Published online Aug 27, 2013. doi: 10.4254/wjh.v5.i8.445
Revised: July 29, 2013
Accepted: August 4, 2013
Published online: August 27, 2013
Processing time: 101 Days and 17.2 Hours
AIM: To evaluate serum complement C4a and its relation to liver fibrosis in children with chronic hepatitis C virus (HCV) infection.
METHODS: The study included 30 children with chronic HCV infection before receiving antiviral therapy. Chronic HCV infection was defined by positive anti-HCV, a positive polymerase chain reaction for HCV-RNA for more than 6 mo with absence of any associated liver disease. A second group of 30 age- and sex-matched healthy children served as controls. Serum C4a levels were measured by enzyme-linked immunosorbent assay. Liver fibrosis stage and inflammatory grade were assessed using Ishak scoring system. Serum C4a levels were compared according to different clinical, laboratory and histopathological parameters. Statistical significance for quantitative data was tested by Mann-Whitney U non-parametric tests. For qualitative data, significance between groups was tested by χ2 test. Correlation was tested by Spearman’s test. Results were considered significant if P value ≤ 0.05.
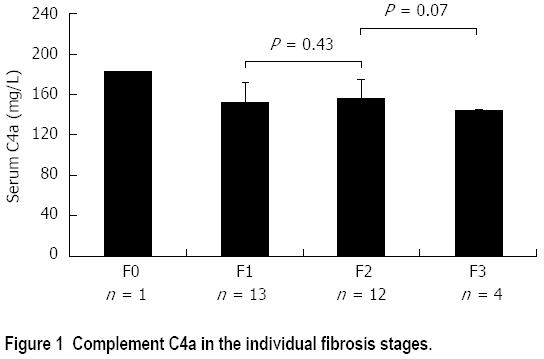
RESULTS: The age of the patients ranged from 3.5 to 18 years and that of controls ranged from 4 to 17 years. C4a mean levels were merely lower in patients (153.67 ± 18.69 mg/L) than that in the controls (157.25 ± 11.40 mg/L) with no statistical significance (P = 0.378). It did not differ significantly in patients with elevated vs those with normal transaminases (152.25 ± 16.62 vs 155.36 ± 21.33; P = 0.868) or with different HCV viremia (P = 0.561). Furthermore, there was no statistical significant difference in serum levels between those with no/mild fibrosis and those with moderate fibrosis (154.65 ± 20.59 vs 152.97 ± 17.72; P = 0.786) or minimal and mild activity (155.1 ± 21.93 vs 152.99 ± 17.43; P = 0.809). Though statistically not significant, C4a was highest in fibrosis score 0 (F0), decreasing in F1 and F2 to be the lowest in F3. When comparing significant fibrosis (Ishak score ≥ 3) vs other stages, C4a was significantly lower in F3 compared to other fibrosis scores (143.55 ± 2.33 mg/L vs 155.26 ± 19.64 mg/L; P = 0.047) and at a cutoff value of less than 144.01 mg/L, C4a could discriminate F3 with 76.9% sensitivity and 75% specificity from other stages of fibrosis.
CONCLUSION: Serum complement C4a did not correlate with any of transaminases, HCV viremia or with the histopathological scores. Although C4a decreased with higher stages of fibrosis, this change was not significant enough to predict individual stages of fibrosis. Yet, it could predict significant fibrosis with acceptable clinical performance.
Core tip: Non-invasive prediction of liver fibrosis is a challenging issue especially in pediatric population. Complement C4a was found, by proteomic analysis, to be associated with liver fibrosis and therefore proposed as a candidate for fibrosis prediction. In adults, serum C4 was found to decrease in hepatitis C virus (HCV) patients with moderate fibrosis and cirrhosis compared to healthy controls. In addition, in advanced HCV-induced liver fibrosis, the net production of C4a was found to be down-regulated. Furthermore, it was found to correlate negatively with alanine transaminase and the histological activity index of the Knodell scoring system. The issue has never been investigated in children before.
- Citation: Behairy BE, El-Mashad GM, Abd-Elghany RS, Ghoneim EM, Sira MM. Serum complement C4a and its relation to liver fibrosis in children with chronic hepatitis C. World J Hepatol 2013; 5(8): 445-451
- URL: https://www.wjgnet.com/1948-5182/full/v5/i8/445.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i8.445
Hepatitis C virus (HCV) is a serious health problem affecting more than 170 million people worldwide[1]. It establishes a chronic infection in up to 85% of cases[2]. Estimates range from less than 1.0% in northern Europe to more than 2.9% in northern Africa[3]. In children younger than 11 years, worldwide seroprevalence of HCV is 0.2% and in those older than 11 years it is 0.4%[1]. Egypt reports the highest prevalence worldwide ranging from 8.7% in upper Egypt to 24.3% in lower Egypt with an average of 13.8%[2,4]. The main (90%) HCV genotype is type 4. Studies of the magnitude of HCV infection in Egyptian children revealed a prevalence of 3% in upper Egypt and 9% in lower Egypt[5].
HCV causes intrahepatic lobular inflammation resulting in fibrosis and eventually cirrhosis[6]. Fibrosis prediction is an essential part of the assessment and management of patients with chronic HCV, worsening of which is probably the best surrogate marker for progression of chronic liver disease[7]. Liver biopsy represents the gold-standard for evaluating fibrosis; however, developing non-invasive tests that can predict liver fibrosis, especially in pediatric population, represents a growing medical need[8].
Conventional biochemical and serological tests are of little value for diagnosis of the degree of liver fibrosis. However, a liver biopsy is sometimes of questionable value because of the heterogeneous distribution of pathological changes in the liver[9]. Blood-based biomarkers offer a number of advantages including safety, cost-savings and widespread accessibility. Although liver biopsy is the gold-standard, it can have life-threatening complications in both adults and children[10,11].
Current serum biomarkers of fibrosis include indirect measures of fibrosis (such as transaminases and platelet count) or direct measures of fibrinogenesis or fibrinolysis (such as hyaluronic acid)[12]. The serum also contains all tissue proteins as leakage markers. Since the liver makes many serum proteins, it is logical to expect that the serum proteome may reflect liver disease[13].
A recent study, using proteomic analysis of serum from adult patients with chronic HCV infection, revealed that complement C4a was a candidate to predict liver fibrosis[14]. Complement C4 is a polymorphic serum protein consisting of two isoforms, C4a and C4b. C4 is expressed primarily in the liver and in macrophages, and its expression is induced in response to acute inflammation or tissue injury[15]. In adults, serum C4 was found to decrease in HCV patients with moderate fibrosis and cirrhosis compared to healthy controls. But in advanced HCV-induced liver fibrosis, the net production of C4a was found to be down-regulated[16]. Furthermore, it was found to correlate negatively with alanine transaminase (ALT) and the histological activity index of the Knodell scoring system[17]. The issue was not investigated in children before.
We aimed to evaluate serum C4a and its relation to liver fibrosis in children with chronic HCV infection.
The study included 30 children with chronic hepatitis C recruited from outpatients and inpatients of Pediatric Hepatology department, National Liver Institute, Menofiya University. Diagnosis was based on serological and virological tests; complete blood count (CBC), liver function tests (LFTs), prothrombin time, anti-HCV antibody (Ab), qualitative and quantitative polymerase chain reaction (PCR) for HCV-RNA. Histopathological findings in liver biopsies, the grade of inflammatory activity and the stage of the disease were also evaluated. A second group of 30 healthy children, served as controls. A signed informed consent was obtained from the parents of all the patients and controls before enrollment in the study. The study was approved by the Research Ethics Committee of the National Liver Institute.
Chronic HCV infection was defined by positive anti-HCV, a positive PCR for HCV-RNA for more than 6 mo, negative hepatitis B viral markers and absence of any associated liver disease. This was supported by the histopathological features of HCV infection in liver biopsy. Patients with decompensated liver disease or cirrhosis were excluded from the study. Control group were defined by apparently healthy individuals with no signs or symptoms of liver disease or any other diseases, normal liver transaminases and negative anti-HCV Ab.
Laboratory investigations, including LFTs, CBC, kidney function tests, serum autoantibodies (anti-nuclear antibodies, anti-smooth muscle antibodies and anti-mitochondrial antibodies) and prothrombin time were performed for all the patients. Viral markers were performed using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer instructions; HCV Ab (Innogenetics, Ghent, Belgium), hepatitis B virus surface antigen, hepatitis B virus core immunoglobulin (Ig)M and IgG Abs (all from Dia Sorin, Saluggia, Italy). Real-time PCR for HCV-RNA was performed using COBAS Ampliprep/COBAS TaqMan, Roche Molecular Systems, Inc., Branchburg, NJ 08876, United States (detection limit was 15 IU/mL). According to the viral load, viremia was classified arbitrarily into low (< 2 × 105 IU/mL), moderate (≥ 2 × 105-2 × 106 IU/mL), and high viremia (≥ 2 × 106 IU/mL)[18]. Serum C4a levels were assayed using ELISA (WKEA Med Supplies Corp, NY 10123, United States) according to the manufacturer instructions. Serum samples of the patients were collected, maximally, within 6 mo of liver biopsy[19].
Liver biopsy was performed using a true cut needle for all the patients. Specimens were fixed in formalin, embedded in paraffin and stained with hematoxylin and eosin, Masson’s trichrome, reticulin and Perl’s stains. Hepatic necroinflammatory activity and liver fibrosis were evaluated according to Ishak staging and grading score. Necroinflammatory activity was classified into minimal (score 1-3), mild (score 4-8), moderate (score 9-12), and severe (score 13-18)[20]. Fibrosis was classified into mild (stage 1), moderate (stages 2-3), and severe fibrosis or cirrhosis (stages 4-6)[5]. Significant fibrosis was defined as Ishak score of 3 or more (presence of bridging fibrosis)[21].
Descriptive results were expressed as mean ± SD or number (percentage) of individuals with a condition. For quantitative data, statistical significance was tested by Mann-Whitney U non-parametric tests. For qualitative data, significance between groups was tested by χ2 test. Correlation was tested by Spearman’s test. Results were considered significant if P value ≤ 0.05. The diagnostic performance was measured as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) and all were expressed as percentages. The cutoff values for optimal clinical performance was determined from the receiver-operating characteristic curve. Statistical analysis was performed using SPSS statistical package version 13 (SPSS Inc., Chicago, IL, United States).
The study included 30 children with chronic HCV infection. They were 12 females and 18 males. Their mean age was 11.12 ± 4.62 ranging from 3.5 to 18 years. A second group of 30 age- and sex-matched (P > 0.05 for both) healthy children served as controls. They were 14 females and 16 males. Their mean age was 10.73 ± 4.19 ranging from 4 to 17 years. The major possible modes of infection were male circumcision and family contact (60% each) followed by surgery (43.4%), blood transfusion (30%) and dental procedures (16.7%). Many children had more than one possible mode of infection. The majority of patients were asymptomatic. Four children had hepatomegaly, one child had splenomegaly and none had jaundice or ascites. Fibrosis stage ranged from F0 to F3 and activity grade ranged from A1 to A7. The majority had moderate fibrosis (56.7%) and mild activity (66.7%), while 8 out of 30 (26.7%) had steatosis (Table 1).
| Parameter | HCV patients (n = 30) |
| Clinical findings | |
| Jaundice | 0 (0.0) |
| Hepatomegaly | 4 (13.3) |
| Splenomegaly | 1 (3.3) |
| Ascites | 0 (0.0) |
| Liver function tests | |
| Total bilirubin (mg/dL) | 1.23 ± 1.051 |
| Direct bilirubin (mg/dL) | 0.30 ± 0.26 |
| Albumin (g/L) | 43.17 ± 7.5 |
| Alanine transaminase (U/L) | 55.57 ± 126.16 |
| Aspartate transaminase (U/L) | 72.10 ± 131.97 |
| Gamma glutamyl transpeptidase (U/L) | 38.90 ± 21.92 |
| Alkaline phosphatase (U/L) | 253.48 ± 97.38 |
| Complete blood count | |
| Hemoglobin (g/L) | 113.6 ± 11.2 |
| White blood cells (× 103/ L) | 8.77 ± 7.52 |
| Platelets (× 103/L) | 383.57 ± 390.87 |
| Fibrosis stage | |
| No (F0) | 1 (3.3) |
| Mild (F1) | 12 (40) |
| Moderate (F2-F3) | 17 (56.7) |
| Activity grade | |
| Minimal | 10 (33.3) |
| Mild | 20 (66.7) |
| Steatosis | 8 (26.7) |
All the patients (except one with F0) had mild to moderate fibrosis and minimal to mild activity in liver biopsy. Yet, nearly half of them had normal transaminases (41.7%, 47.1%, 50.0% and 45.0% for mild fibrosis, moderate fibrosis, minimal activity and mild activity respectively) (Table 2).
| Histopathology | Normal transaminases | Elevated transaminases |
| Fibrosis stage | ||
| No fibrosis (n = 1) | 1 (100) | |
| Mild fibrosis (n = 12) | 5 (41.7) | 7 (58.3) |
| Moderate fibrosis (n = 17) | 8 (47.1) | 9 (52.9) |
| Activity grade | ||
| Minimal activity (n = 10) | 5 (50) | 5 (50) |
| Mild activity (n = 20) | 9 (45) | 11 (55) |
The mean value of serum C4a was lower in patients than in controls (153.67 ± 18.69 mg/L vs 157.25 ± 11.40 mg/L respectively) with no statistically significant difference (P = 0.378). Moreover, there was no statistically significant difference in C4a regarding sex in both the patients (M/F: 150.33 ± 16.22 mg/L vs 158.75 ± 21.64 mg/L; P = 0.234) and controls (M/F: 154.29 ± 8.60 mg/L vs 160.64 ± 13.46 mg/L; P = 0.130). There was no statistically significant difference in mean level of C4a when comparing patients with different fibrosis stages, different activity grades, different levels of viremia and patients with normal transaminases vs those with elevated transaminases (P > 0.05 for all; Table 3). Furthermore, there was no correlation between C4a and any of the studied laboratory parameters or with fibrosis stage and activity grade (Table 4).
| Parameter | C4a (mg/L) | P value |
| Fibrosis stage | 0.786 | |
| No/Mild (n = 13) | 154.65 ± 20.59 | |
| Moderate (n = 17) | 152.97 ± 17.72 | |
| Activity grade | 0.809 | |
| Minimal (n = 10) | 155.1 ± 21.93 | |
| Mild (n = 20) | 152.99 ± 17.43 | |
| Steatosis | 0.186 | |
| Present (n = 8) | 146.13 ± 3.32 | |
| Absent (n = 22) | 156.45 ± 21.19 | |
| Normal transaminases (n = 14) | 155.36 ± 21.33 | 0.868 |
| Elevated transaminases (n = 16) | 152.25 ± 16.62 | |
| Viremia | 0.561 | |
| Low viremia (n = 17) | 156.37 ± 18.91 | |
| Moderate viremia (n = 9) | 152.43 ± 22.19 | |
| High viremia (n = 4) | 145.2 ± 3.96 |
| Parameter | C4a (mg/L) | |
| r | P value | |
| Total bilirubin (mg/dL) | -0.022 | 0.910 |
| Direct bilirubin (mg/dL) | -0.038 | 0.841 |
| Albumin (g/L) | 0.162 | 0.393 |
| Alanine transaminase (U/L) | -0.148 | 0.332 |
| Aspartate transaminase (U/L) | -0.026 | 0.891 |
| Gamma glutamyl transpeptidase (U/L) | 0.000 | 1.000 |
| Alkaline phosphatase (U/L) | 0.176 | 0.446 |
| Hemoglobin (g/dL) | -0.100 | 0.599 |
| White blood cells (× 103/L) | 0.054 | 0.777 |
| Platelets (× 103/L) | 0.228 | 0.226 |
| HCV-RNA (IU/mL) | -0.210 | 0.265 |
| Fibrosis stage | -0.208 | 0.269 |
| Activity grade | -0.114 | 0.548 |
Serum C4a, though not statistically significant, was inversely proportional to the stage of fibrosis. It was the highest (182.52 mg/L) in the patient with F0, decreasing in patients with F1 and F2 (152.33 ± 19.64 mg/L and 155.87 ± 19.46 mg/L respectively) and reaching the lowest level in F3 (143.55 ± 2.33 mg/L) (Figure 1).
Comparing C4a in significant fibrosis (Ishak score≥3) vs other fibrosis stages (F0-F2)
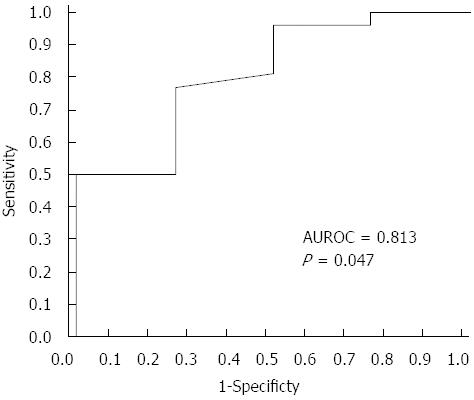
When comparing significant fibrosis with the other stages, C4a was significantly lower in F3 compared to other fibrosis scores (143.55 ± 2.33 mg/L vs 155.26 ± 19.64 mg/L; P = 0.047). C4a at a cutoff level of less than 144.01 mg/L could discriminate F3 with 76.9% sensitivity, 75% specificity, 95.24% PPV and 33.3% NPV from other stages of fibrosis (Figure 2).
The natural history of chronic HCV infection in children differs from that in adults since HCV infection is relatively benign, induces mild changes in the liver with a low level of fibrosis and a low rate of progression and is rarely associated with severe or decompensate liver disease[22]. However, progressive fibrosis and early appearance of end-stage liver disease have been documented[23]. Bortolotti et al[24], reported that hepatitis C in children is usually asymptomatic. Clinically, most of our patients were asymptomatic (73.36%), 13.3% had hepatomegaly, and 3.3% had splenomegally but none had jaundice or ascites. A similar finding was reported by El-Raziky et al[5], since soft enlargement of the liver was found in 2 (11%) children with HCV infection and none had splenomegaly.
In the current study, patients with normal transaminases (46.7%) had both mild (41.7%) to moderate (47.1%) fibrosis and minimal (50%) to mild (45%) activity on histopathological examination. It has been reported that ALT levels are elevated in half of the subjects and histological abnormalities are detectable in three quarters of HCV-RNA positive cases[5]. This means that liver enzymes in chronic HCV infection do not reflect histopathological abnormalities in the majority of cases and liver biopsy would be essential for evaluation of the disease state and extent of liver injury.
There is relatively little information on the histopathology of chronic hepatitis C in children. It has been shown that low ALT levels, low viral load and mild histological changes characterize chronic hepatitis C infection in children[25]. Goodman et al[26], reported that, in a cohort study, grading and staging of liver biopsies from 121 children ages 2 to 16 (mean, 9.8 years) infected with HCV revealed minimal, mild, moderate and severe inflammatory activity in 42%, 17%, 38%, 3% of patients respectively. Five (4%) had bridging fibrosis and 2 (1.7%) had cirrhosis. In the current study, all the patients had liver biopsy. None of them had cirrhosis where fibrosis scores ranged from 0 to 3 and activity grades ranged from 1 to 7. Although universal screening for hepatitis C is not recommended, it is actually the only method to detect HCV in children because carriers are usually asymptomatic. Even transaminases are usually within normal range. Consequently, they would remain undiagnosed until the appearance of symptoms in adolescence or adulthood[27].
The main target of the current study was to evaluate serum complement C4a levels in children with chronic HCV infection and its relation to liver fibrosis. Complement represents a significant non-specific host defense system involved in the protection of the host from virus infection[28]. To escape this protection, viruses are able to express host-homologous proteins, or to borrow cell membrane proteins from the host with complement regulatory activity, protecting viral particles from neutralization by the complement[29]. C4 specific activity appears as a valuable parameter for predicting and monitoring interferon and ribavirin therapy[30]. Deficiencies of complement component C4 isotype C4a has been associated with various autoimmune, inflammatory or infectious diseases as well as with mental disorders and cancer survival[31]. Phenotypic C4 deficiencies are caused by increased protein consumption or genetic deficiencies[32].
Serum C4 levels were found to decrease in adult HCV patients compared to healthy controls as a result of altered transcriptional regulation[30]. In the present study, although there was no significant statistical difference in the mean serum C4a levels between patients and controls, it was lower in patients. Moreover, there was no significant statistical difference in complement C4a level according to different stages of fibrosis, grades of activity or presence or absence of steatosis (P > 0.05 for all). Nonetheless, C4a was in its highest value in F0 and decreased as fibrosis increased with its lowest level in F3. This finding is in agreement with that of Imakiire et al[33], who reported that C4a increases with HCV infection, but decreases with disease progression which reflects the development of an inflammatory process and, evidently, the higher secretion of complement C4a by stimulated macrophages[15]. In addition, we found that C4a was not correlated with any of CBC parameters, liver functions or HCV viremia. Imakiire et al[33] showed that the level of C4a in serum was higher in HCV carriers with persistently normal ALT compared to chronic HCV patients or healthy volunteers.
Buğdaci et al[17] showed a significant negative correlation of C4 with ALT (r = -0.368, P = 0.001) and histological activity index (r = -0.639, P = 0.001) by Knodell score. Such relation was not found in the present study as there was no significant difference in complement C4a levels between patients with normal transaminases and those with elevated ones (P = 0.868). This discrepancy may be due to the difference in the mean age of the studied population (53.88 ± 11.44 years in Buğdaci et al[17], and 11.12 ± 4.62 years in ours), or the smaller number of patients (n = 30) in our study compared to 70 patients in Buğdaci et al[17]. Another important difference is the grade of activity (A1 to A7) and stage of fibrosis (F0 to F3) in ours using Ishak score, compared to that in Buğdaci et al[17], (8 ± 2.75 and 1.66 ± 0.784 for activity and fibrosis respectively) using Knodell score. In agreement with our results, Dumestre-Perard et al[30] reported that there were no statistical significant correlations between specific C4 activity and each of HCV-RNA, ALT, Knodell score, Metavir histological fibrosis and Metavir histological activity (P = 0.29, 0.9, 0.48, 0.96 and 0.22 respectively).
In chronic HCV infection, patients with no or minimal fibrosis at presentation appear to progress slowly and treatment could possibly be delayed or withheld. On the other hand, patients with significant fibrosis (i.e., septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20- year period, so antiviral treatment should be strongly considered[34]. For that we compared C4a in F3 vs other stages of fibrosis. C4a was significantly lower in F3 compared to other fibrosis scores and at a cutoff value of less than 144.01 mg/L it could discriminate F3 with 76.9% sensitivity and 75% specificity (P = 0.047). Although it is accepted to assess serum markers of fibrosis if serum sample is taken within 6 mo of liver biopsy[19], this might be a limitation in the study. For that, serum sampling in the same setting with liver biopsy would be preferred. Another limitation is the relatively small number of patients in the study.
In conclusion, our study demonstrated that complement C4a did not correlate with any of transaminases, HCV viral load or the histopathological scores of liver biopsy. Though C4a decreased in higher stages of fibrosis, this change was not statistically significant enough to predict individual stages of fibrosis. Yet, it could predict significant fibrosis with acceptable clinical performance.
The need for repetition of liver biopsy in patients with chronic hepatitis C virus (HCV), especially in assessing the degree of fibrosis and follow-up of treatment protocols, justifies an intensive search for non-invasive alternatives. Of these alternatives, serum C4a has been proposed. In adults, the reports are contradictory. In this study the authors evaluate C4a as a predictor of HCV-associated liver fibrosis in the pediatric age group.
The natural course of HCV infection in children differs from that in adults since the infection is relatively benign and induces mild changes in the liver with a low level of fibrosis. Patients with no or minimal fibrosis at presentation appear to progress slowly and treatment could possibly be delayed or withheld. On the other hand, patients with significant fibrosis (i.e., septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period so antiviral treatment should be strongly considered.
Complement C4a was proposed by proteomic analytical study in adults as a predictor of HCV-induced liver fibrosis. Further studies of serum level of C4a as a predictor for liver fibrosis were contradictory. Authors evaluated serum C4a as a predictor of HCV-induced liver fibrosis in the pediatric population. Although C4a decreased with higher stages of fibrosis, this change was not statistically significant enough to predict individual stages of fibrosis. Yet, it could predict significant fibrosis (Ishak score ≥ 3) with acceptable clinical performance.
Serum C4a can be used as a non-invasive marker to discriminate patients with significant liver fibrosis who are in need for critical consideration of antiviral therapy from those with no or minimal fibrosis for whom treatment could be delayed or deferred.
Hepatic fibrosis is the final common path of liver injury in most chronic liver diseases and can lead to cirrhosis, which is responsible for the majority of clinical complications. Fibrosis is characterized by excess deposition of extracellular matrix components including different collagens and non-collagenous proteins such as laminin, fibronectin, undulin, cytokines and complement components. The serum contains such tissue proteins as leakage markers.
The submitted manuscript investigates the significance of C4a as a surrogate marker for fibrosis in a children population with HCV infection. The article is interesting. The statistical analysis is good, but the number of patients is small and authors should mention in conclusion that the results were interpreted taking into consideration the small number of patients.
P- Reviewers Delladetsima JK, Dongiovanni P, Mihaila RG, Takaki A S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Yazigi N, Balistreri W. Viral hepatitis. 19th ed. Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Philadelphia (PA): Saunders 2011; 1393-1400. |
| 2. | Welbourn S, Pause A. The hepatitis C virus NS2/3 protease. Curr Issues Mol Biol. 2007;9:63-69. [PubMed] |
| 3. | Webster DP, Klenerman P, Collier J, Jeffery KJ. Development of novel treatments for hepatitis C. Lancet Infect Dis. 2009;9:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Lehman EM, Wilson ML. Epidemic hepatitis C virus infection in Egypt: estimates of past incidence and future morbidity and mortality. J Viral Hepat. 2009;16:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | El-Raziky MS, El-Hawary M, Esmat G, Abouzied AM, El-Koofy N, Mohsen N, Mansour S, Shaheen A, Abdel Hamid M, El-Karaksy H. Prevalence and risk factors of asymptomatic hepatitis C virus infection in Egyptian children. World J Gastroenterol. 2007;13:1828-1832. [PubMed] |
| 6. | Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Novel differential gene expression in human cirrhosis detected by suppression subtractive hybridization. Hepatology. 2003;38:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Valva P, Casciato P, Lezama C, Galoppo M, Gadano A, Galdame O, Galoppo MC, Mullen E, De Matteo E, Preciado MV. Serum apoptosis markers related to liver damage in chronic hepatitis C: sFas as a marker of advanced fibrosis in children and adults while M30 of severe steatosis only in children. PLoS One. 2013;8:e53519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Scheimann AO, Barrios JM, Al-Tawil YS, Gray KM, Gilger MA. Percutaneous liver biopsy in children: impact of ultrasonography and spring-loaded biopsy needles. J Pediatr Gastroenterol Nutr. 2000;31:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Oshrine B, Lehmann LE, Duncan CN. Safety and utility of liver biopsy after pediatric hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2011;33:e92-e97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Adams LA. Biomarkers of liver fibrosis. J Gastroenterol Hepatol. 2011;26:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3341] [Cited by in RCA: 3246] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 14. | Yang L, Rudser KD, Higgins L, Rosen HR, Zaman A, Corless CL, David L, Gourley GR. Novel biomarker candidates to predict hepatic fibrosis in hepatitis C identified by serum proteomics. Dig Dis Sci. 2011;56:3305-3315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Galibert MD, Boucontet L, Goding CR, Meo T. Recognition of the E-C4 element from the C4 complement gene promoter by the upstream stimulatory factor-1 transcription factor. J Immunol. 1997;159:6176-6183. [PubMed] |
| 16. | White IR, Patel K, Symonds WT, Dev A, Griffin P, Tsokanas N, Skehel M, Liu C, Zekry A, Cutler P. Serum proteomic analysis focused on fibrosis in patients with hepatitis C virus infection. J Transl Med. 2007;5:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Buğdaci MS, Karaca C, Alkim C, Kesıcı B, Bayraktar B, Sökmen M. Serum complement C4 in chronic hepatitis C: correlation with histopathologic findings and disease activity. Turk J Gastroenterol. 2012;23:33-37. [PubMed] |
| 18. | Witthöft T, Möller B, Wiedmann KH, Mauss S, Link R, Lohmeyer J, Lafrenz M, Gelbmann CM, Hüppe D, Niederau C. Safety, tolerability and efficacy of peginterferon alpha-2a and ribavirin in chronic hepatitis C in clinical practice: The German Open Safety Trial. J Viral Hepat. 2007;14:788-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Brown KS, Keogh MJ, Tagiuri N, Grainge MJ, Presanis JS, Ryder SD, Irving WL, Ball JK, Sim RB, Hickling TP. Severe fibrosis in hepatitis C virus-infected patients is associated with increased activity of the mannan-binding lectin (MBL)/MBL-associated serine protease 1 (MASP-1) complex. Clin Exp Immunol. 2007;147:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3770] [Article Influence: 125.7] [Reference Citation Analysis (1)] |
| 21. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3221] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 22. | Camarero C, Ramos N, Moreno A, Asensio A, Mateos ML, Roldan B. Hepatitis C virus infection acquired in childhood. Eur J Pediatr. 2008;167:219-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Giacchino R, Bortolotti F, Indolfi G, Verucchi G, Zancan L, Cammj C, D’Antiga L, Marazzi MG, Barbera C, Resti M. Antiviral treatment of hepatitis C in Italian children. Digest Liver Dis. 2009;41:A7-A8. [DOI] [Full Text] |
| 24. | Bortolotti F, Jara P, Diaz C, Vajro P, Hierro L, Giacchino R, de la Vega A, Crivellaro C, Camarena C, Barbera C. Posttransfusion and community-acquired hepatitis C in childhood. J Pediatr Gastroenterol Nutr. 1994;18:279-283. [PubMed] |
| 25. | Wu TC, Chuang WL, Dai CY, Huang JF, Hsieh MY, Hou NJ, Lee LP, Lin WY, Yang JF, Chiu CC. Hepatitis C virus infection among children in aboriginal areas in Taiwan. Trans R Soc Trop Med Hyg. 2008;102:935-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology. 2008;47:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Gerner P, Wirth S, Wintermeyer P, Walz A, Jenke A. Prevalence of hepatitis C virus infection in children admitted to an urban hospital. J Infect. 2006;52:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Morgan B, Harris C. Complement regulators and micro-organisms. Morgan BP, Harris CL, editors. Complement regulatory proteins. San Diego: Academic Press 1999; 207-225. |
| 29. | Lubinski J, Nagashunmugam T, Friedman HM. Viral interference with antibody and complement. Semin Cell Dev Biol. 1998;9:329-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Dumestre-Perard C, Ponard D, Drouet C, Leroy V, Zarski JP, Dutertre N, Colomb MG. Complement C4 monitoring in the follow-up of chronic hepatitis C treatment. Clin Exp Immunol. 2002;127:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Mougey R. A review of the Chido/Rodgers blood group. Immunohematology. 2010;26:30-38. [PubMed] |
| 32. | Braun L, Schneider PM, Giles CM, Bertrams J, Rittner C. Null alleles of human complement C4. Evidence for pseudogenes at the C4A locus and for gene conversion at the C4B locus. J Exp Med. 1990;171:129-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Imakiire K, Uto H, Sato Y, Sasaki F, Mawatari S, Ido A, Shimoda K, Hayashi K, Stuver SO, Ito Y. Difference in serum complement component C4a levels between hepatitis C virus carriers with persistently normal alanine aminotransferase levels or chronic hepatitis C. Mol Med Rep. 2012;6:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, Lefkowitch JH, Ludwig J, Okuda K. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 337] [Article Influence: 11.6] [Reference Citation Analysis (0)] |