Published online Sep 8, 2015. doi: 10.4254/wjh.v7.i19.2220
Peer-review started: March 22, 2015
First decision: April 27, 2015
Revised: June 20, 2015
Accepted: June 30, 2015
Article in press: July 2, 2015
Published online: September 8, 2015
Processing time: 174 Days and 18.6 Hours
AIM: To investigate factors that accurately predict hepatocellular carcinoma (HCC) development after antiviral therapy in chronic hepatitis C (CHC) patients.
METHODS: CHC patients who received pegylated interferon and ribavirin were enrolled in this cohort study that investigated the ability of alpha-fetoprotein (AFP) to predict HCC development after interferon (IFN) therapy.
RESULTS: Of 1255 patients enrolled, 665 developed sustained virological response (SVR) during mean follow-up period of 5.4 years. HCC was occurred in 89 patients, and 20 SVR patients were included. Proportional hazard models showed that HCC occurred in SVR patients showing AFP ≥ 5 ng/mL before therapy and in non-SVR patients showing AFP ≥ 5 ng/mL before and 1 year after therapy besides older age, and low platelet counts. SVR patients showing AFP ≥ 5 ng/mL before therapy and no decrease in AFP to < 5 ng/mL 1 year after therapy had significantly higher HCC incidence than non-SVR patients showing AFP ≥ 5 ng/mL before therapy and decreased AFP (P = 0.043). AFP ≥ 5 ng/mL before therapy was significantly associated with low platelet counts and high values of alanine aminotransferase (ALT) in stepwise logistic regression analysis. After age, gender, platelet count, and ALT was matched by propensity score, significantly lower HCC incidence was shown in SVR patients showing AFP < 5 ng/mL before therapy than in those showing AFP ≥ 5 ng/mL.
CONCLUSION: The criteria of AFP < 5 ng/mL before and 1 year after IFN therapy is a benefical predictor for HCC development in CHC patients.
Core tip: What is current knowledge: (1) Alpha-fetoprotein (AFP) values can predict development of hepatocellular carcinoma (HCC) after interferon therapy in patients with hepatitis C virus; and (2) The predictive value of AFP on HCC development after interferon therapy and its criteria remained uncertain. What is new here: AFP values before interferon therapy have strong predictive value on HCC development after interferon therapy. The simple criteria defined by AFP values before and 1 year after interferon therapy might work efficient to predict HCC development after interferon therapy.
- Citation: Takeuchi Y, Ikeda F, Osawa T, Araki Y, Takaguchi K, Morimoto Y, Hashimoto N, Sakaguchi K, Sakata T, Ando M, Makino Y, Matsumura S, Takayama H, Seki H, Nanba S, Moritou Y, Yasunaka T, Ohnishi H, Takaki A, Nouso K, Iwasaki Y, Yamamoto K. Alpha-fetoprotein before and after pegylated interferon therapy for predicting hepatocellular carcinoma development. World J Hepatol 2015; 7(19): 2220-2228
- URL: https://www.wjgnet.com/1948-5182/full/v7/i19/2220.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i19.2220
Hepatitis C virus (HCV) infection is the predominant cause of liver cirrhosis and hepatocellular carcinoma (HCC) in many countries, including Japan, the United States, and Europe[1-3]. HCV infection is resolved only rarely once it becomes chronic[4]. Chronic hepatitis C (CHC) may occasionally progress to liver cirrhosis and HCC after approximately 30 years without any disease-related symptoms[5,6].
Interferon (IFN) therapy is effective for eliminating the virus and reducing the HCC incidence in CHC patients[7-11]. However, HCC sometimes develops even in patients with a sustained virological response (SVR) due to interferon (IFN) therapy[12]. Recent advances in anti-HCV therapy have improved therapeutic efficacy and compliance, with more than half of patients obtaining SVR. Therefore, the importance of predicting HCC development after viral eradication in SVR patients is increasing.
