Published online May 27, 2014. doi: 10.4254/wjh.v6.i5.340
Revised: January 20, 2014
Accepted: March 3, 2014
Published online: May 27, 2014
Processing time: 187 Days and 17.4 Hours
AIM: To investigate CYP2E1 IgG4 autoantibody levels and liver biochemical markers in adult patients after anesthesia with desflurane.
METHODS: Forty patients who were > 18 years old and undergoing elective surgery under general anesthesia with desflurane were studied. Alpha-glutathione-S-transferase (αGST) and IgG4 antibodies against CYP2E1 were measured preoperatively and 96 h postoperatively, as well as complete blood count, prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), aspartate aminotransferase (SGOT), alanine aminotransferase (SGPT), g-glutamyl-transpeptidase (gGT), alkaline phosphatase, total serum proteins, albumin and bilirubin. A separate group of 8 patients who received regional anesthesia was also studied for calibration of the methodology used for CYP2E1 IgG4 and αGST measurements. Student’s t-test and the Mann-Whitney U test were used for comparison of the continuous variables, and Fisher’s exact test was used for the categorical variables. All tests were two-tailed, with statistical significance set as P < 0.05.
RESULTS: None of the patients developed postoperative liver dysfunction, and all patients were successfully discharged from the hospital. No statistically significant difference was observed regarding liver function tests (SGOT, SGPT, γGT, bilirubin, INR), αGST and CYP2E1 IgG4, before and after exposure to desflurane. After dividing patients into two subgroups based on whether or not they had received general anesthesia in the past, no significant difference in the levels of CYP2E1 IgG4 was observed at baseline or 96 h after desflurane administration (P = 0.099 and P = 0.051, respectively). Alpha-GST baseline levels and levels after the intervention also did not differ significantly between these two subgroups (P > 0.1). The mean αGST differences were statistically elevated in men by 2.15 ng/mL compared to women when adjusted for BMI, duration of anesthesia, number of times anesthesia was administered previously and length of hospital stay. No significant difference was observed between patients who received desflurane and those who received regional anesthesia at any time point.
CONCLUSION: There was no difference in CYP2E1 IgG4 or αGST levels after desflurane exposure; further research is required to investigate their role in desflurane-induced liver injury.
Core tip: Several case reports of hepatotoxicity following anesthesia with desflurane have been published in the literature, implicating cytochrome P450 2E1-IgG4 autoantibodies. This study investigates the possible changes in CYP2E1 IgG4 autoantibody levels and other biochemical markers of liver injury in 40 adult patients who received anesthesia with desflurane for elective surgery. Samples were obtained before and 96 h after exposure to desflurane, and no significant difference was observed in levels of CYP2E1 IgG4, a-glutamyl-S-transferase, aspartate aminotransferase, alanine aminotransferase, g-glutamyl-transpeptidase or alkaline phosphatase levels, regardless of patients’ previous exposure to volatile anesthetics.
- Citation: Batistaki C, Michalopoulos G, Matsota P, Nomikos T, Kalimeris K, Riga M, Nakou M, Kostopanagiotou G. CYP2E1 immunoglobulin G4 subclass antibodies after desflurane anesthesia. World J Hepatol 2014; 6(5): 340-346
- URL: https://www.wjgnet.com/1948-5182/full/v6/i5/340.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i5.340
Volatile anesthetics, mostly halothane, have been implicated as causative agents of drug-induced liver injury (DILI)[1-5], which has been reported to occur in approximately 1 out of 10000 adult patients receiving general anesthesia with halothane[4,5]. Three main mechanisms have been implicated in the development of hepatotoxicity: (1) a hypersensitivity reaction; (2) the production of hepatotoxic metabolites; and (3) hypoxia[4]. A hypersensitivity reaction is believed to be the most important of these mechanisms, it is also known as idiosyncratic drug-induced hepatitis (IDDIH) and is caused by the production of trifluoroacetylated (TFA) hepatic proteins during the metabolism of halothane by the cytochrome P450 2E1 (CYP2E1)[4]. These proteins act as neoantigens and are responsible for the production of autoantibodies against liver tissue[6-8]. In 1996, it was demonstrated that autoantibodies that react with CYP2E1 were significantly elevated in 45%-70% of patients with halothane hepatitis[7,9], particular including CYP2E1 IgG4 and 58 kDa endoplasmic reticulum protein (ERp58) autoantibodies[10,11].
With the development of newer volatile anesthetics, the risk of developing DILI has decreased, mainly due to the reduced metabolism of newer agents by CYP450 2E1 (20%-30% for halothane, compared to 2% for enflurane, 1% for sevoflurane, 0.2%-0.6% or less for isoflurane and 0.02% for desflurane)[12,13]. Njoku et al[14] (1997) demonstrated that desflurane produces lower levels of TFA hepatic proteins, reflecting the decreased metabolism of the agent by CYP2E1. Despite the fact that desflurane seems to have the best safety profile in this regard and is believed to have minimal, if any, hepatotoxic effect, there have been several case reports published implicating desflurane as a causative agent of DILI[11,15-20].
Aspartate aminotransferase (SGOT), alanine aminotransferase (SGPT), g-glutamyl-transpeptidase (gGT), alkaline phosphatase (ALP), albumin, bilirubin and coagulation status are all classical biochemical markers of liver injury, and they have all been reported to change significantly in all published case reports of hepatocellular damage due to desflurane anesthesia[11,15-20]. Additionally, alpha-glutathione S-transferase is believed to be a sensitive marker of hepatocellular injury because it is distributed equally in centrilobular and periportal hepatocytes, in contrast to SGOT/SGPT[21,22]. This distribution is important because liver biopsies of patients with halothane hepatitis have demonstrated that the main histological characteristic of this condition is centrilobular hepatic necrosis[4].
Based on case reports of desflurane hepatotoxicity and the possible mechanisms of DILI in the case of volatile anesthetics, the aim of this study was to investigate the possible production or alteration of CYP2E1 IgG4 autoantibody levels in addition to other biochemical markers of liver injury in patients who received general anesthesia with desflurane.
The Institutional Review Board and Ethics Committee of “Attikon” University Hospital, where the study was conducted, approved the study, and written informed consent was obtained from all patients. Forty randomly selected adult patients who had received general anesthesia with desflurane for elective surgery between 01/2008 and 07/2011 were included. The exclusion criteria included the following: age < 18 years old; American Society of Anesthesiologists classification > III; history of liver disease of any etiology; history of chronic hepatitis Β or C, recent viral disease of unknown etiology; history of exposure to hepatotoxic doses of acetaminophen, non-steroidal anti-inflammatory drugs or antiepileptic drugs; current treatment with hepatotoxic drugs or immunosuppressants; autoimmune or connective tissue disorders; history of alcohol or illicit drug use; pregnancy; malignancies; intra-abdominal procedures; and operating time of more than 6 h.
Patients’ complete medical history and medications were recorded during preoperative assessment. Samples of serum were collected from all patients preoperatively, immediately postoperatively and every 24 h until the 4th postoperative day (96 h after the completion of the surgery). At these time points, complete blood count, prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), aspartate aminotransferase (SGOT), alanine aminotransferase (SGPT), gGT, alkaline phosphatase, total serum proteins, albumin and bilirubin (direct/indirect) were measured. At baseline and 96 h postoperatively, alpha-glutathione-S-transferase (αGST) and IgG4 antibodies against CYP2E1 were measured. A time period of 4 d was selected because the latency period between the exposure and the clinical evidence of liver damage is variable in the current literature on halothane; its approximate value is estimated to be at least 6 d after first exposure and 3 d after multiple exposures[4]. The same applies to the case reports on desflurane-induced hepatotoxicity, in which the period between exposure and clinical presentation varied between 2-17 d[11,15-20]. Therefore, it was assumed that a time period of 4 d would be appropriate to detect early biochemical changes.
Serum was centrifuged at 1000 rounds/min for 10 min and immediately stored at -80 °C until the time of measurements. Complete blood count, coagulation status, SGOT, SGPT, gGT, ALP, serum proteins, and bilirubin were measured directly after sampling, according to the standard perioperative protocol.
Anesthesia in all patients was induced using propofol (2-2.5 mg/kg), fentanyl (3 μg/kg) and cis-atracurium (0.2 mg/kg). Maintenance of anesthesia was achieved with desflurane 6% (in an oxygen/air mixture of 50%) to achieve bispectral index values of 40-50, in addition to a remifentanil infusion as required. Intraoperative monitoring of all patients included systemic blood pressure measurement (invasive when needed), continuous ECG, pulse oxymetry, ETCO2 measurement and bispectral index. Postoperative analgesia included intravenous patient-controlled analgesia with morphine (bolus 1 mg, lockout 10 min) and acetaminophen (1 gr three times daily). In cases of minor surgery without significant postoperative pain, only an acetaminophen-codeine combination was administered (500 mg/30 mg), three times daily. Patients were closely monitored by the anesthesiology team for the efficacy of postoperative analgesia, hemodynamics, oxygenation, nausea and vomiting, two times a day, until the 4th postoperative day. All side effects and complications that occurred during that period were recorded.
A separate group of 8 patients who underwent orthopedic procedures under regional anesthesia was also studied to calibrate the methodology of CYP2E1 IgG4 and αGST measurements. All patients received combined spinal-epidural anesthesia using ropivacaine 0.75% combined with fentanyl, as required. The same serum sampling methodology was followed for these patients. Postoperative analgesia included only morphine administered epidurally (initial dose immediately after surgery) and subsequent acetaminophen-codeine administration (as for the general anesthesia patients).
The determination of serum CYP2E1 IgG4 antibody levels was performed according to a previously described sandwich enzyme-linked immunosorbent assay[21] with slight modifications. Briefly, Immulon 2HB 96-well microtiter plates (ISC BioExpress) were incubated overnight with 0.5 μg/100 μL human CYP2E1 (human CYP2E1 plus P450 reductase plus cytochrome b5 Supersomes (BD Biosciences, Woburn, MA, United States). After washing, plates were incubated with 100 μL serum for 3 h at room temperature and subsequently with 100 μL of a 1:1000 diluted mouse anti-human IgG4 HRP-conjugated second antibody for 2 h at room temperature. The final product was determined by incubation for 20 min with 100 μL of a 1:1 mixture of Color Reagent A (H2O2) and Color Reagent Tetramethylbenzidine (R&D Systems, Minneapolis, United States). The reaction was stopped by the addition of 2 N H2SO4, and the optical density was determined at 450 nm.
The determination of serum αGST was performed using a commercially available ELISA according to the manufacturer’s instructions (Eagle Biosciences, Inc, Boston, United States).
The Shapiro-Wilk test was performed to test for normal distribution of continuous variables. The results are given as the mean ± SD or as the median and interquartile range (IQR) according to normality of continuous variables. All qualitative variables are presented as absolute and relative frequencies. Student’s t test or its non-parametric equivalent, the Mann-Whitney U test, was used for comparison of continuous variables. Fisher’s exact test was employed for comparison of categorical variables.
Mean differences in the liver function tests being studied before and after the intervention (general anesthesia) were investigated by the application of multivariate linear regression models. A stepwise backward-forward technique was applied for the selection of the dependent variables.
All tests were two-tailed, and statistical significance was established at 5% (P < 0.05). Data were analyzed using Stata ™ (Version 10.1 MP, Stata Corporation, College Station, TX, United States).
Of the 40 patients included in the study, one was excluded due to the diagnosis of malignancy. The demographic characteristics of patients, such as their age, height, weight and body mass index, are presented in Table 1. Previous anesthetic exposure (to general anesthesia), type and duration of anesthesia, and total length of hospital stay after the operation, are also presented in Table 1. All patients remained hemodynamically stable throughout the procedure, without periods of sustained hypotension (of more than 20% below baseline values) or hypoxia that might interfere with liver function. No patient in this study developed postoperative hepatotoxicity, and all patients were successfully discharged from the hospital.
| Demographic characteristics | Mean ± SD/median (IQR) or frequencies1 | Range2 |
| Age (yr) | 42 (29-62) | 20-75 |
| Gender (M/F) | 22(56%) 17(44%) | - |
| Height (cm) | 172.62 (10.55) | 152-192 |
| Weight (Kg) | 82.7 (20.55) | 35-140 |
| BMI (Kg/m2) | 27.66 (6.14) | 12.85-41.91 |
| ASA I/II/III | 16 (41%)/22 (56%)/1 (3%) | - |
| Anesthetic data | ||
| Previous anesthesias (no/yes) | 12 (31%)/27 (69%) | - |
| No of previous anesthesias | 1 (0-2) | 0-6 |
| Duration of anesthesia (min) | 150 (90-180) | 30-360 |
| Length of stay (d) | 5 (4-7) | 2-15 |
| Type of operation (n) | Orthopedic: 35 | |
| Fractures: 18 | ||
| Arthroscopies: 12 | ||
| Knee/hip arthroplasties: 5 | ||
| Thyroidectomies (non-malignant): 3 | ||
| Saphenectomy: 1 |
No statistically significant difference was observed in the liver function tests (SGOT, SGPT, γGT, bilirubin, INR), αGST or CYP2E1 IgG4 before and after exposure to desflurane. A significant decrease was only observed in the hematocrit, hemoglobin and albumin levels, postoperatively. All data are presented in detail in Table 2.
| Baseline measurements | Measurements after intervention | P value1 | |
| Blood count | |||
| HCT (%) | 41.2 (37.4-43) | 36.75 (31.65-41.7) | 0.016 |
| HBG (g/dL) | 13.56 (1.944) | 12.01 (1.941) | 0.002 |
| WBC | 7907.94 (2218.9) | 8330 (1826.7) | 0.446 |
| PLT (x 103) | 251 (217-284) | 241 (205-276) | 0.259 |
| Liver function tests | |||
| SGOT (IU/L) | 20 (17-25) | 21.5 (17-28) | 0.489 |
| SGPT (IU/L) | 19 (14-39) | 19.5 (13-30) | 0.987 |
| ALP (IU/L) | 64 (46-77) | 54 (47-63) | 0.222 |
| γGT (IU/L) | 22.5 (13-35) | 23 (13-30) | 0.532 |
| Direct bilirubin (mg/dL) | 0.2 (0.13-0.21) | 0.185 (0.1-0.2) | 0.277 |
| Indirect bilirubin (mg/dL) | 0.5 (0.3-0.7) | 0.4 (0.3-0.6) | 0.840 |
| Total bilirubin (mg/dL) | 0.6 (0.5-1) | 0.6 (0.4-0.7) | 0.610 |
| Proteins (g/dL) | 6.3 (6-6.7) | 5.9 (5.35-6.45) | 0.082 |
| Albumin (g/dL) | 4.1 (3.7-4.3) | 3.5 (3.15-3.95) | 0.013 |
| PT (s) | 11.9 (11.23-13) | 12.55 (11.6-13.5) | 0.219 |
| aPTT (s) | 27.97 (3.09) | 29.83 (5.09) | 0.125 |
| INR | 0.97 (0.92-1.08) | 1.02 (0.9-1.1) | 0.346 |
| IgG4 (g/L) | 0.04 (0.02 -0.5) | 0.065 (0.02-0.415) | 0.576 |
| αGST (ng/mL) | 3.5 (1.69-4.38) | 3.03 (1.11-4.87) | 0.489 |
Multivariate linear regression models were applied using the mean differences of IgG4 and αGST adjusted for gender, BMI, duration of anesthesia, number of times anesthesia was administered previously, and length of hospital stay. A stepwise backward-forward technique was applied for the selection of dependent variables. No significant differences were detected in the mean differences of CYP2E1 IgG4 (before and after general anesthesia) or SGOT, SGPT, γGT, bilirubin and INR (P > 0.05). However, significant differences were detected between the mean differences of αGST according to gender, length of hospital stay and duration of general anesthesia. Alpha-glutamyl-S-transferase mean differences were statistically elevated in men by 2.15 ng/mL compared to women when adjusted for BMI, duration of anesthesia, number of times anesthesia was administered previously, and the length of hospital stay. An increase in the duration of anesthesia by one minute was associated with a mean increase in the αGST mean difference of 0.0323 ng/mL. These results are presented in Table 3.
Of the 8 patients who received regional anesthesia, one was excluded due to a malignancy that was diagnosed after the operation. Four women and 3 men were studied, of median age 70 years old (range 65-70) and ASA II and III. Three of them had received general anesthesia in the past [median 1.5 (range 1-2)], and 4 had not. Although the number of patients who received regional anesthesia was too small to serve as a control group, we compared the median differences (before and after anesthesia, general or regional) of the liver enzymes (SGOT, SGPT, ALP, γGT, αGST) and CYP2E1 IgG4, using the Mann-Whitney U test. There were no significant differences observed for any of the variables studied in this analysis.
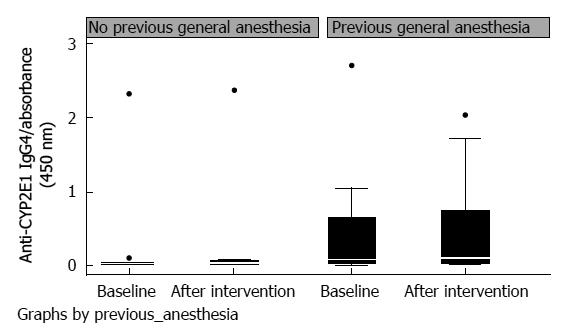
Twelve of the patients studied had not received general anesthesia previously. In this subgroup analysis, mean values for CYP2E1 IgG4 levels, at baseline and after desflurane exposure, were 0.025 (0-0.04) and 0.035 (0-0.07), respectively (P = 0.73). Twenty-seven patients had received general anesthesia previously; of these patients, 19 had surgery in childhood. Their CYP2E1 IgG4 levels before and after desflurane exposure were 0.08 (0.02-0.66) and 0.105 (0.03-0.74), respectively (P = 0.59). The changes in IgG4 levels after a new exposure to desflurane for patients who had and had not previously received general anesthesia were not significantly different (P = 0.79). However, CYP2E1 IgG4 levels in patients who had a medical history of previously receiving general anesthesia compared to those who had not, showed borderline statistical significance 96 h after anaesthesia (with P = 0.099 at baseline and P = 0.051 96 h post anesthesia) (Table 4, Figure 1). Alpha-GST baseline levels and levels after the intervention did not differ significantly between patients who had previously received general anesthesia and those who had not (P > 0.1) (Table 4).
| No previous anesthesia (n = 12) | Previous anesthesia (n = 27) | P value1 | |
| IgG4 levels | |||
| Baseline | 0.025 (0-0.04) | 0.08 (0.02-0.66) | 0.099 |
| 96 h after anesthesia | 0.035 (0-0.07) | 0.105 (0.03-0.74) | 0.051 |
| αGST levels | |||
| Baseline | 2.94 (1.69-3.94) | 3.54 (1.42-5.87) | 0.505 |
| 96 h after anesthesia | 3.825 (3.02-5.4) | 2.65 (1.05-4.33) | 0.147 |
Severe postoperative liver injury has been reported as a rare complication after anesthesia with halogenated anesthetics[1-4,14-21,23]. The main mechanism responsible is believed to be the production of trifluoroacetylated proteins through the metabolism of halogenated anesthetics from CYP2E1, which act as neoantigens and induce the production of autoantibodies against liver tissue[14,21]. Although newer volatile anesthetics, desflurane especially, are reported to be safe due to their low metabolism by CYP2E1[14], there are several case reports implicating desflurane as the causative agent of IDDIH[11,15-20]. In some cases, it was assumed that the mechanism might be prior sensitization of patients from previous exposures to halothane or other halogenated volatile anesthetics[16].
Njoku et al[14] showed that patients with IDDIH had significantly increased CYP2E1 IgG4 autoantibodies compared to control subjects who had never been exposed to halogenated agents. Similarly, in another study, it was observed that the levels of CYP2E1 autoantibodies were asymptomatically increased in pediatric anesthesiologists compared to general anesthesiologists, due to an increased environmental exposure to volatiles[24]. In another study by the same researchers[21] a comparison was made between the levels of CYP2E1 IgG4 in four separate groups: patients with anesthetic-induced IDDIH, pediatric anesthesiologists, general anesthesiologists and healthy controls; CYP2E1 IgG4 levels were significantly higher in patients who developed IDDIH than in all of the other groups. However, no comparison with our results can be made, because there was no direct quantification of the CYP2E1 IgG subclasses. In case reports of desflurane hepatotoxicity, hepatotoxicity occurred between the 2nd and 17th postoperative day and was related to at least one prior administration of anesthesia[11,15-20]. The problem is that CYP2E1 IgG4 levels were quantified and reported to be elevated in only two of these cases[18,20].
In the present study, no patient developed clinical or biochemical indications of liver injury, and there was no statistically significant increase in CYP2E1 IgG4 96 h after receiving anesthesia with desflurane. The hypothesis that previous exposure to halogenated anesthetics may sensitize patients to desflurane, leading them to develop IDDIH after subsequent desflurane exposure, has been stated by various researchers[14]. A cross-sensitization theory between inhalational anesthetics, in addition to the possibility of “immunological memory” of patients who were sensitized to an antigen, may be possible mechanisms to explain this effect[14]. To investigate this possibility, we also performed a subgroup analysis of patients, according to their previous exposure to halogenated anesthetics. Patients who had previously received general anesthesia were studied for their baseline values of CYP2E1 IgG4 and for their tendency to increase these levels after a new exposure to desflurane. The findings did not demonstrate any differences in the degree of increase of CYP2E1 IgG4 between naïve patients and those who had previously received any type of general anesthesia.
Regarding αGST, a multivariate regression model analysis showed that an increase in the duration of anesthesia by one minute was associated with a mean increase in αGST mean difference of 0.0323 ng/mL. This finding may be related to hepatic blood flow alterations and low-grade centrilobular hypoperfusion associated with the longer duration of anesthesia[25-27]. This possibility is in accordance with the findings of another study of patients undergoing partial hepatectomy under anesthesia with desflurane versus propofol, where it was found that αGST levels 120 min after hepatic vascular declamping were significantly higher in desflurane patients[25]. Further research is required on this subject to investigate alterations of αGST with respect to liver blood flow and duration of anesthesia.
Limitations of this study include the small sample size of patients and the absence of detailed data regarding previous anesthetic exposure. Although the number of patients who previously received general anesthesia is known, the exact agents used (inhalational or intravenous) are unknown. However, most of the patients received anesthesia in childhood, where the use of inhalational agents is a common practice. Additionally, the lack of a longer period of measurement after anesthesia and the absence of more frequent measurements (i.e., more time points) for CYP2E1 IgG4 and αGST are also limitations because there are cases of patients developing alterations after a longer time.
Our results suggest that there was no statistically significant difference between the levels of CYP2E1 IgG4 autoantibodies before and after anesthesia with desflurane. There was no evidence that the levels of αGST or any of the other biochemical markers of liver function were altered after anesthesia with desflurane and no signs that previous anesthetic exposure might affect these levels.
In conclusion, our findings suggest there are no significant differences between the levels of CYP2E1 IgG4 antibodies and αGST before and 96 h after anesthesia with desflurane and no difference between patients with previous anesthetic exposure versus naïve patients. Further research is required to investigate the actual role of CYP2E1 IgG4 in the pathogenesis of halogenated anesthetic-induced liver injury, especially in patients with multiple anesthetic exposures.
Batistaki Chrysanthi and Michalopoulos George contributed equally in study design and conduct and in manuscript preparation. The authors would also like to acknowledge Mrs Agathi Karakosta, MD, MSC in Biostatistics, for statistical analysis of data.
Volatile anesthetics, mostly halothane, have been implicated as causative agents of Drug Induced Liver Injury (DILI). The most important mechanism for DILI is believed to be a hypersensitivity reaction, which is due to the production of trifluoroacetylated hepatic proteins during metabolism of halothane by the cytochrome P450 2E1 (CYP2E1). With the development of newer volatile anesthetics, the risk of developing DILI has been decreased, due to the minor metabolism of newer agents by the CYP450 2E1 (20%-30% for halothane, in comparison to 0.02% for desflurane). Despite the fact that desflurane seems to have the best safety profile with this regard, and is believed to have minimal, if any, hepatotoxic effect, there have been several case reports published, implicating desflurane as the causative agent of DILI.
Desflurane is a widely used volatile anesthetic which seems to have the best safety profile regarding liver injury. Despite that, there have been some cases of DILI after desflurane anesthesia and in all of them there was a history of at least one previous general anesthesia. It is very important to identify persons at risk of developing DILI in order to avoid this serious complication. IgG4 CYP2E1 autoantibodies have been implicated in the development of DILI although their specific role is not clear. The measurement of IgG4 CYP2E1 autoantibodies before and after anesthesia with desflurane in patients with and without previous exposure to volatile anesthetics could give some useful information.
In the present study, no patient developed clinical or biochemical indications of liver injury and it was demonstrated that there was no statistically significant increase of CYP2E1 IgG4, 96 h after receiving anesthesia with desflurane. Patients who had previously received general anesthesia were studied as for their baseline values of CYP2E1 IgG4, as well as for their tendency to increase these levels after a new exposure to desflurane. Findings did not demonstrate any significant differences in the degree of increase of CYP2E1 IgG4 between naïve patients and those who had previously received any kind of general anesthesia.
The study results suggest that desflurane anesthesia is not associated with an increase of IgG4 CYP2E1 autoantibodies and that their role has to be further investigated.
Dr. Batistaki C and her colleagues investigated the levels of CYP2E1 IgG4 autoantibody levels and conventional biochemical variables in adult patients before and after anesthesia with desflurane, and found that there was no significant difference in hepatic biochemical variables and CYP2E1 IgG4 levels in patients who received general anesthesia with desflurane. This was an interesting study and the findings also provided them some useful reference in real clinical practice.
P- Reviewers: Cassard-Doulcier AM, Chen EQ S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Brody GL, SWEET RB. Halothane anesthesia as a possible cause of massive hepatic necrosis. Anesthesiology. 1963;24:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Lindenbaum J, Leifer E. Hepatic necrosis associated with halothane anesthesia. N Engl J Med. 1963;268:525-530. [PubMed] |
| 3. | Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis. Hepat Mon. 2011;11:3-6. [PubMed] |
| 4. | Lewis JH. Liver disease caused by anesthetics, toxins and herbal preparations, Sleisenger and Fordtran’s Gastrointestinal and Liver Diseases. 9th ed. USA: MD consult 2010; 1447-1459. |
| 5. | Martin JL. Inhaled anesthetics. Metabolism and toxicity. In Miller’s anesthesia. 7th ed. Philadelphia: Elsevier 2010; . |
| 6. | Kenna JG, Satoh H, Christ DD, Pohl LR. Metabolic basis for a drug hypersensitivity: antibodies in sera from patients with halothane hepatitis recognize liver neoantigens that contain the trifluoroacetyl group derived from halothane. J Pharmacol Exp Ther. 1988;245:1103-1109. [PubMed] |
| 7. | Bourdi M, Chen W, Peter RM, Martin JL, Buters JT, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem Res Toxicol. 1996;9:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, Stribling R, Crippin JS, Flamm S, Somberg KA. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573-582. [PubMed] |
| 10. | Balle J, Claësson MH. Psychosocial conditions and intervention in cancer patients. Ugeskr Laeger. 1993;155:2634-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Martin JL, Plevak DJ, Flannery KD, Charlton M, Poterucha JJ, Humphreys CE, Derfus G, Pohl LR. Hepatotoxicity after desflurane anesthesia. Anesthesiology. 1995;83:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Jackson GK, Aquavella JV. Clinical experience with hydrophilic lenses in monocular aphakia. Ann Ophthalmol. 1976;8:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Eger EI. New inhaled anesthetics. Anesthesiology. 1994;80:906-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 187] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Njoku D, Laster MJ, Gong DH, Eger EI, Reed GF, Martin JL. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth Analg. 1997;84:173-178. [PubMed] |
| 15. | Berghaus TM, Baron A, Geier A, Lamerz R, Paumgartner G. Hepatotoxicity following desflurane anesthesia. Hepatology. 1999;29:613-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Chung PC, Chiou SC, Lien JM, Li AH, Wong CH. Reproducible hepatic dysfunction following separate anesthesia with sevoflurane and desflurane. Chang Gung Med J. 2003;26:357-362. [PubMed] |
| 17. | Côté G, Bouchard S. Hepatotoxicity after desflurane anesthesia in a 15-month-old child with Mobius syndrome after previous exposure to isoflurane. Anesthesiology. 2007;107:843-845. [PubMed] |
| 18. | Anderson JS, Rose NR, Martin JL, Eger EI, Njoku DB. Desflurane hepatitis associated with hapten and autoantigen-specific IgG4 antibodies. Anesth Analg. 2007;104:1452-1453, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Tung D, Yoshida EM, Wang CS, Steinbrecher UP. Severe desflurane hepatotoxicity after colon surgery in an elderly patient. Can J Anaesth. 2005;52:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Chin MW, Njoku DB, MacQuillan G, Cheng WS, Kontorinis N. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1991. A 33-year-old man with dilatation of the ascending aorta and aortic regurgitation. N Engl J Med. 1991;325:874-882. [PubMed] |
| 21. | Njoku DB, Mellerson JL, Talor MV, Kerr DR, Faraday NR, Outschoorn I, Rose NR. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in pathogenesis of idiosyncratic drug-induced hepatitis. Clin Vaccine Immunol. 2006;13:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Hayes PC, Bouchier IA, Beckett GJ. Glutathione S-transferase in humans in health and disease. Gut. 1991;32:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Njoku DB, Greenberg RS, Bourdi M, Borkowf CB, Dake EM, Martin JL, Pohl LR. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth Analg. 2002;94:243-249, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | de Beer CD, Sear JW. Repeat anaesthesia: hepatic injury. Anaesth Intensive Care Med. 2007;8:41-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Laviolle B, Basquin C, Aguillon D, Compagnon P, Morel I, Turmel V, Seguin P, Boudjema K, Bellissant E, Mallédant Y. Effect of an anesthesia with propofol compared with desflurane on free radical production and liver function after partial hepatectomy. Fundam Clin Pharmacol. 2012;26:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Schmidt CC, Suttner SW, Piper SN, Nagel D, Boldt J. Comparison of the effects of desflurane and isoflurane anaesthesia on hepatocellular function assessed by alpha glutathione S-transferase. Anaesthesia. 1999;54:1207-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |