time of the surgery, such as hypertension, diabetes and older age[60,61].
Recommendation: LT can be considered the definitive treatment for HRS-1 patients. HRS-1 patients must receive treatment with vasopressors before LT because it could improve the subsequent results. Patients requiring dialysis for long periods of time (> 4 wk) must be considered for combined liver-kidney transplantation (Evidence Level II-2, grade of recommendation B, Agreement 4.6 ± 0.5).
LT is considered the treatment of choice for HRS-1[62]. It is the only therapy able to reverse this condition completely, resolving circulatory dysfunction and the consequences of cirrhosis and liver failure[63]. Thus, survival can be dramatically improved after LT[55]. In fact, the 180 d survival rate was 97% in a recent study[56]. In another recent study, the one- and three-year survival rates were 80.3% and 76.6%, respectively[55]. Interestingly, the impact of pharmacologic treatment on the outcomes after LT was recently evaluated. In the study by Boyer et al[56], the use of terlipressin plus albumin had no impact on post-transplant survival. However, this is not an argument for neglecting the importance of the treatment of HRS-1, especially considering that the time elapsed between enlistment and transplantation could be weeks. In this regard, an effort to recover renal function before LT is advisable. Although it seems to be the most frequent scenario, not every patient recovers renal function after LT. In a very recent study, Wong et al[64] evaluated the survival of liver recipients who experienced reversal of HRS-1 after LT, compared to the survival of patients who did not. In this study, 75.8% of the recipients had a reversal of HRS-1 after LT. The one-year survival rate after LT was 97% for patients who had a reversal of HRS-1 and 60% for those who did not[64]. Thus, for all of the aforementioned reasons, LT is a desirable approach for HRS-1 patients.
Nonetheless, there are at least two obstacles that render access to LT difficult: (1) the scarcity of liver grafts, which dramatically reduces the likelihood of these patients receiving a transplant as rapidly as they should; and (2) the existence of severe comorbidities or conditions that make LT not plausible. Hence, there is an important role for different therapeutic approaches than could be used as alternatives or “bridges” to LT.
Thus, several therapeutic tools have been evaluated. As expected, the quality of these studies, the efficacy of the interventions, and their availability, costs and adverse effects are, of course, very different. It was the purpose of this panel of experts to determine the treatments with greater efficacy, based on studies with the highest quality available.
Based on the available evidence, the expert panel agrees that the best evidence for the treatment of HRS-1 supports the use of vasoconstrictors as a treatment of the choice, specifically terlipressin, based on a recent systematic review[13]. On the other hand, noradrenalin seems to be as effective as terlipressin. In fact, a recent systematic review evaluated the efficacy of noradrenalin compared to terlipressin. Only four studies were included (154 randomized patients). The authors report a similar rate of reversal of HRS-1, 30 d mortality and recurrence. Thus, in this study, its effect on renal function seemed to be completely comparable to that of terlipressin[21], and its use seems to be adequate when terlipressin is not available. Nonetheless, these findings are more difficult to interpret because two studies included patients with HRS-2. In this regard, the expert panel recommends the use of noradrenalin as a second choice if terlipressin is not available. Another strategy based on the use of vasoconstrictors is the combination of octreotide plus midodrine (also in combination with albumin). Very interestingly, a recent study compared the use of terlipressin plus albumin with the combination of octreotide, midodrine and albumin. Notably, the rate of complete response was 55.5% in the terlipressin group and 4.8% in the octreotide-midodrine group (P < 0.001)[28]. Based on these results, the panel of experts did not recommend the use of octreotide plus midodrine for the treatment of HRS-1.
The use of vasopressin was not recommended by the expert panel due to the scarcity and poor quality of the evidence, in addition to the incidence of ischemic side effects[31].
Another issue evaluated by the panel was the use of albumin as a plasma expander. Most of the studies that evaluated the use of vasoconstrictors combined them with albumin. However, Ortega et al[34] conducted a prospective, non-randomized study that compared the use of terlipressin with and without albumin. A complete response was observed in 77% of patients receiving albumin and in 25% of those who did not receive albumin. In contrast, there is a lack of evidence suggesting that the apparent benefit of using albumin combined with vasoconstrictors cannot be substituted for another colloid or crystalloids. However, considering that the benefit of vasoconstrictors has been proved in combination with albumin, this panel decided to recommend its use every time that vasoconstrictors are indicated.
The use of a TIPS has been tested in only a few patients; however, there have been no randomized, controlled studies, and it has not been compared to the use of vasoconstrictors. In contrast, the associated adverse effects, mostly hepatic encephalopathy, have made the use of the TIPS procedure difficult. For these reasons, the expert panel does not recommend its use.
The MARS has also been tested as an alternative for the treatment of HRS-1. However, its benefits have not been consistently demonstrated. In fact, in a recent randomized, controlled trial, MARS was employed for patients with acute or chronic liver disease, including 95 patients with HRS-1. No benefit on survival was demonstrated[51]. Hence, the panel of experts does not recommend its use.
RRT use is considered controversial in cirrhotic patients with HRS-1 when LT is not considered an option because of the morbidity and mortality associated with the procedure and with liver failure[42]. Although the literature is scarce in this topic, RRT seems to prolong short term survival[43], potentially improving the probability of receiving a liver graft. Thus, although disputable, the expert panel decided to recommend the use of RRT only in those patients listed for LT.
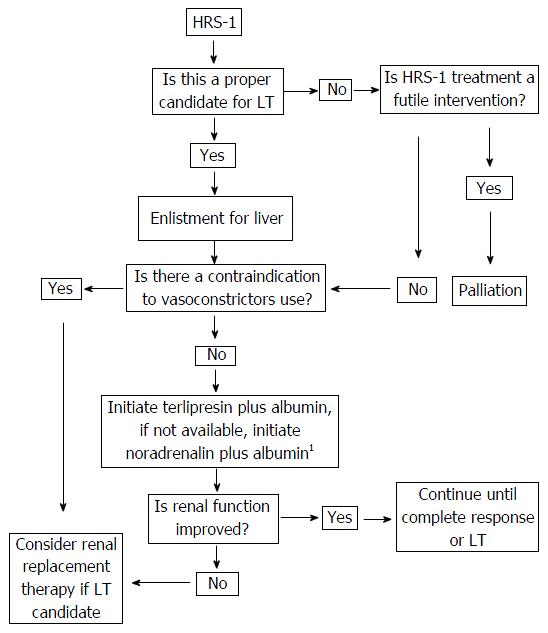
The present consensus is an important effort that intends to organize the available strategies based on the available evidence in the literature, the quality of the evidence and the benefits, adverse effects and availability of the therapeutic tools described. This attempt has been synthesized in the algorithm described in Figure 1. We hope that it will be a useful tool for guiding the management of HRS-1 patients.
Hepatorenal syndrome (HRS) is a severe condition strictly related to the presence of portal hypertension and ascites. It has a very poor prognosis with a mean survival that only reaches two weeks. Several therapeutics alternatives have been evaluated. Based on the best available evidence the authors attempt to define the best therapeutic choice considering its availability and the clinical characteristics of each patient.
Although several therapeutic alternatives have been proposed the quality of the evidence is heterogeneous and it can be difficult to discriminate the best option on each circumstance. A careful evaluation of the evidence is necessary to make appropriate recommendations.
This is the first published consensus about the treatment of HRS. A significant effort has been made to evaluate the quality of the available literature to generate appropriate recommendations.
The information included on this consensus will be a valuable tool to determine the best therapeutic option for HRS based on the best available evidence, the availability of each intervention and the particular condition of the patient.
Delphi-technique method was used to reach consensus. A panel of experts voted on a 1 to 5 Likert scale (where 1 means “totally disagree” and 5 “totally agree”). Approved recommendations (those with average score ≥ 4 on the Likert scale) are presented; Level of evidence: The quality of the evidence is classified in three types. Thus, type I is obtained from well design, randomized trials or systematic reviews. Type II is obtained from studies of lower quality (i.e., non-randomized trials, case-control studies or case series). Type III is obtained from opinion of authorities on the subject matter based on expertise, expert committees, case reports, pathophysiological studies or basic science studies; Levels of recommendation are classified in five categories (A, B, C, D, I) where A corresponds to the stronger recommendation to support a determined intervention and I classifies situations where the evidence is insufficient to generate a recommendation (see Table 3 on the manuscript).
The study is well organized and properly processed. The conclusion is reasonable.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Chile
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Coban M, Ishibashi H, Mocellin S S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 550] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Montoliu S, Ballesté B, Planas R, Alvarez MA, Rivera M, Miquel M, Masnou H, Cirera I, Morillas RM, Coll S. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616-622; quiz e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] |
| 4. | Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1141] [Article Influence: 47.5] [Reference Citation Analysis (1)] |
| 6. | Sawaya GF, Guirguis-Blake J, LeFevre M, Harris R, Petitti D. Update on the methods of the U.S. Preventive Services Task Force: estimating certainty and magnitude of net benefit. Ann Intern Med. 2007;147:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Arab JP, Candia R, Zapata R, Muñoz C, Arancibia JP, Poniachik J, Soza A, Fuster F, Brahm J, Sanhueza E. Management of nonalcoholic fatty liver disease: an evidence-based clinical practice review. World J Gastroenterol. 2014;20:12182-12201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 8. | Mazur JE, Cooper TB, Dasta JF. Terlipressin in hepatorenal syndrome. Ann Pharmacother. 2011;45:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 401] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 10. | Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Dobre M, Demirjian S, Sehgal AR, Navaneethan SD. Terlipressin in hepatorenal syndrome: a systematic review and meta-analysis. Int Urol Nephrol. 2011;43:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, Siringo S, Castellino P. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev. 2012;9:CD005162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Fagundes C, Ginès P. Hepatorenal syndrome: a severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis. 2012;59:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 15. | Barbano B, Sardo L, Gigante A, Gasperini ML, Liberatori M, Giraldi GD, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Duvoux C, Zanditenas D, Hézode C, Chauvat A, Monin JL, Roudot-Thoraval F, Mallat A, Dhumeaux D. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, Sharma AK, Choudhary NS, Chawla Y, Nain CK. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Nassar Junior AP, Farias AQ, D’Albuquerque LA, Carrilho FJ, Malbouisson LM. Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. PLoS One. 2014;9:e107466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Tandon P, Bain VG, Tsuyuki RT, Klarenbach S. Systematic review: renal and other clinically relevant outcomes in hepatorenal syndrome trials. Aliment Pharmacol Ther. 2007;25:1017-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Hassanein TI, Abdeen O, El-Tahawi M, Hart M, Khanna A, R . M. Octeotride, midodrine and albumin triple therapy is effective in reversing hepatorenal syndrome. Hepatology. 2001;34:A54. |
| 24. | Salerno F, Cazzaniga M, Merli M, Spinzi G, Saibeni S, Salmi A, Fagiuoli S, Spadaccini A, Trotta E, Laffi G. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011;55:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Karwa R, Woodis CB. Midodrine and octreotide in treatment of cirrhosis-related hemodynamic complications. Ann Pharmacother. 2009;43:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Hiremath SB, Lokikere SD, Madalageri NK. Efficace of midodrine plus octeotride in hepatorenal syndrome: A meta-analysis. IJRAP. 2012;3:576-581. |
| 27. | Tavakkoli H, Yazdanpanah K, Mansourian M. Noradrenalin versus the combination of midodrine and octreotide in patients with hepatorenal syndrome: randomized clinical trial. Int J Prev Med. 2012;3:764-769. [PubMed] |
| 28. | Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 29. | Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Kiser TH, Fish DN, Obritsch MD, Jung R, MacLaren R, Parikh CR. Vasopressin, not octreotide, may be beneficial in the treatment of hepatorenal syndrome: a retrospective study. Nephrol Dial Transplant. 2005;20:1813-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Eisenman A, Armali Z, Enat R, Bankir L, Baruch Y. Low-dose vasopressin restores diuresis both in patients with hepatorenal syndrome and in anuric patients with end-stage heart failure. J Intern Med. 1999;246:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 34. | Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jiménez W, Arroyo V. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichaï P, Abergel A, Halimi C, Pauwels M, Bronowicki JP. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Umgelter A, Reindl W, Wagner KS, Franzen M, Stock K, Schmid RM, Huber W. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: a prospective uncontrolled trial. Crit Care. 2008;12:R4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Brensing KA, Textor J, Strunk H, Klehr HU, Schild H, Sauerbruch T. Transjugular intrahepatic portosystemic stent-shunt for hepatorenal syndrome. Lancet. 1997;349:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 41. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2066] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 42. | Wilkinson SP, Weston MJ, Parsons V, Williams R. Dialysis in the treatment of renal failure in patients with liver disease. Clin Nephrol. 1977;8:287-292. [PubMed] |
| 43. | Keller F, Heinze H, Jochimsen F, Passfall J, Schuppan D, Büttner P. Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): the role of hemodialysis. Ren Fail. 1995;17:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Capling RK, Bastani B. The clinical course of patients with type 1 hepatorenal syndrome maintained on hemodialysis. Ren Fail. 2004;26:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | The 2011 kidney disease: Improving global outcomes (kdigo) clinical practice guideline for acute kidney injury (aki). Kidney Inter. 2012;2 Suppl:107-110. |
| 46. | Davenport A, Will EJ, Davidson AM. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med. 1993;21:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 206] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Detry O, Arkadopoulos N, Ting P, Kahaku E, Margulies J, Arnaout W, Colquhoun SD, Rozga J, Demetriou AA. Intracranial pressure during liver transplantation for fulminant hepatic failure. Transplantation. 1999;67:767-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Nöldge-Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol. 2001;12 Suppl 17:S75-S82. [PubMed] |
| 49. | Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Lavayssière L, Kallab S, Cardeau-Desangles I, Nogier MB, Cointault O, Barange K, Muscari F, Rostaing L, Kamar N. Impact of molecular adsorbent recirculating system on renal recovery in type-1 hepatorenal syndrome patients with chronic liver failure. J Gastroenterol Hepatol. 2013;28:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 52. | Cholongitas E, Senzolo M, Patch D, Shaw S, O’Beirne J, Burroughs AK. Cirrhotics admitted to intensive care unit: the impact of acute renal failure on mortality. Eur J Gastroenterol Hepatol. 2009;21:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Wolff B, Machill K, Schumacher D, Schulzki I. MARS dialysis in decompensated alcoholic liver disease: a single-center experience. Liver Transpl. 2007;13:1189-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Mitzner SR, Klammt S, Peszynski P, Hickstein H, Korten G, Stange J, Schmidt R. Improvement of multiple organ functions in hepatorenal syndrome during albumin dialysis with the molecular adsorbent recirculating system. Ther Apher. 2001;5:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Lee JP, Kwon HY, Park JI, Yi NJ, Suh KS, Lee HW, Kim M, Oh YK, Lim CS, Kim YS. Clinical outcomes of patients with hepatorenal syndrome after living donor liver transplantation. Liver Transpl. 2012;18:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Boyer TD, Sanyal AJ, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Gülberg V, Sigal S, Bexon AS, Teuber P. Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl. 2011;17:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Xu X, Ling Q, Zhang M, Gao F, He Z, You J, Zheng S. Outcome of patients with hepatorenal syndrome type 1 after liver transplantation: Hangzhou experience. Transplantation. 2009;87:1514-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transplant. 2006;21:478-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 61. | Ruiz R, Kunitake H, Wilkinson AH, Danovitch GM, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141:735-741; discussion 741-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 62. | Arroyo V, Terra C, Ginès P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Gonwa TA, Morris CA, Goldstein RM, Husberg BS, Klintmalm GB. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome--experience in 300 patients. Transplantation. 1991;51:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 194] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Wong F, Leung W, Al Beshir M, Marquez M, Renner EL. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl. 2015;21:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |