Published online Dec 27, 2012. doi: 10.4254/wjh.v4.i12.382
Revised: August 9, 2012
Accepted: November 14, 2012
Published online: December 27, 2012
AIM: To evaluate the effects of surgical weight loss (Roux-en-Y gastric bypass with a modified Fobi-Capella technique) on non alcoholic fatty liver disease in obese patients.
METHODS: A group of 26 morbidly obese patients aged 45 ± 2 years and with a body mass index > 40 kg/m2 who underwent open surgical weight loss operations had paired liver biopsies, the first at surgery and the second after 16 ± 3 mo of weight loss. Biopsies were evaluated and compared in a blinded fashion. The presence of metabolic syndrome, anthropometric and biochemical variables were also assessed at baseline and at the time of the second biopsy.
RESULTS: Percentage of excess weight loss was 72.1% ± 6.6%. There was a reduction in prevalence of metabolic syndrome from 57.7% (15 patients) to 7.7% (2 patients) (P < 0.001). Any significance difference was observed in aspartate aminotransferase or alanine aminotransferase between pre and postsurgery. There were improvements in steatosis (P < 0.001), lobular (P < 0.001) and portal (P < 0.05) inflammation and fibrosis (P < 0.001) at the second biopsy. There were 25 (96.1%) patients with non alcoholic steatohepatitis (NASH) in their index biopsy and only four (15.3%) of the repeat biopsies fulfilled the criteria for NASH. The persistence of fibrosis (F > 1) was present in five patients at second biopsy. Steatosis and fibrosis at surgery were predictors of significant fibrosis postsurgery.
CONCLUSION: Restrictive mildly malabsorptive surgery provides significant weight loss, resolution of metabolic syndrome and associated abnormal liver histological features in most obese patients.
- Citation: Vargas V, Allende H, Lecube A, Salcedo MT, Baena-Fustegueras JA, Fort JM, Rivero J, Ferrer R, Catalán R, Pardina E, Ramón y Cajal S, Guardia J, Peinado-Onsurbe J. Surgically induced weight loss by gastric bypass improves non alcoholic fatty liver disease in morbid obese patients. World J Hepatol 2012; 4(12): 382-388
- URL: https://www.wjgnet.com/1948-5182/full/v4/i12/382.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i12.382
The term non alcoholic fatty liver disease (NAFLD) includes a spectrum of fatty liver diseases ranging from simple steatosis to steatohepatitis [non alcoholic steatohepatitis (NASH)] and cirrhosis[1]. The more progressive forms of NAFLD have been related to metabolic syndrome and obesity[2]. The epidemic of obesity has increased the prevalence of NAFLD and it is already the most common liver disorder in developed countries. Morbid obese patients have a high proportion of NAFLD. Most patients undergoing bariatric surgery have varying degrees of steatosis: as many as 36% have NASH and up to 4% have unsuspected cirrhosis. Only a small percentage of patients undergoing bariatric surgery have normal hepatic histology[3].
The optimal treatment of NASH has yet to be elucidated. In general, efforts have been developed to correct or improve insulin resistance and, in obese patients, weight loss has been prescribed. The effects of weight loss on NAFLD lesions have been studied and the reported effects of this therapy have been variable. Diet induced weight loss improved steatosis but did not always demonstrate an effect on histological parameters since in most studies repeated liver biopsy was not performed[3-8].
Also the effect of bariatric surgery could be confounding. Initially, it was described that rapid weight loss can exacerbate steatohepatitis in morbidly obese patients, especially after bariatric surgery[9]. This effect was more marked when malabsorptive procedures, like jejunoileal bypass or biliopancreatic diversion, were used. More recently, restrictive procedures, such as laparoscopic adjustable gastric banding, have demonstrated significant improvement in histopathological scoring[10-12], but in some studies the improvement in steatosis was accompanied by a progression of lobular inflammation[13].
We have less information about the effects on patients when mixed procedures were used[14]. The most frequently performed is Roux-en-Y gastric bypass, largely restrictive and mildly malabsorptive. In this procedure, the restriction is induced by a small neogastric pouch and a tight stoma and malabsorption by Roux-en-Y configuration of the small intestine[15]. The purpose of this study was to determine whether significant weight loss achieved through a standard mixed procedure - Roux-en-Y gastric bypass[16,17] - of bariatric surgery resulted in improvements in liver histopathology.
Since May 2004, a prospective protocol has been followed for patients with morbid obesity that had a Roux-en-Y gastric bypass. Up to September 2005, twenty-six obese patients with a body mass index of more than 40 kg/m2 who had significant medical, physical or psychosocial disabilities were considered for entry into the study. All patients underwent extensive preoperative assessment that included alcohol consumption, anthropometric measurements and laboratory tests. Laboratory tests included liver function tests, lipid profile, fasting plasma glucose, fasting insulin and hepatitis B and C serological analysis. Diagnosis of type 2 diabetes was based on the American Diabetes Association criteria[18]. Insulin sensitivity was estimated using the homeostatic model assessment method (HOMA)[19]. A diagnosis of metabolic syndrome was based on Adult Treatment Panel III criteria[20]. At the time of the second biopsy, the clinical assessment and anthropometric and biochemical measures were repeated. Percentage of excess weight loss was calculated by dividing the weight change between paired biopsies by the excess weight before surgery, multiplied by 100.
Any patient with a history of alcoholism, consuming more than 200 g of alcohol per week, with evidence of hepatitis B or C or with a history of another specific liver disease, was included in the study.
An open Roux-en-Y gastric bypass with a modified Fobi-Capella technique (ring 7 cm; alimentary limb 225 cm; biliopancreatic limb 60 cm) was performed in all patients.
An index biopsy was taken at the time of surgery with a Hepafix needle. In all patients, a follow up biopsy was obtained as a percutaneous biopsy using a Hepafix needle. All biopsies were at least 2 cm in length and contained at least eight portal tracts. Informed written consent was also obtained from all patients at the time of the index biopsy as part of an approved prospective study of bariatric surgery. Informed written consent also was obtained from all subjects before the second biopsy and the study was conducted according to the ethical guidelines of the Helsinki Declaration.
All liver biopsy specimens were stained with hematoxylin eosin, picrosiriums for fibrosis and periodic acid Schiff (PAS) with diastase to help clarify the degree of inflammation.
A single hepatopathologist (HA), blinded to the patient, clinical and laboratory data and to whether the biopsy was the pre or post operative biopsy, examined all tissue sections at the same time and assessed liver histology using a systemic approach of necroinflammatory grading and fibrosis staging as described by Brunt et al[21] and modified by Kleine et al[22]. Individual histological features were observed and scored separately (Table 1): Steatosis: 0: None; 1: Up to 33%; 2: 33%-66%; 3: > 66%. Hepatocyte ballooning: 0: None; 1: Occasional, Zone 3; 2: “Obvious” Zone 3; 3: Marked, predominantly Zone 3. Mallory bodies: 0: No Mallory bodies; 1: Fewer than two in 10 to 20 × fields; 2: More than two in 10 to 20 × fields. Glycogenated nuclei: 0: Absent; 1: Occasional; 2: Several. Lobular inflammation (inflammatory foci per 20 × with a 20 × ocular): 0: None; 1: 1 to 2/20; 2: Up to 4/20; 3: More than 4/20. Portal inflammation: 0: None; 1: Mild; 2: Moderate; 3: Severe. Fibrosis score: Stage 0: No fibrosis; Stage 1: Zone 3 perisinusoidal/pericellular fibrosis; focally or extensively present; Stage 2: Zone 3 perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis; Stage 3: Zone 3 perisinusoidal/pericellular fibrosis and portal fibrosis with a focal or extensive bridging fibrosis; Stage 4: Cirrhosis.
| Steatosis: |
| 0 : None |
| 1: up to 33% |
| 2: 33%-66% |
| 3: > 66% |
| Hepatocyte ballooning |
| 0: None |
| 1: Occasional, Zone 3 |
| 2: “Obvious” Zone 3 |
| 3: Marked, predominantly Zone 3 |
| Mallory bodies |
| 0: No Mallory bodies |
| 1: Fewer than two in 10 to 20 × fields |
| 2: More than two in 10 to 20 × fields |
| Glycogenated nuclei |
| 0: Absent |
| 1: Occasional |
| 2: Several |
| Lobular inflammation (inflammatory foci per 20 × with a 20 × ocular) |
| 0: None |
| 1: 1 to 2/20 |
| 2: Up to 4/20 |
| 3: More than 4/20 |
| Portal inflammation |
| 0: None |
| 1: Mild |
| 2: Moderate |
| 3: Severe |
| Fibrosis score: |
| Stage 0: No fibrosis |
| Stage 1: Zone 3 perisinusoidal/pericellular fibrosis; focally or extensively present |
| Stage 2: Zone 3 perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis |
| Stage 3: Zone 3 perisinusoidal/pericellular fibrosis and portal fibrosis with a focal or extensive bridging fibrosis |
| Stage 4: Cirrhosis |
Finally, all were graded and staged for NASH according to the system proposed at the American Association for the Study of Liver Diseases single topic conference in September 2002[23].
Also, for each patient, the following variables were assessed at baseline and at the moment of second liver biopsy: age, waist circumference, weight, percentage excess weight loss, body mass index (BMI), alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, gamma-glutamyl transferase level, bilirubin level, serum triglyceride level, cholesterolemia, serum high density lipoprotein cholesterol, serum low density lipoprotein cholesterol, blood glucose level, fasting insulin level, HOMA, steatosis amount, portal and lobular inflammation, fibrosis score and grade of NASH. The relationship between persistence of liver fibrosis after surgery (F > 1) and various risk factors was studied using a univariate analysis. In the univariate analysis, 2 groups were compared according to the presence or absence of significant fibrosis (F > 1) in the liver biopsy.
Statistical analysis of clinical and laboratory data was assessed using a paired samples t test. For histological comparisons pre-surgery and post-surgery, paired t tests were confirmed with Wilcoxon signed rank tests.
There were 26 patients (7 male and 19 female) with paired biopsies. Patient characteristics are shown in Table 2. There were no patients with cirrhosis and no complications from the gastric bypass or deaths during this study. The second biopsy was obtained 16.3 ± 3 mo (range: 12-22 mo) after bariatric surgery and no complications (bleeding, biloma, etc.) were observed during this postoperative percutaneous liver biopsy.
| Pre bariatric surgery | Post bariatric surgery | P value | |
| Weight (kg) | 130.8 ± 20.1 | 82.3 ± 13.7 | < 0.001 |
| BMI (kg/m2) | 49.3 ± 4.8 | 30.9 ± 4.3 | < 0.001 |
| % excess weight loss | 72.1 ± 6.6 | ||
| Waist (cm) | 137.1 ± 12.6 | 97.3 ± 11.0 | < 0.001 |
| Diabetic (%) | 12 (46.1) | 6 (23) | 0.14 |
| Hypertensive (%) | 17 (65.4) | 7 (26.9) | 0.012 |
| Metabolic syndrome | 15 (57.7) | 2 (7.7) | < 0.001 |
| Cholesterol (mmol/L) | 5.44 ± 1.00 | 4.29 ± 0.85 | < 0.001 |
| HDL-C (mmol/L) | 1.24 ± 0.21 | 1.40 ± 0.19 | 0.005 |
| LDL-C (mmol/L) | 3.47 ± 0.76 | 2.46 ± 0.75 | < 0.001 |
| Triglycerides (mmol/L) | 1.60 ± 0.63 | 0.93 ± 0.29 | < 0.001 |
| AST (μkat/L) | 0.35 ± 0.09 | 0.36 ± 0.17 | 0.862 |
| ALT (μkat/L) | 0.49 ± 0.20 | 0.37 ± 0.31 | 0.143 |
| GGT (U/L) | 40.2 ± 17.4 | 19.2 ± 12.8 | < 0.001 |
| Fasting glucose (mmol/L) | 6.46 ± 2.3 | 4.96 ± 0.55 | 0.001 |
| Insulin (pmol/L) | 235.2 ± 7.2 | 56.1 ± 7.2 | 0.006 |
| Insulin resistance (HOMA) | 9.99 ± 13.3 | 1.8 ± 1.4 | 0.006 |
The percentage of excess weight loss was 72.1% ± 6.6% and the average rate of weight loss was 0.69 ± 0.22 kg/wk. Other clinical demographic and weight loss data are shown in Table 1. Weight loss was accompanied by significant favorable changes in anthropometric measures, significant decreases in blood pressure and major improvements in biochemical markers of metabolic syndrome, plasma glucose, insulin levels, insulin sensitivity and cholesterol levels (Table 1). Fifteen of the 26 patients (57.7%) fulfilled criteria for metabolic syndrome and only 2 (7.7%) fulfilled these criteria at the follow-up. Preoperatively, 7 (27%) patients had abnormal alanine aminotransferase or aspartate aminotransferase (> 0.58 μkat/L); postoperatively only 3 had abnormal alanine aminotransferase or an aspartate aminotransferse levels (> 0.58 μkat/L). There were no significant differences in aminotransferase levels with weight loss.
Significant histopathological improvement was seen in steatosis (P < 0.001), ballooning degeneration (P < 0.001), Mallory bodies (P = 0.005), glycogen nuclei (P = 0.001), lobular inflammation (P < 0.001), portal inflammation (P = 0.005) and fibrosis (P < 0.001) (Figure 1 and Table 3).
| Scores | P value | |||||
| Feature | 0 | 1 | 2 | 3 | 4 | |
| Steatosis | < 0.001 | |||||
| Pre | 0 | 13 | 8 | 5 | - | |
| Post | 24 | 2 | 0 | 0 | - | |
| Ballooning degeneration | < 0.001 | |||||
| Pre | 10 | 8 | 7 | 1 | - | |
| Post | 25 | 1 | 0 | 0 | - | |
| Mallory bodies | 0.005 | |||||
| Pre | 18 | 8 | 0 | |||
| Post | 26 | 0 | 0 | |||
| Glycogen nuclei | 0.001 | |||||
| Pre | 8 | 7 | 11 | |||
| Post | 13 | 8 | 5 | |||
| Lobular inflammation | < 0.001 | |||||
| Pre | 1 | 23 | 2 | 0 | ||
| Post | 15 | 11 | 0 | 0 | ||
| Portal inflammation | 0.05 | |||||
| Pre | 1 | 23 | 2 | 0 | ||
| Post | 7 | 19 | 0 | 0 | ||
| Fibrosis | 0.001 | |||||
| Pre | 1 | 17 | 6 | 2 | 0 | |
| Post | 9 | 12 | 4 | 1 | 0 | |
We classified subjects as having NASH if their biopsy scored at least 1 for both grade and stage. There were 25 patients (96%) with NASH in their index biopsy. By contrast, only 4 (15.3%) of the follow up biopsies demonstrated NASH (P < 0.001). Table 4 shows scoring for the grade and stage of NASH in liver biopsies performed at surgery and during follow up. None of the second biopsies revealed progression of grade or stage of liver disease.
| Scores | |||||
| 0 | 1 | 2 | 3 | P value | |
| Grade | |||||
| At surgery | 1 (3.8) | 12 (46.1) | 11 (42.3) | 2 (7.7) | 0.001 |
| Follow up biopsy | 22 (84.6) | 4 (15.4) | 0 (0) | 0 (0) | |
| Stage | |||||
| At surgery | 1 (3.8) | 17 (65.4) | 6 (23.1) | 2 (7.7) | 0.001 |
| Follow up biopsy | 9 (34.6) | 12 (46.1) | 4 (15.4) | 1 (3.8) | |
The changes in steatosis are perhaps one of the more significant features in this analysis. Steatosis score improved overall by two or more grades in 12 patients and by one grade in 14 patients. Although portal inflammation improved significantly, it disappeared in only seven patients; after surgery, some degree of portal inflammation persisted in 19 patients.
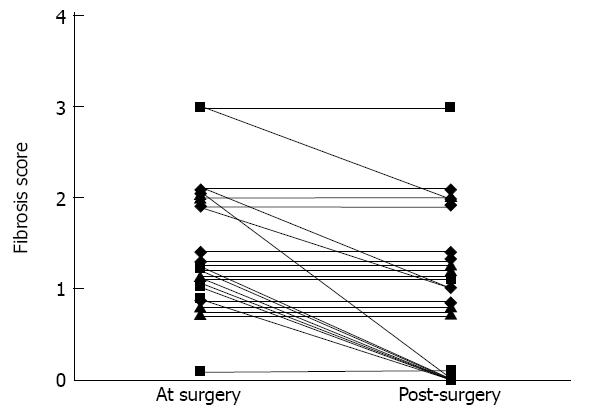
Although the change in fibrosis was significant, it was not constant. Fibrosis score improved overall by two stages in 1 patient and by one stage in 10 patients. In 15 patients, fibrosis remained stable and we did not observe any patient with worsening of liver fibrosis (Figure 2). Eight patients had significant fibrosis (F > 1) before surgery. At the second liver biopsy, five patients still had significant fibrosis (Figure 3). In univariate analysis, patients with significant liver fibrosis after surgery had a significantly higher steatosis score, higher fibrosis score and lower AST level at the time of surgery than patients without (Table 5). After bariatric surgery, one of two patients with metabolic syndrome and none of the 6 patients with diabetes had a fibrosis score greater than 1 in the liver biopsy. There was no difference in the interval between surgery and biopsy in patients with or without significant fibrosis [432 ± 133 d vs 530 ± 122 d respectively, P = not significant (NS)]. Equally, there was no difference in the average rate of weight loss in patients with or without significant fibrosis (0.81 ± 0.26 kg/wk vs 0.66 ± 0.21 kg/wk respectively, P = NS).
| Variable | F 0-1 post surgery (n = 21) | F > 1 post surgery (n = 5) | P value |
| Mean steatosis score at surgery | 1.5 ± 0.7 | 2.6 ± 0.5 | 0.002 |
| Mean fibrosis score at surgery | 1.1 ± 0.4 | 2.4 ± 0.5 | < 0.001 |
| Mean serum AST level at surgery (IU ± SD) | 22.8 ± 11.6 | 11.6 ± 4.0 | 0.034 |
In this study, we have demonstrated that, in patients with morbid obesity, weight loss induced by a mixed bariatric surgery (combination of malabsorptive and restrictive, Roux-en-Y gastric bypass with a modified Fobi-Capella technique) dramatically improved nonalcoholic fatty liver disease lesions observed in these patients.
There are various types of bariatric procedures. Initially, the most commonly used were primarily malabsorptive. Using these techniques, most of the obesity-related liver abnormalities did not improve, or even worsened. Jejunoileal bypass performed in the 1950-1970s was abandoned due to various complications, including significant hepatic lesions and even cirrhosis[24-26]. Numerous mechanisms, such as malabsorption of essential micronutrients, bacterial overgrowth and rapid weight loss with resulting massive influx of free fatty acids, have been implicated in the pathogenesis of NAFLD associated with jejunoileal bypass[27]. For example, Requarth et al[28] reported the long term morbidity after jejunoileal bypass in 453 patients and 24 of these developed acute liver failure (7%) and the 15 years probability of established cirrhosis was 8.1%.
More recently, restrictive or mixed techniques of bariatric surgery have been used. Lately, most of the published studies have focused on the effect of restrictive techniques on NAFLD. Thus, Dixon et al[10] used a laparoscopic adjustable banding technique that achieved a weight loss of around 50% and observed important improvements in steatosis, necroinflammatory changes and fibrosis.
The bariatric surgery technique used by us is mixed, restrictive and mildly malabsorptive. The techniques which, like ours, use a gastric bypass, produce a greater weight loss than gastric banding and also are longer lasting[29,30]. It would be interesting to know if these different types of surgical approaches are accompanied by similar effects on the treatment of liver lesions seen in obesity.
Using a bariatric surgery technique that combines restriction with mild malabsorption, we obtained a significant weight loss in patients. In addition, we have not only achieved a drastic remission of steatosis, but also improvements in lobular inflammatory activity and fibrosis. In our study, the percentage of excess weight loss (72%) was clearly superior to that observed in the Dixon study (52%), previously cited[10]. Although other studies have been done with techniques other than merely restrictive, good results have also been found in improvement of NAFLD[12,31].
It has been suggested that weight loss induced by malabsorptive procedures could disguise NASH improvements after bariatric surgery because, in some patients, impairment in liver fibrosis could be observed. This effect was observed in the study performed by Kral et al[32] that used biliopancreatic diversion. They found a constant improvement in metabolic syndrome but the effect on liver fibrosis varied. In their study, there was a frank improvement of fibrosis in patients with pre surgery advanced fibrosis (grades 1-2) and, in contrast, “de novo” fibrosis appeared in patients that did not have presurgery fibrosis. We have not observed this effect and the small number of samples in which we observed persistence of fibrosis was in those patients with a presurgery fibrosis greater than stage 1. The difference in the results can be explained because Kral et al[32] found alcohol ingestion as a predictive factor of increasing fibrosis. Our series did not include alcoholic patients and this argues in favor of the fact that the increasing fibrosis seen in the Kral study was not related to the type of intervention, but to alcoholic ingestion post surgery.
In our study we saw a global improvement in portal fibrosis. Nevertheless, there were patients whose fibrosis did not improve; in 15 patients it remained stable. Barker et al[14] performed liver biopsy at surgery and postoperatively in 19 patients, mostly women without alcohol ingestion with a bariatric surgery technique similar to that used in our study, and found a frank improvement in fibrosis lesions, except in 4 patients whose lesions remained stable. Probably this slightly greater improvement in fibrosis can be explained by a longer interval between surgery and performance of the second biopsy. It was 21 mo in Barker’s study and 16 mo in our work.
Similarly to Barker et al[14] and other studies about liver improvement of NASH with treatment[10,33], we also evidenced a persistence of portal inflammation. In most of our patients it was mild at surgery and significantly improved when it was analyzed globally; but in 19 patients some degree of portal inflammation persisted. This phenomenon is frequent and it has been suggested that these portal changes do not have a direct relationship with metabolic syndrome or insulin resistance[10,33].
When the factors that could predict persistence of significant liver fibrosis were analyzed, biochemical and clinical factors at the time of biopsies had low statistical power. The most important predictive factors were histological. Liver fibrosis and steatosis score at surgery were statistically associated with the persistence of significant fibrosis in liver biopsy post surgery. It has been demonstrated that steatosis at surgery and insulin resistance influence persistence of steatosis after bariatric surgery[34], but the influence of steatosis on the persistence of fibrosis has not been clearly demonstrated. In our work, we found that, not only liver fibrosis at surgery, but also steatosis score has an influence on the persistence of fibrosis. It is known that a fatty liver is more vulnerable to factors that lead to fibrosis[35] and in the presence of chronic liver diseases, steatosis may exacerbate liver injury[36]. The persistence of liver fibrosis in our patients mediated by various factors may be helped by the previous existence of a severe degree of steatosis.
Finally, patients with a persistence of significant fibrosis had a lower level of aminotransferases than those patients whose fibrosis was maintained or improved. This finding probably has low relevance, because in both groups aminotransferase levels were within normal range and it is also known that ALT values progressively decrease after BMI > 30 kg/m2; therefore, it frequently happens that patients with morbid obesity have normal values of aminotransferase[27].
In conclusion, we have demonstrated that bariatric surgery, using a restrictive and mildly malabsorptive procedure, has a strong effect on improvement of liver abnormalities associated with non alcoholic fatty liver disease in the morbidly obese, although any significant changes were observed in aminotransferase enzymes.
Non alcoholic steatohepatitis (NASH) is one of the most common liver disease in patients with morbid obesity, and is associated with metabolic syndrome. The effects of the most current treatments for NASH (diet induced weight loss, bariatric surgery, etc.) are confounding. Therefore, it will be of interest to know the effects of Roux-en-Y gastric bypass on NASH.
NASH is a very frequent disease affecting morbidly obese but it does not have an specific treatment to improve it, so the hotspot for NASH is to find a treatment for it.
The finding provides further evidence on the conclusions that Roux-en-Y gastric bypass associated weight loss enhances the resolution of metabolic syndrome and improves liver histological features in morbidly obese patients.
This paper, together with other related publications, can be collectively instructive to bariatric surgeons and nutritionists in their practice in treat morbidly obese patients.
This paper shows interesting results even though the sample size is small. Perhaps the more interesting finding to highlight is the improvement in the fibrosis score after surgery. And it is a very interested study that discusses the benefits of surgically induced effects on obese patients with NAFLD.
Peer reviewers: Papandreou Dimitrios, PhD, MD, RD, Assistant Professor of Nutrition, Department of Health Science, University of Nicosia, Cyprus, Head of Pediatric Obesity Unit, Aristotle University of Thessaloniki, School of Medicine, Ahepa General Hospital, P. Mela 22 GR 54622, Greece; Stacee Marie Lerret, PhD, RN, CPNP, Liver Transplant Coordinator, Division of Gastroenterology, Hepatology and Nutrition Children’s Hospital of Wisconsin, Medical College of Wisconsin, 8701 West Watertown Plank Road, Milwaukee, WI 53226, United States; Dong-Yan Jin, Associate Professor, Department of Biochemistry, The University of Hong Kong, 3/F Laboratory Block, Faculty of Medicine Building, 21 Sassoon Road, Pokfulam, Hong Kong, China
S- Editor Zhang DN L- Editor Roemmele A E- Editor Zhang DN
| 1. | Choudhury J, Sanyal AJ. Clinical aspects of fatty liver disease. Semin Liver Dis. 2004;24:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Andersen T, Gluud C, Franzmann MB, Christoffersen P. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991;12:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 255] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 404] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408-1413. [PubMed] |
| 7. | Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD. Fatty liver--an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Luyckx FH, Lefebvre PJ, Scheen AJ. Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab. 2000;26:98-106. [PubMed] |
| 10. | Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 526] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Jaskiewicz K, Raczynska S, Rzepko R, Sledziński Z. Nonalcoholic fatty liver disease treated by gastroplasty. Dig Dis Sci. 2006;51:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Stratopoulos C, Papakonstantinou A, Terzis I, Spiliadi C, Dimitriades G, Komesidou V, Kitsanta P, Argyrakos T, Hadjiyannakis E. Changes in liver histology accompanying massive weight loss after gastroplasty for morbid obesity. Obes Surg. 2005;15:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Luyckx FH, Desaive C, Thiry A, Dewé W, Scheen AJ, Gielen JE, Lefèbvre PJ. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 317] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Blackburn GL, Mun EC. Effects of weight loss surgeries on liver disease. Semin Liver Dis. 2004;24:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Fobi MA. Vertical Banded Gastroplasty vs Gastric Bypass: 10 years follow-up. Obes Surg. 1993;3:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass? Am J Surg. 1996;171:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 156] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30 Suppl 1:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 19. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24514] [Article Influence: 612.9] [Reference Citation Analysis (1)] |
| 20. | Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4680] [Cited by in RCA: 4491] [Article Influence: 195.3] [Reference Citation Analysis (0)] |
| 21. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2887] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 22. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8239] [Article Influence: 412.0] [Reference Citation Analysis (5)] |
| 23. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 24. | Hocking MP, Duerson MC, O'Leary JP, Woodward ER. Jejunoileal bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 124] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Jewell WR, Hermreck AS, Hardin CA. Complications of jejunoileal bypass for morbid obesity. Arch Surg. 1975;110:1039-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kirkpatrick JR. Jejunoileal bypass. A legacy of late complications. Arch Surg. 1987;122:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Haynes P, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease in individuals with severe obesity. Clin Liver Dis. 2004;8:535-547, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Requarth JA, Burchard KW, Colacchio TA, Stukel TA, Mott LA, Greenberg ER, Weismann RE. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995;130:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Howard L, Malone M, Michalek A, Carter J, Alger S. Gastric Bypass and Vertical Banded Gastroplasty- a Prospective Randomized Comparison and 5-Year Follow-up. Obes Surg. 1995;5:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Capella JF, Capella RF. An assessment of vertical banded gastroplasty-Roux-en-Y gastric bypass for the treatment of morbid obesity. Am J Surg. 2002;183:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, Eid GM, Ramanathan R, Taylor DS, Schauer PR. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610-617; discussion 618-620. [PubMed] |
| 32. | Kral JG, Thung SN, Biron S, Hould FS, Lebel S, Marceau S, Simard S, Marceau P. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008-1017. [PubMed] |
| 34. | Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, Louvet A, Dharancy S, Cocq P, Jany T. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 283] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Clouston AD, Jonsson JR, Powell EE. Steatosis as a cofactor in other liver diseases: hepatitis C virus, alcohol, hemochromatosis, and others. Clin Liver Dis. 2007;11:173-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |