Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1058
Peer-review started: February 28, 2021
First decision: May 2, 2021
Revised: May 12, 2021
Accepted: July 30, 2021
Article in press: July 30, 2021
Published online: September 27, 2021
Processing time: 205 Days and 10 Hours
The dying liver causes the suffocation of the kidneys, which is a simplified way of describing the pathophysiology of hepatorenal syndrome (HRS). HRS is characterized by reversible functional renal impairment due to reduced blood supply and glomerular filtration rate, secondary to increased vasodilators. Over the years, HRS has gained much attention and focus among hepatologists and nephrologists. HRS is a diagnosis of exclusion, and in some cases, it carries a poor prognosis. Different classifications have emerged to better understand, diagnose, and promptly treat this condition. This targeted review aims to provide substantial insight into the epidemiology, pathophysiology, diagnosis, and management of HRS, shed light on the various milestones of this condition, and add to our current understanding.
Core Tip: The dying liver causes the suffocation of the kidneys, a simplified way of describing the pathophysiology of hepatorenal syndrome (HRS). This targeted review aims to provide substantial insight into the epidemiology, pathophysiology, diagnosis, and management of HRS, shed the light on the various milestones of this condition, and add to our current understanding.
- Citation: Nassar M, Nso N, Medina L, Ghernautan V, Novikov A, El-Ijla A, Soliman KM, Kim Y, Alfishawy M, Rizzo V, Daoud A. Liver kidney crosstalk: Hepatorenal syndrome. World J Hepatol 2021; 13(9): 1058-1068
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1058.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1058
Hepatorenal syndrome (HRS) has been a challenge for clinicians and patients for many years. It is imperative to have a proper understanding of risk factors, patient popu
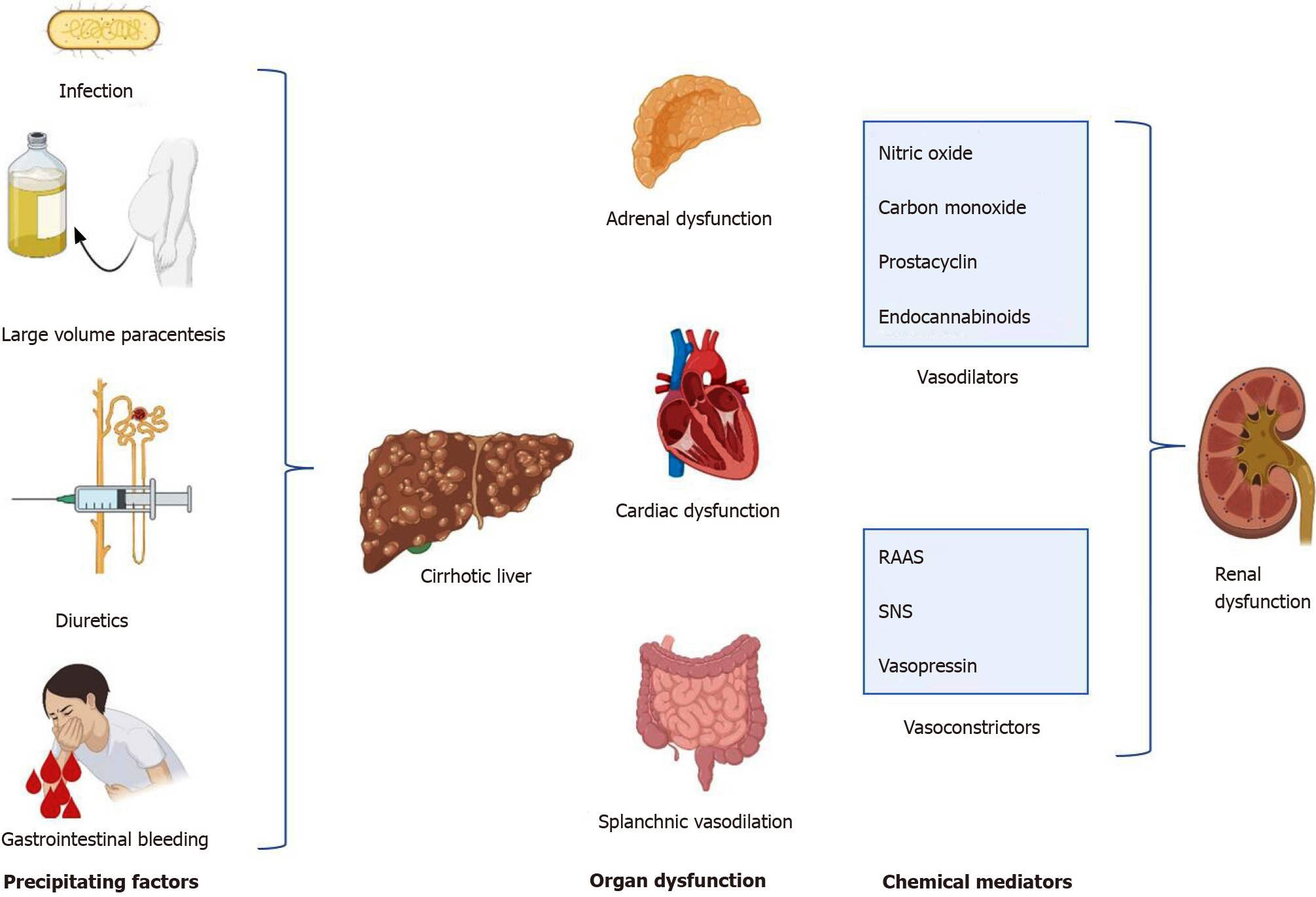
Older and more recent studies have revealed that acute kidney injury (AKI) is diagnosed in almost 50% of hospitalized cirrhotic patients, and HRS-AKI represents 11% to 20% of those cases[1]. HRS occurs in approximately 10% to 40% of patients with ascites and advanced liver cirrhosis[2,3], with the one-year probability of developing HRS estimated to be 18% and the five-year probability 39%[4]. Fortunately, the prevalence of the syndrome is not elevated when no precipitating factors are detected. The most common precipitating events contributing to the development of HRS are infections, gastrointestinal hemorrhage, and large-volume paracentesis (LVP)[4,5].
AKI-HRS is associated with a 30% increase in the risk of mortality during hospital stays. A comprehensive meta-analysis revealed mortality rates of 58% at 1 mo and 63% at one year[3]. Broader knowledge is needed to identify the potential predictors of HRS and stratify the individual risk score. To this end, three independent predictors have been implicated in multivariate analysis: No evidence of enlarged liver, elevated plasma renin activity, and hyponatremia[5].
HRS is a reversible functional renal impairment seen in hepatic cirrhosis with portal vein hypertension and is caused by multiple pathophysiological changes[6]. Renal dysfunction commonly occurs in cirrhotic patients and is associated with high morbidity and mortality[5].
Historically, there were two types of HRS. Type 1 was defined as a fast deterioration of renal function over two weeks with a serum creatinine level > 2.5 mg/dL, while type 2 was described as a subtle impairment over months. According to the more recent definition proposed by the Acute Kidney Injury Network in 2007 and supported by the International Club of Ascites (ICA) and Acute Dialysis Quality Initiative in 2011, HRS was divided into subgroups based on the underlying pathologic process[1]: HRS-AKI and non-HRS AKI. The distinction between these is that HRS-AKI is a functional renal impairment that is reversible with liver transplantation, whereas non-HRS AKI is a structural pathology of the renal parenchyma caused by various injuries. ICA specific criteria for HRS-AKI were defined as an increase in serum creatinine of ≥ 0.3 mg/dL or ≥ 1.5 times the baseline creatinine or a 50% increase within 48 h from baseline, no response to diuretic discontinuation, the presence of cirrhosis with ascites, no evidence of shock, no history of administering nephrotoxic medications, and no signs of organic renal disease[3,5].
Several mechanisms are involved in the pathophysiology of HRS, such as circulatory dysfunction and splanchnic arterial vasodilation, increased vasoconstrictor effects on renal vasculature, cardiac impairment, systemic inflammation, and adrenal insufficiency[1]. Portal hypertension in cirrhosis causes a structural strain on the endothelium, leading to the release of endogenous vasodilators, such as nitric oxide, prostacyclin, carbon monoxide, and endocannabinoids[5,7]. Gut bacterial translocation in the mesenteric lymph nodes and then into the bloodstream, along with nitric oxide and other vasodilators, also contributes to intense splanchnic vasodilation and pooling of large plasma volume into the splanchnic vascular bed[2,4]. This creates low effective circulatory volume, which stimulates the baroreceptors in the carotid body and aortic arch. As a result, counterregulatory systemic vasoconstrictor pathways, such as the sympathetic nervous system, renin-angiotensin-aldosterone system (RAAS), and the non-osmotic release vasopressin, are triggered[6,8]. Consequently, hyperdynamic circulation occurs with increases in cardiac output, heart rate, sodium and water retention, and renal vasoconstriction, leading to the development of ascites and subsequent renal dysfunction. RAAS and vasopressin act on sodium and exacerbate free water retention, further worsening the developing ascites and aggravating renal impairment[1].
In the incipient stages, the kidneys maintain an adequate glomerular filtration rate (GFR) due to renal prostaglandins, which keep the afferent arterioles dilatated. However, cirrhosis progression intensifies both splanchnic and systemic vasodilation and contributes to decreased mean arterial pressure, prolonged renal vasoconstriction with reduced renal blood flow, and GFR[5]. Overall, a state of renal hypoperfusion occurs. Therefore, HRS is a prerenal type of renal failure, which is not responsive to fluids.
Cardiac dysfunction in HRS is caused by the diseased liver itself and less commonly by the same etiologic factor of cirrhosis (e.g., alcohol). Myocardial impairment is complex and has several contributory mechanisms: Increased neurohumoral activity leading to myocardial hypertrophy and fibrosis with affected relaxation and inhibitory effects of the cytokines on the ventricular function[6]. Generally, inotropic and chronotropic functions become altered in hepatocardiorenal syndrome[9].
Non-infectious systemic inflammatory response syndrome was identified in almost half of the patients with AKI-HRS[5]. On the other hand, HRS is often preceded by bacterial infections. Inflammation in cirrhosis is induced by macrophage activation, oxidative stress, and inflammatory molecules[9]. Pathogen-associated molecular patterns emerge from the translocation of gut bacteria and damage-associated molecular patterns from the damaged hepatocytes. In turn, these inflammatory molecules activate cytokine release, leading to increased vasodilator production, with the result being reduced systemic arterial resistance and mean arterial pressure[6].
Relative adrenal insufficiency (RAI) is observed in less than half of the patients with advanced cirrhosis and may develop into HRS. The mechanisms are not well established; however, depletion of the substrates for cortisol production and dysfunction of the hypothalamus-pituitary axis by the pro-inflammatory cytokines have been implied[6]. Other mechanisms have been theorized to contribute to the HRS, mainly the hepatorenal reflex. The hepatorenal reflex is thought to be the result of abnormal hepatic blood flow directly affecting kidney hemodynamics. Evidence to support this theory is reinforced by the transjugular intrahepatic portosystemic shunt placement, which leads to the HRS’s amelioration by reducing portal hypertension[8].
Reduction in GFR and decreases in renal blood flow progress along with the degree of cirrhosis. The following objective evidence indicates that renal impairment in cirrhotic patients is functional: No evidence of morphological changes and largely preserved tubular function on kidney biopsy, resolution of AKI-HRS following liver transplant, and successful cadaveric transplantation of kidneys from patients with HRS[1] (Figure 1).
The diagnostic criteria for the HRS were first developed in 1994, and since then, it has undergone multiple modifications[10]. In the previous years, AKI in cirrhotic patients was defined as a serum creatinine level of ≥ 1.5 mg/dL[11]. The latest guidelines of the ICA reveal that the definition of AKI in this population has changed based on modifications of the Kidney Disease Improving Global Outcomes (KDIGO) criteria[12]. The removal of this static value has led to the earlier identification of this condition in patients with chronic liver disease (CLD)[12].
AKI is now defined as an increase of serum creatinine of ≥ 0.3 mg/dL within 48 h and/or increase of ≥ 50% from the patient's baseline within 7 d (or within the past 3 mo before admission, if a value within the previous week is not available). Furthermore, the ICA classifies AKI in three stages based on serum creatinine levels. Stage 1 is when there is an increase of ≥ 0.3 mg/dL or an increase of ≥ 1.5-fold to 2-fold from the baseline; stage 2 is when there is an increase of > 2-fold to 3-fold from the baseline; stage 3 is when there is an increase of > 3-fold from the baseline or serum creatinine is ≥ 4.0 mg/dL with an acute increase of ≥ 0.3 mg/dL or the initiation of renal replacement therapy (RRT)[11].
The use of urine output as a criterion for AKI in CLD was subsequently removed[11]. Despite this, in a retrospective study, Amathieu et al[13] found that the addition of urine output as a criterion, along with serum creatinine for identification of AKI in patients with CLD, showed increased sensitivity for the identification of this pathology and that the presence of transient oliguria was associated with an increase in mortality rates[13]. Therefore, in this population, an acute decrease in urine output should be considered, particularly in patients with transient oliguria.
HRS is diagnosed when a patient with cirrhosis and ascites has stage ≥ 2 AKI per the ICA guidelines, has no response to diuretic withdrawal or a trial of treatment with albumin for volume expansion (1 g/kg per day with a maximum of 100 g/d) for a total of 2 d, and has no evidence of other etiologies causing kidney injury (i.e. absence of shock, no recent use of nephrotoxic drugs, no macroscopic signs of structural kidney injury, such as the presence of proteinuria, microhematuria, or abnormal findings on renal ultrasonography)[10,12,14,15].
HRS was previously classified as HRS type 1 and HRS type 2, based on the acuity of kidney function deterioration. HRS type 1 was defined as a doubling of serum creatinine above 2.5 mg/dL within 2 wk, and type 2 was defined as a slower increase in serum creatinine to a value > 1.5 mg/dL. These definitions have been renamed from HRS type 1 to HRS-AKI and HRS type 2 to HRS-chronic kidney disease[12].
New biomarkers have been identified for HRS diagnosis, including the urinary neutrophil gelatinase-associated lipocalin (NGAL) and the serum cystatin C. The use of these biomarkers has been shown to help diagnose HRS early and prognostic assessment in patients with decompensated cirrhosis[16]. A systematic review by Puthumana et al[17] revealed that both interleukin (IL) 18 and NGAL might be useful in the differentiation between AKI due to acute tubular necrosis (ATN) and HRS. These and other markers have not been included in the diagnostic criteria at the time of this review but might be considered in the future.
A proper understanding of HRS's underlying pathophysiology is crucial in preventative strategies used in today’s clinical practice. Discussed below are some strategies found beneficial for the prevention of HRS. The focus of all strategies is on reversing the poor perfusion to the kidney due to a combination of renal vessels’ constriction and decreased renal blood flow in response to systemic vasodilation.
Diuretic therapy may cause intravascular volume contraction and result in compensatory vasoconstriction, further worsening an already impaired renal function. In severely decompensated patients, diuretic therapy may induce HRS. The current recommendation for patients with ascites is to receive spironolactone treatment not exceeding 400 mg daily and divided doses of furosemide not exceeding 160 mg daily. In hospitalized patients, the addition of albumin to diuretic regimens may prevent diuretic-induced changes in creatinine and BUN[18].
Large-volume paracentesis can lead to the deterioration of kidney function. Plasma renin activity, baseline creatinine measurements, and daily monitoring should be performed, which helps identify patients deemed to be at high risk of developing post-paracentesis HRS. Such patients should receive supplementation with albumin, with the recommended dosing 6–8 gm/L of ascitic fluid removed[19].
It is a known fact that SBP is a common precipitant of HRS. Prompt recognition and treatment of SBP and managing the patient in a monitored setting are crucial in preventing HRS development. For patients with impaired renal function and bilirubin levels of > 4 mg/dL, IV albumin infusion at 1.5 mg/kg should be initiated[20].
In a study by Ibrahim et al[21], published in the European Journal of Gastroenterology and Hepatology, prolonged therapy with Rifaximin showed benefits due to decreased cirrhosis-related complications, SBP, and recurrent hepatic encephalopathy, along with hemodynamic and renal improvement in patients with alcoholic hepatitis. Further
Finally, another study by Dong et al[22] reported a lower incidence of acute renal injury in patients treated with Rifaximin for at least 90 d.
HRS-AKI is considered a diagnosis of exclusion, and the ICA defines it as AKI (an increase in serum creatinine of 0.3 mg/dL or more within 48 h) in the setting of cirrhosis and ascites, with failure to improve after 48 h of diuretic withdrawal and volume expansion with albumin, in the absence of shock, nephrotoxic drugs, and signs of structural kidney injury (proteinuria > 500 mg/d, microhematuria > 50 RBC/HPF, or abnormal renal imaging)[23-25].
AKI is reported in about 20–30% of hospitalized cirrhotic patients[24,26], with six-fold higher mortality[26], and although HRS is unique to cirrhosis, AKI in cirrhotic patients can be due to other causes, including prerenal azotemia and ATN[23,24]. Other causes such as glomerulonephritis and post-renal AKI should also be considered[24]. As these causes differ markedly in their treatment options and prognosis, early differentiation is key to improving outcomes[23,24,27].
In studies involving cirrhotic patients, hypovolemic AKI was reported as the most common cause of AKI stage IA (stage I with sCr < 1.5 mg/dL), which has better survival (90 d survival rate of 82%) than AKI stage IB (stage I with sCr ≥ 1.5 mg/dL) (90 d survival rate of 55%), where HRS and ATN were more frequent[23]. It was also reported that acute, chronic liver failure was more likely with AKI stage IB[24].
Prerenal AKI: Renal hypoperfusion without tubular or glomerular lesion usually occurs in GI bleeding, dehydration, and/or diuretic use[28]. It is differentiated from the other causes of AKI by improvement after volume replacement with albumin and/or fluids and diuretics withdrawal[23,29].
ATN: Tubular cell necrosis is usually the result of an ischemic (in the setting of shock) or toxic (e.g., nephrotoxic drugs) insult[28]. As with HRS, there is no improvement with withdrawing diuretics and giving albumin[29]. Intrinsic AKI is excluded using the ICA-HRS criteria[29].
The use of UNa and FeNa to differentiate causes of AKI is deemed less useful in cirrhosis: Prerenal AKI and HRS have urinary Na excretion < 20 mEq/L and FeNa < 1%, whereas ATN classically has UNa > 40 mEq/L and FeNa > 1%[28].
Limitations to this rule are that patients with cirrhosis can be on diuretics, which will falsely increase UNa[28]. Additionally, as cirrhosis is a sodium acid state, some ATN cases were reported to have FeNa < 1%[24,28].
The presence of urinary casts may not be helpful either in cirrhosis, as granular and epithelial cell casts (classically seen in ATN) can be present as nonspecific findings in cirrhosis due to hyperbilirubinemia[28].
The use of urinary biomarkers to differentiate the various AKI etiologies in cirrhotic patients is promising: NGAL (a glycoprotein that is overexpressed by injured kidney tubular epithelia) is the most studied, but other urinary markers such as IL-8, albumin, and liver fatty acid-binding protein have also been investigated and show similar performance[24]. Higher levels were found in intrinsic AKI/ATN, compared to HRS. Meanwhile, prerenal had the lowest levels[23,24]. One study done on 94 patients with decompensated cirrhosis showed a median urine neutrophil gelatinase-associated lipocalin (uNGAL) of 1217.50 in ATN, 465.00 in HRS, and 95.50 in prerenal AKI (P < 0.001)[23]. It also determined the optimal cutoffs for the various diagnoses: ATN is likely with uNGAL more than 650 ng/mL (100% sensitivity, 83.78% specificity), HRS is likely with uNGAL between 299-650 ng/mL (87.9% sensitivity, 96.3% specificity), while prerenal is likely with uNGAL less than 299 ng/mL[23].
uNGAL and IL-8 were also found to predict prognosis, where the higher the biomarker levels, the higher is the short-term mortality[23,24].
It is to be noted that leucocytes can also produce uNGAL. Hence, levels of uNGAL can be elevated in the setting of urinary tract infection and should be cautiously interpreted in these settings[24].
The definitive treatment for HRS is liver transplantation[30]. The goal of therapy is to maintain adequate kidney function before the patient undergoes a liver transplant, which can be achieved by optimizing the mean arterial blood pressure and cardiac output[31,32]. Patients should be screened thoroughly for signs of infection, and if necessary, empiric antibiotic therapy should be started[33]. Therapy for the treatment of viral hepatitis, if present, should be continued. Diuretic therapy should be stopped, as these have been identified to be a provoking factor for HRS development.
Patients should receive volume expansion with albumin, as it has shown to significantly reduce mortality in this population, which has not been seen with other volume expanders. The protective effects of albumin are thought to be also driven by its anti-inflammatory and antioxidative effects[5]. Several vasodilators have been studied in the past as potential treatment options for HRS, including dopamine, prostaglandins, and endothelin receptor blockers, which have not been effective in improving kidney function[34,35]. The use of vasoconstrictors, such as terlipressin, norepinephrine, or a combination of midodrine, octreotide, and albumin showed improved renal function and are considered the first line of therapy for HRS[36,37]. The rationale behind its use is the reversal of splanchnic vasodilatation thought to cause renal impairment in this population. The choice of therapy depends on different factors, including which drugs are available at the time of treatment, if the patient is admitted to the intensive care unit or medical floors, and if they qualify for a liver transplant[32].
In patients who have no response to pharmacological alternatives, non-pharmacological approaches should be considered. This includes transjugular intrahepatic portosystemic shunt, RRT, and molecular adsorbent recirculating system (MARS)[33].
Terlipressin and albumin: Terlipressin (a vasopressin analog) and albumin are the most effective medical therapy for HRS[30]. It has been associated with reducing mortality and increased renal function in patients with type 1 HRS (HRS-AKI as per new definition)[38]. It is the most commonly used combination of vasoconstrictive agents (however, not available worldwide) with its efficacy ranging between 25% and 75%[36]. Several studies have compared the efficacy of albumin alone vs albumin combined with terlipressin, demonstrating that their combination is significatively more efficacious[39].
Terlipressin should be administered either by intravenous bolus (0.5–1 mg every 4–6 h) or continuous infusion with an initial dose of 2 mg/d. If there is no appropriate response to therapy (defined as a decrease of at least 25% of creatinine levels), the intravenous bolus dose may be increased up to 2 mg every 4 h or the continuous infusion increased to a maximum of 12 mg/d. Albumin should be administered by intravenous bolus for the first 2 d, with doses of 1 g/kg (with a maximum dose of 100 g/d) and later continued with 25-50 g/d until the terlipressin therapy is stopped[32,36].
Terlipressin has been associated with an increased risk of cardiovascular events and ischemia induction[32,36,38,40]. However, it has a relatively good safety profile, as adverse events are reported in < 1% of patients[41]. Factors that help determine response to therapy are increased mean arterial pressure of ≥ 5 mmHg and decreased serum bilirubin levels to < 10 mg/dL on day 3 of therapy[42]. In a recent phase 3 trial conducted by Wong et al[43], the combination of terlipressin and albumin was reported to be significantly more effective when compared to placebo. However, its use was associated with significant adverse events, including respiratory failure.
Norepinephrine and albumin: Norepinephrine is an acceptable alternative to terlipressin[30]. It is used as a continuous intravenous infusion rate of 0.5–3 mg/h[30]. Its use is limited as the patient needs a central venous catheter for its administration; therefore, it is usually administered in the intensive care setting[33]. Terlipressin is superior to norepinephrine at decreasing RRT's need and increasing survival in this population[44].
Midodrine, octreotide, and albumin: The combination of midodrine, octreotide, and albumin improves hemodynamics, leading to increased GFR and decreased mortality[45,46]. Midodrine is dosed at 7.5 mg every 8 h and can be increased to a maximum dose of 15 mg every 8 h. Octreotide can be given as a continuous infusion at a rate of 50 μg/h or subcutaneously at doses of 100 μg to 200 μg every 8 h. Albumin is added to an intravenous bolus, with doses of 1 g/kg[32]. In a study by Wang et al[47], terlipressin was reported to be superior to octreotide for improved kidney function but did not show superiority to norepinephrine. This combination is usually used in countries where terlipressin is not yet available[36]. The use of this combination is acceptable in non-intensive care settings, such as inpatient medical floors[30].
Transjugular intrahepatic portosystemic shunts: Transjugular intrahepatic portosy
Renal replacement therapy: The indications for RRT in patients with HRS are the same as those without it[10]. RRT may be effective until liver transplantation is available[36,51]. In a retrospective study by Sourianarayanane et al[52], where 380 patients were reviewed, there was no significant improvement in the survival rates of patients undergoing RRT who did not receive liver transplantation. Other studies suggest that mechanical ventilation might play a role as an independent risk factor for worse outcomes at the time of initiation of RRT. Furthermore, RRT initiation in these patients might be futile, compared to those who are not mechanically ventilated[53].
Molecular adsorbent recirculating system (MARS): Albumin dialysis with MARS has been shown to decrease creatinine levels in patients with HRS. However, there have been no significant changes in survival rates among patients receiving this treatment[36,51,54].
Emerging therapies: Serelaxin, a recombinant human relaxin 2, is a molecule that acts on renal vasculature, increasing perfusion. It has been suggested that Serelaxin could be used for the treatment of HRS, given that in animal models, it has been shown to exert renal vasodilatation[5].
Pentoxifylline is a phosphodiesterase inhibitor that has also been suggested as a potential therapeutic option. A small study showed that it is safe to use along with albumin, midodrine, and octreotide[55]. However, further studies are needed to evaluate the effectiveness of these therapies.
HRS is a significant illness linked to poor prognosis in patients with cirrhosis[56]. Patients with Type I HRS have a mortality rate of 80% 2 wk after the confirmation of the disease, increasing to a 100% within months. Patients with type II HRS have a median survival of 3–6 mo after presentation[57]. In 24-47% of patients with chronic ascites and liver disease, RAI is observed, influencing HRS progression[58].
The most crucial objectives in HRS treatment are to reverse AKI and enable additional medications to be provided to the patients before orthotopic liver transplant (OLT). A recently published study reported that patients with HRS who received treatment before OLT had a significantly higher three-year survival rate, lower incidence of renal dysfunction and serious and acute infections, and lower number of days in the ICU and the hospital, as compared to patients who received transplants without HRS and had normal renal function[59]. HRS is closely linked to hyponatremia, and when serum sodium levels fall below 130 mmol/L, the incidence of HRS due to hypo
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lenz K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Amin AA, Alabsawy EI, Jalan R, Davenport A. Epidemiology, Pathophysiology, and Management of Hepatorenal Syndrome. Semin Nephrol. 2019;39:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Bertino G, Privitera G, Purrello F, Demma S, Crisafulli E, Spadaro L, Koukias N, Tsochatzis EA. Emerging hepatic syndromes: pathophysiology, diagnosis and treatment. Intern Emerg Med. 2016;11:905-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Busk TM, Bendtsen F, Møller S. Hepatorenal syndrome in cirrhosis: diagnostic, pathophysiological, and therapeutic aspects. Expert Rev Gastroenterol Hepatol. 2016;10:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 4. | Low G, Alexander GJ, Lomas DJ. Hepatorenal syndrome: aetiology, diagnosis, and treatment. Gastroenterol Res Pract. 2015;2015:207012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 6. | Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Ospina TJR, Restrepo GJC. Pathophysiology, Diagnosis and Management of Hepatorenal Syndrome. Colomb J Gastroenterol. 2016;31:146-153. |
| 8. | Erly B, Carey WD, Kapoor B, McKinney JM, Tam M, Wang W. Hepatorenal Syndrome: A Review of Pathophysiology and Current Treatment Options. Semin Intervent Radiol. 2015;32:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Kazory A, Ronco C. Hepatorenal Syndrome or Hepatocardiorenal Syndrome: Revisiting Basic Concepts in View of Emerging Data. Cardiorenal Med. 2019;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 10. | Chmielewski J, Lewandowski RJ, Maddur H. Hepatorenal Syndrome: Physiology, Diagnosis and Management. Semin Intervent Radiol. 2018;35:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 12. | Ojeda-Yuren AS, Cerda-Reyes E, Herrero-Maceda MR, Castro-Narro G, Piano S. An Integrated Review of the Hepatorenal Syndrome. Ann Hepatol. 2021;22:100236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Amathieu R, Al-Khafaji A, Sileanu FE, Foldes E, DeSensi R, Hilmi I, Kellum JA. Significance of oliguria in critically ill patients with chronic liver disease. Hepatology. 2017;66:1592-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Velez JCQ, Therapondos G, Juncos LA. Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. 2020;16:137-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | de Mattos ÁZ, de Mattos AA, Méndez-Sánchez N. Hepatorenal syndrome: Current concepts related to diagnosis and management. Ann Hepatol. 2016;15:474-481. [PubMed] |
| 16. | Gomaa SH, Shamseya MM, Madkour MA. Clinical utility of urinary neutrophil gelatinase-associated lipocalin and serum cystatin C in a cohort of liver cirrhosis patients with renal dysfunction: a challenge in the diagnosis of hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2019;31:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Puthumana J, Ariza X, Belcher JM, Graupera I, Ginès P, Parikh CR. Urine Interleukin 18 and Lipocalin 2 Are Biomarkers of Acute Tubular Necrosis in Patients With Cirrhosis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1003-1013.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 18. | Wong F, Blendis L. New challenge of hepatorenal syndrome: prevention and treatment. Hepatology. 2001;34:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Saló J, Ginès A, Ginès P, Piera C, Jiménez W, Guevara M, Fernández-Esparrach G, Sort P, Bataller R, Arroyo V, Rodés J. Effect of therapeutic paracentesis on plasma volume and transvascular escape rate of albumin in patients with cirrhosis. J Hepatol. 1997;27:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 21. | Ibrahim ES, Alsebaey A, Zaghla H, Moawad Abdelmageed S, Gameel K, Abdelsameea E. Long-term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2017;29:1247-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Dong T, Aronsohn A, Gautham Reddy K, Te HS. Rifaximin Decreases the Incidence and Severity of Acute Kidney Injury and Hepatorenal Syndrome in Cirrhosis. Dig Dis Sci. 2016;61:3621-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Udgirkar S, Rathi P, Sonthalia N, Chandnani S, Contractor Q, Thanage R, Jain S. Urinary neutrophil gelatinase-associated lipocalin determines short-term mortality and type of acute kidney injury in cirrhosis. JGH Open. 2020;4:970-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Solé C, Pose E, Solà E, Ginès P. Hepatorenal syndrome in the era of acute kidney injury. Liver Int. 2018;38:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Acevedo JG, Cramp ME. Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol. 2017;9:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (3)] |
| 26. | Tariq R, Hadi Y, Chahal K, Reddy S, Salameh H, Singal AK. Incidence, Mortality and Predictors of Acute Kidney Injury in Patients with Cirrhosis: A Systematic Review and Meta-analysis. J Clin Transl Hepatol. 2020;8:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M, Fernández J, Rodés J, Arroyo V, Ginès P. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488-496.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 29. | Rognant N. Acute kidney injury in patients with chronic liver disease. World J Hepatol. 2015;7:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Nanda A, Reddy R, Safraz H, Salameh H, Singal AK. Pharmacological Therapies for Hepatorenal Syndrome: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2018;52:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J Gastroenterol. 2019;25:3684-3703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (4)] |
| 32. | Dundar HZ, Yılmazlar T. Management of hepatorenal syndrome. World J Nephrol. 2015;4:277-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 33. | Facciorusso A. Hepatorenal Syndrome Type 1: Current Challenges And Future Prospects. Ther Clin Risk Manag. 2019;15:1383-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Clewell JD, Walker-Renard P. Prostaglandins for the treatment of hepatorenal syndrome. Ann Pharmacother. 1994;28:54-55. [PubMed] |
| 35. | Bennett WM, Keeffe E, Melnyk C, Mahler D, Rösch J, Porter GA. Response to dopamine hydrochloride in the hepatorenal syndrome. Arch Intern Med. 1975;135:964-971. [PubMed] |
| 36. | Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK. Hepatorenal Syndrome. Clin J Am Soc Nephrol. 2019;14:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 37. | Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Allegretti AS, Israelsen M, Krag A, Jovani M, Goldin AH, Schulman AR, Winter RW, Gluud LL. Terlipressin vs placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst Rev. 2017;6:CD005162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, Ganger D, Jamil K, Pappas SC; REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology. 2016;150:1579-1589.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 40. | Bucsics T, Krones E. Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf). 2017;5:127-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Krag A, Borup T, Møller S, Bendtsen F. Efficacy and safety of terlipressin in cirrhotic patients with variceal bleeding or hepatorenal syndrome. Adv Ther. 2008;25:1105-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, Solá E, Baccaro ME, Terra C, Arroyo V, Ginès P. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA, Sharma P, Sanyal AJ, Mayo MJ, Frederick RT, Escalante S, Jamil K; CONFIRM Study Investigators. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021;384:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 306] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 44. | Arora V, Maiwall R, Rajan V, Jindal A, Muralikrishna Shasthry S, Kumar G, Jain P, Sarin SK. Terlipressin Is Superior to Noradrenaline in the Management of Acute Kidney Injury in Acute on Chronic Liver Failure. Hepatology. 2020;71:600-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 45. | Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Kalambokis G, Economou M, Fotopoulos A, Al Bokharhii J, Pappas C, Katsaraki A, Tsianos EV. The effects of chronic treatment with octreotide vs octreotide plus midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Wang H, Liu A, Bo W, Feng X, Hu Y. Terlipressin in the treatment of hepatorenal syndrome: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e0431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 50. | Song T, Rössle M, He F, Liu F, Guo X, Qi X. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Mindikoglu AL, Pappas SC. New Developments in Hepatorenal Syndrome. Clin Gastroenterol Hepatol. 2018;16:162-177.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Sourianarayanane A, Raina R, Garg G, McCullough AJ, O'Shea RS. Management and outcome in hepatorenal syndrome: need for renal replacement therapy in non-transplanted patients. Int Urol Nephrol. 2014;46:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Witzke O, Baumann M, Patschan D, Patschan S, Mitchell A, Treichel U, Gerken G, Philipp T, Kribben A. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19:1369-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V; RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 55. | Stine JG, Wang J, Cornella SL, Behm BW, Henry Z, Shah NL, Caldwell SH, Northup PG. Treatment of Type-1 Hepatorenal Syndrome with Pentoxifylline: A Randomized Placebo Controlled Clinical Trial. Ann Hepatol. 2018;17:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (3)] |
| 57. | Arroyo V, Terra C, Ginès P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018;38 Suppl 1:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 59. | Restuccia T, Ortega R, Guevara M, Ginès P, Alessandria C, Ozdogan O, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V, Rodés J. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 60. | Angeli P, Wong F, Watson H, Ginès P; CAPPS Investigators. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 274] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 61. | Wang L, Long Y, Li KX, Xu GS. Pharmacological treatment of hepatorenal syndrome: a network meta-analysis. Gastroenterol Rep (Oxf). 2020;8:111-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Cho HC, Jung HY, Sinn DH, Choi MS, Koh KC, Paik SW, Yoo BC, Kim SW, Lee JH. Mortality after surgery in patients with liver cirrhosis: comparison of Child-Turcotte-Pugh, MELD and MELDNa score. Eur J Gastroenterol Hepatol. 2011;23:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |