Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1530
Peer-review started: March 21, 2022
First decision: July 25, 2022
Revised: May 21, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: August 27, 2022
Processing time: 157 Days and 19.8 Hours
Sexual dysfunction (SD) is a prevalent but very commonly ignored aspect in the treatment of liver diseases and cirrhosis. The etiology of SD is multifactorial and therefore treatment strategies are complex, especially in females. Phosphodiesterase inhibitors are useful and effective in erectile dysfunction in males but in females, no single drug is available for SD, therefore multimodal treatment is required depending upon the cause. The foremost and fundamental requirement in both genders is to be stress-free and have adequate control of liver diseases. Improved quality of life is helpful in improving SD and vice versa is also true. Therefore, patients suffering from liver diseases should come forward and ask for treatment for SD, and physicians should actively enquire about SD while history taking and evaluating these patients. SD results in deterioration of quality of life, and both are modifiable and treatable aspects of liver diseases, which are never addressed actively, due to social taboos and fears of SD treatment in the presence of liver diseases. The diagnosis of SD does not require costly investigations, as the diagnosis can be established based on validated questionnaires available for both genders, therefore detailed targeted history taking using questionnaires is essential. Data are emerging in this area but is still at an early stage. More studies should be dedicated to SD in liver diseases.
Core Tip: Liver diseases and cirrhosis related sexual dysfunction (SD) is present in a significantly high proportion of both genders but is often underestimated, ignored, or overlooked. Due to its multifactorial causations, detailed history taking, examination, and addressing potential causes are required for the diagnosis and management of SD. More randomized controlled trials should be planned for both genders regarding the newer treatment options.
- Citation: Jagdish RK. Sexual dysfunctions and their treatment in liver diseases. World J Hepatol 2022; 14(8): 1530-1540
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1530.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1530
Sexual dysfunction (SD) in liver diseases has a multifactorial origin, and this is often the most ignored aspect in both male and female patients. This review attempts to break the myths and social taboos on the sexual aspect, and the quality of life in patients with liver diseases, with simplified diagnostic criteria, common causes, treatment, and other management options. The aim is to provide support for the sexual life of liver disease patients as SD can have a negative effect on the quality of life and can cause psychological issues such as less emotional satisfaction and general unhappiness and depression[1]. It should be noted that, infertility issues related to cirrhosis are separate to SD, therefore are not discussed in this review.
In men, SD is categorized into disorders of desire (low libido), arousal (erectile dysfunction), or orgasm (premature or delayed ejaculation, or anorgasmia), according to their occurrence in the cycle of sexual response[2]. The most common presentations are premature ejaculation, which is arbitrarily defined as ejaculation within one minute (60 s) of vaginal penetration, or erectile dysfunction (ED), which is the inability to obtain and maintain a penile erection in order to achieve satisfactory sexual activity. ED is considered to be common in cirrhosis; predominantly due to endocrine dysfunction and sarcopenia. There is a paucity of data on the precise prevalence of and predictive factors for ED in cirrhosis. Phosphodiesterase type 5 (PDE5) inhibitors were found to be useful for ED in the non-cirrhotic and cirrhotic population in a recent randomized controlled trial (RCT) of tadalafil in cirrhotics[1].
In females, SD is more complex and predominantly manifests as low libido, vaginal dryness, dyspareunia, orgasm inability, or menstrual abnormalities[3].
Patients with cirrhosis have a high prevalence of ED ranging from 25% to 92% in various studies[4-8] as shown in Table 1. In a recent RCT[1], it was found to be 70.3%, whereas in females, studies on prevalence are limited, but it has been shown that the prevalence of abnormal menstrual cycles was seen in 58%, decreased sexual interest in 42%, and no sexual activity in 56% of females[3,9]. In a study of women with non-alcoholic liver disease, reduced sexual desire was found in 33%, reduced arousal in 18%, not feeling orgasm in 25%, and dyspareunia in 21%, related to decreased vaginal lubrication. Prospective studies are needed especially in female patients.
| Disease etiology and age | Prevalence | Study | Ref. | Journal, year | Method used |
| Cirrhosis of liver | 70.3%. ED increases with increase in CTP score | Tadalafil improves erectile dysfunction and quality of life in men with cirrhosis: A randomized placebo controlled trial | Jagdish et al[1], India | Hepatology International, 2021 | IIEF-5 |
| Chronic viral liver diseases | 60%, age < 50 years, 88% age > 50 years. ED | Erectile dysfunction in patients with chronic viral liver disease: Its relevance to protein malnutrition | Toda et al[4], Japan | J Gastroenterology, 2005 | IIEF-5 and medical outcomes study short form 36 (SF-36) |
| Hepatitis B-aged 40-59 yr (SD 50.2 ± 5.7) | Total 24.6%, (CHB-8.6%, HBV-LC-41.2%) | Erectile dysfunction in patients with liver disease related to chronic hepatitis B | Kim et al[5], Korea | Clinical and Molecular | Erectile function of patients was evaluated by the Korean version of IIEF-5 |
| Hepatitis C, age -20-80 yr (SD; 50 ± 17.19) yr | 30% | Erectile dysfunction in patients with chronic hepatitis C virus infection | Hunter et al[6], Egypt | Arab J Gastroenterol, 2014 | An Arabic validated version of the five-item IIEF-5 |
| Alcoholic liver diseases (age < 56 yr) | 61% | Sexual dysfunction in men with alcoholic liver cirrhosis. A comparative study | Jensen et al[7], UK | Liver, 1985 | All groups had a significantly (P less than 0.025) raised prevalence of sexual dysfunction when compared to men without chronic disease |
| Chronic liver disease (mean age 54.8 ± 10.8 yr) | 50.6% | Assessment of sexual function in patients with chronic liver disease | Simsek et al[8], Turkey | Int J Impot Res, 2005 | International index of erectile function |
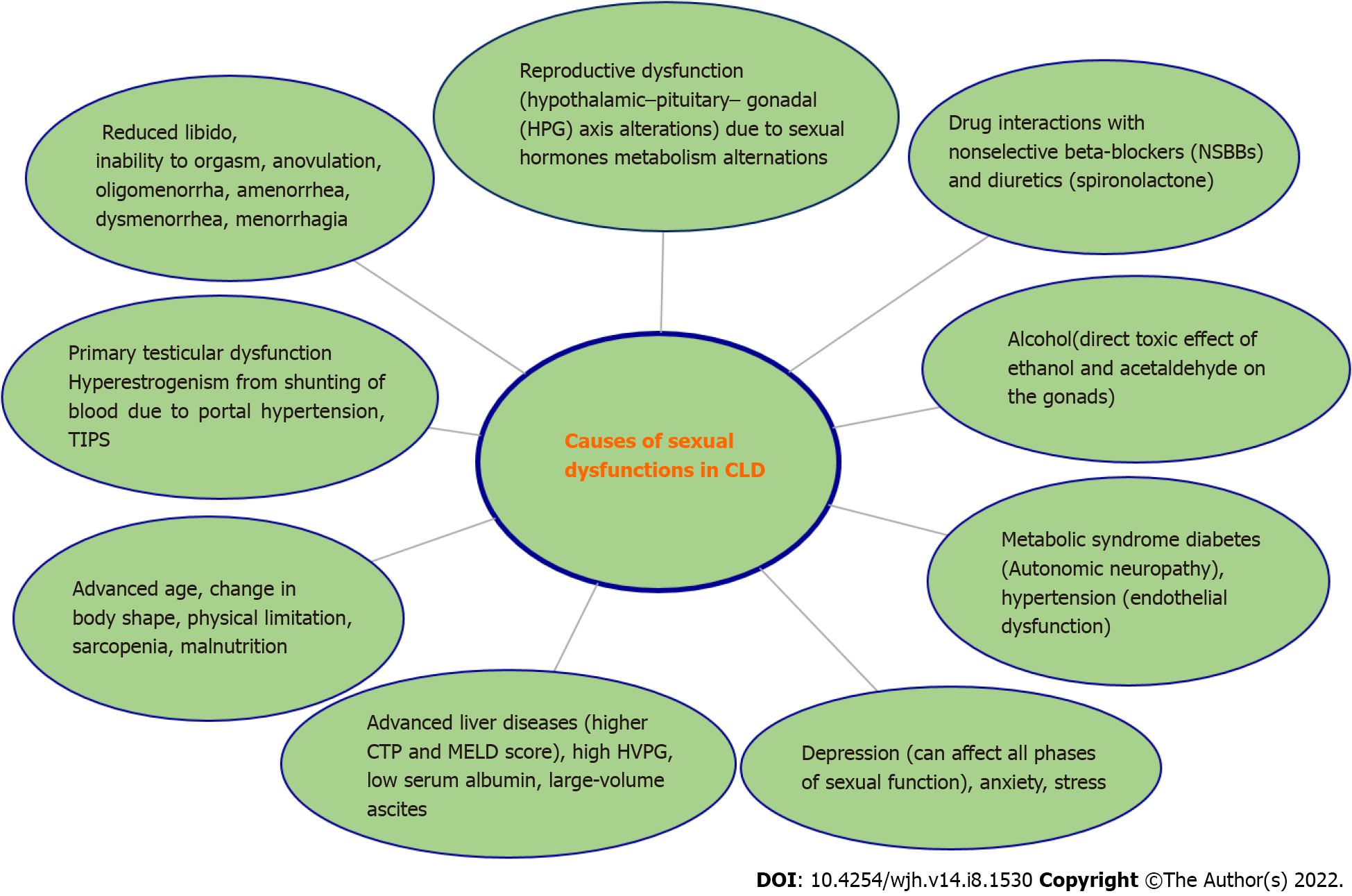
The causation of SD in both genders is multifactorial, although there are only a few studies in females as compared to males. Female sexual function involves hormonal, neurological, vascular, psychological, and emotional aspects. Dysfunction may be triggered or maintained by any of these, or by the interplay between them. The hormonal interplay is grossly disturbed in chronic liver diseases (CLD) along with multiple other non-hormonal factors contributing to SD in CLD. Possible contributing factors to SD in both genders[10,12] in liver diseases are shown in Figure 1.
Reduced quality of life due to depression (which can affect all phases of sexual function), anxiety, and stress are important factors. ED itself is associated with poor health-related quality of life[11,13] and depressive symptoms in both cirrhotic and non-cirrhotic patients.
Low serum albumin is a significant factor in ED and a low international index of erectile function (IIEF) score in liver diseases has been reported in multiple studies[4]. Hypoalbuminaemia results in water retention and loss of muscle volume which decreases sexual desire, and physical function. The ratio of albumin-bound to free testosterone may be influenced by decreased production of albumin which might then influence sexual desire and sleep-related erection. A study by Paternostro et al[14] showed that liver dysfunction, diabetes mellitus, hypertension, and high hepatic venous pressure gradient (HVPG), are key risk factors for ED in male cirrhotic patients.
Sarcopenia, as measured by the appendicular skeletal muscle index, was found to be associated with ED in a recent study[1,12]. This appears to be clinically significant as patients with low muscle mass are more likely to have low albumin, low power, frailty, and poor sexual performance. The concept of sexual frailty is similar to frailty in other organ systems. Musculoskeletal health needs to be highlighted in the evaluation of cirrhosis along with sexual health. More randomized trials should be conducted to understand the pathogenesis and underlying mechanisms and possible treatment options in this area.
Clinical features of hypogonadism, such as ED, infertility, decreased libido, and testicular atrophy, are often seen in patients with advanced liver disease, and it has been shown that the severity of liver cirrhosis correlates with the degree of ED[4].
Effects of drugs: Propranolol has a negative impact on erectile function, which is widely used to treat portal hypertension[15,16], but the doses which are required to cause ED are higher than the doses commonly used in CLD. Although Paternostro et al[14] found that beta-blockers were not associated with ED, beta-blockers generally cause vasodilation, and inhibit the release of renin, angiotensin II, and aldosterone production. These effects result in decreased adrenergic outflow from the sympathetic nervous system, resulting in SD[17]. However, a large meta-analysis[18] did not support the notion that beta-blocker (BB) therapy is associated with a relevant risk of SD, which was also supported by a recent RCT[1]. Spironolactone, an anti-mineralocorticoid and aldosterone antagonist, has anti-androgenic properties and can cause decreased libido and SD[16,17].
Studies have shown that free and albumin-bound testosterone, rather than total testosterone concentration, correlated positively with sexual desire and sleep-related erection in healthy subjects[19]. Therefore, it is possible that the reduced production of albumin may affect the ratio of free testosterone to albumin-bound testosterone, as well as the total amount of testosterone, possibly modifying cell or tissue response to this sex hormone in cirrhotic patients. Sex hormone-binding globulin (SHBG) levels[20,21] are elevated in patients with cirrhosis due to increased hepatic production, but the pathogenesis of this remains unclear. Rising levels of SHBG have been shown to correlate with the severity of fibrosis in patients with chronic liver disease[22]. Due to the binding of testosterone to SHBG, the total serum testosterone value can remain normal or occasionally be raised in this patient group, despite reduced levels of free (presumed biologically active) testosterone[23].
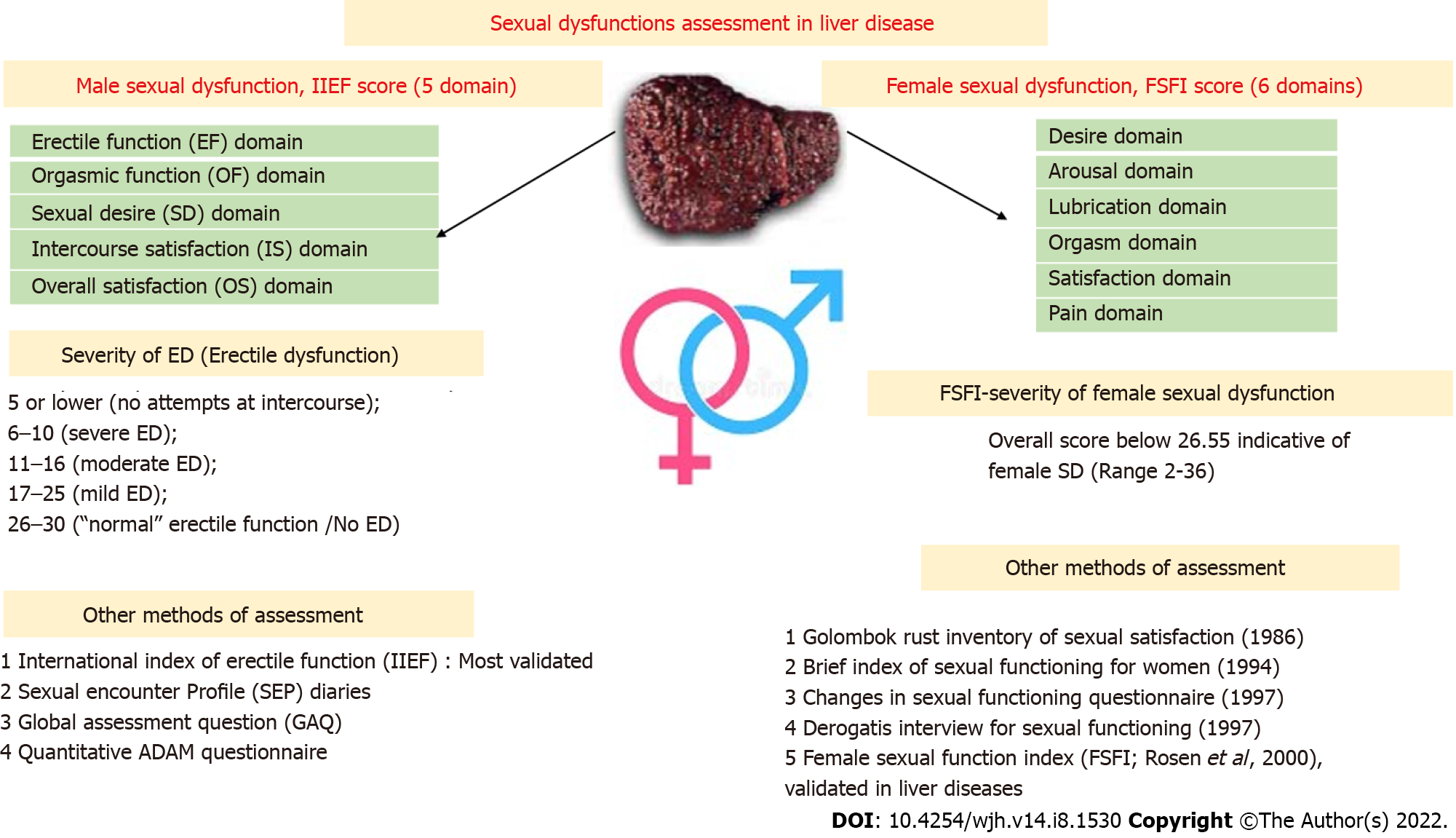
There are various well-validated questionnaires for SD and its risk factor assessment. The female sexual function index (FSFI) and the IIEF in males are the two most validated objective tools for SD severity assessment, with various domains and questions pertaining to each domain, which are then scored into numerical values[10] (Figure 2).
The FSFI: A total of six domains are assessed, with a total set of 19 questions. All domains have the highest score of 6, and the total maximum score is 36, where a score of 0 represents no sexual activity during the preceding month. An overall score below 26.55 is indicative of female SD[10,24]. Female sexual dysfunction (FSD) is classified into three main clinical types. For each diagnosis, the disorder is experienced at least 75% of the time for at least six months (except for medication-induced FSD), resulting in significant distress. The three types, some or all of which may be present, are (1) Sexual interest/arousal disorder, which is defined as reduced or absent sexual interest, responsiveness, erotic thoughts and sexual pleasure; (2) Female orgasmic disorder (absence, infrequency, reduction, delay of orgasm); and (3) Genito-pelvic pain/penetration disorder (difficulty in vaginal penetration, marked vulvovaginal or pelvic pain during penetration, fear or anxiety about pain in anticipation of, during, or after penetration, and tightening or tensing of pelvic floor muscles during attempted penetration).
The IIEF[25]: A 15-item questionnaire that assesses five domains of male sexual function using 5- to 6-point Likert scales, with 0 or 1 signifying a low frequency or ability and 5 signifying a high frequency or ability. These domains include erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. The erectile function domain possible scores range from 1 to 30. The severity of ED is defined using the IIEF-EF domain’s score: 5 or lower, (no attempts at intercourse at all); 6 to 10, severe ED; 11 to 16, moderate ED; 17 to 21, mild to moderate ED; 22 to 25, mild ED; and 26 to 30, is normal erectile function[1]. The IIEF has also been validated in the Indian population[26].
Other questionnaires: The quantitative ADAM (qADAM) questionnaire[27] is a new tool in quantifying the severity of hypogonadism, sexual encounter profile (SEP), and Global Assessment Question for assessing sexual performance[1]. The total qADAM score ranges between 10 and 50, with 10 being the most symptomatic and 50 being the least symptomatic.
The SEP diaries record yes/no responses after sexual encounters. There are five questions, but two questions are most frequently used to assess SD. SEP2 is "Were you able to insert your penis into your partner’s vagina?" and SEP3 is "Did your erection last long enough for you to have successful intercourse?"
Approximately 92% of patients with cirrhosis and SD showed a significant impact on their quality of life as assessed by the SF-36 score[28]. Other scores related to the quality of life are the Generalized Anxiety Disorder 7 (GAD-7) questionnaire[29], where a GAD-7 score of ≥ 10 indicates a probable diagnosis of GAD, and the Patient Health Questionnaire (PHQ)[30] where a PHQ-9 score ≥ 10 is an indication of major depression. Both anxiety and depression are implicated in the causation of ED and SD. In a recent study, we have shown that treatment of ED with tadalafil results in a significant decline in the GAD-7 and PHQ-9 scores, with a significant improvement in scores of five of the eight domains of SF-36 when compared with placebo[1].
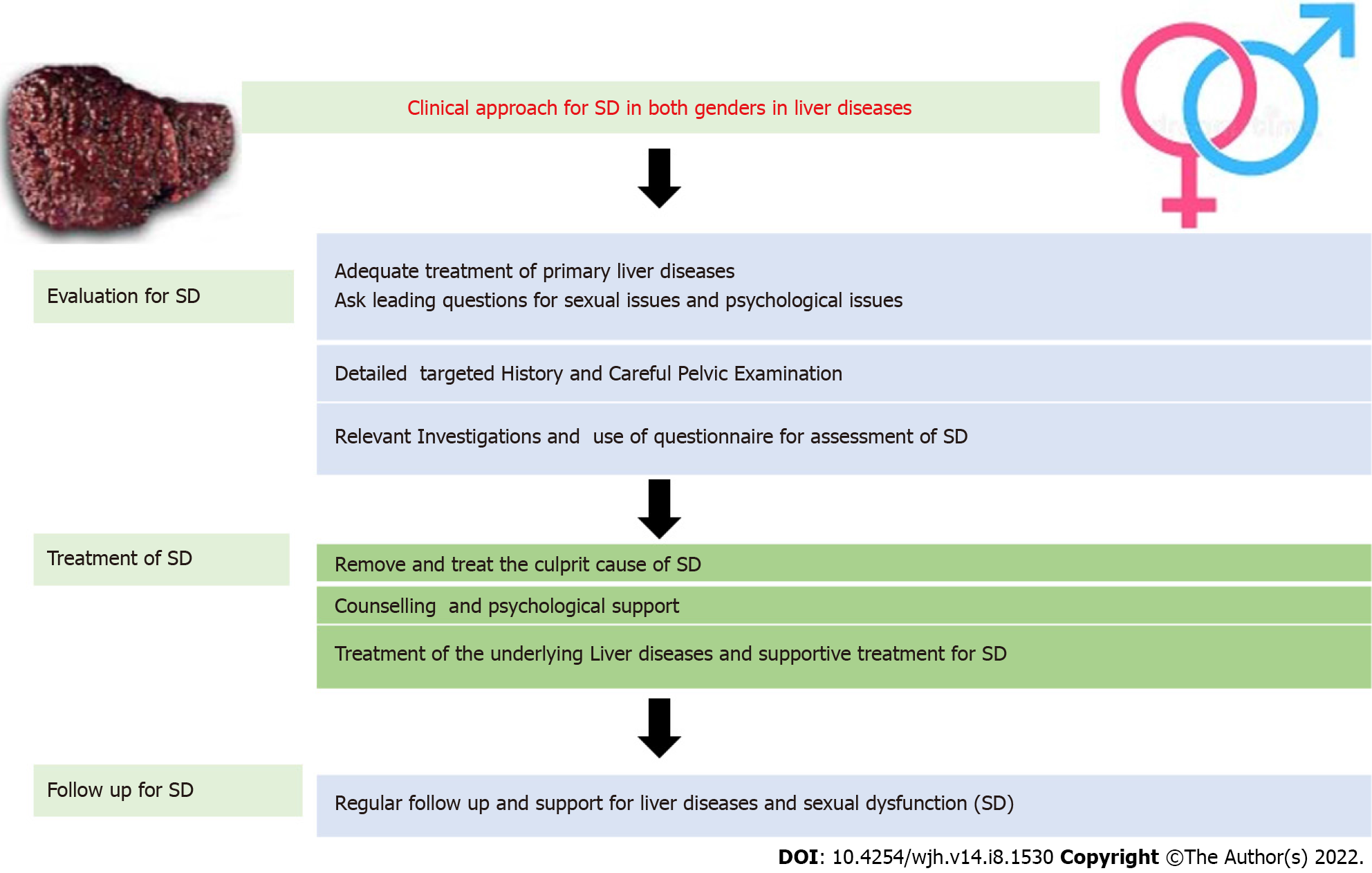
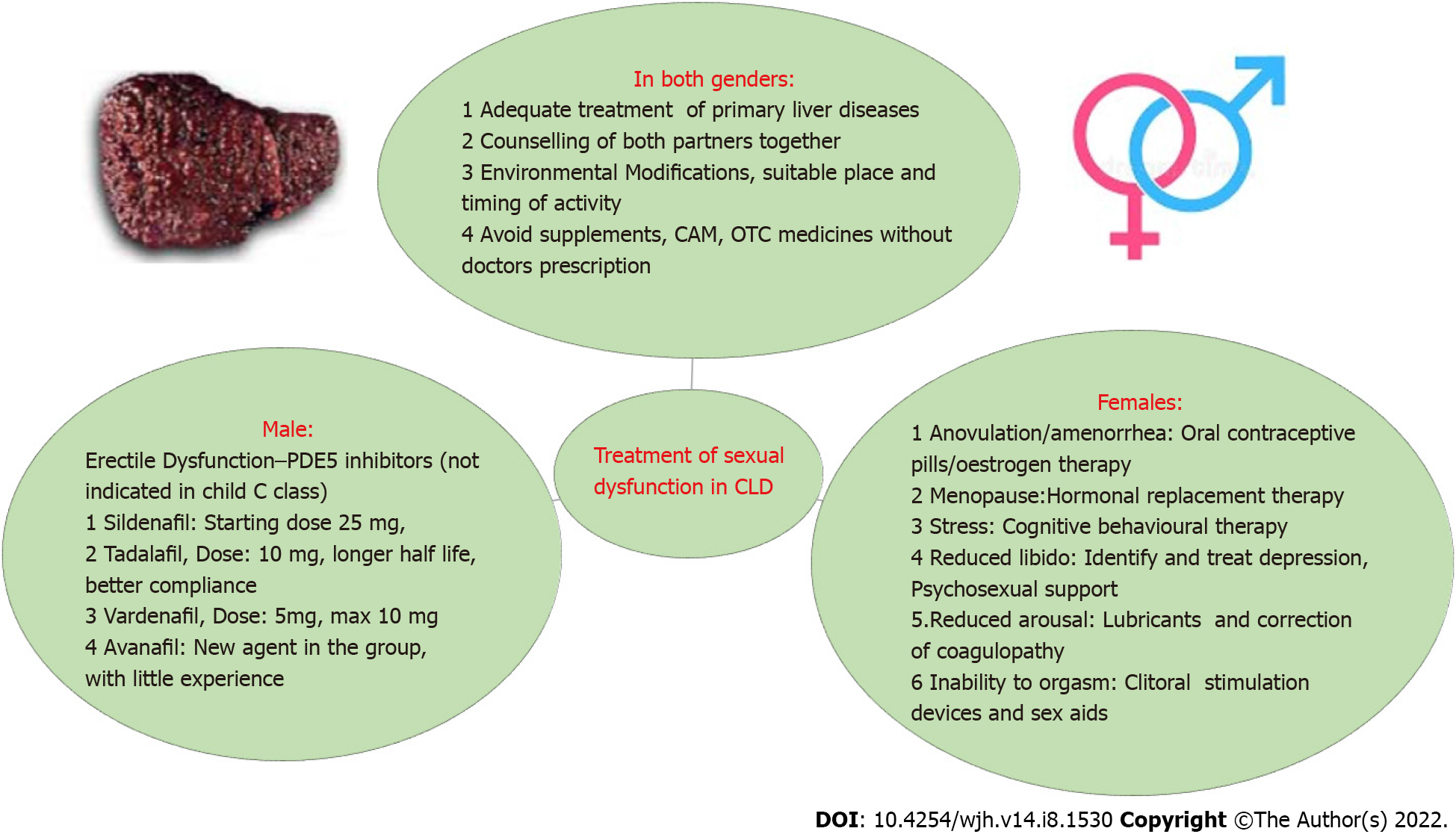
Adequate management of the primary liver disease is of paramount importance, as most of the treatment targeting SD may not be feasible or even contraindicated in Child C cirrhosis[31]. Therefore, adequate control of liver diseases and co-morbidities is warranted. The clinical approach and treatment of SD in liver diseases are summarized in Figures 3 and 4.
Counselling for partners: Counselling for both partners to understand the facts and myths, particularly regarding liver diseases and sexual activity can be advised.
Environmental modifications: Partners may accordingly be advised to change to a suitable environment, to change positions, or poses, as per the stage of the disease. Pelvic floor exercises and general exercises may be recommended to improve frailty, including sexual frailty.
Avoid stamina supplements: Avoid complementary alternative medications, herbs, and over the counter drugs available in many countries to boost stamina. Most of these drugs have not been studied and are strictly discouraged for use as they can be harmful in the presence of liver diseases.
Female SD is subjective dissatisfaction, the management of which can vary among patients. A single drug is not the answer to female SD in contrast to her male counterpart's ED.
Management of female SD in cirrhotics is complex and depends on identifying and treating the predominant underlying causes, but often requires multimodality involvement for optimal results. Evaluation of the underlying causes includes a detailed history, hormonal analysis, pelvic ultrasound, gynaecological examination, and psychological evaluation, along with the status and stage of liver diseases. A new-onset SD can be a sign of advanced liver disease, coagulopathy, or other metabolic or cardiovascular disease.
Various treatment strategies[32,33] are shown in Figure 3. Modifications in lifestyle are recommended, such as possible pelvic floor exercises depending on cirrhotic stage, and cessation of smoking and alcohol consumption. If it is causing poor performance, even in a passive role in sexual activity, blood transfusion or intravenous iron therapy can be used to correct anaemia. Coagulation correction and management of bleeding should be kept in mind. Forced acts may risk bleeding. Arousal enhancement strategies include relationship couples counselling, sex education using videos and educational literature, sexual positions yoga, romantic songs, romantic dressing, making the environment suitable for sexual pleasure, and increasing foreplay time. Cognitive behavioural therapy is a type of psychotherapy that helps to remove sexual inhibitions and helps in enhancing interpersonal relationships and sexual involvement. Medical devices such as clitoral therapy devices for sexual arousal and orgasmic dysfunctions can be helpful in some cases. Oestrogen is effective in the treatment of dyspareunia related to menopause. Strict contraception should be used to avoid pregnancy, as pregnancy should be planned after discussion with the treating doctor for the best outcome. Transdermal testosterone was tried in postmenopausal women with hypoactive sexual desire disorder with variable results[34]. To date, there are no FDA-approved treatments available for FSD; thus, prospective studies and RCTs should be conducted in these patients[35].
Studies of liver transplant recipients have shown that 72% of females became sexually active after LT. 95% of females younger than 46 years had a regular menstrual cycle by the end of the first year of liver transplantation, but irregular bleeding and amenorrhea were present in 26% and 26%, respectively[36,37]. An interval of at least 1-2 years after successful LT is recommended before considering pregnancy. Mycophenolate mofetil should be stopped before planning a pregnancy due to its teratogenicity.
The basic mechanism of sexual arousal and further physiological changes involves nitric oxide (NO) and related mechanisms[38]. Phosphodiesterase (PDE) inhibitors competitively bind to PDE5 and inhibit c-GMP hydrolysis, thus enhancing the effects of NO. This increase in c-GMP in smooth muscle cells results in a prolonged erection. In view of the lack of direct effect of PDE5 inhibitors on corpus cavernosum and smooth-muscle relaxation, adequate sexual stimulation is necessary for an erection to occur. Therefore, foreplay and stress relief are required. The approved agents for the treatment of ED are sildenafil, tadalafil, vardenafil, and avanafil, but tadalafil[39] appears to be the best option due to its long half-life and flexibility in the timing of administration.
A recent RCT[1] demonstrated that therapy with tadalafil (10 mg/d) for 12-wk significantly improved erectile function, anxiety, depression, and quality of life, and was well tolerated by men with cirrhosis (CTP score 10) and ED, with no significant side effects. It also improves depression and quality of life related to ED[12,40,41].
Intramuscular testosterone supplementation[42]: Testosterone has been shown to be beneficial in increasing muscle mass and improving sarcopenia, which can indirectly benefit SD as sarcopenia has been shown to be associated with ED. This may help with sexual frailty. However, direct data on sexual function are not available.
Role of albumin: No direct evidence is available on the role of albumin, but it seems to be logical as it adequately controls the primary liver disease, which may help in sexual frailty. Our hypothesis suggests that reduced serum albumin may affect the ratio of free albumin to bound testosterone with a possible altered testosterone response. Therefore, improving albumin concentration might be helpful in ED and testosterone is more likely to be beneficial in patients with high albumin levels. Therefore, regular albumin therapy and high protein intake may be indirectly helpful in improving SD. This hypothesis needs to be confirmed in prospective trials.
Improvement in SD has not been consistently reported after liver transplantation. This requires more randomized trials for a better understanding. There are a few contradictory studies; therefore, further investigation in both genders is needed. Improvement in erectile function is associated with the absence of hypogonadism before living donor liver transplant[3,10]. However, after transplantation, up to 25% of patients report persistent SD, and approximately one-third of patients describe the appearance of de novo SD. The use of PDE-5 inhibitors has been reported in post-renal transplant recipients with ED and no drug interactions with immunosuppressants or side effects have been observed.
PDE5 inhibitors are generally well tolerated in the treatment of ED[1]. The most common reported adverse drug reactions include headaches, back pain, myalgia, flushing, nasal congestion, sore throat, and dyspepsia. In general, the pain is of mild to moderate severity, typically occurring 12-24 h post-administration and usually resolving within 48 h, with or without medical treatment, such as acetaminophen.
In one study[14], higher MELD scores and higher HVPG values were found in patients with ED. PDE-5 inhibitors have been administered to lower portal pressure in cirrhosis but the results are conflicting[1,43-48] (Table 2).
| HVPG change | Intervention | Ref. |
| No significant change in HVPG | Tadalafil 10 mg | Jagdish et al[1], 2021, Hept International |
| N = 10, in seven patients, HVPG decreased and in three it increased but the decrease was not significant | Sildenafil 50 mg | Clemmesen et al[43], 2008, World J Gastroenterology |
| Lowers portal pressure in the acute setting by about 20% | 75-100 mg of the phosphodiesterase-5-inhibitor udenafil | Kreisel et al[44], 2015, Dig Liver Dis |
| Decreased HVPG in 4/5 patients | Vardenafil 10 mg | Deibert et al[45], 2006, Aliment Pharmacol Ther |
| No effect on HVPG | Sildenafil | Tandon et al[46], 2010, Clin Gastroenterol Hepatol |
| HVPG decrease from 10 mmHg to 7 mmHg (case report) | Sildenafil 20 mg bd | Bremer et al[47], 2007, J Med Case Rep |
| HVPG decreased by 13%, and portal flow increased by 28% (case report) | Vardenafil | Deibert et al[48], 2018, World J Gastroenterology |
Sexual dysfunction in liver diseases is one of the most ignored health issues and more prospective studies on this issue are needed which is potentially treatable. In particular, PDE inhibitors, especially tadalafil, are helpful for ED, and in females, treatment is multifactorial. Adequate control of primary liver diseases and causative agents of SD is warranted. All patients with liver diseases and cirrhosis should be evaluated, as SD is modifiable and treatable, and results in improved quality of life. More prospective and randomized controlled trials are needed on this topic, to understand the global epidemiology of the disease burden, possible underlying mechanisms, particularly related to non-hormonal aspects and sarcopenia, and possible newer treatment options.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Q, China; Zhang Y, China; Hong-Shui Ma, China S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Jagdish RK, Kamaal A, Shasthry SM, Benjamin J, Maiwall R, Jindal A, Choudhary A, Rajan V, Arora V, Bhardwaj A, Kumar G, Kumar M, Sarin SK. Tadalafil improves erectile dysfunction and quality of life in men with cirrhosis: a randomized double blind placebo controlled trial. Hepatol Int. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 2. | Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1015] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | Sorrell JH, Brown JR. Sexual functioning in patients with end-stage liver disease before and after transplantation. Liver Transpl. 2006;12:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Toda K, Miwa Y, Kuriyama S, Fukushima H, Shiraki M, Murakami N, Shimazaki M, Ito Y, Nakamura T, Sugihara J, Tomita E, Nagata C, Suzuki K, Moriwaki H. Erectile dysfunction in patients with chronic viral liver disease: its relevance to protein malnutrition. J Gastroenterol. 2005;40:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Kim M, Kim SY, Rou WS, Hwang SW, Lee BS. Erectile dysfunction in patients with liver disease related to chronic hepatitis B. Clin Mol Hepatol. 2015;21:352-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Hunter SS, Gadallah A, Azawi MK, Doss W. Erectile dysfunction in patients with chronic hepatitis C virus infection. Arab J Gastroenterol. 2014;15:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Jensen SB, Gluud C. Sexual dysfunction in men with alcoholic liver cirrhosis. A comparative study. Liver. 1985;5:94-100. [PubMed] |
| 8. | Simsek I, Aslan G, Akarsu M, Koseoglu H, Esen A. Assessment of sexual functions in patients with chronic liver disease. Int J Impot Res. 2005;17:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 9. | Bach N, Schaffner F, Kapelman B. Sexual behavior in women with nonalcoholic liver disease. Hepatology. 1989;9:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Neong SF, Billington EO, Congly SE. Sexual Dysfunction and Sex Hormone Abnormalities in Patients With Cirrhosis: Review of Pathogenesis and Management. Hepatology. 2019;69:2683-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Jagdish RK, Kamaal A, Shasthry SM, Benjamin J, Maiwall R, Jindal A, Choudhary A, Rajan V, Arora V, Bhardwaj A, Kumar G, Kumar M, Sarin SK. Erectile dysfunction in cirrhosis: Its prevalence and risk factors. J Clin Exp Hepatol. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Liu Q, Zhang Y, Wang J, Li S, Cheng Y, Guo J, Tang Y, Zeng H, Zhu Z. Erectile Dysfunction and Depression: A Systematic Review and Meta-Analysis. J Sex Med. 2018;15:1073-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Paternostro R, Heinisch BB, Reiberger T, Mandorfer M, Schwarzer R, Seeland B, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Erectile dysfunction in cirrhosis is impacted by liver dysfunction, portal hypertension, diabetes and arterial hypertension. Liver Int. 2018;38:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Fogari R, Zoppi A. Effect of antihypertensive agents on quality of life in the elderly. Drugs Aging. 2004;21:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Smith PJ, Talbert RL. Sexual dysfunction with antihypertensive and antipsychotic agents. Clin Pharm. 1986;5:373-384. [PubMed] |
| 17. | Nunes KP, Labazi H, Webb RC. New insights into hypertension-associated erectile dysfunction. Curr Opin Nephrol Hypertens. 2012;21:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Schiavi RC, Schreiner-Engel P, White D, Mandeli J. The relationship between pituitary-gonadal function and sexual behavior in healthy aging men. Psychosom Med. 1991;53:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Kent JR, Scaramuzzi RJ, Lauwers W, Parlow AF, Hill M, Penardi R, Hilliard J. Plasma testosterone, estradiol, and gonadotrophins in hepatic insufficiency. Gastroenterology. 1973;64:111-115. [PubMed] |
| 21. | Galvão-Teles A, Burke CW, Anderson DC, Marshall JC, Corker CS, Bown RL, Clark ML. Biologically active androgens and oestradiol in men with chronic liver disease. Lancet. 1973;1:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 97] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Nguyen HV, Mollison LC, Taylor TW, Chubb SA, Yeap BB. Chronic hepatitis C infection and sex hormone levels: effect of disease severity and recombinant interferon-alpha therapy. Intern Med J. 2006;36:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Gluud C. Serum testosterone concentrations in men with alcoholic cirrhosis: background for variation. Metabolism. 1987;36:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1650] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 25. | Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3900] [Cited by in RCA: 4101] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 26. | Dogra PN, Saini AK, Seth A. Erectile dysfunction after anterior urethroplasty: a prospective analysis of incidence and probability of recovery--single-center experience. Urology. 2011;78:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Mohamed O, Freundlich RE, Dakik HK, Grober ED, Najari B, Lipshultz LI, Khera M. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res. 2010;22:20-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 29. | Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11947] [Cited by in RCA: 18879] [Article Influence: 993.6] [Reference Citation Analysis (0)] |
| 30. | Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21545] [Cited by in RCA: 28943] [Article Influence: 1206.0] [Reference Citation Analysis (0)] |
| 31. | Allen AM, Hay JE. Review article: the management of cirrhosis in women. Aliment Pharmacol Ther. 2014;40:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 33. | Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172; quiz 1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 34. | Achilli C, Pundir J, Ramanathan P, Sabatini L, Hamoda H, Panay N. Efficacy and safety of transdermal testosterone in postmenopausal women with hypoactive sexual desire disorder: a systematic review and meta-analysis. Fertil Steril. 2017;107:475-482.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Nappi RE, Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Expert Opin Pharmacother. 2015;16:875-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation. 1996;62:476-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Ziogas IA, Hayat MH, Tsoulfas G. Obstetrical and gynecologic challenges in the liver transplant patient. World J Transplant. 2020;10:320-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Gupta M, Kovar A, Meibohm B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol. 2005;45:987-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Kirilovsky J, Hyafil F, Labaudinière R. The discovery of tadalafil: a novel and highly selective PDE5 inhibitor. 1: 5,6,11,11a-tetrahydro-1H-imidazo[1',5':1,6]pyrido[3,4-b]indole-1,3(2H)-dione analogues. J Med Chem. 2003;46:4525-4532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Danoff A, Khan O, Wan DW, Hurst L, Cohen D, Tenner CT, Bini EJ. Sexual dysfunction is highly prevalent among men with chronic hepatitis C virus infection and negatively impacts health-related quality of life. Am J Gastroenterol. 2006;101:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, Abbiati R; Italian Study Group for quality of life in cirrhosis. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 355] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 42. | Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 43. | Clemmesen JO, Giraldi A, Ott P, Dalhoff K, Hansen BA, Larsen FS. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J Gastroenterol. 2008;14:6208-6212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, Neagu M, Rössle M, Zipprich A, Caca K, Ferlitsch A, Dilger K, Mohrbacher R, Greinwald R, Sauerbruch T. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rössle M, Kreisel W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther. 2006;23:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Tandon P, Inayat I, Tal M, Spector M, Shea M, Groszmann RJ, Garcia-Tsao G. Sildenafil has no effect on portal pressure but lowers arterial pressure in patients with compensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8:546-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Bremer HC, Kreisel W, Roecker K, Dreher M, Koenig D, Kurz-Schmieg AK, Blum HE, Roessle M, Deibert P. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: a case report. J Med Case Rep. 2007;1:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Deibert P, Lazaro A, Stankovic Z, Schaffner D, Rössle M, Kreisel W. Beneficial long term effect of a phosphodiesterase-5-inhibitor in cirrhotic portal hypertension: A case report with 8 years follow-up. World J Gastroenterol. 2018;24:438-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (1)] |