Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.108100
Revised: April 24, 2025
Accepted: May 26, 2025
Published online: June 27, 2025
Processing time: 82 Days and 18.2 Hours
The optimal duration of antimicrobial treatment for acute cholangitis complicated by gram-positive coccus (GPC) bacteremia remains unclear. The Tokyo Guide
To evaluate the efficacy and safety of shorter antimicrobial treatments for acute cholangitis complicated by GPC bacteremia.
Adult patients with acute cholangitis complicated by GPC bacteremia who underwent endoscopic retrograde cholangiopancreatography between July 2003 and December 2023 were included. Patients were categorized into two groups based on the duration of effective antimicrobial treatment: (1) Short-course treatment (SCT) (< 14 days); and (2) Long-course treatment (LCT) (≥ 14 days). The outcomes assessed included mortality, recurrence, reinfection with the same organism related to the cholangitis, and length of hospital stay.
A total of 44 patients were included in the study: (1) 19 patients in the SCT group; and (2) 25 patients in the LCT group. The median duration of antimicrobial treatment was 9 days [interquartile range (IQR): 2.5-11.0 days] and 16 days (IQR: 15.0-19.0 days) in the SCT and LCT groups, respectively, with a statistically significant difference (P < 0.05). No significant differences were observed in 30-day mortality, cholangitis recurrence, or reinfection with the same organisms within 3 months. However, the length of hospital stay was shorter in the SCT group (median: 12.0 days vs 14.0 days, P = 0.092).
For acute cholangitis complicated by GPC bacteremia, shorter antimicrobial treatment may be a viable option following appropriate biliary drainage. Further studies with larger sample sizes are warranted.
Core Tip: The optimal duration of antimicrobial treatment for acute cholangitis with gram-positive coccus bacteremia has not been well investigated. The Tokyo Guidelines 2018 recommended at least 14 days of treatment, citing the potential risk of infective endocarditis. However, the actual risk appears to be low, and a discrepancy with real-world practice has therefore been proposed. This study evaluated mortality, relapse, reinfection with the same organism, and length of hospital stay between the short-course treatment (SCT) and long-course treatment groups. No significant differences were observed in the treatment outcomes; however, the length of hospital stay tended to be shorter in the SCT group.
- Citation: Kim Y, Ishikawa K, Nakamura K, Ikusaka H, Yokosuka R, Yamazaki T, Suzuki Y, Okuyama S, Takagi K, Fukuda K. Comparison between short-course and long-course antimicrobial treatments for acute cholangitis with gram-positive coccus bacteremia after endoscopic retrograde cholangiopancreatography. World J Hepatol 2025; 17(6): 108100
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/108100.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.108100
Acute cholangitis is a common, life-threatening infection that places a significant burden on patients and the acute medical care system[1-4]. Antibiotic therapy combined with appropriate biliary drainage is the standard treatment for acute cholangitis. Guidelines from Japan and Western countries generally recommend a 4-7 day course of antibiotics[5,6]. For patients with Gram-negative bacteremia, the guidelines do not provide specific recommendations, and a 4-7 days treatment duration, similar to the guidelines for patients without bacteremia, is considered. Some studies have suggested that short-term treatment (within one week) may be safe in such instances[7].
In contrast, for patients with gram-positive bacteremia, the Tokyo Guidelines 2018 (TG18) recommend a longer course of antibiotic therapy that extends beyond 14 days, owing to concerns about complications such as infective endocarditis (IE)[5]. However, there is limited information regarding the bacterial strains or treatment duration in the reported cases of IE following acute cholangitis[8]. In clinical practice, the duration of antibiotic therapy varies widely, often based on individual preferences rather than evidence, suggesting a discrepancy between the guideline-recommended duration and the actual treatment duration[5]. Prolonged treatment duration increases the risk of side effects, mortality, and antibiotic resistance, thereby imposing additional economic burden on the healthcare system[9-12].
Therefore, further research on the optimal treatment duration is required. However, few studies have investigated the optimal duration of antibiotic therapy for cases complicated by gram-positive coccus (GPC) bacteremia. To address this gap, we conducted a retrospective comparative study to evaluate the efficacy of shorter treatment durations.
This single-center, retrospective study was conducted between July 22, 2003, and August 31, 2023, at St. Luke’s International Hospital in Tokyo, Japan, an acute tertiary-care hospital with 520 beds. The study protocol was approved by the hospital’s Ethics Committee (Institutional Review Board No. 24-R014).
This retrospective cohort study evaluated the outcomes of adult patients with acute cholangitis complicated by GPC bacteremia who underwent endoscopic retrograde cholangiopancreatography (ERCP). The inclusion criteria were as follows: (1) GPC detected in blood cultures; (2) Diagnosis of acute cholangitis, either definite or suspected, based on TG18; (3) ERCP performed within four days of GPC detection in blood cultures; and (4) Successful drainage achieved through ERCP. The exclusion criteria were cholangitis caused by a malignancy or other conditions that precluded successful drainage after ERCP. Patients lost to follow-up were excluded from the study.
The variables recorded in this study included age, sex, comorbidities, intravascular devices used, and the site of acquisition. Vital signs, laboratory data, imaging results, echocardiographic findings during treatment, culture results, susceptibility results, specific antimicrobials used for treatment, etiology of cholangitis, and time to biliary drainage from diagnosis were recorded. The appropriate antimicrobial treatments were assessed using antibiotic susceptibility tests. TG18 Grade Scores and Quick Sepsis-Related Organ Failure Assessments (qSOFA) were also derived from the medical charts to assess the severity of cholangitis. The qSOFA score was calculated using the Japan Coma Scale (JCS) when the Glasgow Coma Scale score was not available. A JCS score higher than 0 was defined as altered mental status.
Short-course treatment (SCT) was defined as the total duration of susceptible antimicrobial treatment for the causative organisms of < 14 days, whereas long-course treatment (LCT) was defined as ≥ 14 days. When antibiotics were transitioned from intravenous to oral administration, ‘the duration’ included the days of both oral and intravenous antibiotic use until they were completely discontinued.
The primary outcome was the 30-day mortality. Secondary outcomes included cholangitis recurrence, reinfection with the same organism (including IE) related to cholangitis within 3 months, and length of hospital stay.
Categorical variables were analyzed using the χ2 test or Fisher's exact test. Continuous variables were analyzed using the Student's t-test, Wilcoxon rank-sum test, or Welch’s two-sample t-test. Statistical significance was set at P < 0.05. All statistical analyses were performed using the R 4.4.2 GUI 1.80 Big Sur ARM build (8462) (R Foundation for Statistical Computing, Vienna, Austria).
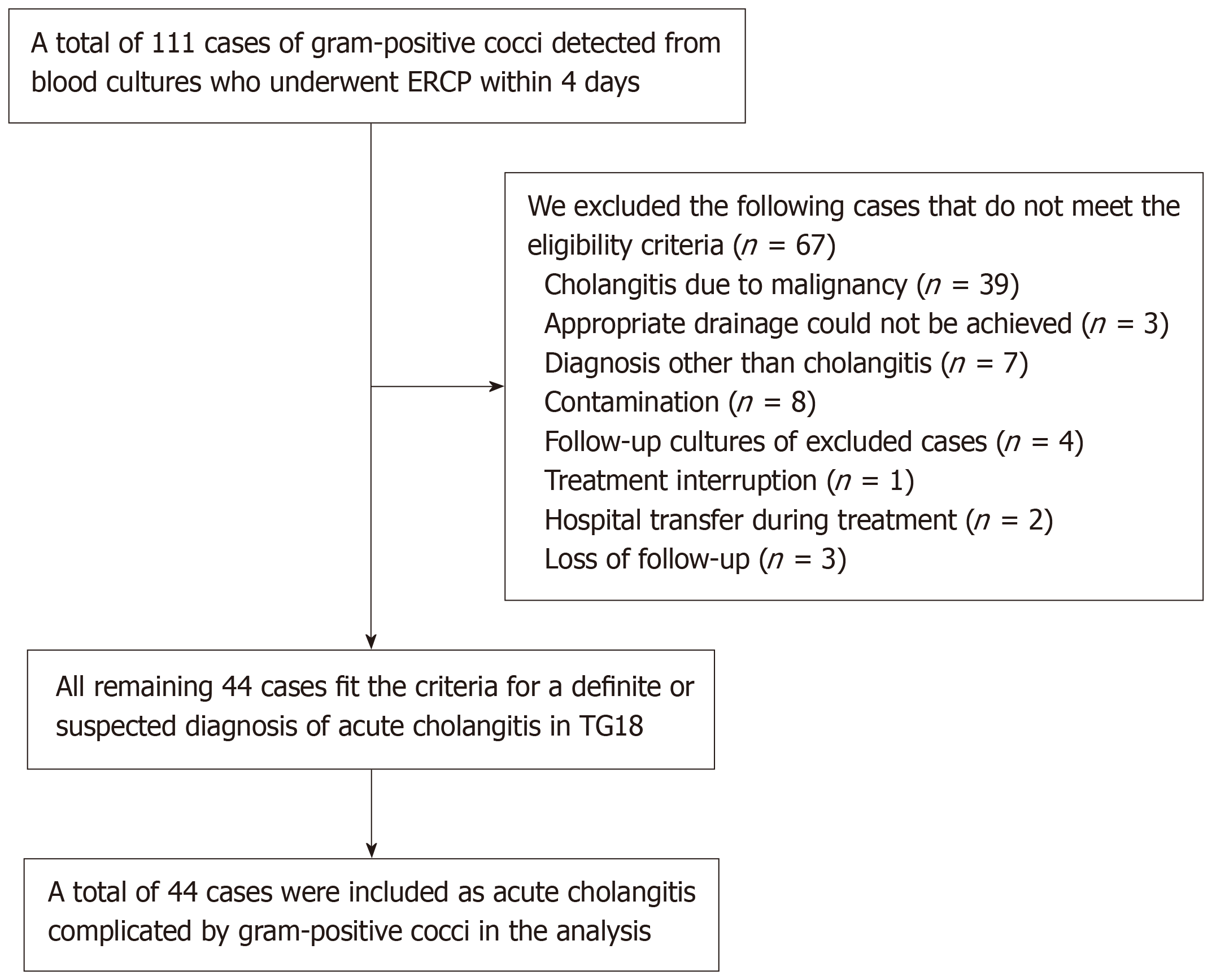
A total of 111 patients with GPC detected in their blood cultures who underwent ERCP within four days were identified. The following patients were excluded: (1) 39 patients with cholangitis due to malignancy; (2) 3 patients with cholangitis in which appropriate drainage could not be achieved, seven with bacteremia unrelated to cholangitis; (3) 7 patients with the diagnosis other than cholangitis; (4) 8 patients with contamination (4 cases of Staphylococcus epidermidis, 3 cases of coagulase-negative Staphylococcus, and 1 case of Staphylococcus capitis subspecies ureolyticus); (5) 4 patients for whom follow-up cultures were obtained; (6) 1 patient with cholangitis owing to treatment interruption; (7) 2 patients were transferred during treatment; and (8) 3 were lost to follow-up. The remaining 44 patients who met the criteria for a definite or suspected diagnosis of acute cholangitis, as defined by TG18, were included in the analysis (Figure 1).
Patient characteristics of the study cohort are shown in Table 1. The median age of the patients was 76.5 years [interquartile range (IQR): 69.8-84.0 years], and 27 (61.4%) were male. Nineteen and 25 patients were classified into the SCT and LCT groups, respectively. The median duration of effective antimicrobial treatment was 9.0 (IQR: 2.5-11.0 days) days and 16 (IQR: 15.0-19.0 days) days in the SCT and LCT groups, respectively (P < 0.001) (Figure 1). The duration of intravenous therapy was 8.0 (IQR: 0.0-10.0 days) days in the SCT group and 12.0 (IQR: 8.0-16.0 days) days in the LCT group. The background disease and the use of immunosuppressants or steroids were similar between the two groups.
| Characteristics | Short-course treatment | Long-course treatment | P value |
| Age (median, IQR) | 78.0 (71.0-85.0) | 75.0 (69.0-84.0) | 0.667 |
| Sex (male) | 13 (68.4) | 14 (56.0) | 0.599 |
| Co-morbidities | |||
| Cerebrovascular disease | 4 (21.1) | 6 (24.0) | 1.000 |
| Chronic pulmonary disease | 4 (21.1) | 6 (24.0) | 1.000 |
| Congestive heart failure | 2 (10.5) | 3 (12.0) | 1.000 |
| Chronic liver disease | 1 (5.3) | 3 (12.0) | 0.622 |
| Chronic kidney disease (serum creatinine > 1.5 mg/dL) | 2 (10.5) | 4 (16.0) | 0.684 |
| Diabetes mellitus | 9 (47.4) | 9 (36.0) | 0.653 |
| Rheumatic disease | 1 (5.3) | 0 (0) | 0.432 |
| Malignancy | 1 (5.3) | 4 (16.0) | 0.370 |
| Charlson Comorbidity Index (median, IQR) | 5.0 (4.5-7.0) | 6.0 (4.0-8.0) | 0.839 |
| Use of other immunosuppressive agents or chemotherapy | 0 (0) | 1 (4.0) | 1.000 |
| Use of glucocorticoids | 0 (0) | 2 (8.0) | 0.498 |
| Tokyo Guideline 18 severity grading | 0.924 | ||
| Grade Ⅰ | 7 (36.8) | 9 (36.0) | |
| Grade Ⅱ | 4 (21.1) | 4 (16.0) | |
| Grade Ⅲ | 8 (42.1) | 12 (48.0) | |
| Quick Sepsis-Related Organ Failure Assessments (median, range) | 1 (0-3) | 2 (0-3) | 0.149 |
| intensive care unit admission | 3 (15.8) | 7 (28.0) | 0.474 |
| Use of vasopressor | 2 (10.5) | 8 (32.0) | 0.148 |
| White blood cells at diagnosis (/mm3) (mean, range) | 9620 (2800-22900) | 12220 (3500-33100) | 0.217 |
| C-reactive protein at diagnosis (mg/dL) (mean, range) | 7.09 (0.04-23.5) | 7.01 (0.05-29.0) | 0.915 |
| Total bilirubin at diagnosis (mg/dL, mean, range) | 3.36 (1.0-7.4) | 3.18 (0.5-11.0) | 0.633 |
| Body temperature (°C) (mean, range) | 37.9 (36.7-39.7) | 38.3 (36.6-40.7) | 0.229 |
| Cause | 0.691 | ||
| Stone | 16 (84.2) | 20 (80.0) | |
| Diverticulum | 1 (5.3) | 3 (12.0) | |
| Stent obstruction | 1 (5.3) | 2 (8.0) | |
| Others | 1 (5.3) | 0 (0) | |
| Acute cholecystitis | 9 (47.4) | 9 (36.0) | 0.653 |
| Acute pancreatitis | 5 (26.3) | 3 (12.0) | 0.262 |
| Heart valve disease | 7 (36.8) | 8 (32.0) | 0.988 |
| Nosocomial onset | 1 (5.3) | 2 (8.0) | 1.000 |
| Intravascular devices | 2 (10.5) | 2 (8.0) | 1.000 |
| Auscultation of murmur | 0.844 | ||
| Yes | 3 (16.7) | 2 (8.7) | |
| No | 15 (83.3) | 21 (91.3) | |
| Persistent fever for 7 days or more | 0 (0) | 0 (0) | |
| Echocardiography | 7 (36.8) | 18 (72.0) | 0.042 |
| Number of positive blood culture | 0.005 | ||
| 1 | 13 (68.4) | 6 (24.0) | |
| 2 | 6 (31.6) | 19 (76.0) | |
| Follow-up of blood culture | 1.000 | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 4 (100) | 13 (100) | |
| Causative organism | |||
| Polymicrobial | 15 (79.0) | 17 (68.0) | 0.507 |
| Enterococcus sp. | 13 (68.4) | 16 (64.0) | 1.000 |
| Enterococcus faecalis | 4 (21.1) | 3 (12.0) | 0.443 |
| Enterococcus faecium | 3 (15.8) | 2 (8.0) | 0.638 |
| Streptococcus sp. | 6 (31.6) | 8 (32.0) | 1.000 |
| Others | 1 (5.3) | 2 (8.0) | 1.000 |
| Antibiotics | |||
| Penicillin | 9 (47.4) | 23 (92.0) | 0.002 |
| Cephalosporin | 13 (68.4) | 16 (64.0) | 1.000 |
| Carbapenem | 2 (10.5) | 4 (16.0) | 0.684 |
| Clindamycin | 8 (42.1) | 7 (28.0) | 0.511 |
| Glycopeptide | 2 (10.5) | 11 (44.0) | 0.021 |
| Fluoroquinolone | 5 (26.3) | 7 (28.0) | 1.000 |
| Linezolid | 1 (5.3) | 0 (0) | 0.432 |
| Metronidazole | 2 (10.5) | 1 (4.0) | 0.570 |
| Aminoglycoside | 1 (5.3) | 0 (0) | 0.432 |
| Time to drainage | 0.710 | ||
| ≤ 24 hours | 15 (78.9) | 21 (84.0) | |
| > 24 hours | 4 (21.1) | 4 (16.0) | |
| Duration of effective antibiotics days (median, IQR) | 9.0 (2.5-11.0) | 16.0 (15.0-19.0) | < 0.001 |
| Duration of effective intravenous antibiotics days (median, IQR) | 8.0 (0.0-10.0) | 12.0 (8.0-16.0) | 0.001 |
The primary cause of acute cholangitis was common bile duct stones, followed by periampullary diverticulum and bile duct stent obstruction, with no notable differences between the two groups. Regarding the severity of acute cholangitis, there were no significant differences in the Tokyo Guideline Grades (I/II/III) [7 (36.8%)/4 (21.1%)/8 (42.1%) in the SCT group vs 9 (36.0%)/4 (16.0%)/12 (48.0%) in the LCT group], in the qSOFA score, the admission of intensive care unit or the use of vasopressor. Auscultation of a murmur was similarly observed in 3 (16.7%) patients and 2 (8.7%) patients in the SCT and LCT groups, respectively, although data were unavailable for 1 patient and 2 patients in each group. There were no patients with persistent fever for 7 days or more. Transthoracic echocardiography during treatment was performed in 7 patients (36.8%) in the SCT group and 18 patients (72.0%) in the LCT group, with a significant difference (P = 0.042); however, no vegetation was detected in either group. The number of positive blood culture sets (1/2 sets) were 13 patients (68.4%) and 6 patients (31.6%) in the SCT group and 6 patients (24.0%) and 19 patients (76.0%) in the LCT group, respectively, with a significant difference (P = 0.005). Follow-up blood cultures were obtained from 4 patients in the SCT group and 13 patients in the LCT group, all of which were negative.
Mixed infections were common among the causative organisms, occurring in 15 patients (79.0%) and 17 (68.0%) patients in the SCT and LCT groups, respectively. Enterococcus spp. was the most frequently detected GPC, identified in 13 patients (68.4%) and 16 patients (64.0%) in the SCT and LCT groups, respectively. Notably, the detection rates did not differ significantly between the groups. Penicillin (9 cases, 47.4% vs 23 cases, 92.0%, P = 0.002) and glycopeptides (2 cases, 10.5% vs 11 cases, 44.0%, P = 0.021) were used significantly more frequently in the LCT group than in the SCT group. Additionally, the time to biliary drainage was statistically comparable between the groups.
The outcomes are presented in Table 2. The 30-day mortality and reinfection rates with the same organisms within 3 months were 0.0% in both groups. The length of hospital stay tended to be shorter at 12.0 (IQR: 7.5-13.0 days) days in the SCT group compared with 14.0 (IQR: 9.0-18.0 days) days in the LCT group (P = 0.092). The rates of cholangitis recurrence within 3 months were 5.3% (1/19) and 8.0 % (2/25) in the SCT and LCT groups, respectively, with no significant difference.
| Outcomes | Short-course treatment (< 14 days) | Long-course treatment (≥ 14 days) | P value |
| 30-day mortality | 0 (0) | 0 (0) | |
| Recurrence of cholangitis within 3 months | 1.000 | ||
| Yes | 1 (5.3) | 2 (8.0) | |
| No | 18 (94.7) | 23 (92.0) | |
| Reinfection with the same organism within 3 months | 0 (0) | 0 (0) | |
| Length of hospital stay (median, interquartile range) | 12.0 (7.5-13.0) | 14.0 (9.0-18.0) | 0.092 |
To the best of our knowledge, this is the first study to examine and compare the duration of treatment with effective antibiotics, specifically for GPC bacteremia-associated cholangitis. In this study, the SCT group showed comparable treatment outcomes to the LCT group and tended to have a shorter hospital stay. Although 1 patient had cholangitis relapse in the SCT group and 2 patients had relapse in the LCT group, these relapses were due to stent obstruction, and no bloodstream infections from the same bacterial strain were noted. The number of positive blood cultures was significantly higher in the LCT group, which may have influenced both the choice of antimicrobials and the treatment duration. Patients in the LCT group also had a significantly higher use of penicillin and glycopeptide antibiotics. This is likely influenced by the definition of the duration of effective antimicrobial administration, patient background, and other factors.
Regarding the guideline recommendations for GPC bacteremia-associated cholangitis, the Netherlands[13], the Infectious Diseases Society of America[6], and the World Society of Emergency Surgery[14] guidelines recommend a treatment duration of 3-7 days after implementation of adequate source control measures without specifically mentioning bacteremia cases. The Surgical Infection Society[15] suggests limiting the duration of intra-abdominal infections with bacteremia to 7 days if patients are no longer bacteremic. However, the TG18 recommends at least two weeks of antibiotic therapy for GPC bacteremia, citing evidence from a study by Gomi et al[8], which reported an IE incidence of 0.26% in acute cholangitis. Notably, data on the bacterial strains, patient characteristics, and treatment specificities are limited. For instance, in Japan, only 9% of specialists administer antibiotics for > 14 days[5].
Although no previous studies have focused exclusively on GPC, some have reported that shorter courses of GPC may be sufficient to treat bacteremia-associated cholangitis. For instance, Doi et al[7] compared SCT ≤ 7 days and LCT ≥ 8 days in 58 cases of GPC bacteremia, including 11 cases receiving SCT and 47 cases receiving LCT, and found no significant differences in treatment outcomes, suggesting that SCT could be a viable option. Furthermore, Satake et al[16] reported similar outcomes for SCT ≤ 3 days and LCT ≥ 4 days in grade I and II stone-associated cholangitis cases with appropriate biliary drainage, which included 8 cases of GPC bacteremia (3 cases receiving SCT and 5 cases receiving LCT). Lastly, Masuda et al[17] compared SCT of ≤ 3 days and LCT of 4-7 days in cases of bacteremia-associated cholangitis with appropriate biliary drainage, including 17 cases of Enterococcus sp. and Streptococcus sp. (4 cases receiving SCT and 13 cases receiving LCT), and found no differences in treatment outcomes.
Considering the causative organisms, Enterococcus sp. is the most commonly detected GPC in acute cholangitis[18–20]. Risk factors and predictive scores for IE in Enterococcus sp. bacteremia have been reported in several studies. The number of positive blood cultures, Origin of the bacteremia, previous Valve disease, Auscultation of heart murmur (NOVA) score predicts the presence of IE in Enterococcus sp. bacteremia, with risk factors including, in order of increasing risk, the number of positive blood cultures (3/3 blood cultures or majority if more than three), unknown origin of bacteremia, previous valvular heart disease, and auscultation of a heart murmur[21]. The DeNOVA score predicted the presence of IE with higher specificity than the NOVA score, although it was limited to Enterococcus faecalis. The suggested risk factors for the DeNOVA score include long duration of symptoms (≥ 7 days), embolization, ≥ 2 positive cultures, unknown origin of infection, valvular disease, and auscultation of a heart murmur[22]. Other factors such as a prosthetic heart valve, known intrinsic valvular disease, previous history of IE, community-acquired infection, and monococcal bacteremia have also been reported[23]. However, if enterococcal bacteremia originates in the gastrointestinal tract, the risk of IE might be low, and a treatment duration of 1-2 weeks has been suggested for uncomplicated enterococcal bacteremia (nosocomial cases in patients without endovascular devices, new murmurs, heart failure, or embolic findings, and without persistent bacteremia)[23]. In addition, extended treatment and hospitalization durations are associated with increased risks of antibiotic-related adverse effects, resistant bacteria, and additional healthcare costs[9-12,24]. Although IE is a serious condition, its low incidence, and the gap between real-world practice and guideline recommendations highlight the need to identify populations that may benefit from a shorter treatment duration.
This study had some limitations that should be noted when interpreting our findings. First, this was a single-center retrospective study with a small sample size and was not a non-inferiority trial. Given the frequency of IE, the sample size was insufficient to suggest that a shorter duration of antimicrobial treatment is safe. In addition, regarding the length of hospital stay, our data were not sufficient to show a significant difference, however, a larger study may have detected this. Second, bile cultures could not be analyzed owing to substantial amounts of missing data. Third, although most patient characteristics were statistically comparable between the two groups, the number of positive blood culture sets, rate of echocardiography performed during treatment, and the use of penicillin and glycopeptides were significantly higher in the LCT group, which may have affected the duration and outcome of the antimicrobial treatment. Fourth, echocardiography and follow-up blood culture tests were not performed for all patients. Additionally, we could not identify some physical findings owing to unavailable data. Therefore, we may have underestimated the risks and biases associated with IE. Finally, we could not apply diagnostic systems for IE similar to Duke’s criteria owing to limited available data. However, none of the patients showed persistent fever, and the origin of the bacteremia was clearly biliary rather than unknown, which is considered a risk factor for enterococcal IE. Furthermore, no cases of recurrent infection with the same organism occurred during the follow-up, supporting the conclusion that IE was unlikely. Despite these limitations, this is the first study to compare and investigate the duration of antimicrobial treatment for GPC bacteremic cholangitis and to provide valuable real-world data.
Our findings suggest that in patients with acute cholangitis and GPC bacteremia, a shorter duration of antimicrobial treatment less than 14 days may be a viable option if adequate biliary drainage is achieved. Further research with larger sample sizes is required to determine the appropriate treatment duration.
| 1. | Lee CC, Chang IJ, Lai YC, Chen SY, Chen SC. Epidemiology and prognostic determinants of patients with bacteremic cholecystitis or cholangitis. Am J Gastroenterol. 2007;102:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Sung YK, Lee JK, Lee KH, Lee KT, Kang CI. The clinical epidemiology and outcomes of bacteremic biliary tract infections caused by antimicrobial-resistant pathogens. Am J Gastroenterol. 2012;107:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Ortega M, Marco F, Soriano A, Almela M, Martínez JA, López J, Pitart C, Mensa J. Epidemiology and prognostic determinants of bacteraemic biliary tract infection. J Antimicrob Chemother. 2012;67:1508-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Melzer M, Toner R, Lacey S, Bettany E, Rait G. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad Med J. 2007;83:773-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Gomi H, Solomkin JS, Schlossberg D, Okamoto K, Takada T, Strasberg SM, Ukai T, Endo I, Iwashita Y, Hibi T, Pitt HA, Matsunaga N, Takamori Y, Umezawa A, Asai K, Suzuki K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, de Santibañes E, Shikata S, Noguchi Y, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Mukai S, Higuchi R, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 6. | Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1020] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 7. | Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect. 2018;24:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Gomi H, Takada T, Hwang TL, Akazawa K, Mori R, Endo I, Miura F, Kiriyama S, Matsunaga N, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Updated comprehensive epidemiology, microbiology, and outcomes among patients with acute cholangitis. J Hepatobiliary Pancreat Sci. 2017;24:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8908] [Cited by in RCA: 7362] [Article Influence: 2454.0] [Reference Citation Analysis (0)] |
| 10. | Curran J, Lo J, Leung V, Brown K, Schwartz KL, Daneman N, Garber G, Wu JHC, Langford BJ. Estimating daily antibiotic harms: an umbrella review with individual study meta-analysis. Clin Microbiol Infect. 2022;28:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 11. | Rice LB. The Maxwell Finland Lecture: for the duration-rational antibiotic administration in an era of antimicrobial resistance and clostridium difficile. Clin Infect Dis. 2008;46:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | World Health Organization. Antimicrobial resistance. Geneva: World Health Organization. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. |
| 13. | Sieswerda E, Bax HI, Hoogerwerf JJ, de Boer MGJ, Boermeester M, Bonten MJM, Dekker D, van Wijk RG, Juffermans NP, Kuindersma M, van der Linden PD, Melles DC, Pickkers P, Schouten JA, Rebel JR, van Zanten ARH, Prins JM, Wiersinga WJ. The 2021 Dutch Working Party on Antibiotic Policy (SWAB) guidelines for empirical antibacterial therapy of sepsis in adults. BMC Infect Dis. 2022;22:687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, Ansaloni L, Bala M, Balogh ZJ, Beltrán MA, Ben-Ishay O, Biffl WL, Birindelli A, Cainzos MA, Catalini G, Ceresoli M, Che Jusoh A, Chiara O, Coccolini F, Coimbra R, Cortese F, Demetrashvili Z, Di Saverio S, Diaz JJ, Egiev VN, Ferrada P, Fraga GP, Ghnnam WM, Lee JG, Gomes CA, Hecker A, Herzog T, Kim JI, Inaba K, Isik A, Karamarkovic A, Kashuk J, Khokha V, Kirkpatrick AW, Kluger Y, Koike K, Kong VY, Leppaniemi A, Machain GM, Maier RV, Marwah S, McFarlane ME, Montori G, Moore EE, Negoi I, Olaoye I, Omari AH, Ordonez CA, Pereira BM, Pereira Júnior GA, Pupelis G, Reis T, Sakakhushev B, Sato N, Segovia Lohse HA, Shelat VG, Søreide K, Uhl W, Ulrych J, Van Goor H, Velmahos GC, Yuan KC, Wani I, Weber DG, Zachariah SK, Catena F. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 15. | Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, Chang PK, O'Neill PJ, Mollen KP, Huston JM, Diaz JJ Jr, Prince JM. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg Infect (Larchmt). 2017;18:1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 16. | Satake M, Yamaguchi Y. Three-day antibiotic treatment for acute cholangitis due to choledocholithiasis with successful biliary duct drainage: A single-center retrospective cohort study. Int J Infect Dis. 2020;96:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Masuda S, Imamura Y, Ichita C, Jinushi R, Kubota J, Kimura K, Makazu M, Sato R, Uojima H, Koizumi K. Efficacy of Short-Course Antibiotic Therapy for Acute Cholangitis With Positive Blood Cultures: A Retrospective Study. Cureus. 2024;16:e58883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Ahmed M. Acute cholangitis - an update. World J Gastrointest Pathophysiol. 2018;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (11)] |
| 19. | Sokal A, Sauvanet A, Fantin B, de Lastours V. Acute cholangitis: Diagnosis and management. J Visc Surg. 2019;156:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Tanaka A, Takada T, Kawarada Y, Nimura Y, Yoshida M, Miura F, Hirota M, Wada K, Mayumi T, Gomi H, Solomkin JS, Strasberg SM, Pitt HA, Belghiti J, de Santibanes E, Padbury R, Chen MF, Belli G, Ker CG, Hilvano SC, Fan ST, Liau KH. Antimicrobial therapy for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:59-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Bouza E, Kestler M, Beca T, Mariscal G, Rodríguez-Créixems M, Bermejo J, Fernández-Cruz A, Fernández-Avilés F, Muñoz P; Grupo de Apoyo al Manejo de la Endocarditis. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Berge A, Krantz A, Östlund H, Nauclér P, Rasmussen M. The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection. 2019;47:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Rosselli Del Turco E, Bartoletti M, Dahl A, Cervera C, Pericàs JM. How do I manage a patient with enterococcal bacteraemia? Clin Microbiol Infect. 2021;27:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Jeong HT, Song JE, Kim HG, Han J. Changing Patterns of Causative Pathogens over Time and Efficacy of Empirical Antibiotic Therapies in Acute Cholangitis with Bacteremia. Gut Liver. 2022;16:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |