Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.104533
Revised: April 17, 2025
Accepted: June 4, 2025
Published online: June 27, 2025
Processing time: 183 Days and 22.2 Hours
Chronic hepatitis B virus (HBV) infection remains a major health burden world
Core Tip: Despite the advancements in hepatitis B virus (HBV) immunology, the mechanism by which hepatocytes exert their innate immune responses against HBV infection remains poorly understood. This article focused on the complex interplay between hepatocytes and the innate immune response during HBV infection and summarized the recent findings about how HBV evading the innate response to promoter viral persistence. These insights may be helpful for developing novel anti-HBV immune-based therapies.
- Citation: Chen P, Zhao J, Chen NK, Chen ZY. Hepatocyte-intrinsic innate immunity in hepatitis B virus infection: A focused review. World J Hepatol 2025; 17(6): 104533
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/104533.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.104533
Although there have been effective prophylactic vaccines for tens of years, chronic hepatitis B (CHB) virus (HBV) infection remains a major public health problem[1]. The World Health Organization estimated that, in 2022, about 296 million people had CHB worldwide, and 1.1 million people died of hepatitis-B-related complications, especially liver cirrhosis and hepatocellular carcinoma[2].
HBV is a small, enveloped DNA virus with unique replication mechanisms characterized by a protein-primed reverse transcription process that relates to but differs from retroviral replication[3,4]. Upon entering the host hepatocytes, the virus releases its partially double-strand, relaxed circular DNA (rcDNA) genome into the nucleus, where the rcDNA is converted to covalently closed circular DNA (cccDNA), which acts as the transcription template for all the viral RNAs, including the pregenomic RNA (pgRNA) and subgenomic mRNAs encoding seven viral antigens: DNA polymerase (P protein); core protein (HBc); precore protein (or HBe); X protein (HBx); and three coterminal surface proteins (large, middle and small HBs). The viral replication begins with the encapsidation of pgRNA into the progeny capsid, where the pgRNA is reverse transcribed by the co-packaged P protein to new rcDNA genomes[3,5]. The majority of newly formed rcDNA-containing-capsids are enveloped and released from the cell as progeny virions, but some mature capsids can be recycled back to the nucleus, where their rcDNA is released and reconverted to cccDNAs to replenish the cccDNA pool[6-9].
It is believed that adaptive immunity is largely responsible for HBV clearance upon infection[10]. The robust HBV-specific cytotoxic CD8+ T cells response directly contributes to the spontaneous resolution of infection[11]; while CD4+ T cells facilitate the activation, proliferation and maintenance of CD8+ T cells[12]. In addition, the neutralizing antibody response can prevent HBV from entering hepatocytes. In most acutely infected people, the cellular and humoral immune responses can clear the infectious virions from the body and induce life-long immunity[13,14]. Unfortunately, for a minority of infected people, the virus persists; probably due to the weak or impaired immune responses[15]. Multiple mechanisms for adaptive immunity evasion have been established. For example, high viral load and long-term exposure to excessive circulating HBs and HBe leads to T cell exhaustion in CHB patients[16].
However, if and how HBV induces an innate immune response remains controversial. HBV has been designated as a stealth virus that does not activate innate antiviral responses[17]. Nevertheless, the current prevailing view is that the innate immune system can sense HBV infection but the virus has evolved efficient strategies to antagonize the antiviral activity exerted by innate immunity[18-21]. Accumulating evidence supports that the innate immune system is also important to control HBV infection, by which it limits viral entry, replication and packaging, and instructs the adaptive immune system[22]. For instance, HBV replication in humans is generally undetectable until about 1 month after in
The dynamic interaction between virus and host immune defenses determines the pathogenesis of HBV[25]. While viral clearance is association with T-cell-mediated cytolytic elimination of infected hepatocytes, emerging evidence underscores the critical role of noncytolytic control mechanisms driven by innate immune components. Pattern re
Harnessing the host’s intrinsic antiviral defenses–through targeted immunomodulation (e.g., PRR agonists and cy
There are three types of host cells involved in anti-HBV innate immunity: Hepatocytes; hepatic nonparenchymal cells, such as liver sinusoidal endothelial cells and Kupffer cells; and conventional innate immune cells, such as dendritic cells and natural killer cells[29]. Hepatocytes, which are the parenchyma cells in the liver, are the target of HBV infection and the hepatocyte-intrinsic innate immunity provides the first line of defense against viral invasion[19]. This review focuses on summarizing how HBV triggers and escapes the hepatocyte-intrinsic innate immune response.
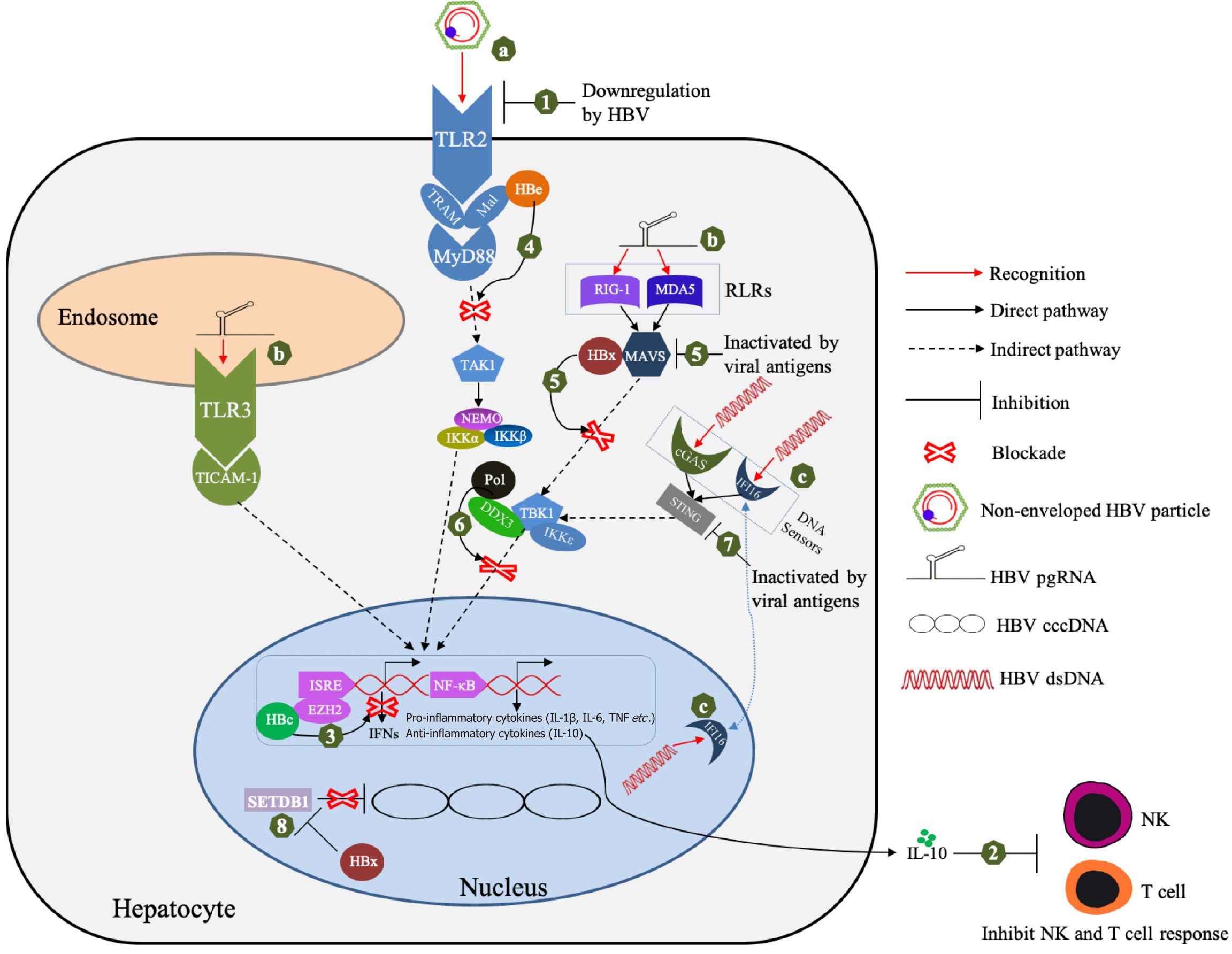
During infection, the innate immune system senses the pathogen-associated molecular patterns (PAMPs) of viruses via diverse host PRRs, including toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), and DNA receptors like cyclic GMP-AMP synthase (cGAS) and the absent in melanoma 2-like receptors (ALRs)[26]. The PRRs recruit their distinct adaptor molecules, triggering the downstream antiviral signaling cascade, inducing IFNs and other inflammatory cytokines.
The human TLRs, comprising 10 members, localize either on the cell surface or in intracellular vesicles[29]. TLR2 locates to the cellular membrane and can recognize a wide range of PAMPs, including viral glycoproteins and bacteria cell wall components such as lipoteichoic acid, peptidoglycan and porins[30]. It was demonstrated in an in vitro study that primary human hepatocytes (PHHs) can sense HBV particles via TLR2, leading to the activation of anti-HBV innate immune response[28]. Further evidence has suggested that the TLR2 signaling pathway is activated by HBc; the glycoprotein at the center of HBV particles[31]. TLR3 is located on endosomal membranes and is responsible for recognition of viral double-stranded RNA[32]. Based on the results from PH5CH8 cell line, Li et al[33] concluded that poly (I-C), a double-strand RNA analog, can activate TLR3-dependent antiviral signaling pathways in hepatocytes. TLR3 Ligands inhibit HBV replication via activation of innate immunity in PHHs[34].
RLRs, including RIG-I and melanoma differentiation-associated protein 5 (MDA-5), are the major innate sensors for viral RNAs within cytosol[35]. Several studies have suggested that different RLR members recognize the viral RNAs derived from different HBV genotypes[20]. Sato et al[36] found that PHHs can sense HBV genotype A, B and C infection via RIG-I-mediated recognition of the 5’-epsilon (ε) stem-loop structure in HBV pgRNA, leading to the production of type III IFNs; while Lu et al[37] reported that HBV genotype D is sensed by MDA-5, but not RIG-I, based on the results from hepatoma cell line Huh-7 transfected with a HBV replicon plasmid.
NLRs, another class of intracellular sensors for PAMPs, comprise 22 members in humans; among which, NLR family pyrin domain-containing 3 (NLRP3), located in hepatocytes and other cell types, is the most well-studied inflammasome sensors[38]. Xie et al[39] reported that HBx can activate the NLRP3 inflammasome in human hepatocyte cell line HL7702 under oxidative stress. Based on the analysis of liver biopsies from patients with CHB, NLRP3 inflammasomes are activated in the liver[40]. HBx plays key roles in NLRP3-associated liver inflammation by inducing the release of reactive oxygen species[40-42].
As a typical DNA virus, at least two types of HBV dsDNA (namely rcDNA and cccDNA) may be recognized by intracellular DNA sensors[19]. Several in vitro studies have illustrated that these HBV dsDNA, either as the whole genomes or DNA fragments, can activate anti-HBV innate immunity via the cGAS-STING pathway[43-46]. Recently, IFI16, a member of the ALRs, has been identified as a unique innate sensor in hepatocyte nuclei for HBV cccDNA[47]. Binding of IFI16 to cccDNA not only leads to activation of antiviral innate immune response but also performs direct epigenetic inhibition of cccDNA transcription by targeting an IFN-stimulated response element present in cccDNA[47].
In sharp contrast to the strong IFN response to hepatitis C virus infection, the lack of detectable hepatic expression of immune-related genes, especially IFN genes and ISGs, in chimpanzees with acute HBV infection leads to the hypothesis that HBV may passively avoid innate immunity[17,48]. After entering host hepatocytes, the viral rcDNA genome, rather than exposed to intracellular DNA sensor, is encapsulated within the capsid, which protects rcDNA from innate recognition[21]. Next, the nucleocapsid-encapsulated rcDNA is transported to the cell nuclei and converted to cccDNA[49]. In hepatocyte nuclei, the cccDNA that serves as the viral original replication template is organized as a minichromosome and mimics the host chromosomes structurally. This appears to be a key strategy to avoid the sensing of cccDNA[21]. Viral RNAs are major targets for innate sensor recognition, but all the subgenomic HBV RNAs transcribed from cccDNA are similar to host mRNAs by their 5’-cap and 3’-poly (A) tail, thus they are ignored by RNA sensors[21]. Among all the viral RNA transcripts, only pgRNA contains an unusual ε stem-loop structure that is prone to recognition[21,50]. However, the pgRNA is rapidly encapsidated by HBc, sequestering it from cytosolic PRRs.
HBV may actively suppress the hepatocyte-intrinsic innate immune response by multiple mechanisms, including downregulating PPRs and their adaptor proteins, interfering with inflammatory signaling pathways, as well as altering epigenetic modification[51,52] (Figure 1). During chronic infection, HBV suppresses expression of innate sensors, leading to an impaired innate response and IFN production[53]. Analysis of liver biopsies from a large cohort of CHB patients has shown that many innate immunity-related genes, including ISGs that encode TLRs/adaptor proteins, type I/III IFN and other proinflammatory chemokines/cytokines are significantly downregulated[54]. By comparing the cytokine expre
HBV proteins, including P protein, HBc, HBe, HBs and HBx, may contribute to inhibiting innate immune response by diverse mechanisms[18,19,59]. Lei et al[60] reported that IFN-α resistance is association with lower levels of IL-1β in CHB patients, while in vitro experiments indicated that HBV P protein is responsible for suppression of inflammasome activation and IL-1β production. To counteract the TLRs/RLRs pathways sensing viral dsRNA, HBV P protein can directly bind to DEAD-box RNA helicase 3 (DDX3) and block its interaction with TANK-binding kinase 1 (TBK1)/IκB kinase epsilon (IKKε) complex, subsequently inhibiting IFN regulatory factor 3 activation and IFN-β production[61,62]. P protein can suppress nuclear factor (NF)-κB signaling by inhibiting activity of IKKs via interaction with heat shock protein 90β[63,64]. Besides interfering with RNA-sensing pathway, HBV P protein can block the innate DNA-sensing pathways, which results in suppression of IFN-β synthesis by direct interaction with the stimulator of interferon genes (STING) and subsequent disruption of its K63-linked ubiquitination[65]. HBc was shown to inhibit the expression of IFN-induced GTP-binding protein MxA at transcriptional level by directly interacting with the promoter region of MxA, thus contributing to impairment of the antiviral effect exerted by IFN[66]. At an early time after infection, HBc from incoming HBV can quickly recruit the histone-methyltransferase EZH2 onto targeted gene promoters, resulting in the epigenetic downregulation of IFNs and ISG expression in hepatocytes[18,67].
HBe and HBs are the two secreted viral proteins involved in multiple immune evasion processes[18]. They have been reported to inhibit IFN-β synthesis by suppressing MAVS expression and interrupting the binding of MAVS to RIG-I[53]. They are able to antagonize the activation of TLR pathways in murine hepatocytes and hepatic nonparenchymal cells[18,68]. Mechanistically, HBe, but not HBc, can interact with the specific Toll/IL-1 receptor (TIR)-containing proteins TRAM and Mal, thus disrupting the homotypic TIR: TIR interaction that is critical for TLR-mediated signaling, finally su
As a multifunctional viral regulator of cellular signal transduction and transcription pathways, HBx can negatively regulate the innate response through diverse strategies. To abolish extracellular IFN-α-mediated signal transduction, HBx can downregulate IFN-α receptor expression in hepatocytes[72]. To antagonize the intracellular RNA-sensing pathway, HBx can interfere with RIG-I/MAVS signaling by ubiquitination of MAVS[73,74]. Surprisingly, HBx can also function as a deubiquitinating enzyme to cleave K63-linked polyubiquitin chains from many critical proteins involving in the signaling cascades of IFN-I induction, including IRF3, IRF7, RIG-I, RIG-I 2CARD, TRAF3 and IKKi, thus blocking subsequent antiviral signal transduction[75]. In addition, HBx suppresses the transcription of TRIM22 (a mediator of IFN-induced antiviral response) by inducing a single CpG (+71) methylation in its 5′-UTR, demonstrating an epigenetic regulation-related mechanism of IFN resistance[76]. HBx can also act as an epigenetic modulator for the chromatin structure of HBV cccDNA minichromosome. For example, it can relieve the transcriptional silencing of cccDNA mediated by SET domain bifurcated histone lysine methyltransferase 1, a major histone methyltransferase responsible for meth
Recently, the N6 methyladenosine (m6A) epitranscriptomic modification of viral pgRNA and host PTEN (phosphatase and tensin homolog; a nIRF-3 nuclear translocation) mRNA was identified a novel strategy to antagonize the innate response[79,80]. As discussed earlier, the 5’-ε stem-loop structure of HBV pgRNA is a target for RIG-I recognition. However, the m6A modification on 5’-ε of pgRNA recruits the m6A reader proteins YTHDF2 and YTHDF3, thus sequestering HBV pgRNA from cytosolic RIG-I[80]. As for PTEN mRNA, its m6A modifications induced by HBV infection destabilize the transcript with a corresponding decrease in PTEN protein levels, leading to inhibition of IRF-3 nuclear import and subsequent IFN synthesis[79]. Specifically, HBx was identified as a key modulator for regulating the HBV-induced m6A modification of pgRNA and PTEN mRNA. During HBV infection, HBx recruits the m6A methyltransferases (METTL3 and 14) onto HBV cccDNA and PTEN chromosomal loci, and achieves cotranscriptional m6A modification of their corresponding RNA transcripts; namely, HBV pgRNA and PTEN mRNA, respectively[80,81].
IFN-α, the only approved anti-HBV immunotherapeutic, can occasionally result in functional cure of CHB in some patients, but it suffers severe systemic side effects as well as poor response rate[82]. This has necessitated development of novel anti-HBV immunotherapies with minimal side effects and increased therapeutic effects. The latest clinical progress in anti-HBV immunotherapeutic referring to innate immunity is summarized (Table 1).
| Drug name | Target | Sponsor | Phase | Ref. |
| Vesatolimod | TLR7 agonist | Gilead | II | [87] |
| Ruzotolimod | TLR7 agonist | Roche | II | [88] |
| JNJ-64794964 (JNJ-4964/AL-034/TQ-A3334) | TLR7 agonist | Chia Tai Tianqing/Janssen | II | [95] |
| Selgantolimod | TLR8 agonist | Gilead | II | [89,90] |
| Cavrotolimod | TLR9 agonist | Bluejay | I | [96] |
| Inarigivir | RIG-I agonist | Spring bank/Gilead | II (terminated) | [91] |
| Recombinant IL2 | IL2 | University of Science and Technology of China | Investigator-Initiated Trial | [85] |
Apart from IFNs, the efficacy and safety of several other cytokines, including IL-12 and IL-2, have been evaluated in CHB patients. As early as 2000–2001, two phase I/II clinical studies have demonstrated that treatment of CHB with IL-12 is safe and tolerable, and appears to be active against HBV infection in terms of leading to HBV DNA and HBeAg clearance in some patients, but it had no advantage in comparison to currently available treatments[83,84]. A recent clinical study reported that the sequential administration of recombinant IL-2 is effective in nonresponder patients after IFN-α therapy by restoring HBV-specific CD8+ T-cell responses, suggesting the CD8 cis-targeted sequential IL-2 therapy may be a promising treatment for refractory CHB[85,86].
TLR and RIG-I agonists represent a novel promising immunotherapeutic strategy against chronic HBV infection. TLR agonists such as vesatolimod (formerly GS-9620; TLR7 agonist), ruzotolimod (formerly RO7020531 or RG-7854; TLR7 agonist) and selgantolimod (formerly GS-9688; TLR8 agonist) are well tolerated and can lead to serological changes associated with progression to durable functional cure in a small number of CHB patients[87-90]. The RIG-I agonist inarigivir (formerly SB9200) stimulates prolonged IFN-α/β secretion and broad ISGs activation, with clinical data suggesting superior activity for reduction of HBsAg compared to nucleos(t)ide analogs such as entecavir[91]. However, the clinical development of inarigivir was terminated due to safety concerns[92].
Research into the immune escape mechanisms of HBV has potential for developing therapies that disrupt viral persistence. Future research should prioritize targeting the stealth strategies of HBV. Novel PRR agonists or inhibitors of HBV-encoded immune antagonists (e.g., HBx or HBs) could restore innate sensing and amplify antiviral responses.
A deeper understanding of host–virus coevolution is also essential. The ability of HBV to epigenetically silence cccDNA and mimic host chromatin modifications highlights the need for therapies that reverse viral epigenetic camouflage, such as histone deacetylase inhibitors or other transcriptional activators to expose viral DNA to immune detection. The unraveling of heterogeneity in innate immune evasion across infected hepatocytes, by using cutting-edge technologies such as single-cell multiomics, will help to guide personalized therapies.
It is well established that the activation of PRR signaling and promotion of IFN synthesis play important roles in the control of HBV infection. However, to maintain persistent infection, HBV has evolved multiple sophisticated mechanisms to evade the antiviral innate immune response. The comprehensive understanding of the interactions between HBV and innate immune system is essential for developing curative treatment strategies against HBV. Novel immunotherapeutics such as TLR/RIG-I agonists have demonstrated impressive antiviral efficacy in clinical trials, but the challenges such as immune hyperactivation risks and inefficient HBsAg loss highlight the need for combination therapies to achieve sustained functional cure. For example, a recent clinical trial has reported that the combination of TLR7 agonist (ru
The limitations in the field include the lack of physiologically relevant models that recreate the immune evasion of HBV in human hepatocytes within a functional immune microenvironment. Current in vitro systems (e.g., NTCP-reconstituted HepG2 cells) poorly model innate signaling complexity, while animal models (e.g., HBV-infected hu
We would like to thank Dr. Guochuang Chen for helpful and constructive comments during the preparation of the Figures and Tables.
| 1. | Martyn E, Eisen S, Longley N, Harris P, Surey J, Norman J, Brown M, Sultan B, Maponga TG, Iwuji C, Flanagan S, Ghosh I, Story A, Matthews PC. The forgotten people: Hepatitis B virus (HBV) infection as a priority for the inclusion health agenda. Elife. 2023;12:e81070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. Available from: https://iris.who.int/bitstream/handle/10665/376353/9789240090903-eng.pdf?sequence=1. |
| 3. | Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. 2008;134:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 692] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 5. | Niklasch M, Zimmermann P, Nassal M. The Hepatitis B Virus Nucleocapsid-Dynamic Compartment for Infectious Virus Production and New Antiviral Target. Biomedicines. 2021;9:1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 528] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Köck J, Rösler C, Zhang JJ, Blum HE, Nassal M, Thoma C. Generation of covalently closed circular DNA of hepatitis B viruses via intracellular recycling is regulated in a virus specific manner. PLoS Pathog. 2010;6:e1001082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Wu TT, Coates L, Aldrich CE, Summers J, Mason WS. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Liu F, Campagna M, Qi Y, Zhao X, Guo F, Xu C, Li S, Li W, Block TM, Chang J, Guo JT. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS Pathog. 2013;9:e1003613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Loggi E, Gamal N, Bihl F, Bernardi M, Andreone P. Adaptive response in hepatitis B virus infection. J Viral Hepat. 2014;21:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 763] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 12. | Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 722] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 13. | Ma Z, Zhang E, Gao S, Xiong Y, Lu M. Toward a Functional Cure for Hepatitis B: The Rationale and Challenges for Therapeutic Targeting of the B Cell Immune Response. Front Immunol. 2019;10:2308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Corti D, Benigni F, Shouval D. Viral envelope-specific antibodies in chronic hepatitis B virus infection. Curr Opin Virol. 2018;30:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 16. | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 17. | Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79:9369-9380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 346] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Faure-Dupuy S, Lucifora J, Durantel D. Interplay between the Hepatitis B Virus and Innate Immunity: From an Understanding to the Development of Therapeutic Concepts. Viruses. 2017;9:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Thomas E, Baumert TF. Hepatitis B Virus-Hepatocyte Interactions and Innate Immune Responses: Experimental Models and Molecular Mechanisms. Semin Liver Dis. 2019;39:301-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Xu C, Chen J, Chen X. Host Innate Immunity Against Hepatitis Viruses and Viral Immune Evasion. Front Microbiol. 2021;12:740464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 21. | Dandri M, Bertoletti A, Lütgehetmann M. Innate immunity in hepatitis B and D virus infection: consequences for viral persistence, inflammation, and T cell recognition. Semin Immunopathol. 2021;43:535-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Khanam A, Chua JV, Kottilil S. Immunopathology of Chronic Hepatitis B Infection: Role of Innate and Adaptive Immune Response in Disease Progression. Int J Mol Sci. 2021;22:5497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 23. | Villeneuve JP. The natural history of chronic hepatitis B virus infection. J Clin Virol. 2005;34 Suppl 1:S139-S142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 25. | Tsai KN, Kuo CF, Ou JJ. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018;26:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 26. | Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 1098] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 27. | Gehring AJ. New treatments to reach functional cure: Rationale and challenges for emerging immune-based therapies. Best Pract Res Clin Gastroenterol. 2017;31:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Chang J, Block TM, Guo JT. The innate immune response to hepatitis B virus infection: implications for pathogenesis and therapy. Antiviral Res. 2012;96:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Du Y, Wu J, Liu J, Zheng X, Yang D, Lu M. Toll-like receptor-mediated innate immunity orchestrates adaptive immune responses in HBV infection. Front Immunol. 2022;13:965018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 30. | Oliveira-Nascimento L, Massari P, Wetzler LM. The Role of TLR2 in Infection and Immunity. Front Immunol. 2012;3:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 546] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 31. | Cooper A, Tal G, Lider O, Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Gleeson PA. The role of endosomes in innate and adaptive immunity. Semin Cell Dev Biol. 2014;31:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Li K, Chen Z, Kato N, Gale M Jr, Lemon SM. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem. 2005;280:16739-16747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 34. | Lucifora J, Bonnin M, Aillot L, Fusil F, Maadadi S, Dimier L, Michelet M, Floriot O, Ollivier A, Rivoire M, Ait-Goughoulte M, Daffis S, Fletcher SP, Salvetti A, Cosset FL, Zoulim F, Durantel D. Direct antiviral properties of TLR ligands against HBV replication in immune-competent hepatocytes. Sci Rep. 2018;8:5390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20:537-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1164] [Cited by in RCA: 1082] [Article Influence: 216.4] [Reference Citation Analysis (0)] |
| 36. | Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 346] [Article Influence: 34.6] [Reference Citation Analysis (1)] |
| 37. | Lu HL, Liao F. Melanoma differentiation-associated gene 5 senses hepatitis B virus and activates innate immune signaling to suppress virus replication. J Immunol. 2013;191:3264-3276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Chou WC, Jha S, Linhoff MW, Ting JP. The NLR gene family: from discovery to present day. Nat Rev Immunol. 2023;23:635-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 94] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 39. | Xie WH, Ding J, Xie XX, Yang XH, Wu XF, Chen ZX, Guo QL, Gao WY, Wang XZ, Li D. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res. 2020;69:683-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, Vassiliadis T, Loli G, Phillipidis A, Zebekakis P, Germenis AE, Speletas M, Germanidis G. The role of the NLRP3 inflammasome and the activation of IL-1β in the pathogenesis of chronic viral hepatic inflammation. Cytokine. 2018;110:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Chu FX, Wang X, Li B, Xu LL, Di B. The NLRP3 inflammasome: a vital player in inflammation and mediating the anti-inflammatory effect of CBD. Inflamm Res. 2024;73:227-242. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Hurtado-Navarro L, Angosto-Bazarra D, Pelegrín P, Baroja-Mazo A, Cuevas S. NLRP3 Inflammasome and Pyroptosis in Liver Pathophysiology: The Emerging Relevance of Nrf2 Inducers. Antioxidants (Basel). 2022;11:870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Xu D, Tian Y, Xia Q, Ke B. The cGAS-STING Pathway: Novel Perspectives in Liver Diseases. Front Immunol. 2021;12:682736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Verrier ER, Yim SA, Heydmann L, El Saghire H, Bach C, Turon-Lagot V, Mailly L, Durand SC, Lucifora J, Durantel D, Pessaux P, Manel N, Hirsch I, Zeisel MB, Pochet N, Schuster C, Baumert TF. Hepatitis B Virus Evasion From Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase Sensing in Human Hepatocytes. Hepatology. 2018;68:1695-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Cui X, Clark DN, Liu K, Xu XD, Guo JT, Hu J. Viral DNA-Dependent Induction of Innate Immune Response to Hepatitis B Virus in Immortalized Mouse Hepatocytes. J Virol. 2016;90:486-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Dansako H, Ueda Y, Okumura N, Satoh S, Sugiyama M, Mizokami M, Ikeda M, Kato N. The cyclic GMP-AMP synthetase-STING signaling pathway is required for both the innate immune response against HBV and the suppression of HBV assembly. FEBS J. 2016;283:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Yang Y, Zhao X, Wang Z, Shu W, Li L, Li Y, Guo Z, Gao B, Xiong S. Nuclear Sensor Interferon-Inducible Protein 16 Inhibits the Function of Hepatitis B Virus Covalently Closed Circular DNA by Integrating Innate Immune Activation and Epigenetic Suppression. Hepatology. 2020;71:1154-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669-6674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 550] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 49. | Hu J, Tang L, Cheng J, Zhou T, Li Y, Chang J, Zhao Q, Guo JT. Hepatitis B virus nucleocapsid uncoating: biological consequences and regulation by cellular nucleases. Emerg Microbes Infect. 2021;10:852-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Bartenschlager R, Schaller H. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 1992;11:3413-3420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 245] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Broering R, Luo X, Liu J, Lu M. Controversial: Early Innate Responses to Hepatitis B Virus Infection, an Explanation for Viral Persistence? Virol Sin. 2021;36:163-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Wang Z, Liu N, Yang Y, Tu Z. The novel mechanism facilitating chronic hepatitis B infection: immunometabolism and epigenetic modification reprogramming. Front Immunol. 2024;15:1349867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Zhao HJ, Hu YF, Han QJ, Zhang J. Innate and adaptive immune escape mechanisms of hepatitis B virus. World J Gastroenterol. 2022;28:881-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 54. | Lebossé F, Testoni B, Fresquet J, Facchetti F, Galmozzi E, Fournier M, Hervieu V, Berthillon P, Berby F, Bordes I, Durantel D, Levrero M, Lampertico P, Zoulim F. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol. 2017;66:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 55. | Guo P. Suppression of interferon-mediated antiviral immunity by hepatitis B virus: an overview of research progress. Scand J Immunol. 2013;78:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Khan M, Syed GH, Kim SJ, Siddiqui A. Hepatitis B Virus-Induced Parkin-Dependent Recruitment of Linear Ubiquitin Assembly Complex (LUBAC) to Mitochondria and Attenuation of Innate Immunity. PLoS Pathog. 2016;12:e1005693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Hou ZH, Han QJ, Zhang C, Tian ZG, Zhang J. miR146a impairs the IFN-induced anti-HBV immune response by downregulating STAT1 in hepatocytes. Liver Int. 2014;34:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Zhou L, He R, Fang P, Li M, Yu H, Wang Q, Yu Y, Wang F, Zhang Y, Chen A, Peng N, Lin Y, Zhang R, Trilling M, Broering R, Lu M, Zhu Y, Liu S. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat Commun. 2021;12:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 59. | Suslov A, Wieland S, Menne S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr Opin Virol. 2018;30:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Lei Q, Li T, Kong L, Li L, Ding X, Wang X, Zhang X, Qin B. HBV-Pol is crucial for HBV-mediated inhibition of inflammasome activation and IL-1β production. Liver Int. 2019;39:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Yu S, Chen J, Wu M, Chen H, Kato N, Yuan Z. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol. 2010;91:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (3)] |
| 62. | Wang H, Ryu WS. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 2010;6:e1000986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (2)] |
| 63. | Liu D, Wu A, Cui L, Hao R, Wang Y, He J, Guo D. Hepatitis B virus polymerase suppresses NF-κB signaling by inhibiting the activity of IKKs via interaction with Hsp90β. PLoS One. 2014;9:e91658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 64. | Ortega-Prieto AM, Dorner M. Immune Evasion Strategies during Chronic Hepatitis B and C Virus Infection. Vaccines (Basel). 2017;5:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 65. | Liu Y, Li J, Chen J, Li Y, Wang W, Du X, Song W, Zhang W, Lin L, Yuan Z. Hepatitis B virus polymerase disrupts K63-linked ubiquitination of STING to block innate cytosolic DNA-sensing pathways. J Virol. 2015;89:2287-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (3)] |
| 66. | Fernández M, Quiroga JA, Carreño V. Hepatitis B virus downregulates the human interferon-inducible MxA promoter through direct interaction of precore/core proteins. J Gen Virol. 2003;84:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Gruffaz M, Testoni B, Luangsay S, Fusil F, Malika A-G, Mancip J, Petit M-A, Javanbakht H, Cosset F-L, Zoulim F, Duronfel D. The nuclear function of Hepatitis B capsid (HBc) protein is to inhibit IFN response very early after infection of hepatocytes. Hepatology. 2013;58:276A. |
| 68. | Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (4)] |
| 69. | Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (3)] |
| 70. | Wang Y, Cui L, Yang G, Zhan J, Guo L, Chen Y, Fan C, Liu D, Guo D. Hepatitis B e Antigen Inhibits NF-κB Activity by Interrupting K63-Linked Ubiquitination of NEMO. J Virol. 2019;93:e00667-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Deng F, Xu G, Cheng Z, Huang Y, Ma C, Luo C, Yu C, Wang J, Xu X, Liu S, Zhu Y. Hepatitis B Surface Antigen Suppresses the Activation of Nuclear Factor Kappa B Pathway via Interaction With the TAK1-TAB2 Complex. Front Immunol. 2021;12:618196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Cho IR, Oh M, Koh SS, Malilas W, Srisuttee R, Jhun BH, Pellegrini S, Fuchs SY, Chung YH. Hepatitis B virus X protein inhibits extracellular IFN-α-mediated signal transduction by downregulation of type I IFN receptor. Int J Mol Med. 2012;29:581-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Kim SJ, Khan M, Quan J, Till A, Subramani S, Siddiqui A. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS Pathog. 2013;9:e1003722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 74. | Wang X, Li Y, Mao A, Li C, Li Y, Tien P. Hepatitis B virus X protein suppresses virus-triggered IRF3 activation and IFN-beta induction by disrupting the VISA-associated complex. Cell Mol Immunol. 2010;7:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 75. | Jiang J, Tang H. Mechanism of inhibiting type I interferon induction by hepatitis B virus X protein. Protein Cell. 2010;1:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (4)] |
| 76. | Lim KH, Park ES, Kim DH, Cho KC, Kim KP, Park YK, Ahn SH, Park SH, Kim KH, Kim CW, Kang HS, Lee AR, Park S, Sim H, Won J, Seok K, You JS, Lee JH, Yi NJ, Lee KW, Suh KS, Seong BL, Kim KH. Suppression of interferon-mediated anti-HBV response by single CpG methylation in the 5'-UTR of TRIM22. Gut. 2018;67:166-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 77. | Rivière L, Gerossier L, Ducroux A, Dion S, Deng Q, Michel ML, Buendia MA, Hantz O, Neuveut C. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J Hepatol. 2015;63:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 78. | Liu W, Zhang X, Deng Y, Wang D, Li H. Unfolding HBx for an epigenetic switch of HBV cccDNA minichromosome. Protein Cell. 2025;. [DOI] [Full Text] |
| 79. | Kim GW, Imam H, Khan M, Mir SA, Kim SJ, Yoon SK, Hur W, Siddiqui A. HBV-Induced Increased N6 Methyladenosine Modification of PTEN RNA Affects Innate Immunity and Contributes to HCC. Hepatology. 2021;73:533-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 80. | Kim GW, Imam H, Khan M, Siddiqui A. N(6)-Methyladenosine modification of hepatitis B and C viral RNAs attenuates host innate immunity via RIG-I signaling. J Biol Chem. 2020;295:13123-13133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 81. | Kim GW, Siddiqui A. Hepatitis B virus X protein recruits methyltransferases to affect cotranscriptional N6-methyladenosine modification of viral/host RNAs. Proc Natl Acad Sci U S A. 2021;118:e2019455118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 82. | Kang L, Pan J, Wu J, Hu J, Sun Q, Tang J. Anti-HBV Drugs: Progress, Unmet Needs, and New Hope. Viruses. 2015;7:4960-4977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Carreño V, Zeuzem S, Hopf U, Marcellin P, Cooksley WG, Fevery J, Diago M, Reddy R, Peters M, Rittweger K, Rakhit A, Pardo M. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J Hepatol. 2000;32:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Zeuzem S, Carreño V. Interleukin-12 in the treatment of chronic hepatitis B and C. Antiviral Res. 2001;52:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Wang D, Fu B, Shen X, Guo C, Liu Y, Zhang J, Sun R, Ye Y, Li J, Tian Z, Wei H. Restoration of HBV-specific CD8(+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-α therapy. Signal Transduct Target Ther. 2021;6:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 86. | Andreata F, Moynihan KD, Fumagalli V, Di Lucia P, Pappas DC, Kawashima K, Ni I, Bessette PH, Perucchini C, Bono E, Giustini L, Nguyen HC, Chin SM, Yeung YA, Gibbs CS, Djuretic I, Iannacone M. CD8 cis-targeted IL-2 drives potent antiviral activity against hepatitis B virus. Sci Transl Med. 2024;16:eadi1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, Tian X, Cathcart AL, Woo J, Subramanian GM, Andreone P, Kim HJ, Chuang WL, Nguyen MH. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat. 2018;25:1331-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 88. | Yuen MF, Balabanska R, Cottreel E, Chen E, Duan D, Jiang Q, Patil A, Triyatni M, Upmanyu R, Zhu Y, Canducci F, Gane EJ. TLR7 agonist RO7020531 versus placebo in healthy volunteers and patients with chronic hepatitis B virus infection: a randomised, observer-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2023;23:496-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 89. | Gane EJ, Dunbar PR, Brooks AE, Zhang F, Chen D, Wallin JJ, van Buuren N, Arora P, Fletcher SP, Tan SK, Yang JC, Gaggar A, Kottilil S, Tang L. Safety and efficacy of the oral TLR8 agonist selgantolimod in individuals with chronic hepatitis B under viral suppression. J Hepatol. 2023;78:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Janssen HL, Lim YS, Kim HJ, Sowah L, Tseng CH, Coffin CS, Elkhashab M, Ahn SH, Nguyen AH, Chen D, Wallin JJ, Fletcher SP, McDonald C, Yang JC, Gaggar A, Brainard DM, Fung S, Kim YJ, Kao JH, Chuang WL, Brooks AE, Dunbar PR. Safety, pharmacodynamics, and antiviral activity of selgantolimod in viremic patients with chronic hepatitis B virus infection. JHEP Rep. 2024;6:100975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 91. | Yuen MF, Chen CY, Liu CJ, Jeng WJ, Elkhashab M, Coffin CS, Kim W, Greenbloom S, Ramji A, Lim YS, Kim YJ, Fung SK, Kim DJ, Jang JW, Lee KS, Iyer RP, Macfarlane C, Jackson K, Locarnini SA, Chan HLY, Afdhal NH. A phase 2, open-label, randomized, multiple-dose study evaluating Inarigivir in treatment-naïve patients with chronic hepatitis B. Liver Int. 2023;43:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 92. | Mak LY, Seto WK, Yuen MF. Novel Antivirals in Clinical Development for Chronic Hepatitis B Infection. Viruses. 2021;13:1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Janssen HLA, Sonneveld MJ. Combination Therapy for Chronic HBV Infection. N Engl J Med. 2024;391:2163-2168. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 94. | Hou J, Zhang W, Xie Q, Hua R, Tang H, Morano Amado LE, Yang SS, Peng CY, Su WW, Chuang WL, Kim DJ, Avihingsanon A, Kao JH, Leerapun A, Yuen MF, Asselah T, Liang X, Bo Q, Canducci F, Catanese MT, Chen E, Cheng C, Chughlay F, Das S, Glavini K, Guerreiro N, Huang Y, Kakrana P, Kazma R, Patil A, Pavlovic V, Surujbally B, Triyatni M, Upmanyu R, Wat C, Gane E; Piranga Study Group. Xalnesiran with or without an Immunomodulator in Chronic Hepatitis B. N Engl J Med. 2024;391:2098-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 95. | Hu Y, Zhang H, Wu M, Liu J, Li X, Zhu X, Li C, Chen H, Liu C, Niu J, Ding Y. Safety, pharmacokinetics and pharmacodynamics of TQ-A3334, an oral toll-like receptor 7 agonist in healthy individuals. Expert Opin Investig Drugs. 2021;30:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Daniel WL, Lorch U, Mix S, Bexon AS. A first-in-human phase 1 study of cavrotolimod, a TLR9 agonist spherical nucleic acid, in healthy participants: Evidence of immune activation. Front Immunol. 2022;13:1073777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |