Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.104761
Revised: April 9, 2025
Accepted: May 18, 2025
Published online: June 27, 2025
Processing time: 168 Days and 10.2 Hours
Clinically significant Portal hypertension (PH), defined by a hepatic venous pressure gradient (HVPG) greater than 10 mmHg, is a key predictor of decom
Core Tip: Portal hypertension (PH) is the main driver of morbidity and mortality in patients with cirrhosis. Over the last decade, there have been many critical developments in the diagnosis and management of this condition. The following review will provide the reader with a state-of-the-art appreciation of the most recent updates in the non-invasive and invasive diagnostic approaches, including the use of liver stiffness, spleen stiffness, and endoscopic ultrasound. A diagnostic algorithm is proposed to guide clinicians. We also discuss the role of pharmacological, endoscopic, and interventional therapeutic options to prevent and manage manifestations of PH.
- Citation: Xie XY, Benmassaoud A. Advances in the diagnosis and management of clinically significant portal hypertension in cirrhosis: A narrative review. World J Hepatol 2025; 17(6): 104761
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/104761.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.104761
Portal hypertension (PH) is characterized by an increased pressure in the portal venous system, primarily due to elevated intrahepatic vascular resistance and increased portal venous flow, most commonly observed in patients with cirrhosis[1]. Clinically significant PH (CSPH), defined by a hepatic venous pressure gradient (HVPG) greater than 10 mmHg, is the main predictor of decompensation events such as variceal bleeding, ascites formation, and hepatic encephalopathy (HE). Early detection and management of CSPH are essential to prevent these life-threatening complications and improve patient outcomes. This narrative review focuses on the pathophysiology, diagnostic approaches, and management of PH in the context of cirrhosis.
PH can be classified into prehepatic[2], intrahepatic[3,4] or posthepatic etiologies[5]. The causes of PH are presented in greater detail in Table 1.
| Classification | Etiologies |
| Prehepatic | Portal vein thrombosis |
| Splenic vein thrombosis | |
| Congenital venous abnormalities | |
| Intrahepatic | Presinusoidal: Hepatoportal sclerosis; schistosomiasis; myeloproliferative diseases; sarcoidosis; early stage of primary biliary cholangitis; primary sclerosing cholangitis; congenital hepatic fibrosis; arsenic toxicity |
| Sinusoidal: Cirrhosis; alcohol-associated hepatitis; nodular regenerative hyperplasia | |
| Postsinusoidal: Veno-occlusive disease | |
| Posthepatic | Budd-Chiari |
| Heart failure | |
| Pulmonary hypertension | |
| Constrictive pericarditis |
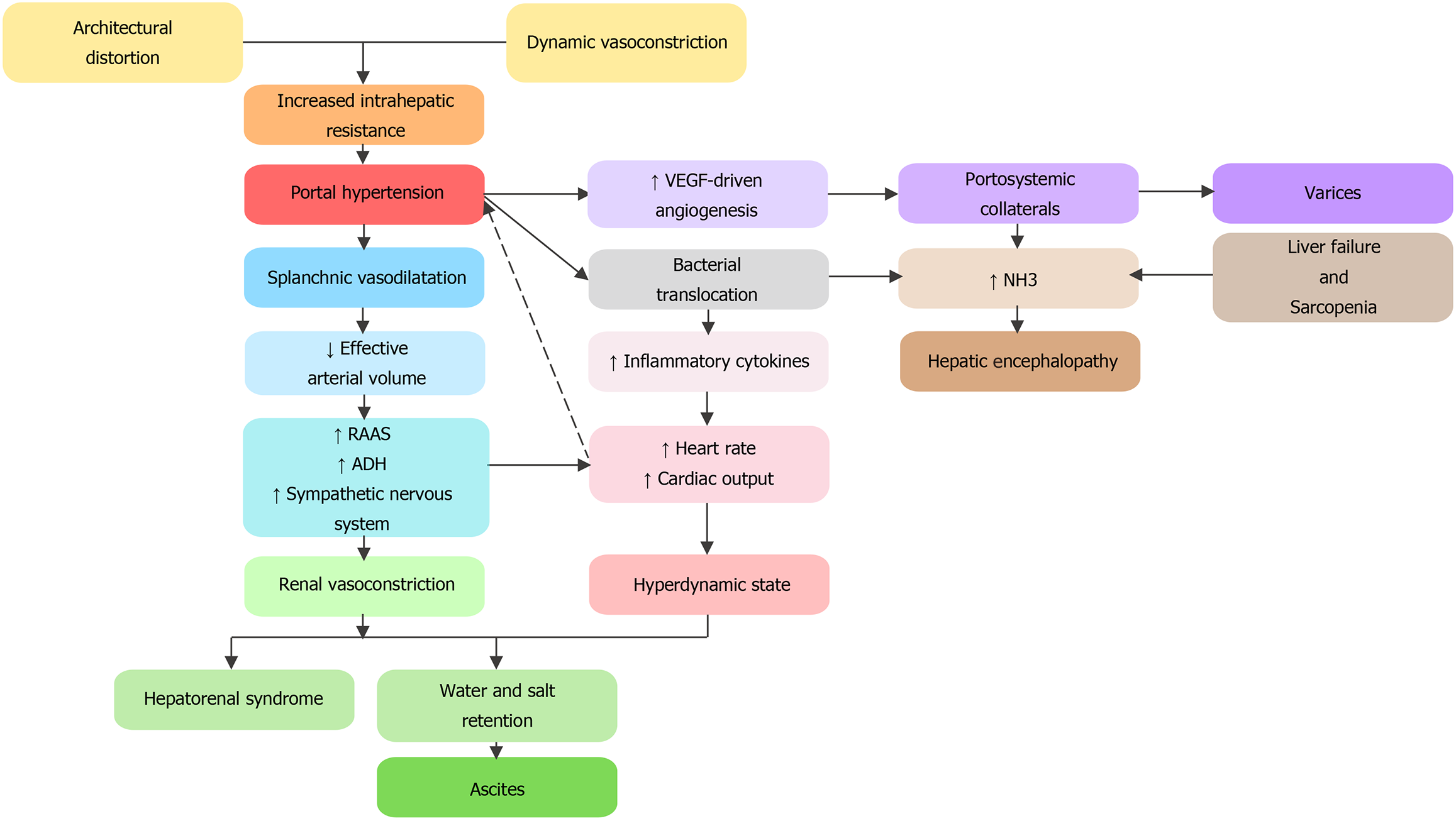
In cirrhosis, architectural modifications through collagen deposition and nodule formation alter sinusoidal blood flow, leading to increased fixed vascular resistance. Injured hepatocytes release cytokines which stimulate contraction of myofibroblasts leading to an increased vascular tone. This dynamic component of hepatic resistance is modulated by an excess production of vasoconstrictors and exacerbated by a locally reduced hepatic nitric oxide (NO) bioavailability. Elevated pressure in the portal system causes shear stress on the splanchnic vessels and leads to excessive production of systemic NO and other vasodilators[3]. Systemic vasodilation leads to a reduced effective arterial volume activating the renin-angiotensin-aldosterone system and the antidiuretic hormone. This leads to a hyperdynamic state where salt and water retention contribute to ascites formation. Increased portal pressure and vascular endothelial growth factor-driven angiogenesis result in the creation of portosystemic collaterals, manifesting as varices[6]. HE results from a complex mechanism of accumulation of toxins and ammonia in the systemic circulation via portosystemic shunting, impaired liver function, bacterial translocation, and sarcopenia (Figure 1)[5].
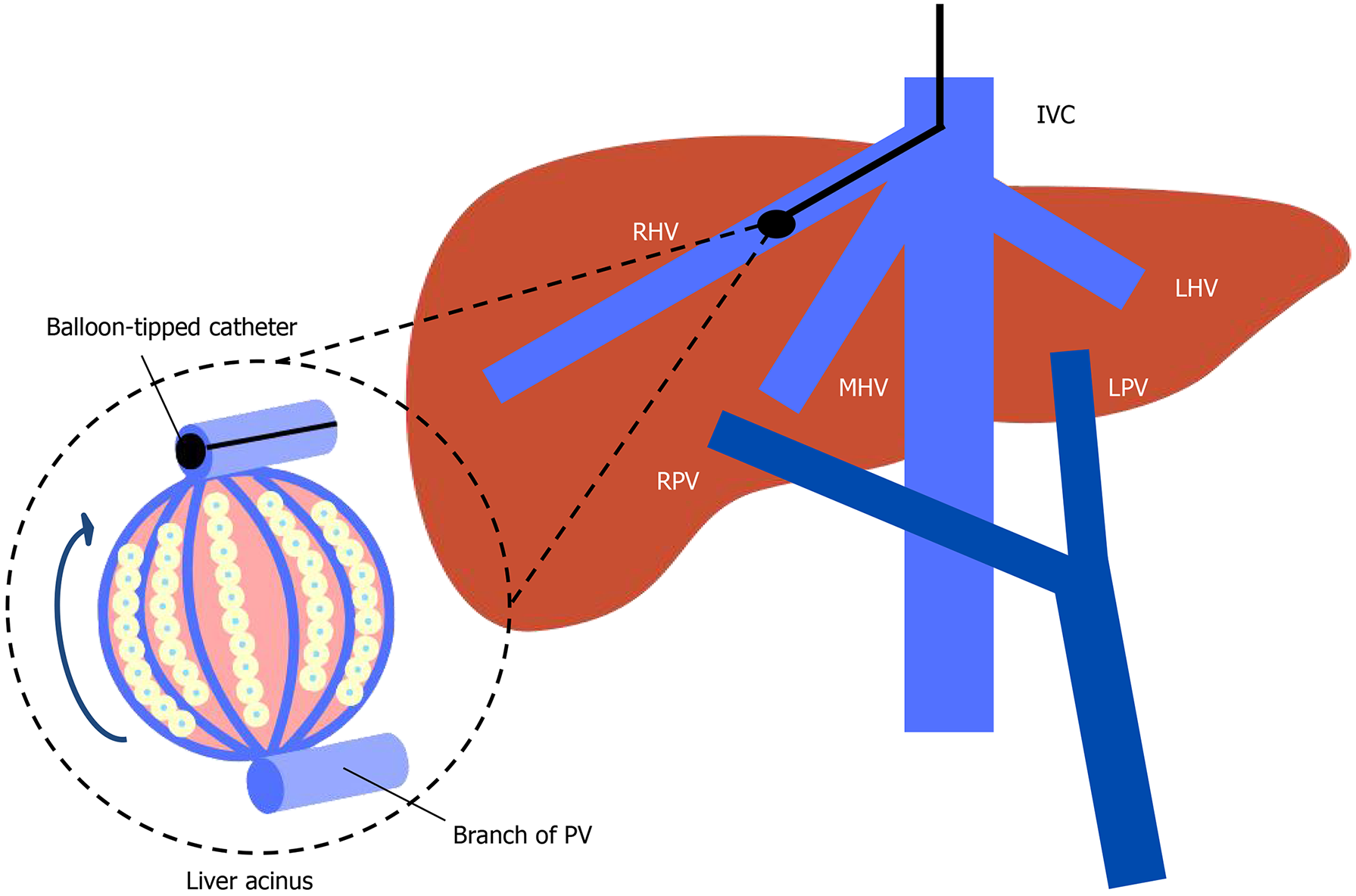
Due to the previous difficulties and complications related to direct measurements of the portal venous pressure, the gold standard to diagnose PH in patients with cirrhosis relies on indirect estimation of portal venous pressure, namely the HVPG[7]. HVPG is calculated from the difference between the wedged hepatic venous pressure (WHVP) and the free hepatic venous pressure (FHVP). Catheterization of the hepatic vein is performed under local anesthesia usually with a transjugular venous approach. After the installation of a venous introducer, a balloon-tipped catheter is inserted under fluoroscopy guidance in the inferior vena cava and advanced into the hepatic vein[8]. When we measure the WHVP by inflating the balloon and occluding a branch of the hepatic vein, the static column of blood equalizes in pressure with the preceding vessels, which are the hepatic sinusoids (Figure 2)[8]. Due to the obstruction of sinusoidal flow by altered architecture in cirrhosis and the lack of pressure equilibration through inter-sinusoidal communications, the pressure within the sinusoids equilibrates with the portal perfusion pressure. Therefore, WHVP provides an indirect estimate of portal pressure in patients with cirrhosis[8]. FHVP is the measure of the non-occluded hepatic vein allowing the measurement of a pressure gradient across the liver. In cirrhosis, an HVPG above 5 mmHg is diagnostic of sinusoidal PH. HVPG above 10 mmHg is diagnostic of CSPH and predicts hepatic decompensation[5]. A HVPG above 12 mmHg is associated with variceal hemorrhage and a HVPG above 20 mmHg is associated with a high risk of mortality in variceal hemorrhage[5]. While highly relevant for the risk stratification of patients, the use of HVPG is limited due to a lack of accessibility outside of highly specialized centers. Furthermore, while HVPG remains the gold standard for diagnosis of PH in viral-related and alcohol-related cirrhosis[5], it has limitations in noncirrhotic PH, where WHVP could be normal or slightly elevated, leading to underestimation of portal pressure[9]. This is particularly relevant in conditions like primary biliary cholangitis and metabolic dysfunction-associated steatotic liver disease (MASLD)[5,10]. PH can develop early in the highly variable natural course of MASLD in pre-cirrhotic patients and HVPG can underestimate portal pressure[11]. Data from the large simtuzumab trial reveal that approximately 1 in 7 patients with MASLD who have developed a liver decompensation event had an HVPG < 10 mmHg[12]. In a European multicenter observational cross-sectional study, patients with MASLD and advanced chronic liver disease (ACLD) present with a higher prevalence of liver decompensation at any HVPG value (< 10 mmHg, 10-12 mmHg or > 12 mmHg) than patients with hepatitis C virus (HCV)-ACLD. None of the patients with HVPG < 10 mmHg in the viral group developed a decompensation event, whereas 9% in the MALSD group developed a decompensation event[13]. These findings suggest the need for an adapted risk stratification in MASLD patients with ACLD.
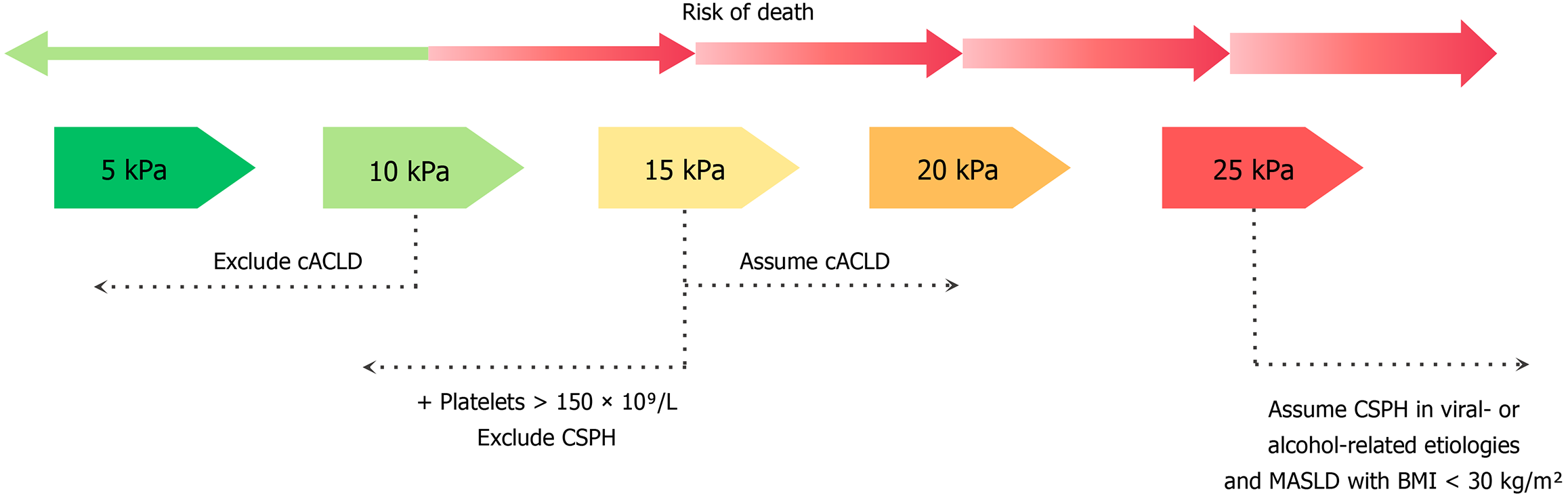
Liver stiffness measurement (LSM) using transient elastography (TE) with the combination of platelet count has been validated as a noninvasive tool for ACLD staging and decompensation risk stratification[7,14]. The rule of five using LSM by TE (5-10-15-20-25 kPa) alone or with platelet count is recommended by the BAVENO VII consensus to rule in or rule out compensated ACLD (cACLD) and CSPH (Figure 3)[7]. LSM above 15 kPa rules in cACLD, and below 10 kPa rules out cACLD. Based on the ANTICIPATE study, LSM above 25 kPa presents a positive predictive value of > 90% for CSPH in patients with chronic viral-related or alcohol-related liver disease or MASLD with a body mass index (BMI) below 30 kg/m2[14]. In patients with MASLD and a BMI above 30 kg/m2, the positive predictive value decreases to 63%[5], highlighting the need for alternative diagnostic approaches in this population. The ANTICIPATE metabolic dysfunction-associated steatohepatitis model which incorporates LSM, platelet count, and BMI, shows promise but requires further validation[15,16]. LSM below 15 kPa and platelet count above 150 × 109/L rule out CSPH. Up to 40%-60% of patients fall into two grey zones associated with indeterminate results[17]: (1) LSM between 20-25 kPa and a platelet count below 150 × 109/L; and (2) An LSM between 15-20 kPa and platelet count below 110 × 109/L, both correlating with probable CSPH. A validation retrospective multicentric cohort study by Wong et al[18] has shown that among the grey zones, viral-related cACLD are associated with a negligible decompensation risk, whereas non-viral cACLD are associated with a higher decompensation risk. However, his study presents a selection bias where 75% of the patients are viral cACLD who have been treated, which questions the applicability of these criteria to other etiologies of cACLD.
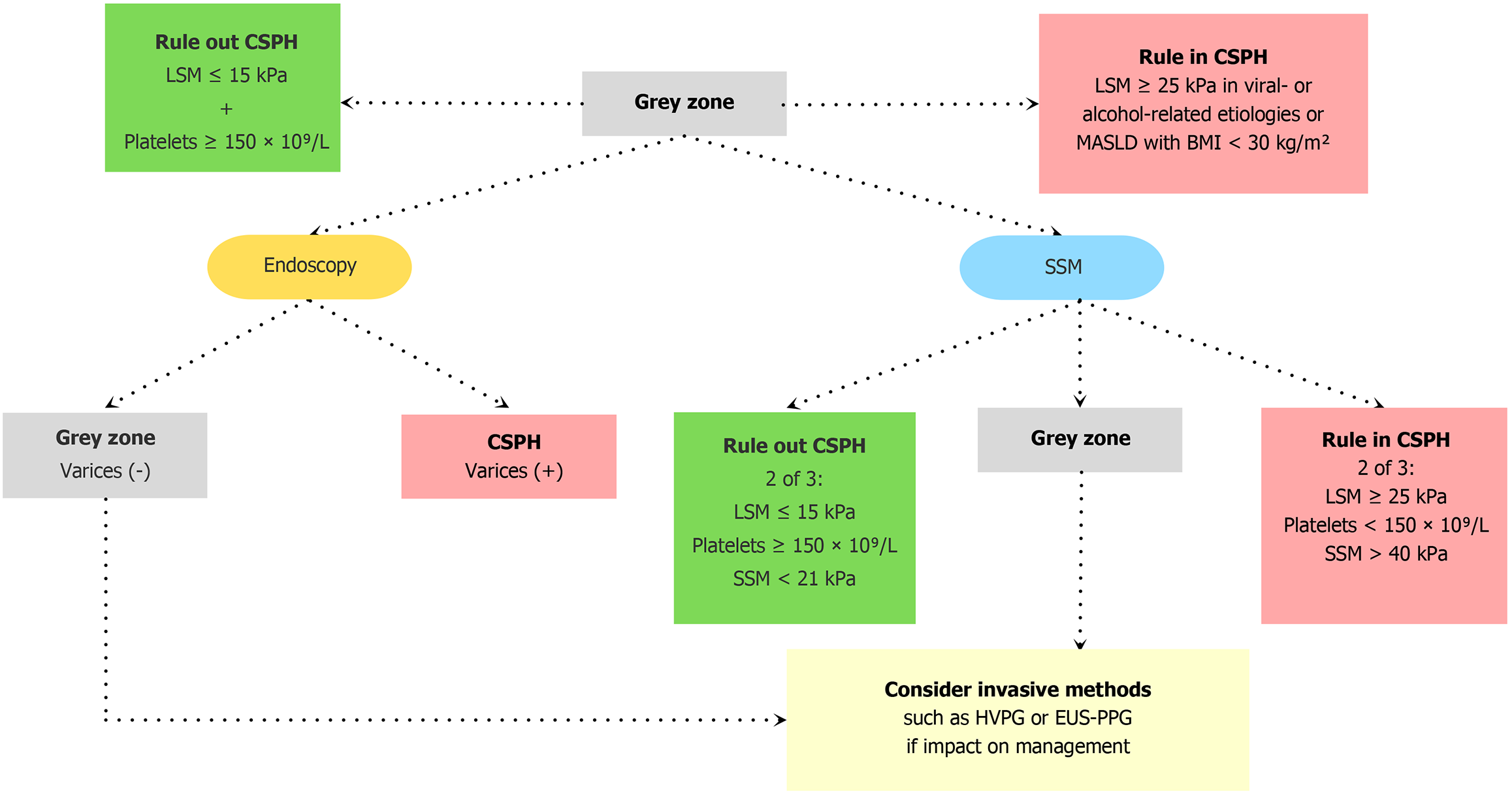
Multiple studies have shown that spleen stiffness measurement (SSM) is a promising tool for predicting CSPH and decompensation[19-23]. In the BAVENO VII consensus, SSM by TE may be used in addition to the rule of five in viral-related cACLD [untreated HCV, untreated and treated hepatitis B virus (HBV)] in a dual cutoff model to rule out CSPH in SSM < 21 kPa or rule in CSPH in SSM > 50 kPa[7]. A retrospective validation cohort study by Dajti et al[17] has shown that the combination of BAVENO VII criteria using LSM with SSM reduces the grey zone from 48% to 32% using a dual cutoff model (SSM < 21 kPa or SSM > 50 kPa) and to 9% using a single cutoff SSM of 40 kPa while maintaining a predictive positive value above 90%. While the 40 kPa cutoff has shown to be suboptimal for ruling out CSPH in non-viral etiologies (negative predictive value < 90%), it has the most efficient performance at ruling in patients with CSPH[17]. This dual approach of combining LSM with SSM is therefore particularly useful in viral-related cACLD. In a recent posthoc analysis of the PREDESCI trial, Dajti et al[24] demonstrated that endoscopic evaluation in patients with inconclusive results after initial CSPH screening by BAVENO VII and American Association for the Study of Liver Diseases (AASLD) criteria (rule in CSPH in LSM ≥ 25 kPa or LSM ≥ 20 kPa + PLT < 150 × 109/L and rule out CSPH in LSM ≤ 15 kPa + PLT ≥ 150 × 109/L) allows to reduce the grey zone to 22%. The non-invasive BAVENO VII with SSM model was comparable to the BAVENO VII/AASLD criteria with endoscopy and correctly predicted decompensation risk at 3 years. See Figure 4 for the proposed algorithm[24]. This remains a proposed framework, without no definite algorithm established. In patients with CSPH and contraindications or intolerance to non-selective beta-blockers (NSBB) as prophylaxis for decompensation, the BAVENO VII consensus recommends avoiding unnecessary endoscopy in patients with an SSM below 40 kPa and who meet the BAVENO VI criteria for endoscopy (LSM ≥ 20 kPa or platelet ≤ 150 × 109/L)[7]. A recent prospective study by Giuffrè et al[25] shows that a decreased SSM has better accuracy in correlating with clinical response to NSBB than LSM and heart rate. Clinical response to NSBB was defined by stability or do
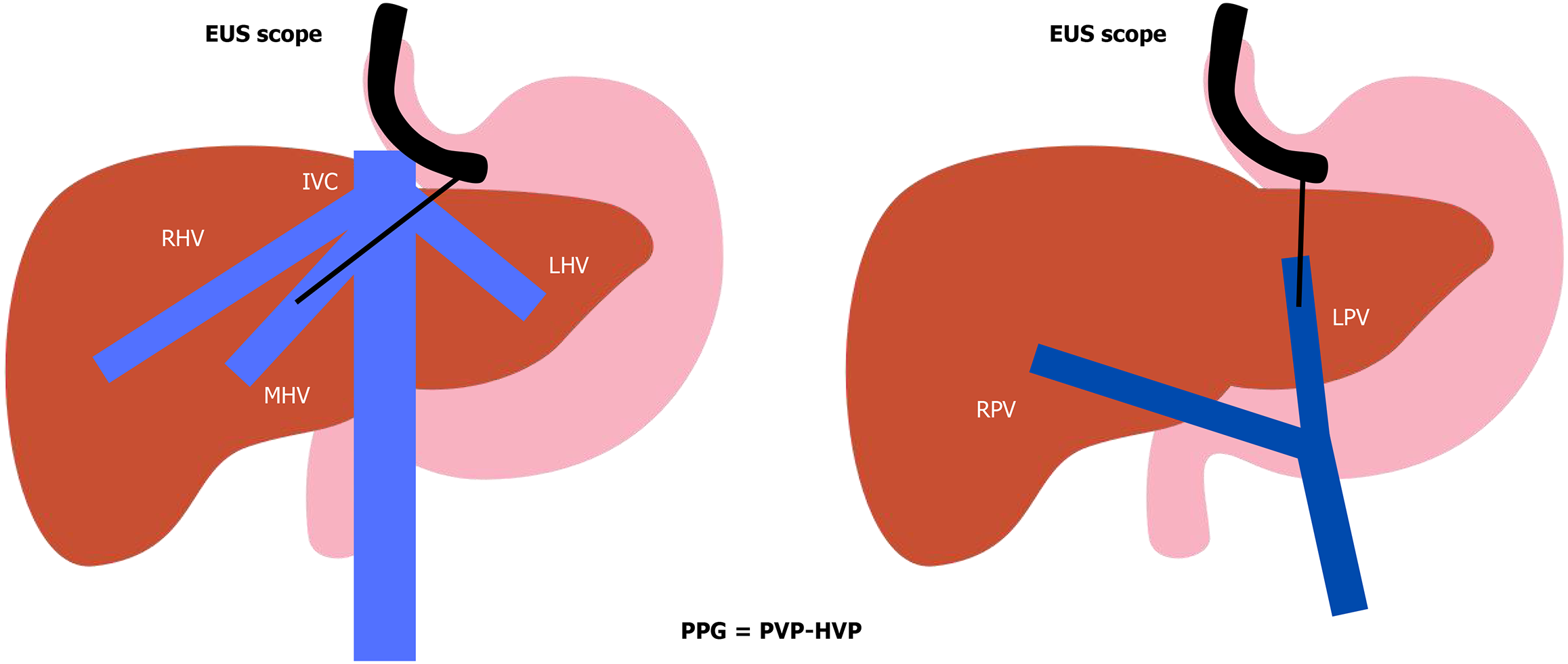
In recent years, endoscopic ultrasound-guided portal pressure gradient (EUS-PPG) measurements have emerged as a promising tool to assess PH (Table 2). In a recent meta-analysis by Dhindsa et al[28], eight cohort studies with a total of 178 patients have shown a technical success rate of 94.6%, a clinical success rate of 85.4%, and a total adverse events rate of 10.9%. The 93.7% of the adverse events are considered mild according to the American Society for Gastrointestinal Endoscopy[28]. Technical success is defined by inserting successfully the needle into the correct vessel and measuring portal and hepatic venous pressures (Figure 5). Clinical success was defined by the correlation of the portal pressure gradient (PPG) measurement with liver biopsy or HVPG. A few other small prospective studies have also shown a great correlation between EUS-PPG and HVPG[29,30]. We recently highlighted that the use of moderate to deep sedation during EUS-PPG procedures can cause variations in abdominal pressures in different phases of the respiratory cycle[31], and lead to inaccurate PPG measures. This can lead to misclassifying the severity of PH[32]. A case series by Chen et al[33] of 3 patients shows the feasibility of EUS-PPG under conscious sedation using ketamine and low-dose midazolam. A large multicentric retrospective study by Kolb et al[34] has reported better performance of EUS-PPG as a predictor of histological cirrhosis compared with fibrosis-4 (FIB-4) and aminotransferase to platelet ratio index (APRI) scores. EUS-PPG has also shown great correlation with esophageal varices, portal hypertensive gastropathy and thrombocytopenia. There is a need for large prospective studies and randomized controlled trials before standardizing the practice of EUS-PPG[25]. An ongoing randomized controlled trial will be comparing endoscopic ultrasound (EUS) and transjugular approaches for liver biopsies and PPG/HVPG measurements (NCT 05118308). We also await the results from the Encounter study (NCT 04987034) which will correlate EUS-PPG with HVPG. EUS-PPG has the potential to revolutionize the management of PH by combining hemodynamic assessment, liver biopsy, and endoscopic evaluation in a single procedure. However, large prospective studies and randomized controlled trials are needed to standardize its use and validate its accuracy.
| HVPG | EUS-guided portal pressure gradient | |
| Technique | Transjugular catheterization of the hepatic vein with a balloon-tipped catheter | Under EUS guidance, fine-needle puncture of the hepatic vein and the portal vein |
| Principle | HVPG = wedged hepatic venous pressure – free hepatic venous pressure | PPG = portal vein pressure – hepatic vein pressure |
| Sedation | Usually under minimal (low-dose midazolam) or no sedation | Usually under moderate to deep sedation |
| Advantages | Well-established as the gold standard for clinically significant portal hypertension assessment | Direct measurement of portal vein pressure. Alternative when HVPG is not accurate such as presinusoidal PH. Can be combined with endoscopic evaluation of varices |
| Limitations | Indirect measurement of portal vein pressure. May underestimate presinusoidal PH such as primary biliary cholangitis and metabolic dysfunction-associated steatotic liver disease. May be contraindicated in severe coagulopathy | Limited availability and expertise. Requires further validation. Moderate to deep sedation can cause hemodynamic variations and lead to inaccurate PPG measures |
Some limitations of the use of LSM and SSM include inflammation, operator-dependent issues, increased BMI, congestion, and presence of ascites. EUS-guided shear wave elastography (EUS-SWE) is an emerging technology for LSM and SSM. A prospective study compared EUS-SWE measurement of SSM in patients with and without CSPH, where CSPH was clinically diagnosed by a hepatologist[35]. In EUS, the spleen is visualized through a transgastric approach and shear wave elastography (SWE), using an acoustic radiation force, induces shear waves in the interested area of the spleen to estimate tissue stiffness. Patients with CSPH had significantly higher SSM (37.6 kPa ± 8.5 kPa vs 29.1 kPa ± 9.9 kPa, P = 0.003)[35]. EUS-SWE measurement of SSM may present theoretical advantages in patients with significant central obesity. In another prospective study, EUS-SWE evaluation of LSM is compared with LSM by TE using EUS-guided liver biopsy as the gold standard. Right lobe SWE strongly correlates to fibrosis stage, and accuracy is comparable to TE[36]. However, left lobe measurements have a 3.5 times higher variance than the right lobe. In a recent multicentric pilot study, EUS-SWE was found to be superior to TE and FIB-4 in predicting advanced liver fibrosis and cirrhosis in patients with MASLD and obesity[37]. Larger prospective studies are necessary to standardize the technique and validate the benefits of this novel approach compared to other noninvasive tools[38].
Decompensation is defined by events that include overt ascites (or pleural effusion with increased serum ascites albumin gradient > 1.1 g/dL), overt HE (West-Haven ≥ II), and variceal bleeding[7]. Precipitating factors for hepatic decompensation are infections, additional liver injuries such as acute alcoholic hepatitis, acute viral hepatitis, HBV flares, drug-induced liver injury, hepatocellular carcinoma (HCC), and major surgery[7].
The current BAVENO VII guidelines recommend removing etiological factors of liver disease by obtaining sustained viral response in HCV or HBV suppression in chronic viral-related ACLD, and long-term alcohol abstinence. Treating these underlying etiologies may lead to significant HVPG reduction and prevent hepatic decompensation[7]. In addition to pharmacological and procedural interventions, patient education and lifestyle modifications play a crucial role in managing PH. In the prospective sport diet study, an uncontrolled pilot study, 60 patients with a BMI ≥ 26 kg/m² and HVPG ≥ 6 mmHg underwent a 16-week lifestyle intervention with a personalized hypocaloric diet and 60 minutes of supervised physical activity per week. A total of 50 patients completed the study. Recruited participants were not restricted to those with MASLD but also included those with viral or alcohol-related liver cirrhosis. Results showed significant weight loss was associated with a significant reduction in HVPG. Greater weight loss ≥ 10% was associated with larger HVPG reductions. The intervention was safe, with no clinical decompensation, and weight loss was maintained at 6 months without worsening liver function. The study concluded that 16 weeks of diet and moderate exercise safely reduced body weight and portal pressure in this patient population[39].
NSBB reduce cardiac output by blocking beta-1 receptors and induce splanchnic vasoconstriction by blocking beta-2 receptors, resulting in an unopposed effect of alpha-1 receptors[5]. The randomized controlled trial PREDESCI in 2019 demonstrates that long-term use of NSBB increases decompensation-free survival in cirrhosis patients with CSPH[40]. Hemodynamic response to NSBB consists of a decrease of 10%-20% in baseline HVPG or to an HVPG < 12 mmHg[41]. Carvedilol has an additional intrinsic activity of anti-alpha-1 adrenergic activity, inducing splanchnic vasodilatation, which reduces PH. Carvedilol is more effective at reducing HVPG and better tolerated than propranolol[40]. Multiple trials support the greater effectiveness of carvedilol at reducing HVPG than traditional NSBB[42-44]. A prospective study by Reiberger et al[45] shows that carvedilol is effective at reducing HVPG in non-responders to propranolol. According to the current guidelines from the AASLD, the use of NSBB and preferably carvedilol to an optimal dose of 12.5 mg per day[5]. A randomized controlled trial by Tripathi et al[46] compares carvedilol and endoscopy variceal ligation (EVL) in preventing first variceal bleed and demonstrates lower first variceal bleeding with carvedilol but no difference in mortality on intention-to-treat analysis. Patients with cACLD and CSPH who present contraindications or intolerance to NSBB should undergo endoscopic screening for varices every 2 years (if underlying liver disease etiology remains) or every 3 years (after removal of underlying cause)[5]. Patients with cACLD and low-risk varices (< 5 mm, without red signs, not Child-Pugh C) while having contraindications to NSBB, should repeat endoscopy every year to reassess varices needing treatment[5]. In patients who cannot take NSBBs, primary prophylactic EVL should be performed for high-risk varices, and ligation should be repeated every 2-4 weeks until eradication. Endoscopy should then be repeated at 6 months and every 12 months afterward to reassess varices needing treatment[5]. Patients with cACLD who are on NSBBs for the prevention of decompensation do not need to undergo endoscopic screening to detect varices as there will be no impact on management[7].
There are currently no medications that are approved for reducing PH except for beta-blockers. Statins have been demonstrated in various studies to lower PH in patients with cirrhosis. A meta-analysis by Kim et al[47] reviewing 10 cohort studies and 3 randomized controlled trials demonstrates that statin use is associated with a 46% lower risk of hepatic decompensation, 46% lower mortality, and 27% lower risk of variceal bleeding or progression of PH. The BAVENO VII consensus encourages the use of statins in patients with cirrhosis and approved indications for statins. Statins should be prescribed at a lower dose (simvastatin at a maximum dosage of 20 mg/day) in Child-Pugh B and C cirrhosis patients and should be monitored for muscular and hepatic toxicity. The benefits of statins in Child-Pugh C have not been well-established[48] and their use should be limited[7].
Ascites: Ascites occurs in 50% of cirrhotic patients within 10 years[3]. Sodium restriction 2 g/day and diuretics are the first-line therapy to target a negative sodium balance and net fluid loss[49]. Fluid restriction is only recommended in moderate or severe hyponatremia. Aldosterone antagonists and loop diuretics should be tapered to the lowest dose to achieve minimal ascites to prevent adverse effects[49]. Monitoring of body weight and serum creatinine should be regularly performed. Large-volume paracentesis (LVP) combined with hyper oncotic albumin is the mainstay of therapy in grade 3 ascites[49]. A prospective study by Tan et al[50] suggests that LVP < 8 L per session and adequate albumin infusion are associated with better preservation of renal function and survival within 2 years. Expert opinion recommends LVP < 8 L and 6-8 g of albumin infusion per liter of removed ascites to reduce the risk of post-paracentesis circulatory dysfunction[49].
HE: HE can be classified into type A occurring in acute liver failure, type B in portosystemic shunt, and type C in cirrhosis[51]. Precipitating events include constipation, infections, gastrointestinal bleeding, dehydration, and diuretics overuse. Alternative causes should be ruled out. Ammonia should be measured in patients with potential concomitant causes of delirium, a normal value would rule out HE[51]. The Animal Naming Test is a screening tool for covert HE in cirrhotic patients without a history of overt HE that needs further validation[51]. Treatment of covert and overt HE is recommended with non-absorbable disaccharides such as lactulose. Albumin dialysis may improve the grade of HE in cirrhosis but needs further validation[52-54].
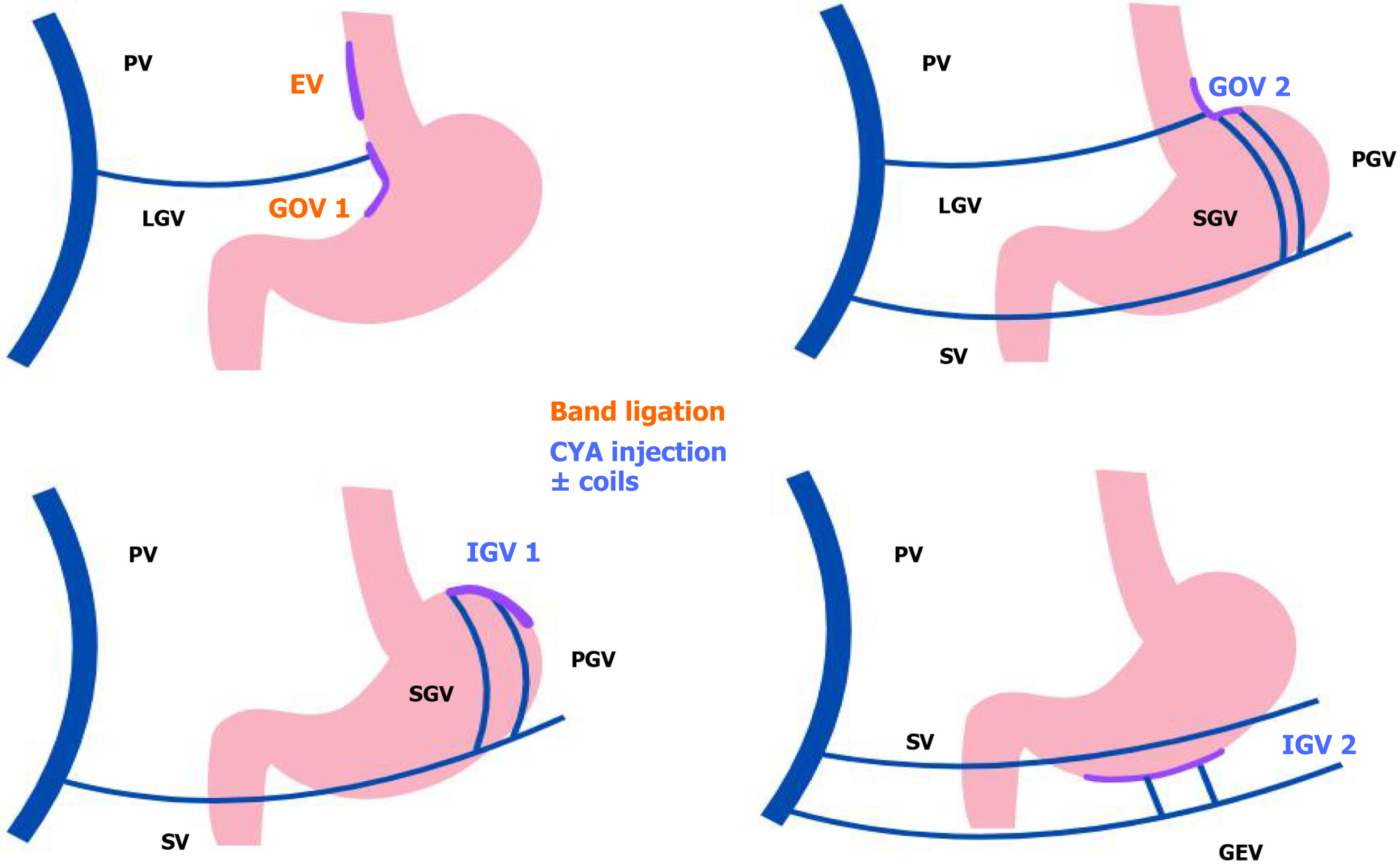
Variceal bleeding: Acute variceal hemorrhage is a potentially fatal complication of PH. Despite advances in therapeutic strategies, the mortality remains between 10%-15% at 6 weeks[5]. According to the current AASLD 2024 guidelines, the goal of hemodynamic resuscitation is to maintain organ perfusion but to avoid exacerbating PH. Blood transfusions should be given with a targeted hemoglobin at 7-8 g/dL[5]. Patients who present altered consciousness and risk of aspiration should be intubated before endoscopy and extubation is recommended as soon as possible after the procedure. Vasoactive agents (octreotide, somatostatin, or terlipressin) should be promptly started and maintained for 2-5 days[5]. Antibiotic prophylaxis with ceftriaxone 1 g/24 hours or adjusted agent depending on local resistance should be initiated at presentation and maintained until discharge or for 5 days. Although the risk of infection is low in Child-Pugh A cirrhosis, there is a lack of evidence supporting the avoidance of antimicrobial therapy in this group[5]. Considering the risk of aspiration pneumonia, pre-intubation or pre-endoscopic nasogastric tube installation should be avoided[5]. Erythromycin infusion before endoscopy contributes to a better visualization in the absence of contraindications[55,56]. Endoscopy should be performed within 12 hours of presentation. EVL is the first-line therapy for acute esophageal variceal bleeding[5]. For isolated gastric varices (IGV) and gastro-esophageal varices type 2 (GOV 2), treatment with tissue adhesives is recommended (Figure 6)[57]. For GOV 1, ligation or tissue adhesive may be used. Endoscopy treatment using argon, radiofrequency ablation, or band ligation can be used for local treatment of portal hypertensive gastropathy[5]. In refractory hemorrhage, endotracheal tube and balloon tamponade of esophageal stenting are recommended as a bridge to a definite therapy[5]. Self-expandable metal stents have shown to be more effective in bleeding control and safer than balloon tamponade, without significant difference in mortality[58,59] although they are not yet Food and Drug Administration-approved in the United States. In patients actively bleeding on endoscopy and meet specific criteria, pre-emptive TIPS should be considered (see section on TIPS). Balloon-occluded retrograde transvenous obliteration may be considered an alternative therapy in patients with GOV 2, IGV 1, or ectopic varices[5,7].
Further decompensation is defined by an additional decompensation event (ascites, variceal bleeding, encephalopathy) and/or jaundice, or recurrence of the same decompensation event (recurrent ascites, recurrent variceal bleeding, recurrent encephalopathy), spontaneous bacterial peritonitis, and/or hepatorenal syndrome (HRS)[7].
Preventing further decompensation in patients with ascites: Refractory ascites (RA) develops in 5%-10% of cirrhotic patients with ascites and is defined by ascites that fails to be mobilized or reoccurs after LVP despite dietary sodium restriction and diuretics[5]. A prospective study by Téllez et al[60] has measured lower renal perfusion pressure below the critical threshold in patients with RA who receive NSBB and suggests using NSBB with caution or avoiding them in RA. A prospective study by Sersté et al[61] has found an association of NSBB with lower survival rates in patients with RA. However, a large post hoc analysis of 3 randomized controlled trials supports the safe use of NSBB in RA[62]. A meta-analysis and multiple retrospective studies have also concluded no significant difference in mortality with the use of NSBB in RA[63-65]. Due to its impact on alpha-1 receptors, carvedilol should be used with caution in patients with RA who often exhibit circulatory dysfunction. Traditional NSBB might be preferable in this context[5]. The current BAVENO VII guidelines support the use of prophylaxis traditional NSBB or carvedilol in patients with ascites and low-risk varices to prevent first variceal bleeding[7]. Patients with ascites and high-risk varices should receive traditional NSBB or carvedilol rather than EVL. NSBB should be temporarily dose-reduced or suspended in patients with ascites and low blood pressure (systolic pressure < 90 mmHg or mean arterial pressure < 65 mmHg) or in the context of HRS[7].
Preventing recurrent HE: Recurrent HE if ≥ 2 episodes occur within 5 months and persistent HE if the patient does not return to his baseline[51]. After a first episode of overt HE, lactulose is recommended as secondary prophylaxis[51]. Rifaximin is recommended as an additional therapy to lactulose after 2 episodes of overt HE within 6 months[51]. Obliteration of portosystemic shunts in patients with cirrhosis and recurrent or persistent HE despite medical treatment should be considered if their model for end-stage liver disease (MELD) score < 11[51].
Preventing recurrent variceal bleeding: The risk of rebleeding after an initial episode of variceal hemorrhage is up to 60% at one year without prophylaxis[5]. The first 6 weeks after the initial bleed represents a high-risk period[66] and secondary prophylaxis should be initiated as soon as the variceal bleed is controlled, within 7 days of admission[5]. Traditional NSBB or carvedilol with EVL are the first-line secondary prophylaxis to prevent recurrent variceal bleeding[7]. A meta-analysis has shown reduced rebleeding and reduced mortality with combination therapy rather than NSBB alone or EVL alone[67]. Patients who underwent preemptive TIPS do not need prophylaxis combination therapy.
EUS-guided management of varices: Endoscopic injection of cyanoacrylate (CYA) glue remains the most commonly practiced approach to achieve hemostasis in gastric varices. However, some types of gastric varices such as IGV 1 and GOV 2 are more challenging to manage due to their location and size. EUS-guided approach allows direct visualization of these varices and permits precise estimation of their size and identification of feeding vessels[68]. For primary prophylaxis of gastric varices, a randomized controlled trial by Sabry et al[69] demonstrates that EUS-guided CYA injection leads to a higher variceal obliteration rate during the index session (77.2% vs 38.1%, P = 0.014), smaller CYA amount needed (1 mL vs 2 mL, P = 0.027), and similar adverse events rate (4.5% vs 14.3%, P = 0.345) compared with direct endoscopic injection. For secondary prophylaxis of gastric varices, a propensity-matched, multicentric study has shown lesser sessions needed to achieve obliteration (1 vs 1.5, P < 0.0001), re-bleeding episodes (13.8% vs 39.1%, P < 0.0001) and re-intervention rates (12.1% vs 50.4%; P < 0.001) with EUS-guided therapy (coils and CYA glue) compared with conventional endoscopic CYA injection[70]. EUS-guided CYA injection for IGV has also shown lesser sessions needed, late rebleeding rates, and postinjection ulcers than conventional endoscopic CYA injection[71]. A small retrospective study has demonstrated the feasibility, safety, and 95% technical success rate for EUS-guided placement of coils in combination with thrombin[72]. A few case reports have described benefits of EUS-guided management of rectal varices[73], ectopic varices[74,75], and parastomal varices[76]. EUS-guided therapy with tissue adhesive with or without coils remains currently in the BAVENO VII consensus’s research agenda[7]. The financial implications and the lack of technical expertise remain challenges of EUS-guided therapy vs conventional endoscopic therapy[77].
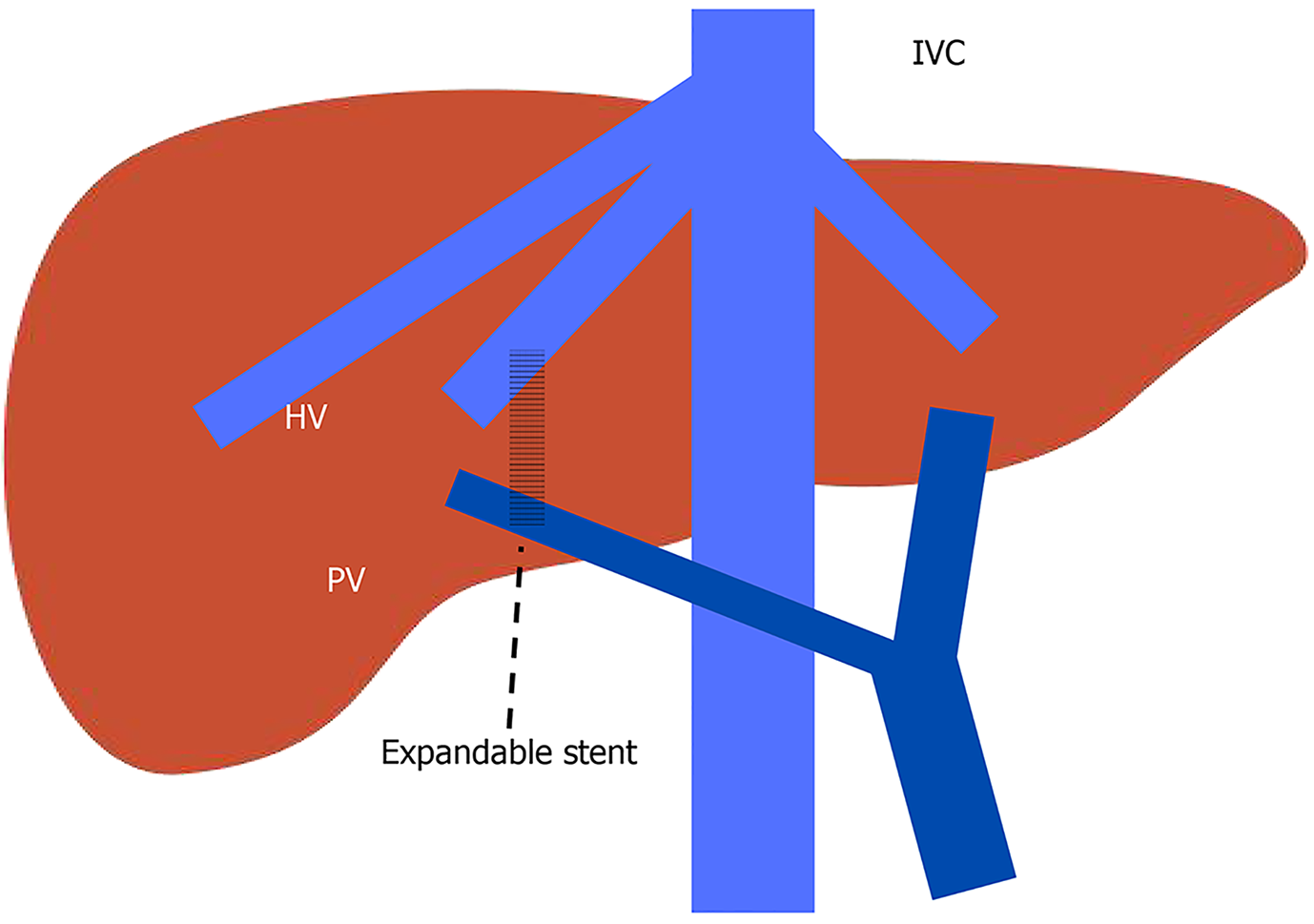
TIPS: TIPS is the creation of an endovascular portosystemic shunt to decrease PPG placed by interventional radiologists under fluoroscopic and ultrasound guidance using a transjugular venous approach under deep sedation or general anesthesia. A hepatic vein is catheterized first and from that vein, the portal vein is punctured followed by dilatation and the installation of a polytetrafluoroethylene-covered stent-graft (Figure 7)[78]. TIPS should be considered for RA regardless of the history of variceal bleeding[7]. TIPS in RA has been shown to improve survival in a meta-analysis by Bai et al[79] reviewing 6 randomized controlled trials. Resolution of ascites post TIPS is not immediate but will eventually be in 80% of patients with TIPS[49]. In patients actively bleeding on endoscopy and meet the criteria of Child-Pugh B > 7 points, Child-Pugh C < 14 points, or HVPG > 20 mmHg, preemptive TIPS is recommended within 72 hours and ideally within 24 hours of presentation[7]. Multiple studies have demonstrated improved 1-year survival with preemptive TIPS[80-83]. In acute variceal hemorrhage, the goal is to reduce PPG < 12 mmHg[7]. The lack of benefits in TIPS can be discussed in patients with Child-Pugh score ≥ 14 or MELD score > 30 and lactate > 12 mmol/L, unless liver trans
EUS-guided Intrahepatic Portosystemic Shunt: TIPS is generally a safe and effective procedure to alleviate PH. However, in patients at high risk of vascular complications, EUS-guided intrahepatic portosystemic shunt (EIPS) may offer theoretical benefits. EIPS does not require vascular access into the inferior vena cava or the right heart, does not use radiation, and can be concomitantly used as direct portal pressure measurement and for gastric varices management[86]. Schulman et al[87] has shown the high feasibility of this technique using a lumen-apposing metal stent with direct portal pressure measurement in a survival animal model. However, the procedure needs further studies in humans, particularly in patients with cirrhosis and a high risk of coagulopathy.
Sarcopenia is classically defined as loss of muscle mass and frailty as the loss of muscle contractile function[88]. Sarcopenia affects 40%-70% of patients with cirrhosis and frailty is present in 18%-43% of this population[89]. Computed tomography imaging is currently considered the gold standard to evaluate sarcopenia by measuring the skeletal muscle index (SMI) or the psoas muscle index[88]. Sarcopenia is correlated with poorer mortality pre-LT[90] and post-LT[91], hepatic decompensation[92], development of acute on chronic liver failure (ACLF)[93], longer hospitalization[94], increased infection[95], and reduced of quality of life[96]. According to the AASLD 2021 Practice Guidance for Mal
LT represents a treatment option for PH-related complications especially when combined with hepatic synthetic dysfunction. Patients with decompensated cirrhosis and a MELD score ≥ 15 should be evaluated for LT as it is associated with an improvement in survival (LT)[100].
Recompensated cirrhosis is defined by the removal of the underlying cause of the liver disease, absence of ascites off diuretics, absence of encephalopathy off lactulose and rifaximin, absence of recurrent variceal bleed for at least 12 months, and sustained improvement in liver function tests[7]. The BAVENO VII consensus currently recommends against removing NSBB unless CSPH is resolved[7]. At this moment, clear definitions are needed to determine the removal of the underlying cause of liver disease in non-alcohol and non-viral etiologies[101].
The management of PH in cirrhosis has seen significant advancements in recent years, with non-invasive diagnostic tools and novel therapeutic approaches improving patient outcomes. However, further research is needed to validate emerging techniques such as EUS-PPG and to refine risk stratification algorithms, particularly in non-viral etiologies of cirrhosis. A multidisciplinary approach remains essential to optimize care for patients with complex and refractory PH.
| 1. | Guo H, Ni H, Xue J, Wang F, Sun X, Wang Z, Niu M. Global scientific trends on portal hypertension and cirrhosis in the 21st century: A bibliometric and visualized analysis. PHC. 2023;2:46-48. [DOI] [Full Text] |
| 2. | Bosch J, Iwakiri Y. The portal hypertension syndrome: etiology, classification, relevance, and animal models. Hepatol Int. 2018;12:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Bloom S, Kemp W, Lubel J. Portal hypertension: pathophysiology, diagnosis and management. Intern Med J. 2015;45:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Nakhleh RE. The pathological differential diagnosis of portal hypertension. Clin Liver Dis (Hoboken). 2017;10:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, Bosch J. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 103] [Article Influence: 103.0] [Reference Citation Analysis (1)] |
| 6. | Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol. 2020;26:6111-6140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (6)] |
| 7. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1486] [Article Influence: 495.3] [Reference Citation Analysis (2)] |
| 8. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 9. | Bochnakova T. Hepatic Venous Pressure Gradient. Clin Liver Dis (Hoboken). 2021;17:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Warnes TW, Roberts SA, Smith A, Cope VM, Vales P, McMahon R. Portal pressure is of significant prognostic value in primary biliary cholangitis. Liver Int. 2023;43:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Mitten EK, Portincasa P, Baffy G. Portal Hypertension in Nonalcoholic Fatty Liver Disease: Challenges and Paradigms. J Clin Transl Hepatol. 2023;11:1201-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, Shiffman ML, Aguilar Schall R, Jia C, McColgan B, Djedjos CS, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Muir AJ, Afdhal NH, Bosch J, Goodman Z. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology. 2019;70:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 275] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 13. | Bassegoda O, Olivas P, Turco L, Mandorfer M, Serra-Burriel M, Tellez L, Kwanten W, Laroyenne A, Farcau O, Alvarado E, Moga L, Vuille-Lessard E, Fortea JI, Ibañez L, Tosetti G, Vanwolleghem T, Larrue H, Burgos-Santamaría D, Stefanescu H, Paternostro R, Cippitelli A, Lens S, Augustin S, Llop E, Laleman W, Trebicka J, Chang J, Masnou H, Zipprich A, Miceli F, Semmler G, Forns X, Primignani M, Bañares R, Puente A, Berzigotti A, Rautou PE, Villanueva C, Ginès P, Garcia-Pagan JC, Procopet B, Bureau C, Albillos A, Francque S, Reiberger T, Schepis F, Graupera I, Hernandez-Gea V. Decompensation in Advanced Nonalcoholic Fatty Liver Disease May Occur at Lower Hepatic Venous Pressure Gradient Levels Than in Patients With Viral Disease. Clin Gastroenterol Hepatol. 2022;20:2276-2286.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, Procopet B, Bosch J, Genesca J, Berzigotti A; Anticipate Investigators. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The "Anticipate" study. Hepatology. 2016;64:2173-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 15. | Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, Stefanescu H, Ma MM, Mandorfer M, Mergeay-Fabre M, Procopet B, Schwabl P, Ferlitsch A, Semmler G, Berzigotti A, Tsochatzis E, Bureau C, Reiberger T, Bosch J, Abraldes JG, Genescà J. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am J Gastroenterol. 2021;116:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Segna D, Mendoza YP, Lange NF, Rodrigues SG, Berzigotti A. Non-invasive tools for compensated advanced chronic liver disease and portal hypertension after Baveno VII - an update. Dig Liver Dis. 2023;55:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 17. | Dajti E, Ravaioli F, Zykus R, Rautou PE, Elkrief L, Grgurevic I, Stefanescu H, Hirooka M, Fraquelli M, Rosselli M, Chang PEJ, Piscaglia F, Reiberger T, Llop E, Mueller S, Marasco G, Berzigotti A, Colli A, Festi D, Colecchia A; Spleen Stiffness—IPD-MA Study Group. Accuracy of spleen stiffness measurement for the diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a systematic review and individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 18. | Wong YJ, Zhaojin C, Tosetti G, Degasperi E, Sharma S, Agarwal S, Chuan L, Huak CY, Jia L, Xiaolong Q, Saraya A, Primignani M. Baveno-VII criteria to predict decompensation and initiate non-selective beta-blocker in compensated advanced chronic liver disease patients. Clin Mol Hepatol. 2023;29:135-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Ma X, Wang L, Wu H, Feng Y, Han X, Bu H, Zhu Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS One. 2016;11:e0165786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Song J, Huang J, Huang H, Liu S, Luo Y. Performance of spleen stiffness measurement in prediction of clinical significant portal hypertension: A meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, Marzi L, Arena U, Pinzani M, Festi D. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 22. | Takuma Y, Morimoto Y, Takabatake H, Toshikuni N, Tomokuni J, Sahara A, Matsueda K, Yamamoto H. Measurement of Spleen Stiffness With Acoustic Radiation Force Impulse Imaging Predicts Mortality and Hepatic Decompensation in Patients With Liver Cirrhosis. Clin Gastroenterol Hepatol. 2017;15:1782-1790.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Wang H, Wen B, Chang X, Wu Q, Wen W, Zhou F, Guo Y, Ji Y, Gu Y, Lai Q, He Q, Li J, Chen J, Hou J. Baveno VI criteria and spleen stiffness measurement rule out high-risk varices in virally suppressed HBV-related cirrhosis. J Hepatol. 2021;74:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Dajti E, Villanueva C, Berzigotti A, Brujats A, Albillos A, Genescà J, García-Pagán JC, Colecchia A, Bosch J; PREDESCI trial investigators; A study by the Baveno Cooperation, an EASL Consortium. Exploring algorithms to select candidates for non-selective beta-blockers in cirrhosis: A post hoc analysis of the PREDESCI trial. J Hepatol. 2025;82:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Giuffrè M, Dupont J, Visintin A, Masutti F, Monica F, You K, Shung DL, Crocè LS; NSBB-Elasto-Response-Prediction Group. Predicting response to non-selective beta-blockers with liver-spleen stiffness and heart rate in patients with liver cirrhosis and high-risk varices. Hepatol Int. 2025;19:460-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Buechter M, Manka P, Theysohn JM, Reinboldt M, Canbay A, Kahraman A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018;50:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Stefanescu H, Marasco G, Calès P, Fraquelli M, Rosselli M, Ganne-Carriè N, de Ledinghen V, Ravaioli F, Colecchia A, Rusu C, Andreone P, Mazzella G, Festi D. A novel spleen-dedicated stiffness measurement by FibroScan® improves the screening of high-risk oesophageal varices. Liver Int. 2020;40:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Dhindsa BS, Tun KM, Fiedler A, Deliwala S, Saghir SM, Scholten K, Ramai D, Girotra M, Chandan S, Dhaliwal A, Bhat I, Singh S, Adler DG. Endoscopic ultrasound-guided portal pressure gradient measurement: a systematic review and meta-analysis. Ann Gastroenterol. 2024;37:356-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Martinez-Moreno B, Martínez Martínez J, Herrera I, Guilabert L, Rodríguez-Soler M, Bellot P, Miralles C, Pascual S, Irúrzun J, Zapater P, Palazón-Azorín JM, Gil Guillén V, Jover R, Aparicio JR. Correlation of endoscopic ultrasound-guided portal pressure gradient measurements with hepatic venous pressure gradient: a prospective study. Endoscopy. 2025;57:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Zhang W, Peng C, Zhang S, Huang S, Shen S, Xu G, Zhang F, Xiao J, Zhang M, Zhuge Y, Wang L, Zou X, Lv Y. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 31. | Reverter E, Blasi A, Abraldes JG, Martínez-Palli G, Seijo S, Turon F, Berzigotti A, Balust J, Bosch J, García-Pagán JC. Impact of deep sedation on the accuracy of hepatic and portal venous pressure measurements in patients with cirrhosis. Liver Int. 2014;34:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Benmassaoud A, Chen YI. Sedation during EUS-guided portal pressure gradient measurement: the elephant in the room. Gastrointest Endosc. 2022;96:690-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Chen Y, Deschenes M, Wong P, Sebastiani G, Chen T, Benmassaoud A. Endoscopic Ultrasound-guided Portal Pressure Gradient Measurement Is Technically Successful and Safe Without Deep Sedation: A Proof-of-Concept Case Series. Tech Innovat Gastroi. 2022;24:402-403. [DOI] [Full Text] |
| 34. | Kolb JM, Monachese M, Rubin RA, Wang TJ, Choi A, Bazarbashi AN, Brahmbhatt B, Zakaria A, Cortes P, Kesar V, Abel WF, Chen WP, McLaren C, Tavangar A, Singal AG, Taunk P, Wallace MB, Kedia P, Lee D, Abbas A, Yeaton P, Cosgrove N, Kesar V, Chang KJ, Ryou M, Samarasena J. Endoscopic Ultrasound-Guided Portosystemic Pressure Gradient Correlates with Clinical Parameters and Liver Histology. Clin Gastroenterol Hepatol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Abimansour JP, Chin JY, Vargas EJ, Kaur J, Abu Dayyeh BK, Law RJ, Garimella V, Levy MJ, Storm AC, Dierkhising R, Allen A, Chandrasekhara V. EUS-based shear wave elastography of the spleen for detection of clinically significant portal hypertension. iGIE. 2024;3:507-511. [DOI] [Full Text] |
| 36. | Diehl DL, Sangwan V, Khurana S, Khara HS, Zhang J, Confer BD. Reproducibility of EUS-guided shear wave elastography for assessment of hepatic fibrosis: a prospective pilot cohort study. Gastrointest Endosc. 2025;101:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Wang TJ, Jirapinyo P, Shah R, Schuster K, Papke DJ, Thompson CC, Doyon L, Lautz DB, Ryou M. EUS-guided shear wave elastography for fibrosis screening in patients with obesity and metabolic dysfunction-associated steatotic liver disease: a pilot study (with video). Gastrointest Endosc. 2025;101:456-462.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Iglesias-García J, Domínguez-Muñoz JE. Standardization of endoscopic ultrasound shear wave elastography. Clin Endosc. 2023;56:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, Bosch J; Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 40. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 41. | Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer JM, Alvarez-Urturi C, Torras X, Balanzó J, Guarner C. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 42. | Sharma M, Singh S, Desai V, Shah VH, Kamath PS, Murad MH, Simonetto DA. Comparison of Therapies for Primary Prevention of Esophageal Variceal Bleeding: A Systematic Review and Network Meta-analysis. Hepatology. 2019;69:1657-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 43. | Dardari L, Taha M, Dahat P, Toriola S, Satnarine T, Zohara Z, Adelekun A, Seffah KD, Salib K, Arcia Franchini AP. The Efficacy of Carvedilol in Comparison to Propranolol in Reducing the Hepatic Venous Pressure Gradient and Decreasing the Risk of Variceal Bleeding in Adult Cirrhotic Patients: A Systematic Review. Cureus. 2023;15:e43253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Turco L, Reiberger T, Vitale G, La Mura V. Carvedilol as the new non-selective beta-blocker of choice in patients with cirrhosis and portal hypertension. Liver Int. 2023;43:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 45. | Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, Heinisch BB, Trauner M, Kramer L, Peck-Radosavljevic M; Vienna Hepatic Hemodynamic Lab. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 46. | Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR, Hayes PC. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 47. | Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1521-1530.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 48. | Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F, Calleja JL, Albillos A, Bosch J; BLEPS Study Group. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160-1170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 49. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 459] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 50. | Tan HK, James PD, Wong F. Albumin May Prevent the Morbidity of Paracentesis-Induced Circulatory Dysfunction in Cirrhosis and Refractory Ascites: A Pilot Study. Dig Dis Sci. 2016;61:3084-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 74.0] [Reference Citation Analysis (1)] |
| 52. | Hassanein TI, Tofteng F, Brown RS Jr, McGuire B, Lynch P, Mehta R, Larsen FS, Gornbein J, Stange J, Blei AT. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 53. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V; RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 54. | Bañares R, Ibáñez-Samaniego L, Torner JM, Pavesi M, Olmedo C, Catalina MV, Albillos A, Larsen FS, Nevens F, Hassanein T, Schmidt H, Heeman U, Jalan R, Moreau R, Arroyo V. Meta-analysis of individual patient data of albumin dialysis in acute-on-chronic liver failure: focus on treatment intensity. Therap Adv Gastroenterol. 2019;12:1756284819879565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Altraif I, Handoo FA, Aljumah A, Alalwan A, Dafalla M, Saeed AM, Alkhormi A, Albekairy AK, Tamim H. Effect of erythromycin before endoscopy in patients presenting with variceal bleeding: a prospective, randomized, double-blind, placebo-controlled trial. Gastrointest Endosc. 2011;73:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Rahman R, Nguyen DL, Sohail U, Almashhrawi AA, Ashraf I, Puli SR, Bechtold ML. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta-analysis and systematic review. Ann Gastroenterol. 2016;29:312-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Song JE, Kim BS. Endoscopic Therapy and Radiologic Intervention of Acute Gastroesophageal Variceal Bleeding. Clin Endosc. 2019;52:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Escorsell À, Pavel O, Cárdenas A, Morillas R, Llop E, Villanueva C, Garcia-Pagán JC, Bosch J; Variceal Bleeding Study Group. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: A multicenter randomized, controlled trial. Hepatology. 2016;63:1957-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 59. | Rodrigues SG, Cárdenas A, Escorsell À, Bosch J. Balloon Tamponade and Esophageal Stenting for Esophageal Variceal Bleeding in Cirrhosis: A Systematic Review and Meta-analysis. Semin Liver Dis. 2019;39:178-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Téllez L, Ibáñez-Samaniego L, Pérez Del Villar C, Yotti R, Martínez J, Carrión L, Rodríguez de Santiago E, Rivera M, González-Mansilla A, Pastor Ó, Bermejo J, Bañares R, Albillos A. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 61. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 62. | Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016;63:1968-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 63. | Wong RJ, Robinson A, Ginzberg D, Gomes C, Liu B, Bhuket T. Assessing the safety of beta-blocker therapy in cirrhosis patients with ascites: A meta-analysis. Liver Int. 2019;39:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, Ferguson JW. Non-selective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015;64:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 65. | Sinha R, Lockman KA, Mallawaarachchi N, Robertson M, Plevris JN, Hayes PC. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol. 2017;67:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 66. | D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 67. | Albillos A, Zamora J, Martínez J, Arroyo D, Ahmad I, De-la-Peña J, Garcia-Pagán JC, Lo GH, Sarin S, Sharma B, Abraldes JG, Bosch J, Garcia-Tsao G; Baveno Cooperation. Stratifying risk in the prevention of recurrent variceal hemorrhage: Results of an individual patient meta-analysis. Hepatology. 2017;66:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 68. | Singh S, Chandan S, Inamdar S, Kadkhodayan KS, Dhar J, Samanta J, Facciorusso A. EUS-Guided Vascular Interventions: Recent Advances. J Clin Med. 2024;13:4835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Sabry F, Seif S, Eldesoky A, Hakim H, Altonbary AY. EUS-guided cyanoacrylate injection into the perforating vein versus direct endoscopic injection in the treatment of gastric varices. Endosc Int Open. 2023;11:E202-E210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Samanta J, Nabi Z, Facciorusso A, Dhar J, Akbar W, Das A, Birda CL, Mangiavillano B, Auriemma F, Crino SF, Kochhar R, Lakhtakia S, Reddy DN. EUS-guided coil and glue injection versus endoscopic glue injection for gastric varices: International multicentre propensity-matched analysis. Liver Int. 2023;43:1783-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 71. | Wang Z, Zeng Z, Chen L, Shi C, Jin J, Zhang F, Zhang Q, Mei X, Kong D. Endoscopic ultrasonography-guided injection of cyanoacrylate in the treatment of gastroesophageal varices type 1: a single-center randomized study. Surg Endosc. 2023;37:8277-8284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 72. | O'Rourke J, Todd A, Shekhar C, Forde C, Pallan A, Wadhwani S, Tripathi D, Mahon BS. EUS-guided thrombin injection and coil implantation for gastric varices: feasibility, safety, and outcomes. Gastrointest Endosc. 2024;100:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Tominaga S, Kobayashi M, Kato H. Endoscopic ultrasound sclerotherapy for refractory hepatic encephalopathy associated with rectal varices. Dig Endosc. 2024;36:382-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Okuno N, Hara K, Haba S, Kuwahara T, Urata M, Kondo T, Yamamoto Y. Endoscopic ultrasound-guided vascular intervention for pancreaticojejunal variceal bleeding. Endoscopy. 2024;56:E329-E330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 75. | Bahdi F, George R, Patel K. EUS-guided coiling and cyanoacrylate injection of ectopic duodenal varices. VideoGIE. 2021;6:35-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Dhar J, Bharath NP, Mahajan G, Bhujade H, Gupta P, Facciorusso A, Samanta J. Bleeding parastomal varices in a case of decompensated cirrhosis with tubercular abdominal cocoon: endoscopic ultrasound-guided angioembolization with coil and glue to the rescue. Endoscopy. 2024;56:E439-E440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Manolakis A, Tsagkidou K, Koumarelas KE. Endoscopic ultrasound-guided therapies in the treatment of gastric varices: An in-depth examination of associated adverse events. World J Gastrointest Endosc. 2024;16:640-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 78. | Lee EW, Eghtesad B, Garcia-Tsao G, Haskal ZJ, Hernandez-Gea V, Jalaeian H, Kalva SP, Mohanty A, Thabut D, Abraldes JG. AASLD Practice Guidance on the use of TIPS, variceal embolization, and retrograde transvenous obliteration in the management of variceal hemorrhage. Hepatology. 2024;79:224-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 79. | Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 80. | Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, Casanovas G, Bosch J, Lv Y, Thabut D, Fan D, Hernández-Gea V, García-Pagán JC; Preemptive TIPS Individual Data Metanalysis, International Variceal Bleeding Study and Baveno Cooperation Study groups. Effects of Early Placement of Transjugular Portosystemic Shunts in Patients With High-Risk Acute Variceal Bleeding: a Meta-analysis of Individual Patient Data. Gastroenterology. 2021;160:193-205.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 81. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 833] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 82. | Hernández-Gea V, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Ibañez-Samaniego L, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud LL, Noronha Ferreira C, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Robic MA, Conejo I, Catalina MV, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Garcia-Pagán JC; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS Improves Outcome in High-Risk Variceal Bleeding: An Observational Study. Hepatology. 2019;69:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 83. | Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Silva-Junior G, Martinez J, Genescà J, Bureau C, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud L, Ferreira CN, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Weiss E, Catalina MV, Erasmus HP, Uschner FE, Schulz M, Brol MJ, Praktiknjo M, Chang J, Krag A, Nevens F, Calleja JL, Robic MA, Conejo I, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Pavesi M, Garcia-Pagán JC, Jansen C, Bañares R; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 84. | Bureau C, Thabut D, Jezequel C, Archambeaud I, D'Alteroche L, Dharancy S, Borentain P, Oberti F, Plessier A, De Ledinghen V, Ganne-Carrié N, Carbonell N, Rousseau V, Sommet A, Péron JM, Vinel JP. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt : A Randomized Controlled Trial. Ann Intern Med. 2021;174:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 85. | Copelan A, Kapoor B, Sands M. Transjugular intrahepatic portosystemic shunt: indications, contraindications, and patient work-up. Semin Intervent Radiol. 2014;31:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 86. | Baliss M, Patel D, Madi MY, Bazarbashi AN. EUS-Guided Vascular Interventions. J Clin Med. 2023;12:2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 87. | Schulman AR, Ryou M, Aihara H, Abidi W, Chiang A, Jirapinyo P, Sakr A, Ajeje E, Ryan MB, Thompson CC. EUS-guided intrahepatic portosystemic shunt with direct portal pressure measurements: a novel alternative to transjugular intrahepatic portosystemic shunting. Gastrointest Endosc. 2017;85:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 395] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 89. | Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 234] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 90. | Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 91. | DiMartini A, Cruz RJ Jr, Dew MA, Myaskovsky L, Goodpaster B, Fox K, Kim KH, Fontes P. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 92. | Tapper EB, Zhang P, Garg R, Nault T, Leary K, Krishnamurthy V, Su GL. Body composition predicts mortality and decompensation in compensated cirrhosis patients: A prospective cohort study. JHEP Rep. 2020;2:100061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Mauro E, Crespo G, Martinez-Garmendia A, Gutierrez-Acevedo MN, Diaz JM, Saidman J, Bermudez C, Ortiz-Patron J, Garcia-Olveira L, Zalazar F, Narvaez A, Spina JC, Orta R, Savluk L, Piano S, Marciano S, Gadano A. Cystatin C and Sarcopenia Predict Acute on Chronic Liver Failure Development and Mortality in Patients on the Liver Transplant Waiting List. Transplantation. 2020;104:e188-e198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 94. | Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB, Kneteman N. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 95. | Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 96. | Ando Y, Ishigami M, Ito T, Ishizu Y, Kuzuya T, Honda T, Ishikawa T, Fujishiro M. Sarcopenia impairs health-related quality of life in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31:1550-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 98. | Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 99. | Benmassaoud A, Roccarina D, Arico F, Leandro G, Yu B, Cheng F, Yu D, Patch D, Tsochatzis E. Sarcopenia Does Not Worsen Survival in Patients With Cirrhosis Undergoing Transjugular Intrahepatic Portosystemic Shunt for Refractory Ascites. Am J Gastroenterol. 2020;115:1911-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 100. | Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 700] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 101. | Reiberger T, Hofer BS. The Baveno VII concept of cirrhosis recompensation. Dig Liver Dis. 2023;55:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |