Published online Jun 27, 2010. doi: 10.4254/wjh.v2.i6.221
Revised: May 14, 2010
Accepted: May 21, 2010
Published online: June 27, 2010
AIM: To study complement activation in 46 patients with alcoholic cirrhosis and ascites but no spontaneous bacterial peritonitis (SBP) and 10 healthy controls.
METHODS: Complement activation was determined by the measurement of soluble membrane attack complex (sMAC) concentrations in ascites and plasma. In patients, metabolic liver function was determined by the galactose elimination capacity and the clinical status assessed by the Model of End-Stage Liver Disease and Child-Pugh scores.
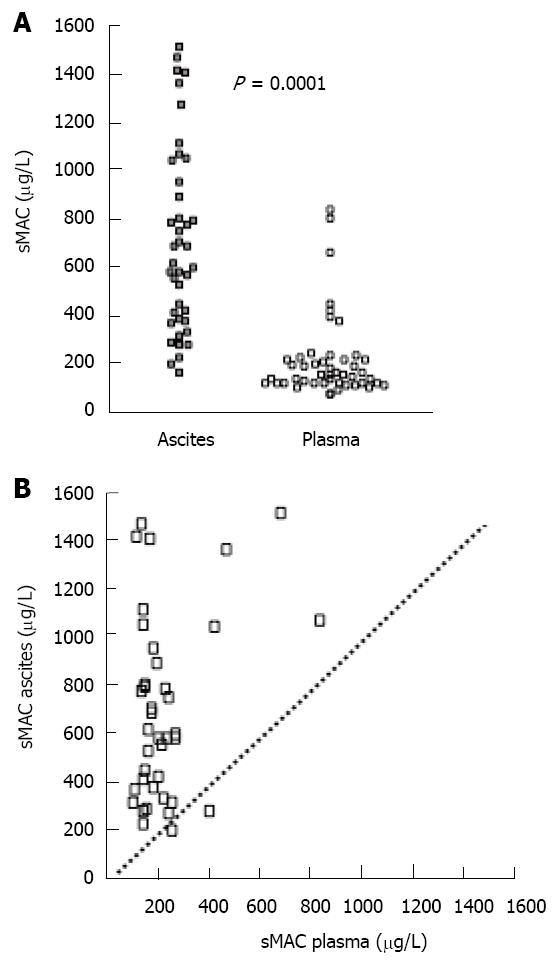
RESULTS: Ascites sMAC levels were markedly higher than in the corresponding plasma sample (median (range): 596 (170 - 1519) vs 160 (77 - 848) μg/L; P < 0.01). Ascites sMAC levels correlated positively with liver status. There was no relationship between ascites sMAC and leukocyte count. No relationship between ascites sMAC and blood C-reactive protein, albumin or neutrophile count was found. Plasma sMAC concentrations were slightly higher in patients than in controls [130 μg/L (70 - 204); P = 0.04]. Neither sMAC in ascites nor plasma was related to mortality.
CONCLUSION: The increased sMAC concentration in ascites and plasma indicate an activation of the complement system in cirrhosis even in the absence of SBP. This was particularly evident in the peritoneal fluid and most marked in patients with preserved liver status. The high ascites sMAC levels may reflect transudation of membrane attack complexes from the liver. Whether this complement activation has any clinical implications remains to be clarified.
- Citation: Bjerre M, Holland-Fischer P, Grønbæk H, Frystyk J, Hansen TK, Vilstrup H, Flyvbjerg A. Soluble membrane attack complex in ascites in patients with liver cirrhosis without infections. World J Hepatol 2010; 2(6): 221-225
- URL: https://www.wjgnet.com/1948-5182/full/v2/i6/221.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i6.221
Bacterial infections are frequent in patients with liver cirrhosis and have major impact on their short and long term morbidity and mortality[1]. Translocation of intestinal bacteria to ascites is among the primary events in the development of spontaneous bacterial peritonitis (SBP), one of the most important infectious complications in patients with advanced cirrhosis and ascites[2,3].
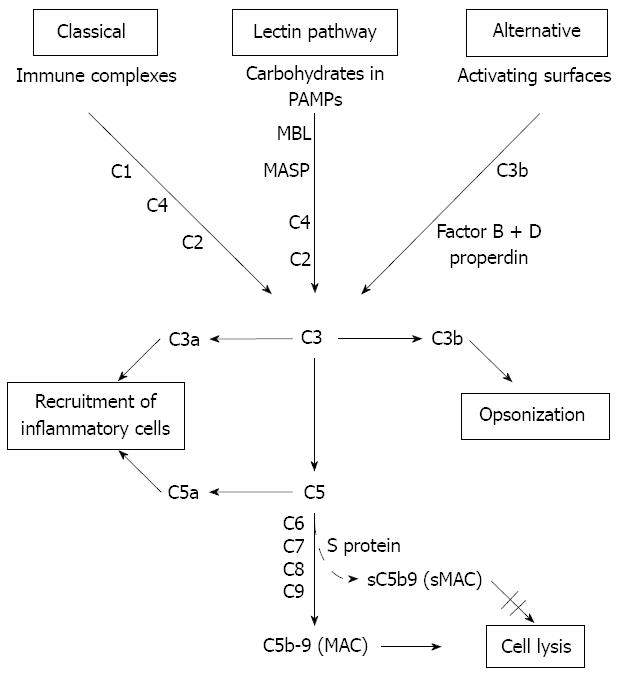
Patients with cirrhosis have immunological dysfunctions which increase their risk of bacterial infections. The complement system is an important mediator of innate and acquired immunity and is activated almost immediately on the appearance of pathogenic microorganisms. Complement proteins, mainly synthesized by the hepatocytes, circulate as inactive forms and are activated by three pathways: the classical, the lectin and the alternative pathway[4-6] (Figure 1). The common final product is the membrane attack complex (MAC), generated by combination of the complement components (C)5b, C6, C7, C8 and C9. MAC forms channels on the surface of cellular membranes leading to cell lysis[7,8]. MAC formed in the absence of target membranes binds to S-protein which inhibits the membrane-damaging effect and creates a stable non-lytic soluble MAC form (sMAC)[9].
Plasma sMAC concentrations may be reduced in patients with cirrhosis as part of their immune incompetence and because of decreased liver function for synthesis of complement proteins. In the light of the frequent occurrence of peritonitis, ascites sMAC might be even lower. Accordingly, low concentrations of complement components in plasma and ascites have been reported to increase the patients’ susceptibility to infection[10].
To further study this hypothesis, we measured corresponding concentrations of sMAC in plasma and ascites in a group of patients with decompensated alcoholic cirrhosis with ascites but without SBP.
We consecutively included non-infected patients (n = 46) with liver cirrhosis and ascites admitted for a diagnostic or therapeutic paracentesis between August 2002 and April 2008. The diagnosis of cirrhosis was established by a combination of biochemical, clinical and ultrasonographic findings and none of the patients had a liver biopsy. We only included patients with no signs of SBP as all patients had ascites neutrophil counts < 250 × 106/L. Etiologies of cirrhosis were alcoholic cirrhosis (n = 39) and autoimmune or cryptogenic cirrhosis (n = 7). None of the patients were diagnosed with chronic viral hepatitis. Fasting EDTA-plasma (all) and ascites (n = 44) were collected concomitantly and stored at -80°C. Evaluation of the severity of hepatic encephalopathy (HE-grade)[11] was Grade 0 (n = 38) and Grade 1 (n = 8). Five patients had previous episodes of SBP. During the period of follow-up (median follow-up 53 mo), 61% (n = 28) of the patients died. Blood samples from ten age and sex-matched healthy subjects served as controls. Informed consent was obtained according to the declaration of Helsinki and the local authorities of ethics approved the study.
Bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, C-reactive protein (CRP) concentrations, coagulation time and total protein concentration in ascites were determined by standard laboratory methods at the Hospital Department of Clinical Biochemistry. Leukocyte counts in blood and ascites were measured by an automated technique followed by manual counting and separation into mono- and polymorph-nuclear leucocytes by a total count > 200 cells/mL.
sMAC concentration was measured by a sensitive time-resolved immunofluorometric assay (TRIFMA) described in details elsewhere[12]. The limit of detection for the assay was 1 μg/L. The intra- and inter-assay coefficients of variance were below 5% and 12% respectively.
Clinical status was assessed according to the Model for End-stage Liver Disease (MELD) Score[13] and the Child-Pugh Score[14]. The Galactose Elimination Capacity (GEC) was used to quantitatively measure metabolic liver function. The GEC was determined from blood galactose concentration-decay curves after iv administration of galactose corrected for urinary excretion as described by Tygstrup[15] .
Comparisons between groups were performed by one-way ANOVA or by Mann-Whitney tests according to the data distribution. sMAC concentrations showed a skewed distribution and values are given as median (range). Spearman correlation with two-tailed probability values was used to estimate the strength of association between variables. Mortality and sMAC was evaluated by ROC analysis and the connection between time to death during the follow-up period was estimated by the Kaplan-Meier method, grouped by sMAC concentrations above or below the median in either plasma or ascites. A value of P < 0.05 was regarded as statistically significant.
There was no difference in gender or age between the patient group and the control group (Table 1). All patients had increased CRP and decreased albumin (P < 0.0001). The patients had slightly increased ALT, ALP (compared with normal range) and bilirubin (P < 0.0001). No patient had an ascites neutrophil count above 250 × 106/L (i.e. none had SBP). However, five patients had a history of SBP.
| Patients (n = 46) | Controls (n = 10) | P value | |
| Sex (% men) | 65 | 63 | NS |
| Age (years) | 54 ± 9 | 55 ± 7 | NS |
| Plasma analysis | |||
| Leukocyte counts (109 cells/L) | 9.9 ± 6.3 | ND | |
| CRP (mg/L) | 233 ± 183 | NR < 10 | |
| Albumin (μmol/L) | 359 ± 78 | 641 ± 36 | < 0.0001 |
| Creatinine (μmol/L) | 81.2 ± 44.6 | 74.7 ± 11 | NS |
| sMAC (μg/L) | 160 (77 - 848) | 130 (70 - 204) | 0.04 |
| Ascitic fluid analysis | |||
| Leukocyte counts (106 cells/L) | 130 (0 - 213) | - | |
| Total protein (g/L) | 11.9 ± 6.6 | - | |
| sMAC (μg/L) | 596 (170 - 1519) | - | |
| Liver function | |||
| ALT (U/L) | 51 ± 75 | NR 7 - 56 | |
| Bilirubin (µmol/L) | 99.4 ± 89.8 | 11.5 ± 4.9 | < 0.0001 |
| ALP (U/L) | 312 ± 230 | NR 38 - 126 | |
| Coagulation time (ratio of expected) | 0.44 ± 0.18 | 1.11 ± 0.15 | < 0.0001 |
| INR | 1.56 ± 0.35 | 0.86 ± 0.05 | < 0.0001 |
| MELD score | 14.1 ± 7.1 | 0.9 ± 2.3 | < 0.0001 |
| Child Pugh score | 10.5 ± 1.8 | ND | |
| GEC (mmol/min) | 1.54 ± 0.4 | ND | |
| GEC (ratio of expected) | 0.56 ± 0.1 | ND |
The patients had markedly increased sMAC concentrations in ascites compared with plasma (P < 0.001) (Figure 2). The average ascites/plasma ratio was 3.5. There was only a weak and borderline significant correlation between sMAC in plasma and ascites (R2 = 0.04, P = 0.05). Furthermore, plasma sMAC was slightly higher in the patients than in controls (P = 0.04) (Table 1).
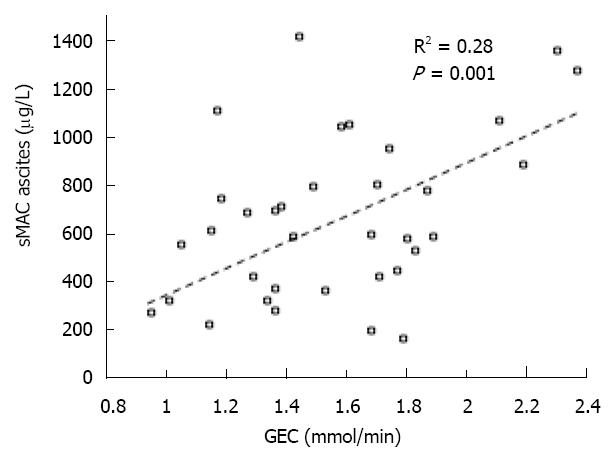
There was a positive correlation between ascites sMAC and the GEC (R2 = 0.28, P < 0.01) (Figure 3). Furthermore, we observed a negative correlation to MELD scores (R2 = -0.13, P = 0.02) and Child-Pugh scores (R2 = -0.07, P = 0.09), i.e. an increase in sMAC levels equals a better liver status. There was a positive correlation between ascites total protein concentration and ascites sMAC (R2 = 0.18, P = 0.007) and between ascites sMAC and P-CRP (R2 = 0.16, P = 0.03). Ascites leukocyte count did not correlate with sMAC concentrations either in ascites or plasma. A negative correlation was found between ascites sMAC and ALT (R2 = -0.07, P = 0.03). None of the correlations were found for plasma sMAC concentration. The mortality rate of patients was not associated with either plasma or ascites sMAC. ROC curves showed no association between sMAC concentration and death; area under the ROC curve for sMAC levels in ascites and in plasma was 0.55 and 0.56 respectively.
The main findings of this study were that ascites sMAC concentrations were markedly higher than the corresponding plasma values in non-infected patients with decompensated alcoholic cirrhosis. Furthermore, sMAC levels in ascites but not in plasma paralleled measures of liver status. Finally, in patients the plasma values were slightly higher than in controls. These findings are new and contrasted our stated a priori assumptions.
Increased sMAC reflects increased complement activation. Therefore, the increased plasma values in the patients is likely to represent a discreet complement activation as part of such patients’ frequent systemic low-grade inflammation[16], confirmed by elevated CRP levels in all patients.
The increased ascites sMAC correlated with better liver status assessed by the Child-Pugh and MELD scores as well as by GEC. In a study of patients, newly- diagnosed cirrhosis GEC was found to be a strong predictor of short and long term all-cause and cirrhosis-related mortality[17]. These observations support the positive relationship of ascites sMAC to each of them and the possibility of preserved liver status being causally related to increased levels of ascites sMAC. None of the patients had SBP (or other infections) at inclusion (ascites leukocyte count, < 250 × 106/L), signifying that the increased ascites complement activation was not due to local bacterial invasion. However, the presence of increased sMAC showed that the complement system in ascites had become activated in vivo. Without leukocyte infiltration a possible explanation for this observation may be complement activation caused by an increased cell-turnover or cell death due to chronic low-grade inflammation in the cirrhotic liver. This notion may be supported by the correlation between ascites sMAC and ALT.
In cirrhosis, there is sinusoidal portal hypertension and protein rich ‘ultrafiltrate’ from Disse’s space passes between hepatocytes and is transsudated across the peritoneal endothelium and into the abdominal cavity[18]. The positive correlation found between ascites total protein and sMAC supports that both were due to that mechanism. The mechanistic link between high ascites sMAC in relationship to good liver status is not clear. The most likely explanation is that a well-preserved liver function is more able to respond to infections with a sufficient activation of the complement components. This may protect against bacterial growth in the opsonin-poor ascites fluid and thus explain why patients with good liver function are less prone to peritonitis. In support, the only patient in our cirrhosis group with an ascites/plasma ratio of sMAC below 1 and a very poor liver status was readmitted with SBP 3 wk after discharge.
Considering this, it may be surprising that ascites sMAC was not related to long-term mortality with the emergence of SBP being associated with markedly increased mortality. Duration of cirrhosis in this prospective study may have an influence. In biopsies from cirrhotic livers, Polihronis et al[19] found variable amounts of MAC and sMAC deposited in the cirrhotic connective tissue. The amount of deposited sMAC did not correlate with the cause of cirrhosis and they found no indication that sMAC was involved in the pathogenesis of hepatic injury.
A study of bacterial translocation in patients with liver cirrhosis without SBP showed that ascites sMAC was increased but only in those ascites which contained an increased bacterial DNA content[20]. Since the presence of bacDNA is not available in our samples a direct comparison is not possible. Five patients were previously diagnosed with SBP but no association to sMAC levels were found and thus other factors are likely to be involved. This is supported by elevated sMAC in ascites reported in other patient groups characterised by cell changes or degeneration (ovarian cancer and patients with non-malignant endometriosis)[21,22]. The high ascites sMAC levels may reflect transudation of membrane attack complexes from the liver. Thus it may be of interest in order to analyse patients with other causes of transudative ascitis.
In conclusion, the high sMAC concentrations in ascites indicate an increased activation of the complement system in cirrhotic patients caused by systemic low-grade inflammation and hepatic activation. The increased sMAC levels correlated with liver function but had no association with survival. The role of sMAC in liver cirrhosis is still unclear and studies in cirrhotic patients with SBP and other infections may help to elucidate this.
Patients with cirrhosis have immunological dysfunctions which increase their risk of bacterial infections. The complement system is an important mediator of innate and acquired immunity and is activated almost immediately on the appearance of pathogenic microorganisms. The activation generates the membrane attack complex (MAC) and in the absence of target membranes a stable non-lytic soluble MAC (sMAC) form. Low concentrations of complement components in plasma and ascites have been reported to increase patients’ susceptibility to infection.
Low concentrations of complement components in plasma and ascites have been reported to increase patients’ susceptibility to infection. However, it is unknown whether liver cirrhosis per se is able to initiate complement activation and, if so, how this affects the clinical outcome of patients.
The main findings of this study were that ascites sMAC concentrations were markedly higher than the corresponding plasma values in non-infected patients with decompensated alcoholic cirrhosis. Furthermore, sMAC levels in ascites but not in plasma paralleled measures of liver status.
Increased sMAC may protect against bacterial growth in the opsonine-poor ascites fluid and thus explain why patients with good liver function are less prone to peritonitis
This manuscript is interesting, original and well designed. The presentation is good and readable, and the statistical methods used are appropriate. It may be accepted for publication with modification.
Peer reviewers: Krishnan Rajeshwari, Professor, Department of Pediatrics, Maulana Azad Medical College, Bahadur Shah Zafar Marg, New Delhi 110002, India; Mauricio Silva, MD, PhD, Post-graduate Course of Hepatology, Universidade Federal de Ciências da Saúde de Porto Alegre, Rua Sarmento Leite 245, Porto Alegre, Rio Grande do Sul, Brazil; Neil Louis Julie, MD, Gastroenterology and Hepatology, 7609 Exeter Rd, Bethesda, MD 20814, United States
| 2. | Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669-674; quiz 675-676. |
| 3. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. |
| 4. | Cooper NR. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151-216. |
| 5. | Müller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321-347. |
| 6. | Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987;262:7451-7454. |
| 7. | Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058-1066. |
| 8. | Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140-1144. |
| 9. | Fosbrink M, Niculescu F, Rus H. The role of c5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol Res. 2005;31:37-46. |
| 10. | Homann C, Varming K, Høgåsen K, Mollnes TE, Graudal N, Thomsen AC, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544-549. |
| 11. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. |
| 12. | Haahr-Pedersen S, Bjerre M, Flyvbjerg A, Mogelvang R, Dominquez H, Hansen TK, Galatius S, Bech J, Madsen JK, Søgaard P. Level of complement activity predicts cardiac dysfunction after acute myocardial infarction treated with primary percutaneous coronary intervention. J Invasive Cardiol. 2009;21:13-19. |
| 13. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. |
| 14. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 15. | Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl. 1966;18:118-125. |
| 16. | Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182-189. |
| 17. | Jepsen P, Vilstrup H, Ott P, Keiding S, Andersen PK, Tygstrup N. The galactose elimination capacity and mortality in 781 Danish patients with newly-diagnosed liver cirrhosis: a cohort study. BMC Gastroenterol. 2009;9:50. |
| 18. | McKEE FW, WILT WG Jr. The circulation of ascitic fluid; interchange of plasma and ascitic fluid protein as studied by means of C14-labeled lysine in dogs with constriction of the vena cava. J Exp Med. 1950;91:115-122. |
| 19. | Polihronis M, Machet D, Saunders J, O’Bryan M, McRae J, Murphy B. Immunohistological detection of C5b-9 complement complexes in normal and pathological human livers. Pathology. 1993;25:20-23. |
| 20. | Francés R, González-Navajas JM, Zapater P, Muñoz C, Caño R, Pascual S, Márquez D, Santana F, Pérez-Mateo M, Such J. Bacterial DNA induces the complement system activation in serum and ascitic fluid from patients with advanced cirrhosis. J Clin Immunol. 2007;27:438-444. |
| 21. | Bjørge L, Hakulinen J, Vintermyr OK, Jarva H, Jensen TS, Iversen OE, Meri S. Ascitic complement system in ovarian cancer. Br J Cancer. 2005;92:895-905. |
| 22. | Kabut J, Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A. Levels of complement components iC3b, C3c, C4, and SC5b-9 in peritoneal fluid and serum of infertile women with endometriosis. Fertil Steril. 2007;88:1298-1303. |