Recent studies on HCC development in CHC patients after IFN therapy have proposed that in addition to liver cirrhosis, old age, and male gender, the initial values of and changes in alpha-fetoprotein (AFP) during IFN therapy can predict HCC development[13-15]. However, the potential of AFP to predict HCC development after IFN therapy remains uncertain in CHC patients. We therefore conducted a large-scale, long-term cohort study of CHC patients receiving therapy with pegylated interferon and ribavirin to determine the ability of AFP to predict HCC development after IFN therapy and to define simple criteria for AFP values that can accurately predict HCC development after IFN therapy.
The study enrolled 1318 CHC patients who had undergone combination therapy with pegylated interferon and ribavirin at the Okayama University Hospital or its affiliated hospitals between 2005 and 2011. We excluded 44 patients who developed HCC before interferon therapy, and 19 patients who developed HCC within 1 year after the completion of therapy. The data of the remaining 1255 patients were used for analysis. Written informed consent was obtained from all patients, and the study was conducted in accordance with the Declaration of Helsinki. All protocols were approved by the ethics committees of the institutes.
All patients had HCV RNA in their serum, as confirmed by the qualitative Amplicor or TaqMan HCV assay (Roche Molecular Diagnostics, Tokyo, Japan). They received antiviral therapy with standard doses of Peg-IFN alpha-2a or 2b with ribavirin. SVR was defined as undetectable HCV RNA in the serum 24 wk after the completion of therapy.
The characteristics of the patients and their biochemical, hematological, and virological data were collected at enrollment. The patients were examined for HCC by abdominal ultrasonography, dynamic computed tomography, and/or magnetic resonance imaging every 3-6 mo before and after therapy. Serum AFP values were measured every 1-6 mo. The surveillance protocols were in accordance with the standard of care in the clinical practice manual of the Japan Society of Hepatology[16]. When HCC was suspected on the basis of the screening examination, additional procedures such as dynamic study, hepatic angiography, and/or tumor biopsy were used to confirm the diagnosis. The end of follow-up was defined as the time of HCC development or the last medical attendance until June 2011. The mean follow-up period was 5.4 years (range: 1.0-8.1 years).
Data are expressed as mean ± SD or median (range). Proportional hazard models were used to estimate the factors associated with HCC development after interferon therapy in SVR and non-SVR patients separately. The cut-off value was defined for each parameter: median for age and AFP, lower limit of normal range for white blood cell, hemoglobin, and platelet count, and upper limit of normal range for alanine aminotransferase. The HCC incidence was estimated by the Kaplan-Meier method and compared among the patient groups using the log-rank test. Serum AFP values at different time points were compared using the paired t test. Factors associated with decreased AFP < 5 ng/mL 1 year after therapy among the patients showing AFP ≥ 5 ng/mL before therapy were analyzed using stepwise logistic regression analysis. The propensity score was estimated for each patient using a logistic regression model. Our study matched subjects using a caliper width within 0.1 of the propensity score. Using this method, comparable patient groups were identified. The baseline characteristics of the propensity score-matched pairs were almost identical. P values < 0.05 were considered significant. The statistical analyses were performed using JMP software (SAS Institute, Cary, NC, United States). The statistical review of the study was performed by a biomedical statistician.
The characteristics of the patients enrolled in the study are shown in Table 1. The mean age was 59 years, and 581 patients (46%) were female. In the study, 1255 patients enrolled and 665 (53%) achieved SVR, whereas 335 had virological relapse and 255 had partial or no virological response. AFP values in SVR patients gradually decreased during IFN therapy from a mean of 7.3 ng/mL before IFN therapy to 3.9 ng/mL at the end of therapy (paired t test, P < 0.0001) and decreased further to 3.4 ng/mL 1 year after therapy (P = 0.025, Figure 1). Of the 274 SVR patients showing AFP values ≥ 5 ng/mL before IFN therapy, the values decreased to < 5 ng/mL in 148 patients 1 year after IFN therapy, whereas decreased AFP values to < 5 ng/mL were not observed in 126 patients. Of the 580 non-SVR patients, 252 had AFP values < 5 ng/mL before IFN therapy and 328 patients had values ≥ 5 ng/mL. Of the 328 patients showing AFP values ≥ 5 ng/mL before IFN therapy, 93 had decreased AFP values to < 5 ng/mL 1 year after IFN therapy, whereas no decrease in values to < 5 ng/mL was observed in 235 patients. Stepwise logistic regression analysis showed that factors associated with AFP ≥ 5 ng/mL before therapy were low platelet count and high alanine aminotransferase (ALT) values (P = 0.0040, and P = 0.028, respectively, Table 2). Non-SVR patients had significantly higher AFP values (12 ng/mL) than SVR patients before IFN therapy (P < 0.0001), and decreased AFP values 7.3 ng/mL at the end of therapy. The changes in AFP values between the end of therapy and 1 year after therapy were scarce (P = 0.20, Figure 1). Stepwise logistic regression analysis showed that decreased AFP values to < 5 ng/mL 1 year after IFN therapy among the SVR patients showing AFP ≥ 5 ng/mL before therapy were significantly associated only with low ALT values 1 year after IFN therapy (P = 0.027, Table 3). As for non-SVR patients showing AFP values ≥ 5 ng/mL before IFN therapy, low ALT values at the end and 1 year after IFN therapy were selected as significant factors associated with decreased AFP values to < 5 ng/mL 1 year after IFN therapy (P = 0.0040, and P = 0.028, respectively).
| Patient characteristics | n = 1255 |
| Age (yr) | 59 (18-79)1 |
| Gender (male/female) | 674/581 |
| HCV genotype (1/2/3) | 846/406/3 |
| White blood cell (/μL) | 5076 ± 15062 |
| Hemoglobin (g/dL) | 14.0 ± 1.52 |
| Platelet count (10000/μL) | 16.7 ± 5.52 |
| Alanine aminotransferase (IU/L) | 56 ± 372 |
| Alpha-fetoprotein (ng/mL) | 4.6 (0.9-223)1 |
| Therapeutic outcome (SVR/relapse/NVR) | 665/335/255 |
| Univariate analysis | Multivariate analysis | |||
| Factors | OR (range1) | P | OR (range1) | P |
| Age (1: ≥ 60 yr) | 1.2 (0.96-1.5) | 0.10 | ||
| Gender (1: male) | 1.2 (0.93-1.5) | 0.19 | ||
| HCV genotype (1: type 1) | 0.72 (0.57-0.92) | 0.007 | ||
| White blood cell (1: ≥ 4000/μL) | 0.78 (0.60-1.0) | 0.061 | ||
| Hemoglobin (1: ≥ 12.5 g/dL) | 0.97 (0.74-1.3) | 0.80 | ||
| Platelet count (1: ≥ 130000/μL) | 0.31 (0.24-0.41) | < 0.0001 | 0.35 (0.27-0.46) | < 0.0001 |
| ALT (1: ≥ 40 IU/L) | 3.0 (2.3-3.8) | < 0.0001 | 2.7 (2.1-3.4) | < 0.0001 |
| Univariate analysis | Multivariate analysis | |||
| Factors | OR (range1) | P | OR (range1) | P |
| SVR patients | ||||
| Age (1: ≥ 60 yr) | 0.84 (0.50-1.4) | 0.50 | ||
| Gender (1: male) | 1.3 (0.77-2.2) | 0.32 | ||
| HCV genotype | 0.89 (0.54-1.5) | 0.64 | ||
| White blood cell (1: ≥ 4000/μL) | 0.79 (0.43-1.5) | 0.45 | ||
| Hemoglobin (1: ≥ 12.5 g/dL) | 0.55 (0.26-1.2) | 0.12 | ||
| Platelet count (1: ≥ 130000/μL) | 1.2 (0.66-2.0) | 0.60 | ||
| ALT (1: ≥ 40 IU/L) | 1.5 (0.82-2.8) | 0.19 | ||
| ALT at end of therapy (1: ≥ 40 IU/L) | 0.86 (0.43-1.7) | 0.68 | ||
| ALT 1 yr after therapy (1: ≥ 40 IU/L) | 0.27 (0.087-0.86) | 0.027 | 0.27 (0.087-0.86) | 0.027 |
| Non-SVR patients | ||||
| Age (1: ≥ 60 yr) | 0.89 (0.55-1.4) | 0.64 | ||
| Gender (1: male) | 1.2 (0.73-1.9) | 0.53 | ||
| HCV genotype | 1.1 (0.59-2.2) | 0.70 | ||
| White blood cell (1: ≥ 4000/μL) | 1.5 (0.86-2.7) | 0.15 | ||
| Hemoglobin (1: ≥ 12.5 g/dL) | 1.6 (0.91-2.8) | 0.10 | ||
| Platelet count (1: ≥ 130000/μL) | 1.8 (1.1-2.9) | 0.019 | 1.5 (0.88-2.4) | 0.14 |
| ALT (1: ≥ 40 IU/L) | 1.2 (0.69-2.1) | 0.53 | ||
| ALT at end of therapy (1: ≥ 40 IU/L) | 0.31 (0.18-0.54) | < 0.0001 | 0.41 (0.22-0.75) | 0.0040 |
| ALT 1 yr after therapy (1: ≥ 40 IU/L) | 0.35 (0.21-0.59) | < 0.0001 | 0.53 (0.30-0.93) | 0.028 |
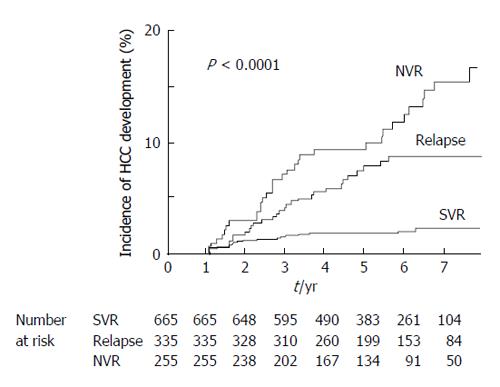
During the mean follow-up period of 5.4 years, HCC was occurred in 20 SVR patients: within 4 years of follow-up in 18 patients (90%) and after approximately 6 years of follow-up in the remaining patients. Twenty-eight patients with relapse and 41 patients with partial or no virological response developed HCC. The duration until detection of HCC was variable in non-SVR patients. As shown in Figure 2, the cumulative HCC incidence was 2.8% at 5 years after IFN therapy in SVR patients, which was significantly lower than that in non-SVR patients (11.1%, log-rank test, P < 0.0001). As related to HCC development within 3, 4, and 5 years after IFN therapy, receiver operating characteristic curves were constructed to determine the best cut-off values for AFP in SVR patients. The results showed AFP values of 4.9, 4.3, and 4.3 ng/mL to be the best cut-off values with areas under the curves of 0.83, 0.81, and 0.82, respectively. The best cut-off values were also determined for non-SVR patients, and the cut-offs were 5.6, 4.7, and 5.3 ng/mL with areas under the curves of 0.81, 0.80, and 0.80, respectively. The desired sensitivity value > 90% was achieved for the cut-off of 5.0 ng/mL, and the specificity was > 50% both in SVR and non-SVR patients. Therefore, AFP values 5 ng/mL were used as the cut-off values for further analyses. Table 4 shows the analysis of predictive factors of HCC development after IFN therapy for CHC patients in Cox proportional hazard models. Multivariate analysis in SVR patients showed old age, low platelet counts, and high AFP values before IFN therapy, but not high AFP values 1 year after IFN therapy, were significant factors associated with HCC development after IFN therapy in the follow-up period in SVR patients (P = 0.0092, P = 0.022, P = 0.017, and P = 0.49, respectively). As for non-SVR patients, male gender, HCV genotype 1, low platelet counts, and high AFP values before therapy and 1 year after IFN therapy were independent factors associated with HCC development after IFN therapy (P = 0.0035, P = 0.021, P = 0.0063, P = 0.011, and P = 0.014, respectively).
| Univariate analysis | Multivariate analysis | |||
| Factors | HR (range1) | P | HR (range1) | P |
| SVR patients | ||||
| Age (1: ≥ 60 yr) | 4.7 (1.7-13) | 0.0028 | 3.8 (1.4-11) | 0.0092 |
| Gender (1: male) | 1.3 (0.53-3.3) | 0.55 | ||
| HCV genotype | 0.84 (0.35-2.0) | 0.71 | ||
| White blood cell (1: ≥ 4000/μL) | 1.4 (0.41-4.8) | 0.58 | ||
| Hemoglobin (1: ≥ 12.5 g/dL) | 1.2 (0.35-4.0) | 0.79 | ||
| Platelet count (1: ≥ 130000/μL) | 0.20 (0.081-0.47) | 0.0003 | 0.35 (0.14-0.88) | 0.022 |
| ALT (1: ≥ 40 IU/L) | 3.0 (0.87-10) | 0.083 | ||
| ALT at end of therapy (1: ≥ 40 IU/L) | 2.4 (0.81-7.2) | 0.12 | ||
| ALT 1 yr after therapy (1: ≥ 40 IU/L) | 0.99 (0.13-7.4) | 0.99 | ||
| AFP (1: ≥ 5 ng/mL) | 28 (3.7-208) | 0.0012 | 13 (1.6-109) | 0.017 |
| AFP at end of therapy (1: ≥ 5 ng/mL) | 8.0 (3.2-20) | < 0.0001 | 1.9 (0.61-5.9) | 0.27 |
| AFP 1 yr after therapy (1: ≥ 5 ng/mL) | 5.5 (2.3-13) | < 0.0001 | 1.5 (0.50-4.2) | 0.49 |
| Non-SVR patients | ||||
| Age (1: ≥ 60 yr) | 1.4 (0.87-2.3) | 0.16 | ||
| Gender (1: male) | 2.3 (1.4-3.9) | 0.001 | 2.1 (1.3-3.6) | 0.0035 |
| HCV genotype | 0.15 (0.037-0.61) | 0.0083 | 0.19 (0.046-0.78) | 0.021 |
| White blood cell (1: ≥ 4000/μL) | 0.64 (0.39-1.0) | 0.076 | ||
| Hemoglobin (1: ≥ 12.5 g/dL) | 0.90 (0.54-1.5) | 0.69 | ||
| Platelet count (1: ≥ 130000/μL) | 0.26 (0.15-0.42) | 0.0025 | 0.47 (0.28-0.81) | 0.0063 |
| ALT (1: ≥ 40 IU/L) | 2.7 (1.5-4.9) | 0.0014 | 1.0 (0.53-2.0) | 0.92 |
| ALT at end of therapy (1: ≥ 40 IU/L) | 2.8 (1.8-4.5) | < 0.0001 | 1.2 (0.69-2.0) | 0.54 |
| ALT 1 yr after therapy (1: ≥ 40 IU/L) | 2.4 (1.5-3.9) | 0.0003 | 1.2 (0.69-2.0) | 0.56 |
| AFP (1: ≥ 5 ng/mL) | 13 (4.9-37) | < 0.0001 | 4.6 (1.4 - 15) | 0.011 |
| AFP at end of therapy (1: ≥ 5 ng/mL) | 6.5 (3.6-12) | < 0.0001 | 0.97 (0.40-2.3) | 0.94 |
| AFP 1 yr after therapy (1: ≥ 5 ng/mL) | 7.8 (4.1-15) | < 0.0001 | 3.0 (1.2-7.1) | 0.014 |
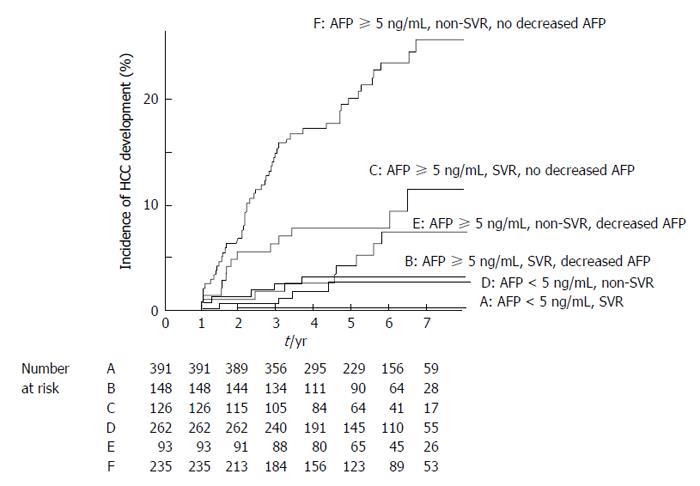
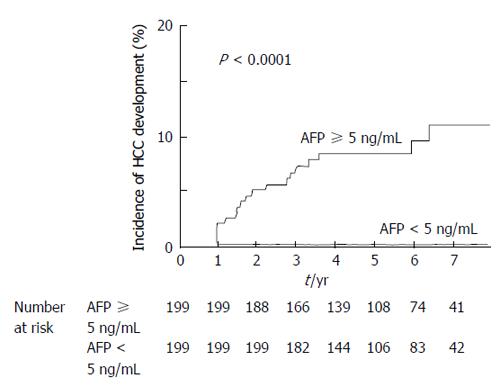
The cumulative HCC incidence was compared among the patient groups classified according to AFP values. Figure 3 showed that both SVR and non-SVR patients showing AFP values < 5 ng/mL before therapy had a significantly lower incidence than those showing values ≥ 5 ng/mL (log-rank test, P < 0.0001). No SVR patient showing AFP values < 5 ng/mL before therapy developed HCC during the follow-up period, except for one 69-year-old female patient. This patient had advanced cirrhotic liver disease, with a platelet count of 104000 cells/μL, AFP values 4.3 ng/mL, and alanine aminotransferase values 22 IU/L before IFN therapy. An HCC 1.2 cm in size was detected in her liver 20 mo after IFN therapy. The cumulative HCC incidence was compared between the patient groups classified according to AFP values before therapy and 1 year after IFN therapy. The incidence was significantly lower in patients with decreased AFP values to < 5 ng/mL than in those without decreased values (P = 0.011 in SVR patients, and P = 0.0026 in non-SVR patients, respectively). It was noteworthy that SVR patients showing AFP values ≥ 5 ng/mL before IFN therapy and no decrease in AFP values to < 5 ng/mL 1 year after IFN therapy had a significantly higher HCC incidence than non-SVR patients showing AFP values ≥ 5 ng/mL before IFN therapy and decreased AFP values to < 5 ng/mL 1 year after therapy (P = 0.043).
Propensity score matching was utilized to clarify the impact of AFP value on HCC development for the SVR patients; the significant factors associated with HCC development age, gender, platelet count, and alanine aminotransferase were matched for the SVR patients by propensity score. The comparison between the patients showing AFP < 5 ng/mL and ≥ 5 ng/mL before interferon therapy showed significantly lower cumulative HCC incidence in those showing AFP < 5 ng/mL than in those showing AFP ≥ 5 ng/mL (P <0.0001, Figure 4).
The present study was a large-scale, long-term cohort study of CHC patients receiving combination therapy with pegylated interferon and ribavirin. The results demonstrate that AFP values have a significant predictive impact on HCC development after IFN therapy besides age and liver fibrosis and elucidated that CHC patients showing AFP values < 5 ng/mL before IFN therapy and/or decreased AFP values to < 5 ng/mL 1 year after IFN therapy may have a low risk of developing HCC, irrespective of the therapeutic outcome. AFP value before and 1 year after IFN therapy is a simple and useful marker for predicting HCC development during the follow-up period after IFN therapy.
Recent advances in anti-HCV therapy have increased the necessity to establish useful predictors of HCC development after viral eradication in SVR patients. It is necessary to identify patients who require close follow-up for HCC development. In general, SVR patients have a significantly lower risk of developing HCC than non-SVR patients. However, previous reports showed that high AFP values before and/or after IFN therapy are associated with HCC development after IFN therapy in CHC patients, although the criteria for AFP varied among reports[13-15].
We investigated the changes in AFP values before, at end, and 1 year after IFN therapy, and clarified the efficacy of the AFP values at different time points with regard to HCC development after IFN therapy in CHC patients. Precise comparisons of risks of HCC development among the patient groups classified by therapeutic outcomes and AFP values before and 1 year after IFN therapy revealed that SVR patients showing AFP values ≥ 5 ng/mL before IFN therapy and no decrease in AFP values to < 5 ng/mL 1 year after IFN therapy required periodical survey of HCC development, because they have a significantly higher risk of developing HCC than non-SVR patients showing AFP values < 5 ng/mL before and/or 1 year after IFN therapy.
AFP is widely used as a serological marker for HCC and germ-cell tumors[17-22]. AFP value 10 ng/mL is generally regarded as the upper limit of normal range, with presence of cancer suspected when AFP value increases above this value. However, AFP value is sometimes elevated in CHC patients without HCC[23-25]. The precise mechanisms of elevation in AFP values remain uncertain. The present study demonstrated that increased AFP values in CHC patients were significantly associated with low platelet counts and higher values of ALT, reflecting advanced liver fibrosis and high hepatitis activity. Furthermore, the predictive value of AFP value on HCC development was evaluated for the SVR patient groups with different AFP values, matched of age, gender, platelet count, and value of ALT with propensity score matching. The results showed that SVR patients showing AFP values ≥ 5 ng/mL before IFN therapy have a significantly higher risk of HCC development than those showing AFP values < 5 ng/mL, responsible for elevated AFP values besides advanced liver fibrosis and high hepatitis activity. These results are consistent with a previous report on the association of AFP values with patient characteristics in CHC patients showing that AFP values correlate with liver fibrosis, steatosis, and hepatitis activity[26]. Elevated AFP values may therefore combine with several risk factors other than cancer.
Interestingly, the results revealed that the changes of AFP values during IFN therapy were correlated with the changes of the values of alanine aminotransferase, and that decreased AFP values < 5 ng/mL 1 year before therapy were significantly associated with normal ALT values 1 year after IFN therapy in both SVR and non-SVR patients. Therefore, it is possible that major effects of IFN therapy on HCC development is associated with decreased AFP values by reduced hepatitis activity.
In conclusion, the results suggest that patients showing AFP values < 5 ng/mL before therapy or decreased AFP values < 5 ng/mL 1 year after therapy have a significantly lower risk of developing HCC than patients showing AFP values ≥ 5 ng/mL 1 year after therapy. Strict follow-up is therefore required for SVR patients without a decrease in AFP values to < 5 ng/mL 1 year after IFN therapy because of high risk of developing HCC after IFN therapy. This simple criteria of AFP concentrations ≥ 5 ng/mL before and 1 year after IFN therapy is thus a useful predictor for HCC development in CHC patients.
Future study might be needed to analyze the development of HCC for CHC patients with oral therapy with direct antiviral agents, and compare with patients in this study.
Improvement in antiviral therapy increases the obtaining a sustained viral response (SVR) in chronic hepatitis C (CHC) patients. However, there are some patients who develop hepatocellular carcinoma (HCC) after SVR. The authors need simple and useful marker for predicting HCC development during the follow-up period after interferon (IFN) therapy.
Recent advances in anti-hepatitis C virus therapy have increased the necessity to establish useful predictors of HCC development after viral eradication in SVR patients. Alpha-fetoprotein (AFP) values have a significant predictive predictive value on HCC development before and/or after interferon therapy in CHC patients besides age and liver fibrosis.
It is necessary to identify patients who required close follow-up for HCC development. In general, SVR patients have a significantly lower risk of developing HCC than non-SVR patients. However, previous reports showed that high AFP values before and/or after IFN therapy are associated with HCC development after IFN therapy in CHC patients, although the criteria for AFP varied among reports. The authors investigated the changes in AFP values before, at end, and 1 year after IFN therapy, and clarified the efficacy of the AFP values at different time points with regard to HCC development after IFN therapy in CHC patients.
The results suggest that patients showing AFP values < 5 ng/mL before therapy or decreased AFP values of < 5 ng/mL 1 year after therapy have a significantly lower risk of developing HCC than patients showing AFP values ≥ 5 ng/mL 1 year after therapy.
Strict follow-up is therefore required for SVR patients without a decrease in AFP values to < 5 ng/mL 1 year after IFN therapy because of high risk of developing HCC after IFN therapy. Simple criteria of AFP values ≥ 5 ng/mL before and 1 year after IFN therapy is a useful predictor for HCC development in CHC patients.
The manuscript is very interesting, and the topic is indeed of broad significance for different kinds of readers. The statistical methods you used are good and well chosen, and the Tables and Figures well realised and clear.
P- Reviewer: Gnocchi D, Wong GLH S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, Ventura M, Vall M, Bruguera M, Bru C. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 508] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 2. | Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 458] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Hasan F, Jeffers LJ, De Medina M, Reddy KR, Parker T, Schiff ER, Houghton M, Choo QL, Kuo G. Hepatitis C-associated hepatocellular carcinoma. Hepatology. 1990;12:589-591. [PubMed] |
| 4. | Yokosuka O, Kojima H, Imazeki F, Tagawa M, Saisho H, Tamatsukuri S, Omata M. Spontaneous negativation of serum hepatitis C virus RNA is a rare event in type C chronic liver diseases: analysis of HCV RNA in 320 patients who were followed for more than 3 years. J Hepatol. 1999;31:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2160] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 6. | Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, Lefkowitch JH, Ludwig J, Okuda K. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 337] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 780] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 8. | Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, Maeda Y, Shirai Y, Fukuzaki T, Kaji I. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 232] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 607] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 11. | Serfaty L, Aumaître H, Chazouillères O, Bonnand AM, Rosmorduc O, Poupon RE, Poupon R. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 278] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Osaki Y, Ueda Y, Marusawa H, Nakajima J, Kimura T, Kita R, Nishikawa H, Saito S, Henmi S, Sakamoto A. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol. 2012;47:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Oze T, Hiramatsu N, Yakushijin T, Miyazaki M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res. 2010;40 Suppl 1:2-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 18. | Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 515] [Article Influence: 21.5] [Reference Citation Analysis (4)] |
| 19. | Cedrone A, Covino M, Caturelli E, Pompili M, Lorenzelli G, Villani MR, Valle D, Sperandeo M, Rapaccini GL, Gasbarrini G. Utility of alpha-fetoprotein (AFP) in the screening of patients with virus-related chronic liver disease: does different viral etiology influence AFP levels in HCC? A study in 350 western patients. Hepatogastroenterology. 2000;47:1654-1658. [PubMed] |
| 20. | Gebo KA, Chander G, Jenckes MW, Ghanem KG, Herlong HF, Torbenson MS, El-Kamary SS, Bass EB. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology. 2002;36:S84-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98:679-690. [PubMed] |
| 22. | Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Liaw YF, Tai DI, Chen TJ, Chu CM, Huang MJ. Alpha-fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver. 1986;6:133-137. [PubMed] |
| 24. | Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis C. Am J Gastroenterol. 1998;93:2452-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Goldstein NS, Blue DE, Hankin R, Hunter S, Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels in patients with chronic hepatitis C. Relationships with serum alanine aminotransferase values, histologic activity index, and hepatocyte MIB-1 scores. Am J Clin Pathol. 1999;111:811-816. [PubMed] |
| 26. | Moritou Y, Ikeda F, Iwasaki Y, Baba N, Takaguchi K, Senoh T, Nagano T, Takeuchi Y, Yasunaka T, Ohnishi H. Predictive impact of polymorphism of PNPLA3 on HCC development after interferon therapy in Japanese patients with chronic hepatitis C. Springerplus. 2013;2:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |