Published online Dec 27, 2010. doi: 10.4254/wjh.v2.i12.434
Revised: November 4, 2010
Accepted: November 11, 2010
Published online: December 27, 2010
AIM: To introduce Granulocyte-colony stimulating factor (G-CSF) as a new therapeutic modality for schistosomiasis through stem cell mobilization, immunomodulation or fibrosis remodeling.
METHODS: In this study, a 5 d course of human recombinant G-CSF (100 μg/kg sc) was applied to Schistosoma mansoni-infected mice at different stages of disease (5 d before infection as well as 3, 5 and 7 wk post-infection). The animals were sacrificed at 10 d as well as 4, 6 and 8 wk post infection. Mice were examined for: (1) Total leukocyte count which is an accepted surrogate marker for the stem cell mobilization into the circulation; (2) Egg count in intestine and liver tissue to assess the parasitic load; and (3) Histopathological changes in Hx/E and Masson trichrome stained sections as well as collagen content in Sirius red-stained liver sections to determine the severity of liver fibrosis.
RESULTS: Mice developed leukocytosis. The egg load and the number of granulomas were not affected by the G-CSF treatment but there was an obvious change in the composition of granulomas towards an increased cellularity. Moreover, fibrosis was significantly decreased in treated groups compared to untreated animals (collagen content either preinfection or at 3 and 5 wk post infection: 5.8 ± 0.5, 4.7 ± 0.5, 4.0 ± 0.7 vs 8.2 ± 0.9; P≤ 0.01).
CONCLUSION: Although G-CSF did not cause direct elimination of the parasite, it enhanced granulomatous reaction and reduced the fibrosis. Further investigation of the underlying mechanisms of these two actions is warranted.
- Citation: Ghanem LY, Dahmen U, Dirsch O, Nosseir MM, Mahmoud SS, Mansour WA. Does granulocyte-colony stimulating factor administration induce damage or repair response in schistosomiasis? World J Hepatol 2010; 2(12): 434-441
- URL: https://www.wjgnet.com/1948-5182/full/v2/i12/434.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i12.434
Schistosomiasis is a parasitic disease that mainly affects the rural population in developing countries. An estimated 200 million people suffer from this disease, 85% of whom live in sub-Saharan Africa, and 650 million people are at risk of infection[1].
Schistosoma mansoni (S. mansoni) is a leading cause of chronic liver disease. Morbidity from this infection is primarily the result of a granulomatous reaction to eggs deposited in the liver leading to the induction of fibrosis and portal hypertension[2]. The immuno-pathology of the granulomatous response has been studied extensively in murine models as there is a close similarity to that in human schistosomiasis[3]. Granulocyte-colony stimulating factor (G-CSF) is a cytokine that shows a variety of biological functions. Its action ranges from mobilization of bone marrow-derived stem cells to immuno-modulation of a variety of host responses and even anti-infectious properties. It is in clinical use for the mobilization of bone marrow-derived stem cells before peripheral stem cells are harvested and stem cell transplantation is performed[4]. A new strategy employing bone marrow-derived stem cells has recently been attempted for organ repair. Stem cell mobilization with G-CSF had a dramatic effect on the enhancement of stem cell "transdifferentiation" into cardiomyocytes in a rat model of myocardial infarction[5]. Petersen et al[6] first reported "transdifferentiation" of hematopoietic stem cells into liver cells. The frequency of transdifferentiation and thereby its biological relevance has been a controversial discussion[7]. Granulocyte-colony stimulating factor can also modulate the immune response by affecting T cells and their cytokine secretion. Some authors[8-10] have described polarization of T cell response towards T helper 2 cell type by G-CSF. Another study[11] documented inhibition of both Th1 and Th2 with reduced INF-γ and IL-4 production by G-CSF. Moreover, it was found that G-CSF is in clinical use to battle cytomegalovirus (CMV) infection in immuno-suppressed patients. Noursadeghi et al[12] reported the effect of G-CSF on the non-specific acute phase response associated with increased resistance to bacterial infection. Schneider et al[13] evaluated the effect of G-CSF in 60 patients undergoing major surgery and observed an increase in anti-infectious mediators, reduction in the incidence and severity of postoperative infections plus attenuation of the postoperative acute phase response. It was demonstrated that G-CSF upregulates lipopolysaccharide binding protein (LBP) in the liver, thus potentially contributing to increased resistance against lipopolysaccharide (LPS)[14]. This result hints at the potential influence of G-CSF on the innate immune system.
The aim of this study is to employ an interesting drug candidate, G-CSF, as a new therapeutic approach that may potentially influence the damage response and consequently the course of liver disease caused by S. mansoni infestation.
Experimental animals and experimental design: CD1 mice, aged 6-7 wk and weighing 15-18 g, were used in this study. Mice were bred at the Schistosome Biological Supply Program (SBSP) (Theodor Bilharz Research Institute, Giza, Egypt). They were fed on a standard pelleted diet (containing 24% protein, 4% fat and about 4%-5% fiber and water ad libitum) according to a recipe prepared by Lowell University, USA. The study met the national guidelines of experimental animal research.
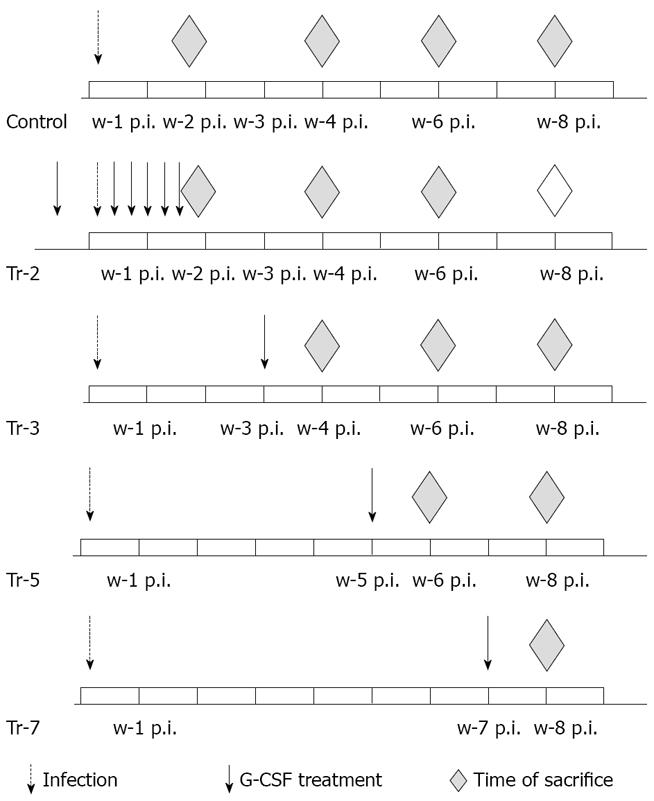
CD1 mice (6-8/subgroup) were infected with cercariae of S. mansoni and treated with G-CSF. In the first group, G-CSF treatment (100 μg/kg) started 5 d prior to infection and continued every other day for 10 d post infection (PI). In other groups, G-CSF treatment started 3, 5, and 7 wk post infection (wpi). Animals were sacrificed on day 10 and week 4, 6 and 8 post infections (Figure 1).
Parasites:S. mansoni cercariae were obtained from infected Biomphalaria alexandrina snails which were reared and maintained at the SBSP. The original strain of S. mansoni was obtained from Lowell University, Lowell, Massachusetts, USA. by passage throughout bred mice and B. alexandrina snails.
Estimating cercarial densities: The cercarial suspension was mixed with a magnetic stirring bar and ten times of 0.1 mL aliquots were removed from the center of the suspension by a syringe and placed into a petri dish. Before counting under a dissecting microscope, one drop of Lugol’s iodine solution was added. The counts were averaged and the cercarial density was determined[15].
Infection: About 70-80 S. mansoni cercariae suspended in 0.2 mL solution was injected subcutaneously into the back of each mouse using a 22 gauge needle syringe[16].
Mice were examined for general condition, body weight, liver weight at time of sacrifice and liver/body weight ratio.
Confirmation of G-CSF effect was based on: (1) Relative amount of mobilized leukocytes. Total leukocytic count was performed using an automated cell analyzer (Sysmex Corporation, KX_21, Japan) and differential counts were made from the slide-mounted blood films stained with Leishman stain; (2) Egg count in intestine and liver[17]. The number of ova/gm intestinal or hepatic tissue was counted after digestion overnight in 5% KOH; and (3) Assessment of disease severity by detailed liver histology including assessment of liver fibrosis.
Histopathology: Following sacrifice of animals by cervical dislocation, the liver was removed, rinsed with phosphate buffered saline and weighed. Liver specimens from the right lobe were processed and stained with hematoxylin and eosin and Masson trichrome stains. Sections were examined for hepatic parenchyma, periovular granulomas as well as inflammatory cell exudation and fibrous tissue deposition in portal tracts together with bile ductular and blood vascular changes. The diameter of granulomas was measured (5 granulomas/section) using the ocular micrometer. This was done only for granulomas containing ova in their centers and not confluent ones. The mean diameter for each group was calculated. Evaluation of liver fibrosis was done by morphometry using 10 μm thick, Sirius Red-stained sections. This is a strong anionic dye that stains collagen by reacting, via its sulphonic acid groups, with basic groups present in the collagen molecule. The elongated dye molecules are attached to the collagen fiber in such a way that their long axes are parallel[18]. Morphometric assessment of the collagen content in portal tracts and around granulomas was performed using a Zeiss microscope connected to a Kontron Image Analysis System. Quantification of hepatic collagen deposition was done under x50 magnification employing the CIRES software program and represented as mean percentage of fibrotic area ± SD in 3 sections/animal.
Statistical analysis was performed using the analysis of variance method (ANOVA)[19]. Significant level was reached at P≤ 0.05.
All groups (treated and untreated) had a good health status throughout the experimental period and apparent manifestations of schistosomiasis did not develop.
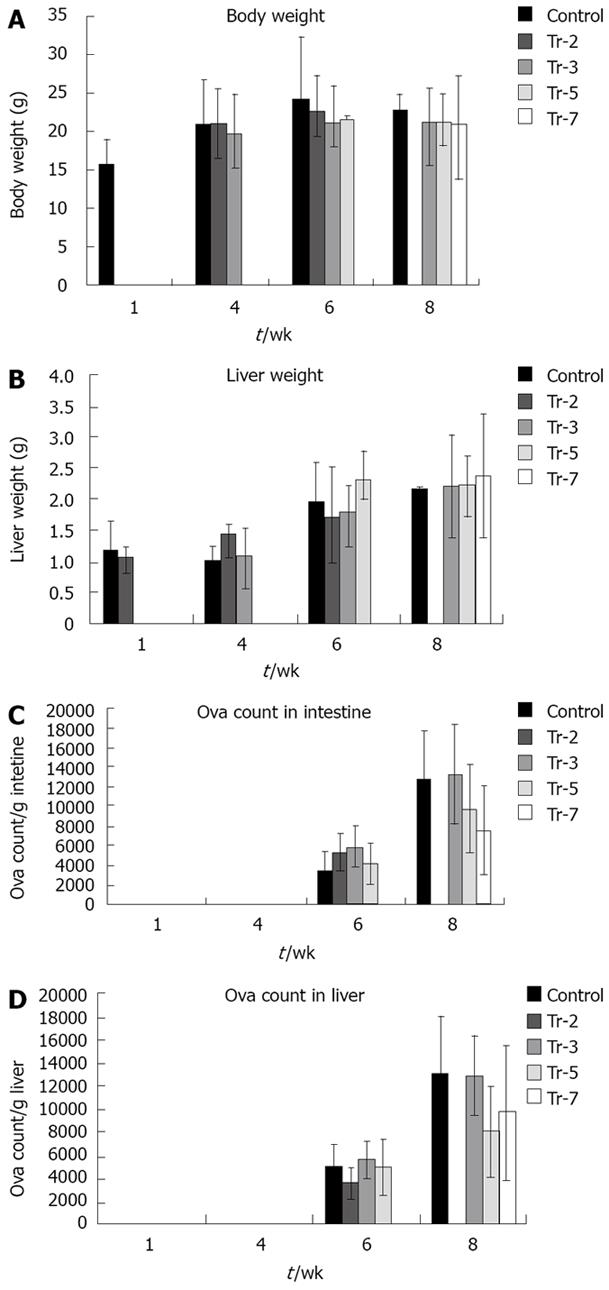
Body weight (Figure 2A) was not affected significantly by G-CSF treatment before infection and 3 wpi in animals sacrificed 4 wpi with an average body weight of 20.95 g, 21.10 g and 19.89 g in untreated, preinfection treated and 3 wpi treated animals respectively. Body weight in mice sacrificed 6 wpi was not affected significantly by treatment, being 24.2 vs 22.75, 21.2, 21.75 g in controls untreated, preinfection treated and treated 3 and 5 wpi respectively. Also, at 8 wpi, the body weight of controls and those receiving treatments did not differ significantly (22.9, 20.9, 21.4 and 21.08 g in controls, treated 3, 5 and 7 wpi respectively).
The effect of treatment on liver weight by treatment was dependent on the stage of infection (Figure 2B). Liver weight was not significantly affected in mice sacrificed 10 d PI to be 1.06 g in the group treated preinfection compared to 1.17 g in untreated controls. A highly significant increase occurred at 4 wpi in groups treated before infection and 3 wpi relative to controls (1.44 and 1.1 g vs 1.01 respectively) (P≤ 0.01). Liver weight was significantly affected at 6 wpi by G-CSF treatment 5 wpi (2.32 g vs 1.97 g of controls) (P≤ 0.01). However, it was not significantly affected 8 wpi to be 2.23, 2.23, 2.37 g in those treated 3, 5 and 7 wpi respectively compared to 2.17 g in control.
All treated groups had significantly higher liver/body weight ratio at 4 wpi (4.85% in untreated controls vs 6.98% in preinfection and 5.47% in 3 wpi treated groups). However, treatment did not affect this parameter in any of the groups sacrificed at either 6 or 8 wpi.
The average total leukocytic count (TLC) in animals receiving G-CSF treatment was significantly higher compared to the untreated group. In mice sacrificed at 6 wpi, the TLC in the untreated group was 9.04 × 103/μL. However, it was 8.4, 14.87 and 10 × 103/μL in animals receiving treatment before infection or treated 3 and 5 wpi respectively. Also, animals sacrificed at 8 wpi showed significantly higher levels (P≤ 0.01) in mice treated 3, 5 and 7 wpi (13.3, 12.06 and 18.36 × 103/μL respectively) compared to the untreated group (9.07 × 103/µL).
The number of eggs retained in the small bowel and the liver of treated and untreated animals (Figure 2C, D) were not affected significantly in mice sacrificed at 6 and 8 wpi. A gradual non significant reduction of liver egg burden was observed at 8 wpi when comparing the untreated and treated groups 3, 5 and 7 wpi (13.2 × 103 eggs/g liver vs 13.0, 8.1 and 9.7 × 103 eggs/g liver weights respectively).
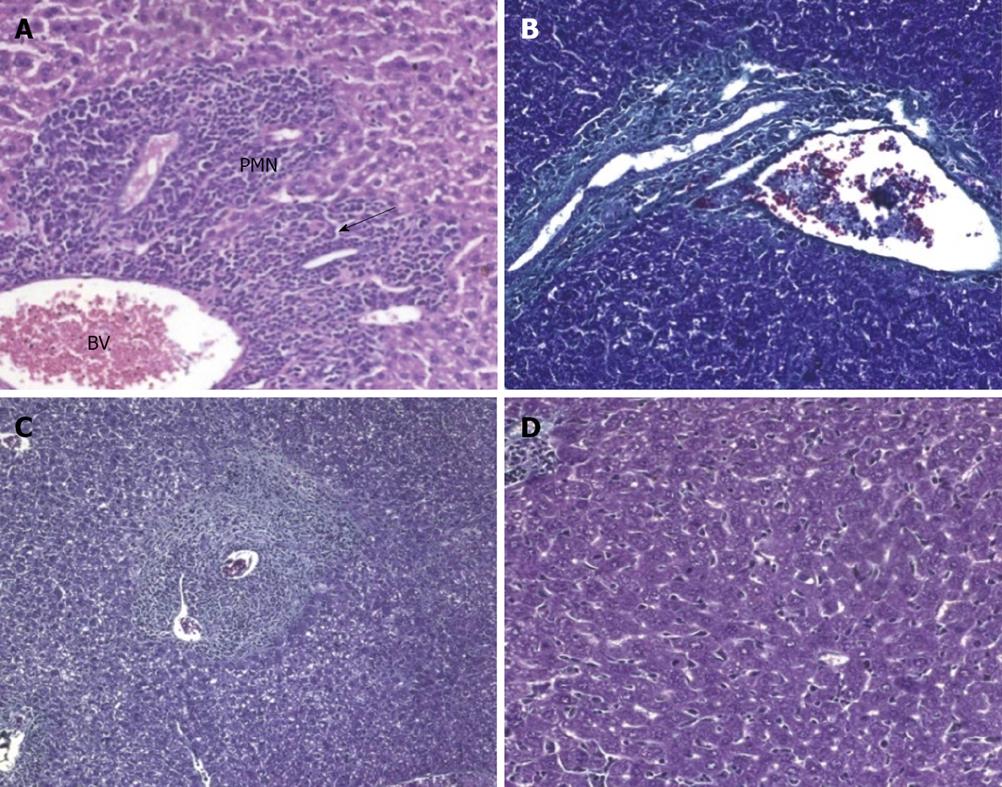
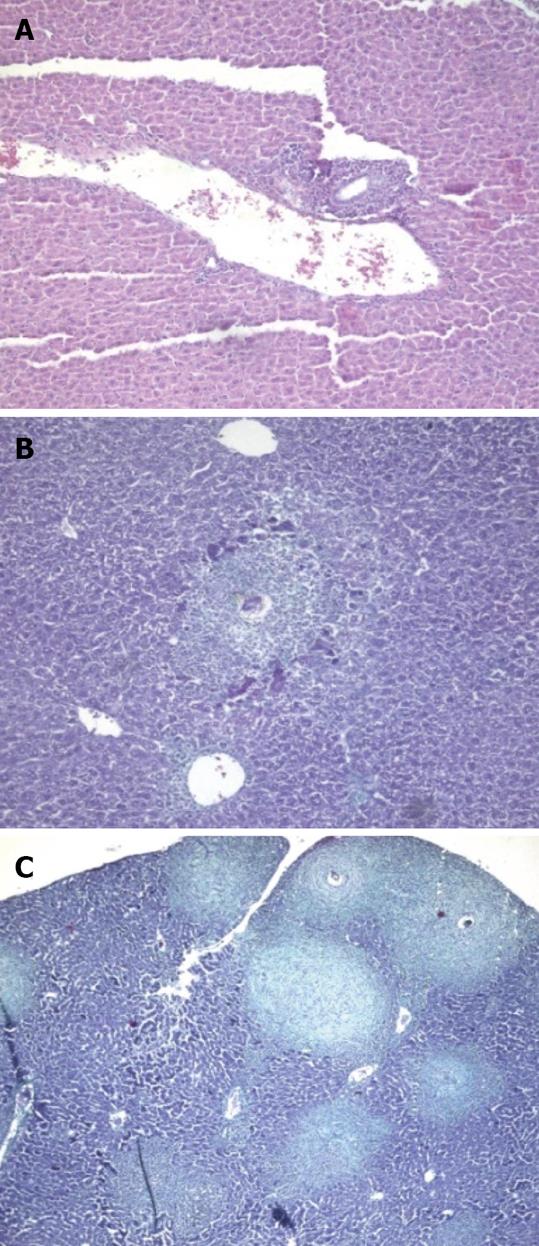
At the acute stage of infection (10 d PI), animals treated before and for a few days after infection showed an increase of infiltrating polymorphonuclear cells (PMNCs) in the portal tract (Figure 3A) in comparison to the untreated group (Figure 4A). Both groups showed dilated portal blood vessels.
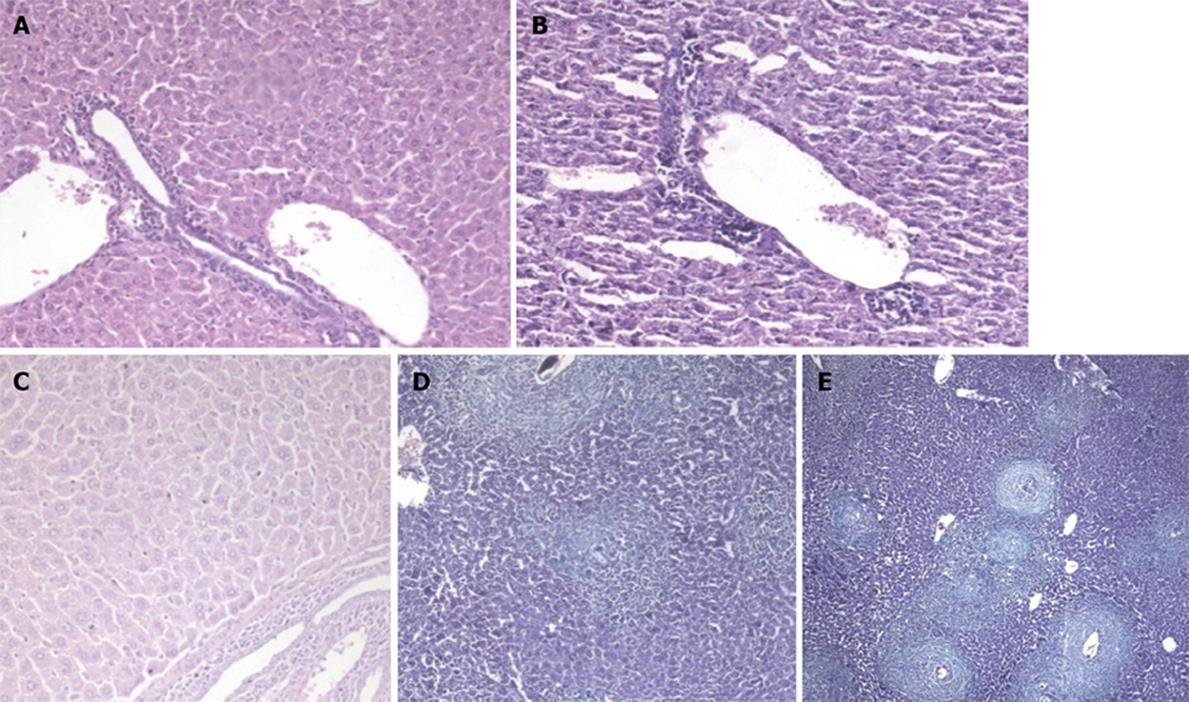
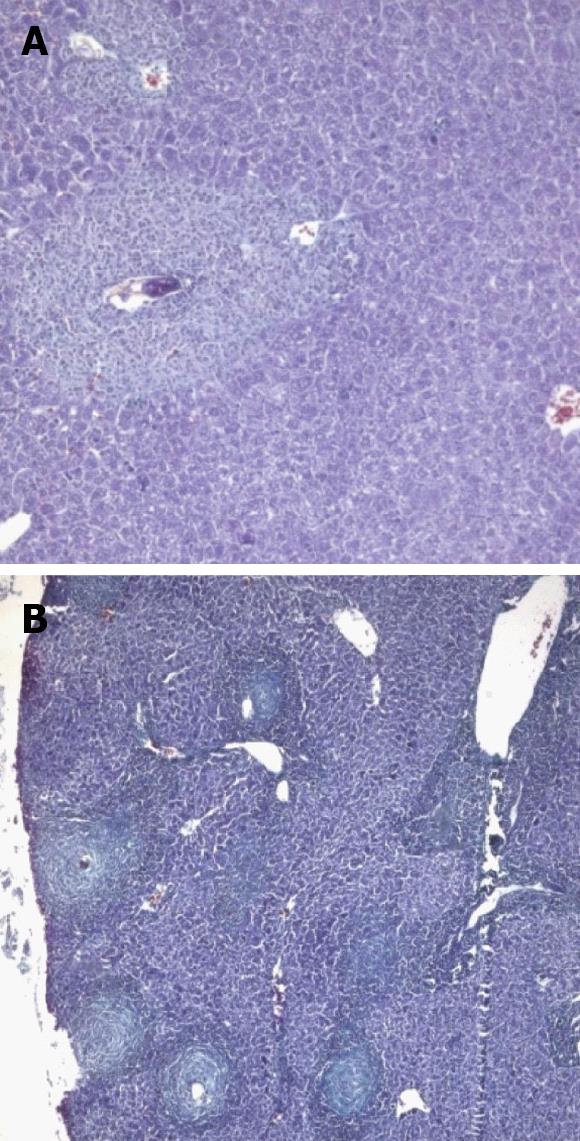
At 4 wpi, animals treated preinfection showed milder inflammation in their portal tracts compared to untreated mice (Figure 4B). A comparable degree of inflammation was presented in animals treated at 3 wpi (Figures 3B and 5A). Kupffer cell hyperplasia and intralobular inflammatory cell infiltration seen in the untreated group (Figure 4C) were not found in treated animals at 3 wpi.
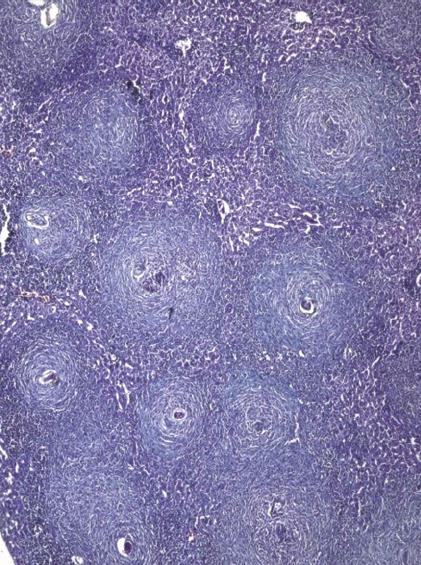
At 6 wpi, a considerable decrease in portal tract inflammatory infiltrate was seen in G-CSF-treated animals compared to untreated ones regardless of the time of treatment. Although the effect of treatment on granuloma diameter was not significant at this stage compared to untreated animals (Figure 4D), cellular granulomas became more predominant (Figures 3C, 5B, 6A) but their number did not change. Hyperplastic Kupffer cells and lobular inflammatory infiltrates continued to be present in animals treated with G-CSF before the infection (Figure 3D) but were absent in those treated at 3 and 5 wpi. Fibrous granulomas were encountered in animals treated at 3, 5 and 7 wpi (Figures 5C, 6B, 7) compared to the untreated group.
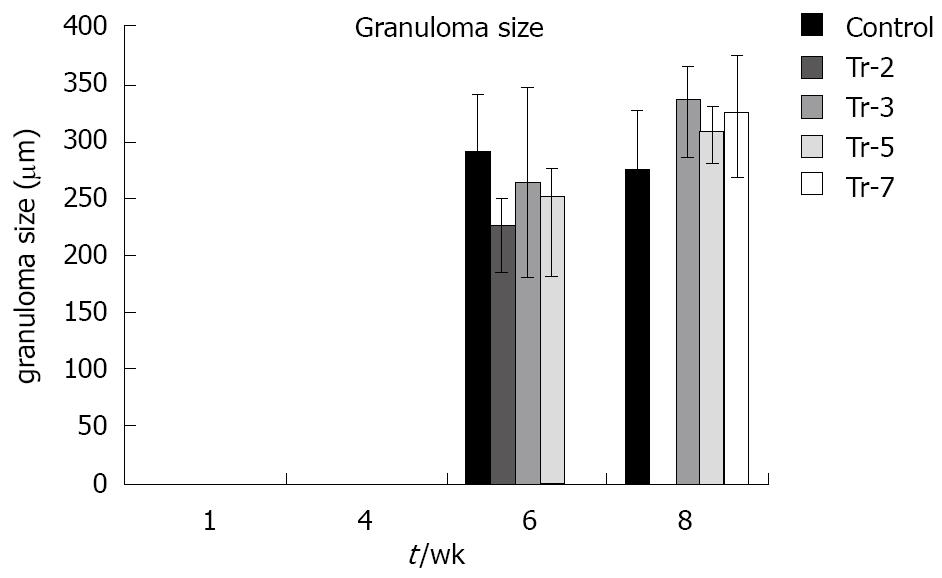
At 8 wpi, an intense portal tract inflammation was observed in G-CSF treated groups similar to untreated animals (Figures 6B, 4E respectively). However, animals treated at 3 wpi showed a decreased inflammatory reaction with a significant increase in granuloma diameter (Figure 5C). It was 275.8 μm in untreated animals versus 336.12, 308.3 and 325 μm in 3, 5 and 7 wpi treated mice respectively (Figure 8). Granulomas showed lobular and portal distribution.
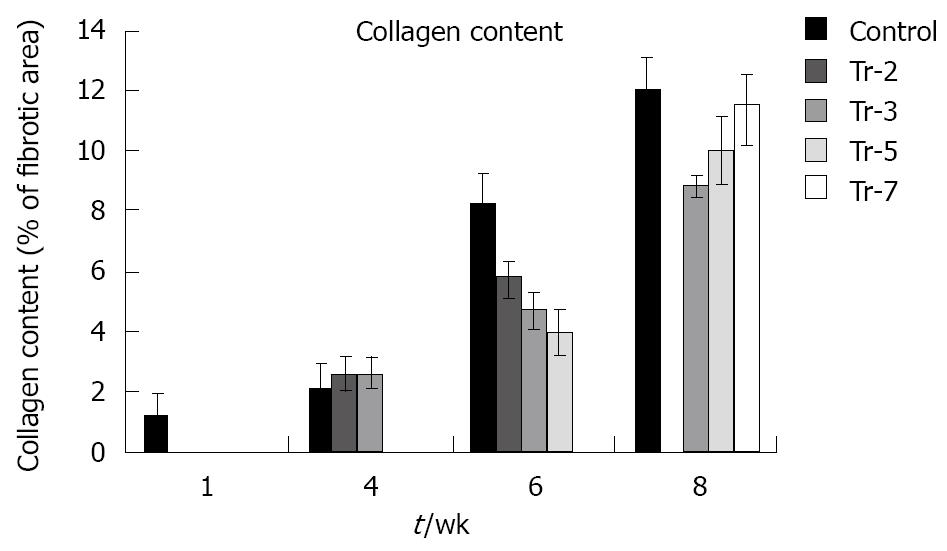
Treatment did not affect collagen deposition in untreated animals at 4 wpi (2.1 ± 0) compared to animals treated preinfection 2.6 ± 0.5 and animals treated at 3 wpi 2.6 ± 0.47. On the other hand, a highly significant decrease in collagen content (P≤ 0.01) was found at 6 wpi in treated animals either preinfection or at 3 and 5 wpi (5.8 ± 0.5, 4.7 ± 0.5, 4.0 ± 0.7 vs untreated group 8.2 ± 0.9). Although treatment did not fully inhibit the progressing collagen deposition, the effect of treatment was also visible in animals at 8 wpi in animals treated at 3, 5 and 7 wpi respectively (8.9 ± 0.4, 10.0 ± 0.8 and 11.6 ± 0.9) compared to untreated ones (12.0 ± 1.0) (Figure 9).
S. mansoni infection is detected by the first line of defense - the innate immune system which does not detect the whole parasite but reacts to molecules released by S. mansoni or molecular changes induced by the parasite. Accordingly, the innate immunity is able to detect the pathogen associated molecular pattern (PAMP) and a primary inflammatory response is induced, eventually resulting in an immune response.
Administration of G-CSF has been shown to protect rodents against LPS toxicity[20] and to induce an upregulation of LBP synthesis in the liver[14] that decrease LPS toxicity through TLR4[21]. Since G-CSF treatment influences the “damage detection” in this model, we evaluated its effect on S. mansoni infection especially since it was reported that parasites directly affect TLR3 and TLR4[22]. In the course of disease, periovular granuloma and the induction of fibrosis cause most of the clinical symptoms. The number of granulomas correlates with the severity of disease but is not affected by G-CSF. As granulomas develop in response to egg deposition in the tissue, we assessed the amounts of eggs retained in liver and intestine. As expected, we found increased egg load during the course of the disease with no significant reduction in treated mice. The egg load is generally employed as an important parameter to assess the efficiency of S. mansoni treatment[23] and consequently a lack of a significant effect of G-CSF treatment on the egg load indicates that G-CSF does not enhance the primary damage response.
The number of granulomas did not change in response to treatment; however, the composition of granuloma was different in G-CSF treated animals with a predominance of cellular ones. This could be caused by GCSF-mediated recruitment of inflammatory cells from extrahepatic sources or due to endogenous hematopoiesis. Lenzi et al[24] presented evidence of internal cellular production by granulomas and expression of G-CSF receptors. Exploration of the respective cytokine profile might help to elucidate the mechanisms of these flared up reactions and whether this reaction was biased to either the Th1 or Th2 type.
Lack of full recovery by elimination of the parasites leads to the secondary damage response associated with the onset of a marked immune reaction, progressive tissue damage and repair with the development of a marked fibrosis. Portal fibrosis eventually results in clinical symptoms due to marked portal hypertension[2].
In this study, the extent of fibrosis, as estimated by the quantitative morphometric analysis of collagen content in Sirius red-stained liver sections, was significantly decreased in animals sacrificed at 6 and 8 wpi in all G-CSF treated groups. Fibrosis develops during the chronic phase of granulomatous inflammation in murine schistosomiasis. Fibrosis seems to represent a protective function during infection by neutralizing and sequestering egg antigens that can potentially damage host tissues[25]. An abrogation of Th2 response is then required to prevent excessive fibrosis. However this mechanism cannot explain the antifibrotic action of G-CSF administered in this experiment as the latter produces Th2 polarization rather than abrogation. It could be speculated that the inhibitory effect of G-CSF on collagen deposition may be related to enhanced fibrotic degradation rather than decreased fibrous synthesis. G-CSF was found in previous studies[26] to enhance the matrix metalloproteinase family (MMP 1 and 9). Whether this is also the mechanism involved in the current experiment is the topic of ongoing experiments.
In conclusion, the most prominent effect of treatment with G-CSF consisted of a slight effect on the inflammatory reaction but a marked reduction of collagen deposition irrespective of the time of starting treatment was initiated. Further work is needed to dissect the molecular events underlying immunomodulation and fibrous remodeling actions of G-CSF covering later stages of the disease for better evaluation of its therapeutic effect in schistosomiasis.
Granulocyte-colony stimulating factor (G-CSF) has a variety of biological functions including mobilization of bone marrow-derived stem cells, immuno-modulation of a variety of host responses and anti-infectious properties.
This article introduces G-CSF as a new therapeutic modality in Schistosoma mansoni (S. mansoni).
Treatment with G-CSF revealed slight affection of the inflammatory reaction and marked reduction of collagen deposition irrespective of the time of starting treatment.
Further work is needed to dissect the molecular events underlying immunomodulation and fibrous remodeling actions of G-CSF covering later stages of the disease for better evaluation of its therapeutic effect in schistosomiasis.
The manuscript describes the effects of human recombinant G-CSF on liver histopathology of mice infected with S. mansoni and prevents progression of granuloma formation. The findings are of interest, but observational.
Peer reviewers: Takuji Tanaka, MD, PhD, The Tohkai Cytopathology Institute, Cancer Research and Prevention (TCI-CaRP), 4-33 Minami-Uzura, Gifu 500-8285, Japan; Helieh Saatara Oz, DVM, MS, PhD, HSRB 414 Center for Oral Health Research, College of Dentistry, University of Kentucky Medical Center, 800 Rose St, Lexington, KY 40536, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
| 1. | Taylor M. Global trends in schistosomiasis control. Bull World Health Organ. 2008;86:738. |
| 2. | Yamashita T, Boros DL. IL-4 influences IL-2 production and granulomatous inflammation in murine schistosomiasis mansoni. J Immunol. 1992;149:3659-3664. |
| 3. | Cheever AW. Quantitative comparison of the intensity of Schistosoma mansoni infections in man and experimental animals. Trans R Soc Trop Med Hyg. 1969;63:781-795. |
| 4. | Dührsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074-2081. |
| 5. | Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:103. |
| 6. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 7. | Dirsch O, Chi H, Gu YL, Ji Y, Broelsch C, Dahmen U. Influence of stem cell mobilization and liver regeneration on hepatic parenchymal chimerism in the rat. Transplantation. 2006;81:1695-1699. |
| 8. | Pan L, Delmonte J Jr, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422-4429. |
| 9. | Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484-2490. |
| 10. | Franzke A, Piao W, Lauber J, Gatzlaff P, Könecke C, Hansen W, Schmitt-Thomsen A, Hertenstein B, Buer J, Ganser A. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102:734-739. |
| 11. | Rutella S, Pierelli L, Bonanno G, Sica S, Ameglio F, Capoluongo E, Mariotti A, Scambia G, d'Onofrio G, Leone G. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood. 2002;100:2562-2571. |
| 12. | Noursadeghi M, Pepys MB, Gallimore R, Cohen J. Relationship of granulocyte colony stimulating factor with other acute phase reactants in man. Clin Exp Immunol. 2005;140:97-100. |
| 13. | Schneider C, von Aulock S, Zedler S, Schinkel C, Hartung T, Faist E. Perioperative recombinant human granulocyte colony-stimulating factor (Filgrastim) treatment prevents immunoinflammatory dysfunction associated with major surgery. Ann Surg. 2004;239:75-81. |
| 14. | Ji Y, Dahmen U, Madrahimov N, Madrahimova F, Xing W, Dirsch O. G-CSF administration in a small-for-size liver model. J Invest Surg. 2009;22:167-177. |
| 15. | Mathieson W, Wilson RA. A comparative proteomic study of the undeveloped and developed Schistosoma mansoni egg and its contents: The miracidium, hatch fluid and secretions. Inter J Parasitol. 2010;40:617-628. |
| 16. | Botros SS, Salama SH, Estafan MY, Gomaa NE, Metwally AA, Ebeid FA Relationship between schistosomicidal drug efficacy, gonadal steroid hormonal changes and state of disease immunopathology in murine Schistosomiasis mansoni. Pharmacological Research. 1990;22:359-370. |
| 17. | Cheever AW, Lenzi JA, Lenzi HL, Andrade ZA. Experimental models of Schistosoma mansoni infection. Mem Inst Oswaldo Cruz. 2002;97:917-940. |
| 18. | Janqueira LCU, Bignolas G, Bretani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447-455. |
| 19. | Snedicor GW, Couchran WG. “Statistical methods”. Seventh Edition. The Iowa State University Press , Ames, Iowa. 1980;. |
| 20. | Görgen I, Hartung T, Leist M, Niehörster M, Tiegs G, Uhlig S, Weitzel F, Wendel A. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992;149:918-924. |
| 21. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. |
| 22. | Semnani RT, Venugopal PG, Leifer CA, Mostböck S, Sabzevari H, Nutman TB. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood. 2008;112:1290-1298. |
| 23. | Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659-667. |
| 24. | Lenzi HL, Romanha W de S, Zorzenon dos Santos RM, Rosas A, Mota EM, Manso PPA, Caputo LFG, Pelajo-Machado M. Four whole-istic aspects of schistosome granuloma biology: fractal arrangement, internal regulation, autopoietic component and closure. Mem Inst Oswaldo Cruz. 2006;101:219-231. |
| 25. | Warren KS. The immunopathogenesis of schistosomiasis: a multidisciplinary approach. Trans R Soc Trop Med Hyg. 1972;66:417-434. |
| 26. | Minatoguchi S, Takemura G, Chen XH, Wang N, Uno Y, Koda M, Arai M, Misao Y, Lu C, Suzuki K. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572-2580. |