Published online Feb 27, 2014. doi: 10.4254/wjh.v6.i2.98
Revised: December 2, 2013
Accepted: December 12, 2013
Published online: February 27, 2014
Processing time: 180 Days and 4.6 Hours
AIM: To investigate the effect of methylsulfonylmethane (MSM), recently reported to have anti-cancer effects, in liver cancer cells and transgenic mice.
METHODS: Three liver cancer cell lines, HepG2, Huh7-Mock and Huh7-H-rasG12V, were used. Cell growth was measured by Cell Counting Kit-8 and soft agar assay. Western blot analysis was used to detect caspases, poly (ADP-ribose) polymerase (PARP), and B-cell lymphoma 2 (Bcl-2) expressions. For in vivo study, we administered MSM to H-ras12V transgenic mice for 3 mo.
RESULTS: MSM decreased the growth of HepG2, Huh7-Mock and Huh7-H-rasG12V cells in a dose-dependent manner. That was correlated with significantly increased apoptosis and reduced cell numbers in MSM treated cells. Cleaved caspase-8, cleaved caspase-3 and cleaved PARP were remarkably increased in the liver cancer cells treated with 500 mmol/L of MSM; however, Bcl-2 was slightly decreased in 500 mmol/L. Liver tumor development was greatly inhibited in the H-ras12V transgenic mice treated with MSM, compared to control, by showing reduced tumor size and number. Cleaved PARP was significantly increased in non-tumor treated with MSM compared to control.
CONCLUSION: Liver injury was also significantly attenuated in the mice treated with MSM. Taken together, all the results suggest that MSM has anti-cancer effects through inducing apoptosis in liver cancer.
Core tip: Methylsulfonylmethane (MSM) is an organic sulfur-containing compound. MSM suppressed hepatic tumor growth through activation of apoptosis. MSM could be a potential candidate as an anti-liver cancer agent.
- Citation: Kim JH, Shin HJ, Ha HL, Park YH, Kwon TH, Jung MR, Moon HB, Cho ES, Son HY, Yu DY. Methylsulfonylmethane suppresses hepatic tumor development through activation of apoptosis. World J Hepatol 2014; 6(2): 98-106
- URL: https://www.wjgnet.com/1948-5182/full/v6/i2/98.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i2.98
Liver cancer is the sixth most common malignancy and the third most common cause of cancer-related mortality worldwide[1]. Approximately 560000 cases are diagnosed each year and 550000 deaths are due to liver cancer. In most countries, 75%-90% of liver cancers are hepatocellular carcinomas[2]. The main risk factors of liver cancer include infection with hepatitis B virus (HBV) or hepatitis C virus (HCV)[3]. Other risk factors include excessive alcohol consumption, nonalcoholic steatohepatitis, autoimmune hepatitis, primary biliary cirrhosis, particularly aflatoxin B and various genetic metabolic diseases[4]. However, mortality is diminishing with the development of vaccine and therapy methods. In particular, therapy using natural extracts with no side effects has been reported. It was recently reported that tetrandrine induces apoptosis in human hepatocellular carcinoma[5] and berbamine induces apoptosis and tumor growth inhibition[6].
Apoptosis is a physiological process for involution and atrophy of various tissues and organs during development and maintenance of tissue homeostasis[7]. The apoptosis pathway is mediated by death receptors that include tumor necrosis factor receptor (TNFR), Fas and TNF-related apoptosis-inducing ligand (TRAIL). These ligands lead to the recruitment and activation of initiator cysteine aspartic proteases (caspases) such as caspases-8 and 10. These lead to the activation of caspase-3. The active caspase-3 involves DNA fragmentation, nuclear fragmentation, membrane blebbing and other morphological and biochemical changes[8]. Otherwise, apoptosis is initiated by the stress-mediated release of cytochrome-c. The cytochrome-c activates initiator caspase, typically caspase-9, which leads to the activation of the executioner caspase-3. In response to apoptotic stimuli, pro-apoptotic members of the B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and Bcl-2 homologous antagonist killer (Bak) become activated and act on the mitochondria to induce the release of cytochrome-c[8].
Methylsulfonylmethane (MSM), an organic sulfur-containing compound, inhibits LPS-induced release of pro-inflammatory mediators in murine macrophages through downregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) signaling[9]. Moreover, the effect of MSM has been reported in cancer. MSM suppresses breast cancer growth by down-regulating signal transducer and activator of transcription 3 (STAT3) and signal transducer and activator of transcription 5b (STAT5b) pathways[10]. Apoptotic effects in other cancer cells, such as esophageal, gastric and liver cancer cells, were reported[11].
To further understand the effect of MSM in prevention of hepatic tumorigenesis, we have examined it in liver cancer cell lines and liver cancer mouse model.
HepG2 and Huh7 cell lines were maintained in a DMEM (HyClone, United States), supplemented with a 10% FBS (HyClone, United States), penicillin/streptomycin (HyClone, United States) in a CO2 incubator at 37 °C. Huh7-H-rasG12V cell lines were generated by stably transfecting H-rasG12V in Huh7 cells. The pCAG-HA-H-rasG12V-neo was constructed as follows. The coding sequences for mutated H-rasG12V were inserted by PCR cloning into the EcoR I site of the pCAG-HA-neo vector and confirmed by restriction mapping and DNA sequencing. Huh7 cells were plated in 6-well culture plates for 24 h prior to transfection. Cells were transfected with 3 μg of pCAG-HA-H-rasG12V-neo construct using a Lipofectamine 2000 reagent (Invitrogen, United States), according to the manufacturer’s instructions. After 48 h, cells were trypsinized and plated in a medium containing 400 μg/mL neomycin (G418). Following selection for 2 wk, total populations of neomycin-resistant cells were pooled and single-cells sorted into 96-well plates with a growth medium containing 400 μg/mL neomycin. Sorted single cells were grown under selection for an additional 2 wk and expanded into stable cell lines. The candidate clones were analyzed by Western blot analysis using a HA (Roche, Germany) antibody.
The generation of H-ras12V transgenic liver cancer mouse model was previously described[12]. We used 3 mo old H-ras12V transgenic male mice. H-ras12V transgenic mice were divided into two groups and administered with PBS (control group, n = 5) and methylsulfonylmethane (MSM, 100 μg/g) (treated group, n = 6) every day for 3 mo. The genotyping of PCR primers for the H-ras12V were 5’-CTAGCGCTGCAGGAATTC-3’ and 5’-GTAGTTTAACACATTATACACT-3’. The mice were housed in a pathogen-free animal facility under standard 12 h light/dark cycle. All animal procedures were conducted in accordance with the guidelines of the institutional Animal Care and Use Committee, Korea Research Institute of Bioscience and Biotechnology (KRIBB).
MSM and crystal violet were purchased from Sigma-Aldrich Co. (United States).
The cell growth after treatment with MSM was measured by Cell Counting Kit-8 (CCK-8) (Dojindo, Japan). HepG2 and Huh7 (Mock, H-rasG12V) cells were suspended at a concentration of 5 × 103 cells/well and cultured in 96-well flat bottomed microplate. After exposure to MSM at different time points (0, 24, 48, 72 and 96 h), CCK-8 (10 μL) was added to each well of a 96-well flat bottomed microplate containing 100 μL of culture medium and MSM (0, 200 μmol/L, 200 mmol/L, and 500 mmol/L) and the plate was incubated for 2 h at 37 °C. Viable cells were counted by absorbance measurements at 450 nm using auto microplate reader (VERSAmax™, United States).
HepG2, Huh7 - Mock, and Huh7-H-rasG12V (5 × 103 cells) were suspended in 1 mL of DMEM containing 0.3% agar in cell-growth medium and plated in triplicates over a first layer of 0.6% agar in cell-growth medium. The cells were grown at 37 °C and 5% CO2. Then the viable colonies were stained with 0.01% crystal violet (Sigma, United States) for 2 h. We treated with MSM (0, 200 μmol/L, 200 mmol/L, and 500 mmol/L) on the top agar on day 0. The MSM contained medium was changed every day.
Apoptosis was also evaluated by flow cytometry after Annexin V-FITC/PI (BD Bioscience, United States) staining. The cells were digested with trypsin and resuspended in 100 μL of binding buffer, 5 μL of Annexin V-FITC and added with 5 μL of PI, and the mixture was incubated at room temperature for 15 min in the dark. The cells were analyzed using a BD FACSCalibur (BD Bioscience, United States) and divided into four groups: normal cells (Annexin V negative and PI positive), early apoptotic cells (Annexin V positive and PI negative), late apoptotic cells (Annexin V positive and PI positive) and necrotic cells (Annexin V negative and PI positive). The percentages of the different cell groups were determined by a scatter plot analysis.
We homogenized liver cancer cell lysates in lysis buffer (20 mmol/L HEPES, 150 mmol/L NaCl, 2 mmol/L EGTA, 1 mmol/L EDTA, 20 mmol/L glycerol phosphate, 1% Triton X-100 and 10% glycerol) with protease (Sigma, United States) and a phosphatase-inhibitor cocktail (Roche, Germany). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. For Western blot analysis, 30 μg protein lysates were separated on 12% sodium dodecyl sulfate polyacrylamide gels and transferred onto nitrocellulose membranes (Millipore, United States). The membranes were primarily blotted with primary antibodies against GAPDH (Lab Frontier, South Korea), HA (Roche, Germany), cleaved caspase-3, cleaved caspase-8, cleaved PARP [Poly (ADP-ribose) polymerase] or Bcl-2 (Cell Signaling Technology Inc., United States) at 4 °C overnight. They were washed five times with 10 mmol/L Tris-HCI (pH 7.5) containing 150 mmol/L NaCl and 0.2% Tween-20 (TBST) and incubated with horseradish peroxidase conjugated goat anti-rabbit IgG or anti-mouse IgG (Pierce, United States) for 1 h at room temperature. After the removal of excess antibodies by washing with TBST, specific binding was detected using a SuperSignal chemiluminescent substrate (Pierce, United States) according to the manufacturer’s instructions.
Huh7-H-rasG12V cells were suspended at a concentration of 0.3 × 106 cells/well and cultured in 6-well plate. After exposure to MSM for 24 h, cells were calculated by trypan blue stain through an electron microscope (Nikon, Japan).
Once a mo during the experimental period, blood samples were taken from orbital venous congestion. Plasma was prepared by centrifugation of the blood at 10000 rpm for 5 min at 4 °C and stored at -70 °C until analysis. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured with an automatic chemistry analyzer (Hitachi 7150, Japan).
The liver was removed from the mice and immediately fixed in a buffer solution of 10% formalin for pathological analysis. Fixed tissues were processed routinely for paraffin embedding and 5 μm sections were prepared and stained with hematoxylin and eosin (H and E). Stained areas were viewed using an optical microscope.
Data were analyzed using SigmaStat 3.1 software. All data are presented as the mean ± the standard error of the mean (SEM) from at least three independent experiments. Comparisons between groups were analyzed by Student’s t-test for paired and unpaired measure. P value < 0.001 was considered statistically significant.
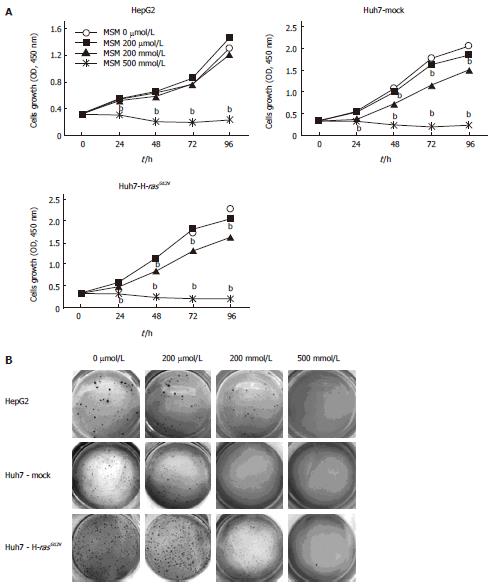
To investigate the effect of MSM in cell growth, cell lines such as HepG2, Huh7-Mock, and Huh7-H-rasG12V were exposed to MSM in a dose-dependent manner. Cell growth was analyzed at 0, 24, 48, 72 and 96 h using CCK-8. HepG2 cell growth was significantly inhibited with treatment of 500 mmol/L but the growth of Huh7-Mock and Huh7-H-rasG12V cells was significantly reduced with treatment of 200 mmol/L and 500 mmol/L of MSM (Figure 1A). To assess the effect of MSM in colony formation, we conducted a soft agar assay. As shown in Figure 1B, colony size and number of HepG2 cell were decreased in a dose-dependent manner. However, Huh7-Mock and Huh7-H-rasG12V cells were remarkably inhibited in 200 mmol/L and 500 mmol/L (Figure 1B). These results suggest that MSM treatment inhibits cell growth significantly in liver cancer cell lines.
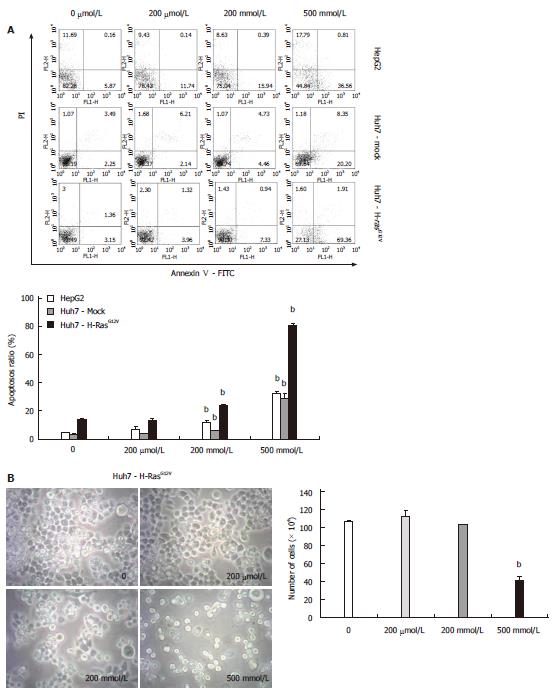
To determine whether MSM induces apoptosis in liver cancer cell lines, we utilized Annexin V/PI staining, observed morphology and counted live cells. The Annexin V/PI staining was performed to examine the reversion of phosphatidylserine, a marker for apoptosis. Our results showed that the proportion of apoptotic cells was induced by treatment of 200 mmol/L and 500 mmol/L in liver cancer cell lines. Particularly, the apoptosis rate was increased 6-fold in all liver cancer cell lines treated with 500 mmol/L compared to control (Figure 2A). We examined the morphology and counted live cells of Huh7-H-rasG12V. Morphological changes were observed in 200 mmol/L and 500 mmol/L. Also, adherent cells were decreased in 200 mmol/L and 500 mmol/L treated with MSM. In addition, live cell number was significantly reduced in 500 mmol/L of MSM compared to control (Figure 2B). The data indicate that MSM treatment induces apoptosis in liver cancer cell lines.
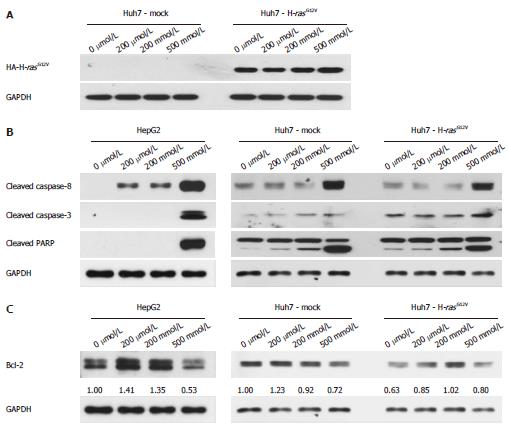
The expression of HA-H-rasG12V in Huh7-H-rasG12V was confirmed by Western blot. HA-H-rasG12V was overexpressed in Huh7-H-rasG12V cell (Figure 3A). To understand the mechanisms involved in MSM-induced apoptosis in liver cancer cell lines, we first determined caspase activity. The protein levels of cleaved caspase-3, cleaved caspase-8 and cleaved PARP were significantly increased in liver cancer cell lines treated with 500 mmol/L (Figure 3B). To investigate whether mitochondrial anti-apoptosis proteins are involved in regulating MSM-induced apoptosis of liver cancer cell lines, we examined the protein level of Bcl-2. The result indicated that Bcl-2 was decreased in liver cancer cell lines treated with 500 mmol/L (Figure 3C). It suggests that MSM induced apoptosis by regulating the expression of cleaved caspase-3, cleaved caspase-8 and cleaved PARP.
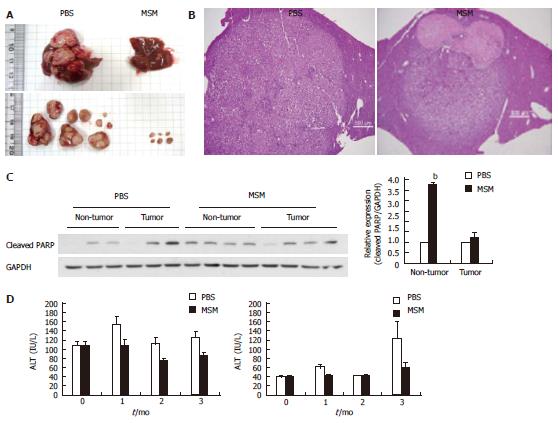
To investigate the suppression effects of MSM in hepatic tumorigenesis, H-ras12V transgenic mice were orally administered with MSM (100 μg/g) for 3 mo. Tumor volume and number were significantly reduced in the MSM treated group compared to the control group (Figure 4A). To determine histological changes, we examined the H and E staining in mouse liver. As shown in representative photomicrographs of liver histology, the tumor size in the MSM treated group was dramatically decreased compared to the control group. Moreover, necrosis was observed in the MSM treated group (Figure 4B). To confirm in vitro data on apoptosis, we examined the protein levels of cleaved PARP. Cleaved PARP was increased in MSM treated non-tumors compared to PBS treated control; however, not in tumor (Figure 4C). To examine the effect of MSM in liver function of H-ras12V transgenic mice, we checked AST and ALT levels. AST levels were significantly lower in the MSM treated group for 3 mo than the control group. ALT levels were also lower in the MSM treated group for 1 mo than the control group (Figure 4D). These data suggest that MSM suppresses liver damage in H-ras12V transgenic mice.
MSM is naturally obtained from various species of fruits, vegetables, grains, animals and animal products. The chemical structure of MSM is a combination of oxygen in dimethyl sulfoxide (DMSO), so also called dimethyl sulfone (DMSO2). The anti-inflammatory effects of MSM on lipopolysaccharide-induced inflammatory responses in murine macrophages[9] and effects of MSM in breast cancer by down regulating STAT3 and STAT5b pathways[10] were reported. However, the effect of MSM has not been studied in liver cancer. In the present study, we found that MSM dramatically inhibits hepatic tumor cell growth. We performed CCK-8 and soft agar assay. Anchorage dependent tumor cell growth inhibition was found in liver cancer cells, such as HepG2, Huh7-Mock and Huh7-H-rasG12V treated with 500 mmol/L of MSM (Figure 1A). Anchorage independent cell growth was also inhibited in the liver cancer cells treated with both 200 mmol/L and 500 mmol/L of MSM (Figure 1B). The results indicate that MSM is effective in inhibiting liver cancer cell growth.
To further investigate the apoptosis in liver cancer cells treated with MSM, we examined apoptotic cells by Annexin V/PI staining. Apoptotic cells were significantly increased by treatment of 500 mmol/L in liver cancer cell lines (Figure 2A) and the morphology of Huh7-H-rasG12V cells was changed in 200 mmol/L and 500 mmol/L. The numbers of live cells were reduced in 500 mmol/L (Figure 2B). The result indicated that MSM caused apoptosis in liver cancer cell lines.
Cancer chemotherapy is known to induce tumor cell death in a variety of cell types in part by promoting the intracellular ROS. Recently, salinomycin-induced apoptosis of human prostate cancer cells was due to accumulated ROS[13]. In our study, ROS levels were significantly increased in 500 mmol/L of MSM in Huh7 cell lines (Supplemental Figure 1), suggesting that MSM treatment regulated ROS levels in liver cancer cell lines.
To clarify the apoptotic mechanism stimulated by MSM, we studied both the death receptor pathway and the mitochondrial pathways[14]. Cell surface death receptors, such as Fas which bind their ligands, initiate signaling to activate caspase-8, caspase-3 to induce apoptosis, and signaling involved in mitochondrial release of cytochrome-c, which activates caspase-9 and caspase-3[15]. We performed Western blot. Bcl-2 was decreased in all of the cell lines treated with 500 mmol/L (Figure 3C) and MSM treatment led to an increased apoptotic response involving caspase-3, caspase-8 and PARP activation in liver cancer cell lines (Figure 3B). The results demonstrate that MSM induces apoptosis through activation of the caspase pathway.
We performed in vivo studies to investigate the liver tumor growth suppressive function of MSM. We orally administered MSM (100 μg/g) to H-ras12V transgenic mice for 3 mo. During the administration, body weight ratio was not changed between MSM treated group and control group (Supplemental Figure 2). However, the AST and ALT levels of MSM treated group were lower than the control group (Figure 4D). In addition, tumor volume and number were noticeably reduced in the MSM treated group (Figure 4A). The expression of cleaved PARP was increased in MSM treated non-tumors compared to PBS treated control; however, similar in tumors between the PBS and MSM treated group (Figure 4C). As shown in photomicrographs of liver histology, tumor size of the MSM treated group was decreased compared to the control group (Figure 4B). All the data suggest that MSM improves liver function and suppresses hepatic tumorigenesis through activation of apoptosis.
MSM was efficacious with treatment of 500 mmol/L in inhibition of hepatic tumor cell growth. In addition, the apoptosis rate was increased 6-fold in all of liver cancer cell lines treated with 500 mmol/L compared to control. These results indicate that MSM is efficacious with treatment of the highest dose in liver cancer cells, consistent with the result that MSM suppresses breast cancer cell growth at 300 mmol/L[10]. MSM is an edible natural organic compound present in many food items and is not associated with any toxic effects, even at higher concentrations[16,17]. MSM administration with high dose (100 μg/g) to H-ras12V transgenic mice for 3 mo did not affect body weight ratio but improved liver function by showing lowered AST and ALT levels and remarkably retarded hepatic tumor growth in the MSM treated group. All the results suggest that MSM could be available for inhibition of hepatic tumor growth. Further research is needed to be feasible in humans.
In summary, we showed that MSM induced growth inhibition and apoptosis in hepatic tumorigenesis. Therefore, MSM could be a potential candidate as an anticancer agent.
Liver cancer is the third most common cause of cancer-related mortality worldwide. However, there are only a few effective ways to prevent or treat liver cancer. Therefore, studies are going on in the area of liver cancer. Methylsulfonylmethane (MSM), an organic sulfur-containing compound, is naturally obtained from various species of vegetables, grains, animals and animal products. Recently, a study has reported that MSM can be used to inhibit breast cancer growth.
MSM is an edible natural organic compound present in many food items and is not associated with any toxic effects, even at higher concentrations. Research is focused on finding the efficacy of higher doses of MSM treatment in cells and mice with H-ras activated liver cancer.
MSM decreased the growth of Huh7-H-rasG12V cells in a dose-dependent manner. That was correlated with significantly increased apoptosis in MSM treated cells. Cleaved caspase-8, cleaved caspase-3 and cleaved PARP were remarkably increased in the liver cancer cells treated with 500 mmol/L of MSM. Liver tumor development was greatly inhibited in the H-ras12V transgenic mice treated with MSM compared to control, by showing reduced tumor size and number.
The results suggest that MSM could be a potential candidate for prevention of liver cancer.
MSM is a very simple organic sulfur-containing compound with a molar mass of 94.13 g/mol. MSM contains only eleven atoms and is found in foods, including fruits, vegetables, grains and beverages.
This study described the efficacy of MSM treatment in cells and mice with H-ras activated liver cancer well. The results are interesting and indicate that MSM could be used for preventing hepatic tumor growth.
P- Reviewers: Chiu IM, Lakatos PL S- Editor: Cui XM L- Editor: Roemmele A E- Editor: Liu XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13556] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 2. | Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049-1055. [PubMed] |
| 3. | Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol. 2005;23:8093-8108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Liu C, Gong K, Mao X, Li W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int J Cancer. 2011;129:1519-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Wang GY, Lv QH, Dong Q, Xu RZ, Dong QH. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J Asian Nat Prod Res. 2009;11:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [PubMed] |
| 8. | Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta. 2011;1813:238-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 9. | Kim YH, Kim DH, Lim H, Baek DY, Shin HK, Kim JK. The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biol Pharm Bull. 2009;32:651-656. [PubMed] |
| 10. | Lim EJ, Hong DY, Park JH, Joung YH, Darvin P, Kim SY, Na YM, Hwang TS, Ye SK, Moon ES. Methylsulfonylmethane suppresses breast cancer growth by down-regulating STAT3 and STAT5b pathways. PLoS One. 2012;7:e33361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Jafari N, Bohlooli S, Mohammadi S, Mazani M. Cytotoxicity of methylsulfonylmethane on gastrointestinal (AGS, HepG2, and KEYSE-30) cancer cell lines. J Gastrointest Cancer. 2012;43:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Wang AG, Moon HB, Lee MR, Hwang CY, Kwon KS, Yu SL, Kim YS, Kim M, Kim JM, Kim SK. Gender-dependent hepatic alterations in H-ras12V transgenic mice. J Hepatol. 2005;43:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kim KY, Yu SN, Lee SY, Chun SS, Choi YL, Park YM, Song CS, Chatterjee B, Ahn SC. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10243] [Cited by in RCA: 9569] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 15. | Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526-534. [PubMed] |
| 16. | Morton JI, Siegel BV. Effects of oral dimethyl sulfoxide and dimethyl sulfone on murine autoimmune lymphoproliferative disease. Proc Soc Exp Biol Med. 1986;183:227-230. [PubMed] |
| 17. | Horváth K, Noker PE, Somfai-Relle S, Glávits R, Financsek I, Schauss AG. Toxicity of methylsulfonylmethane in rats. Food Chem Toxicol. 2002;40:1459-1462. [PubMed] |