Published online Oct 27, 2013. doi: 10.4254/wjh.v5.i10.596
Revised: September 18, 2013
Accepted: October 16, 2013
Published online: October 27, 2013
Processing time: 115 Days and 14.8 Hours
MK615, a compound extracted from the Japanese apricot “Prunus mume” has been reported to have in vitro anti-tumor activities against several cancer cell lines, including hepatocellular carcinoma (HCC). However, the clinical effects and feasibility of administering MK615 for patients with HCC were unknown. We experienced a case with advanced HCC for which MK615 was effective against both lymph node and pulmonary metastases. A 60-year-old female underwent surgical resection of a 9 cm HCC in the right lobe. The pathological diagnosis was moderately differentiated HCC with vascular invasion. The HCC recurred in the liver 8 mo after the surgery. Radiofrequency ablation and transarterial infusion chemotherapy were performed, but the recurrence was not controlled. One year after the intrahepatic recurrence, pulmonary and lymph metastasis appeared. Sorafenib was administered, but was not effective. Then, MK615 was administered as a final alternative therapy after informed consent was obtained from the patient. Three months later, her alpha-fetoprotein level decrease and both the lymph node and pulmonary metastases decreased in size. The patient has survived for more than 17 mo after the MK615 administration, and was in good condition. Although further investigations are necessary to clarify its safety and efficacy in humans, MK615 may be useful for the treatment of HCC, without serious adverse effects.
Core tip: We experienced a case with advanced hepatocellular carcinoma (HCC) for which MK615, a compound extracted from the Japanese apricot “Prunus mume” was effective against both lymph node and pulmonary metastases. MK615 was administered as a final alternative therapy. Three months later, her alpha-fetoprotein level decrease and both the lymph node and pulmonary metastases decreased in size. MK615 has been reported to have in vitro anti-tumor activities against several cancer cell lines, including HCC. Although further investigations are necessary to clarify its safety and efficacy in humans, MK615 may be useful for the treatment of HCC, without serious adverse effects.
-
Citation: Hoshino T, Takagi H, Naganuma A, Koitabashi E, Uehara S, Sakamoto N, Kudo T, Sato K, Kakizaki S. Advanced hepatocellular carcinoma responds to MK615, a compound extract from the Japanese apricot “
Prunus mume ”. World J Hepatol 2013; 5(10): 596-600 - URL: https://www.wjgnet.com/1948-5182/full/v5/i10/596.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i10.596
Hepatocellular carcinoma (HCC) is one of the most intractable cancers, and the clinical outcome is still unsatisfactory despite improvements in the therapeutic strategies for HCC[1-3]. For the patients with advanced HCC with poor liver function, surgical resection, radiofrequency ablation (RFA) or transarterial chemoembolization (TACE) cannot be applied because of their adverse effects, and palliative care is the only recommended treatment for such patients[1-3]. Although these patients might be cured by a liver transplant, such treatment is usually not possible due to the severe donor shortage[4]. For advanced stage HCC patients with preserved liver function, sorafenib is usually indicated, but its side effects, such as cytopenia and liver dysfunction, sometimes require a disruption or discontinuation of the treatment.
MK615 is a compound extracted from the Japanese apricot “Prunus mume”[5] and contains several triterpenoids, such as oleanolic acid and ursolic acid[5]. It has been reported that MK615 inhibits cell growth and induces the death of several tumor cell lines[5-7], including gastric cancer[5], promyelocytic leukemia[5], breast cancer[8], pancreatic cancer[9], HCC[10,11], colon cancer[12], esophageal cancer[13], malignant melanoma[14,15] and lung cancer cells[16]. The activities underlying the anti-tumor effects of MK615 have been reported to include the induction of apoptosis[5,8], autophagy[12,16] and the suppression of aurora A kinase[9,11]. Furthermore, some clinical studies have shown promising effects in some cancer patients[5,12]. Recently, hepatoprotective effects of MK615 have been reported for patients with chronic liver diseases[17]. However, the clinical benefit of MK615 for HCC patients has not been evaluated.
We recently experienced a case with advanced HCC in which MK615 was effective for both lymph node and pulmonary metastases. Although further investigations are necessary to clarify the safety and efficacy of the treatment in human patients, MK615 may be useful for the treatment of HCC, without the serious adverse effects associated with the current treatments.
A 60-year-old underwent surgical resection of a primary HCC lesion in the right lobe that was 9 cm in diameter. The pathological diagnosis was moderately differentiated HCC, ly2, v2, n0 and m0. The HCC recurred in the liver 8 mo later and RFA was performed, but the recurrence was not controlled. Transarterial infusion chemotherapy, including cisplatin and a 5-fluorouracil/cisplatin combination was performed for the intrahepatic recurrence. One year after the recurrence, pulmonary and lymph metastases appeared. Sorafenib was administered, but was not effective.
Then, MK615 was administered as a final alternative therapy after informed consent was obtained from the patient. MK615 (Misatol MER) was kindly provided by AdaBio Co Ltd. (Takasaki, Japan). A total of 6.5 g of Misatol MER was administered twice per day. The administration of MK615 was approved by the internal review board of Takasaki General Hospital, and adopted the protocol for clinical research entitled, “The clinical feasibility study of Misatol MER (MK615) for the patients with advanced stage-hepatocellular carcinoma”.
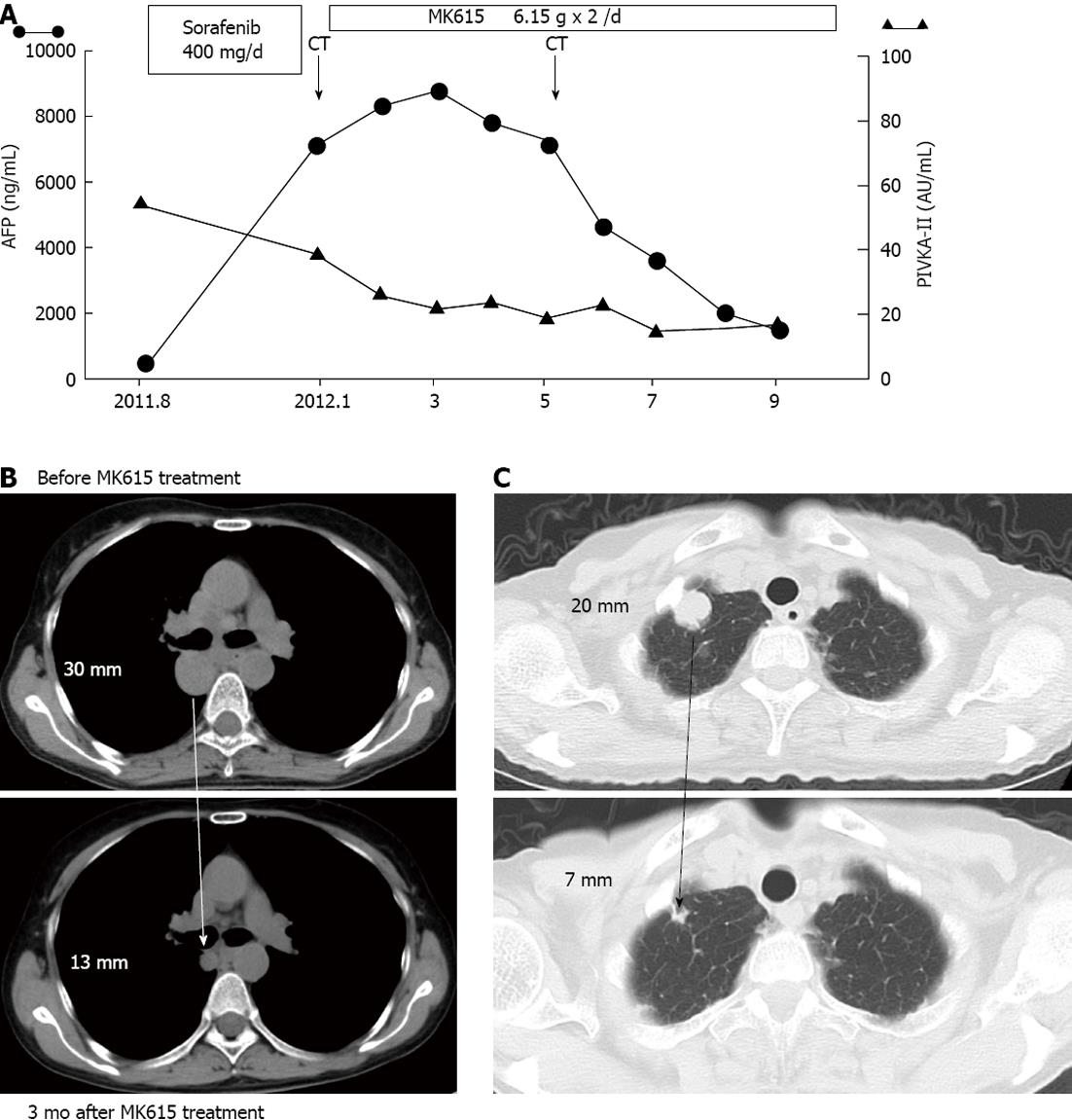
Three months later, the patient’s alpha-fetoprotein (AFP) levels decreased (Figure 1A) and both the lymph node (Figure 1B) and pulmonary (Figure 1C) metastases decreased in size. This patient has survived for more than 17 mo and was in good condition at her latest follow-up examination in August 2013.
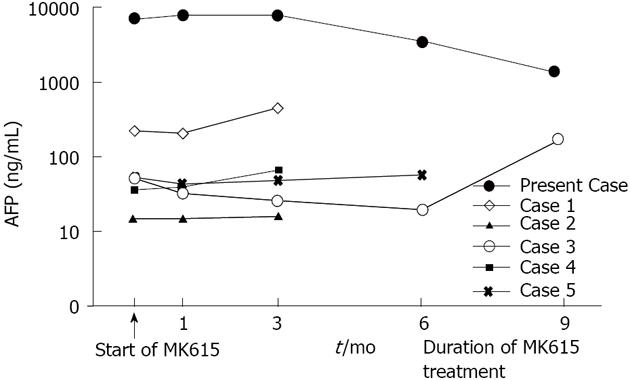
We conducted a preliminary clinical trial of MK615 for HCC. Six patients, including this case, received MK615 treatment as alternative therapy. These patients were not able to receive the conventional treatments, including surgical resection, RFA and TACE, because of advanced HCC and/or poor liver function, and so were administered MK615. The modified RECIST[18] was applied for the evaluation of the therapeutic effect of the treatment. The characteristics of the patients who had taken MK615 for more than 3 mo are shown in Table 1. All cases were of stage IV disease. The overall survival was 4.8 mo from the start of MK615 administration. The changes in AFP are shown in Figure 2. Although there was only one PR case, no serious adverse events were observed.
| Case | Sex | Age | Stage | CP | Previous treatment | Duration (mo) | Response | Cause of death |
| 1 | F | 64 | IVA | A | 5FU + IFN, Sorafenib | 3 | PD | HCC progression |
| 2 | F | 85 | IVA | C | None | 3 | SD | HCC progression |
| 3 | M | 63 | IVA | C | None | 6.5 | SD-PD | HCC progression |
| 4 | M | 57 | IVA | A | TACE | 3 | PD | HCC progression |
| 5 | F | 73 | IVA | C | None | 6 | PD | Survived |
| This case | F | 60 | IVB | A | Surgery, RFA, TAI | 17 | PR | Survived |
MK615 contains ume-derived triterpenoids, including oleanolic acid and ursolic acid. Triterpenoids had been reported to suppress the growth of many cancer cell lines[19-22]. Although the activities underlying the anti-tumor effects have been reported to include the induction of apoptosis[5,8], autophagy[12,16] and the suppression of aurora A kinase[9,11], the exact mechanism(s) of action of MK615 are still being elucidated. A large amount of basic data regarding the effects of MK615 against cancer cells in vitro have been published[5-16]. However, there has been little clinical data with regard to the effects of MK615 against cancer. One study with a small number of clinical cases showed that MK615 was useful for malignant melanoma[15], and the clinical efficacy and safety of MK615 has been reported for patients with chronic liver disease[17]. However, the clinical benefit of MK615 for HCC patients has not been evaluated.
We experienced a case of advanced HCC in which MK615 was effective for both lymph node and pulmonary metastases. Concerning the relationship between MK615 and HCC, Sakuraoka et al[10] reported that MK615 suppresses the proliferative effects of glyceraldehyde-derived advanced glycation end-products on a HCC cell line, HuH7, by decreasing the expression of their cellular receptor (RAGE). Another study reported that MK615 inhibited the growth of two HCC cell lines, HuH7 and Hep3B, in a dose-dependent manner[11]. A cell cycle analysis revealed that MK615 increased the population of cells in the G2/M phase[11] and that MK615 suppressed the expression of Aurora A[11]. These studies demonstrated that MK615 has anti-tumor effects against HCC. Although the mechanism(s) of anti-tumor activity in the present case is unknown, MK615 appears to exert anti-tumor effects on HCC in vivo. This case is the first case demonstrating the clinical efficacy of MK615 against HCC.
We also attempted to treat six patients with advanced stage HCC with poor liver function. Our policy is that if the patients had a chance to be treated with conventional anticancer treatments, the patients should be treated using these treatments, and alternative treatments are reserved only for those with no other options. Although the present case was the only PR, none of the subjects experienced adverse effects of the treatment. Therefore, it is considered that the effects of MK615 may be useful for patients with advanced HCC, particularly for patients with poor functional reserve, and that the treatment is not associated with the severe adverse effects associated with the conventional treatments.
Although further studies are required to demonstrate the safety and efficacy of MK615 for HCC patients, the preliminary results are promising. We are planning to conduct a clinical study of combination therapy using MK615 with other anti-cancer agents, and/or a controlled study with a large number of patients with advanced HCC.
The authors thank Taro Adachi for effective discussion for the accomplishment of this study.
P- Reviewers Reddanna P, Vitale A S- Editor Zhai HH L- Editor A E- Editor Yan JL
| 1. | Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2870] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 3. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4506] [Article Influence: 225.3] [Reference Citation Analysis (0)] |
| 4. | Takada Y, Uemoto S. Living donor liver transplantation for hepatitis C. Surg Today. 2013;43:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Adachi M, Suzuki Y, Mizuta T, Suzuki K, Shiojima K, Arai Y, Masuda K, Uchiyama M, Oyamada T and Clelici M. The “Prunus mume Sieb. et Zucc” (Ume) is a rich natural source of novel anti-cancer substance. Int J Food Properties. 2007;10:375-384. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Al-Jahdari WS, Sakurai H, Yoshida Y, Mobaraki A, Suzuki Y, Nakano T. MK615, a prospective anti-proliferative agent, enhances CD4/CD8 ratio after exposure to irradiation. Int J Radiat Biol. 2011;87:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Hiraishi K, Jimma F, Soma H, Morimoto Y, Adachi T and Adachi M: Potential of MK615, an extract from heat-concentrate of Prunus mume, as a medicinal material. Personalized Medicine Universe. 2013;1:17-28. |
| 8. | Nakagawa A, Sawada T, Okada T, Ohsawa T, Adachi M, Kubota K. New antineoplastic agent, MK615, from UME (a Variety of) Japanese apricot inhibits growth of breast cancer cells in vitro. Breast J. 2007;13:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Okada T, Sawada T, Osawa T, Adachi M, Kubota K. MK615 inhibits pancreatic cancer cell growth by dual inhibition of Aurora A and B kinases. World J Gastroenterol. 2008;14:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sakuraoka Y, Sawada T, Okada T, Shiraki T, Miura Y, Hiraishi K, Ohsawa T, Adachi M, Takino J, Takeuchi M. MK615 decreases RAGE expression and inhibits TAGE-induced proliferation in hepatocellular carcinoma cells. World J Gastroenterol. 2010;16:5334-5341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Okada T, Sawada T, Osawa T, Adachi M, Kubota K. A novel anti-cancer substance, MK615, from ume, a variety of Japanese apricot, inhibits growth of hepatocellular carcinoma cells by suppressing Aurora A kinase activity. Hepatogastroenterology. 2007;54:1770-1774. [PubMed] |
| 12. | Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K. New anti-proliferative agent, MK615, from Japanese apricot “Prunus mume” induces striking autophagy in colon cancer cells in vitro. World J Gastroenterol. 2007;13:6512-6517. [PubMed] |
| 13. | Yamai H, Sawada N, Yoshida T, Seike J, Takizawa H, Kenzaki K, Miyoshi T, Kondo K, Bando Y, Ohnishi Y. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int J Cancer. 2009;125:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Tada K, Kawahara K, Matsushita S, Hashiguchi T, Maruyama I, Kanekura T. MK615, a Prunus mume Steb. Et Zucc (‘Ume’) extract, attenuates the growth of A375 melanoma cells by inhibiting the ERK1/2-Id-1 pathway. Phytother Res. 2012;26:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Matsushita S, Tada KI, Kawahara KI, Kawai K, Hashiguchi T, Maruyama I, Kanekura T. Advanced malignant melanoma responds to Prunus mume Sieb. Et Zucc (Ume) extract: Case report and in vitro study. Exp Ther Med. 2010;1:569-574. [PubMed] |
| 16. | Sunaga N, Hiraishi K, Ishizuka T, Kaira K, Iwasaki Y, Jimma F, Adachi M, Mori M. MK615, A Compound extract from the Japanese apricot “Prunus mume” inhibits in vitro cell growth and interleukin-8 expression in non-small cell lung cancer cells. J Cancer Sci Ther. 2011;S11:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Hokari A, Ishikawa T, Tajiri H, Matsuda T, Ishii O, Matsumoto N, Okuse C, Takahashi H, Kurihara T, Kawahara K. Efficacy of MK615 for the treatment of patients with liver disorders. World J Gastroenterol. 2012;18:4118-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 497] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Hollósy F, Mészáros G, Bökönyi G, Idei M, Seprödi A, Szende B, Kéri G. Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells. Anticancer Res. 2000;20:4563-4570. [PubMed] |
| 20. | Li J, Guo WJ, Yang QY. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J Gastroenterol. 2002;8:493-495. [PubMed] |
| 21. | Wang X, Zhang F, Yang L, Mei Y, Long H, Zhang X, Zhang J, Qimuge-Suyila X. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J Biomed Biotechnol. 2011;2011:419343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Lin CC, Huang CY, Mong MC, Chan CY, Yin MC. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011;59:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |