Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2150
Peer-review started: March 24, 2021
First decision: June 15, 2021
Revised: June 24, 2021
Accepted: October 17, 2021
Article in press: October 17, 2021
Published online: December 27, 2021
Processing time: 277 Days and 12.4 Hours
Noninvasive measures to estimate liver fibrosis in lieu of biopsy in nonalcoholic liver disease (NAFLD) can broadly differentiate high vs low degrees of condition extent. However, an “indeterminate score” necessitates further clinical investigation and biopsy becomes essential, highlighting the need for identification of other noninvasive factors with accuracy for this midlevel extent and its prognosis. Lean NAFLD cases are of particular interest regarding this issue, as they present as otherwise healthy, and will benefit greatly from the less invasive assessment.
To estimate the agreement of two noninvasive assessment tools in lean NAFLD patients, and assess factors related to indeterminate scores.
Ultrasound-diagnosed NAFLD patients, without sign of other chronic liver disease (n = 1262), were enrolled from a tertiary private medical centre between 2016-2019. After grouping by body mass index (obese, overweight, and lean), each participant underwent FibroScan. NAFLD fibrosis score (NFS) was used for subclassification (lower, higher, and indeterminate). No patient underwent liver biopsy. The kappa statistic was used to assess inter-rater agreement between the three groups on liver fibrosis degree assessed via FibroScan and NFS. Indeterminate score among the three groups was assessed to identify factors that predict its determination.
The NAFLD study cohort was composed of lean (159/1262, 12.6%), overweight (365/1262, 29%) and obese (737/1262, 58.4%) individuals. The lean patients were significantly younger (49.95 ± 15.3 years, P < 0.05), with higher serum high density lipoprotein (52.56 ± 16.27 mg/dL, P < 0.001) and lower prevalences of type 2 diabetes mellitus, hypertension and hyperlipidaemia. All groups showed a predominance of lower fibrosis degree. The lean NAFLD patients showed a significantly lower NFS (P < 0.001). Degree of agreement between FibroScan and NFS was fair between the lean and obese NAFLD categories, and moderate in the overweight category. NFS was predictive of indeterminate score. Age was a factor among all the body mass index (BMI) categories; other associated factors, but with less strength, were serum alanine aminotransferase in the overweight category and BMI in the obese category.
Lean NAFLD patients showed lower degree and prevalence of liver fibrosis by NFS; however, follow-up biopsy is still needed.
Core Tip: Nonalcoholic fatty liver disease (NAFLD) has emerged as a leading cause of chronic liver disease and its complications. Evaluation of fibrosis in NAFLD is of the utmost importance to early application of targeted intervention. The utilization of liver biopsy has diminished, due to patient unacceptance, sampling error, and availability of noninvasive measures of fibrosis. In this study of NAFLD cases, lean patients showed a relatively healthy metabolic profile, lower fibrosis degree and less frequent “indeterminate score“ than overweight and obese patients, among which increased age and serum alanine aminotransferase level were predictive factors for determination.
- Citation: Khayyat YM. Determination of “indeterminate score” measurements in lean nonalcoholic fatty liver disease patients from western Saudi Arabia. World J Hepatol 2021; 13(12): 2150-2160
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2150.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2150
Nonalcoholic fatty liver disease (NAFLD) is a growing cause of liver-related mortality which, in recent decades, has surpassed other known causes of chronic liver diseases. It is now considered in the differential diagnoses of both overweight and lean individuals, in association with a well-established panel of metabolic abnormalities. Traditionally, the NAFLD diagnosis has been made by transabdominal ultrasound and its extent determined by the invasive assessment method of percutaneous liver biopsy. This method, despite its accuracy in staging of fibrosis, is still limited by sampling error and a hazardous risk profile of procedure-related complications, regardless of whether the approach is targeted or non-targeted[1].
Visceral obesity was long considered the sole reason for suspicion of underlying NAFLD; however, it is now recognized that lean individuals develop NAFLD. Several inflammatory cytokines have been linked to the potent effect of visceral obesity and its effects on liver fibrosis, such as the NACHT, LPR and PYD-domain containing proteins (NALPs)[2] and on hypoadiponectemia (as well as its role in liver fibrosis)[3]. The reported incidence of NAFLD among the general population is 12.1%, and within that population, lean individuals account for 40.8% and their cases do not represent healthy or benign forms of the condition[4,5]. The lean NAFLD cases add a remarkable burden to the overall landscape of NAFLD. As such, the increased clinical awareness and research focus has led to generation of novel noninvasive tests based upon mathematical modelling, serum biomarkers and liver stiffness transient elastography, providing safe alternative assessment tools by which to evaluate liver fibrosis in lieu of biopsy[6]. Such tests can be applied by specialists and non-specialists alike, particularly for the primary staging of NAFLD[7]. They have been demonstrated to have good performance, with high negative predictive values compared to liver biopsy. They are also particularly informative for NAFLD patients with high risk of advanced fibrosis, through repeated assessment by transient elastography that provides good accuracy of prediction of liver and non-liver related mortality[8].
These less invasive methods of assessment, however, are limited by uncertainty regarding the evaluation of a category of cases that falls between the low and high grades of fibrosis; such cases are scored as “indeterminate” and that label prompts further evaluation by liver biopsy (simultaneously highlighting the limited utility of the noninvasive methods early in the disease process)[9]. Complicating this situation is the fact that the increasing emergence of lean NAFLD cases has in turn increased the demand for noninvasive testing. The study described herein was, thus, designed to first determine the prevalence of indeterminate scored cases among a representative group of lean NAFLD patients, then to comparatively assess findings from bedside transient elastography or FibroScan, and ultimately to identify factors that may predispose lean NAFLD patients to obtaining an indeterminate score by noninvasive liver fibrosis tools.
This study was conducted at a tertiary hospital, between 2016 and 2019. Patients at least 15 years of age who received diagnosis of NAFLD (based on findings from imaging studies in accordance with ultrasonography criteria of fatty liver[10]) and those presenting components of metabolic syndrome (i.e. type 2 diabetes mellitus, hypertension, hyperlipidaemia, central obesity) were recruited. Patients were denied study enrolment if they were under 15-years-old, showed evidence of concurrent active medical disease that is known to impair liver function or of other secondary causes of chronic liver disease, had incomplete data, died during the study recruitment period, or refused participation in the study. Patient data collected upon enrolment included general medical history, liver disease-related history [covering other causes of chronic liver disease, such as risk factors for acquiring viral hepatitis (hepatitis B and hepatitis C virus)], medications (including over-the-counter and herbal remedies), active alcohol use or abuse, and recreational drug use. All enrolled patients were directly assessed for other causes of chronic liver disease, including hemochromatosis, Wilson’s disease, and alpha 1 antitrypsin clinical manifestations, as well as autoimmune liver diseases, including autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, and hepatic vascular disease. All enrolled patients underwent complete physical examination, yielding anthropometric data on height and weight [by standard measurement protocols, used to assess body mass index (BMI)] as well as data on stigmata of chronic liver disease.
Each enrolled patient was fasted for 3 h and then subjected to FibroScan assessment using FibroScan 502 Touch instrument (Echosens©, Paris, France). A medium probe was applied when the skin capsule distance was ≤ 2.5 cm and an XL probe for ≥ 2.5 cm. For each patient, a median score was calculated from the values obtained from 10 successful scans performed at a single localized area.
For each enrolled patient, NAFLD fibrosis score (NFS)[11] was calculated by the following formula: -1.675 + 0.037 × age (in years) + 0.094 × BMI (as kg/m2) + 1.13 × IFG/diabetes (with yes = 1, no = 0) + 0.99 × aspartate aminotransferase/alanine aminotransferase ratio - 0.013 × platelet count (as × 109/L) - 0.66 × albumin (as g/dL).
After exclusion of other causes of chronic liver disease, the enrolled patients were divided into the following three groups according to their BMI: obese (BMI ≥ 30); overweight (BMI: 25-30); and lean (BMI ≤ 25). The noninvasive parameters of liver fibrosis were used to classify the BMI cohorts into low and high degree of liver fibrosis categories[12-14], with the former assigned to patients with FibroScan values < 7.9 kPa and NFS < –1.455 and the latter assigned to patients with FibroScan values > 9.5 kPa and NFS > 0.675; “indeterminate” was assigned for liver fibrosis when the measurement values fell between the low and high categorizations.
All enrolled patients received testing for liver chemistry panel (after 4-6 h of fasting), serum glycosylated haemoglobin, and serum fasting lipid profile. Adherence to diabetic, hypertension and lipid lowering medications were verified through interviews with the patient interviews and/or immediate family relatives, as well as hospital dispensing records.
All statistical analyses were performed with SPSS software (version 26.0; IBM Corp., Armonk, NY, United States). Descriptive statistics and frequencies were calculated. Group differences were examined using one-way analysis of variance or its nonparametric equivalent, the Kruskal-Wallis test. In terms of post-hoc tests, Bonferroni correction was applied. Relationships between categorical variables were analysed with the chi-square test of independence. The kappa statistic was used to assess inter-rater agreement between the three groups on liver fibrosis degree assessed via FibroScan and NFS. Lastly, prediction of indeterminate NFS was determined by binary logistic regression modelling, with a P-value of < 0.005 indicating statistical significance. The statistical methods used and data interpretation were verified by an external biostatistician.
The study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the Ethics Committee of International Medical Centre (Approval No. 2019-11-115).
A total of 1753 patients were recruited during the study period, with 1262 meeting the criteria for enrolment and inclusion in the final analysis. A total of 491 patients had been excluded for the following reasons: incomplete data (n = 103); chronic hepatitis B (n = 185); chronic hepatitis C (n = 71); underwent weight management surgery (n = 66); active neoplastic disorders (n = 11); coexisting medical conditions known to cause liver function test alterations (n = 33); use of hepatotoxic medications(n = 8); and death during the study recruitment period (n = 13).
The entire study cohort was comprised of 159 lean NAFLD patients (12.6%), 365 overweight NAFLD patients (29.0%), and 737 obese NAFLD patients (58.4%). Tables 1 and 2 summarize the metabolic parameters and diseases among the three groups. The lean NAFLD group was of significantly younger age than the overweight and obese groups (P = 0.012).
| Variable | Lean | Overweight | Obese | P1 |
| mean ± SD | mean ± SD | mean ± SD | ||
| Age in yr | 49.95 ± 15.34 | 51.34 ± 14.33 | 53.34 ±13.43 | 0.0122 |
| BMI | 23.14 ± 1.95 | 27.70 ± 1.71 | 35.38 ± 4.62 | 0.174 |
| HbA1c, % | 6.07 ± 1.41 | 6.51 ± 1.61 | 6.46 ± 1.39 | 0.290 |
| ALT in U/L | 37.14 ± 66.48 | 32.52 ± 32.16 | 30.73 ± 30.72 | 0.924 |
| AST in U/L | 28.30 ± 23.81 | 26.44 ± 26.96 | 25.04 ± 20.91 | 0.093 |
| GGT in U/L | 60.40 ± 81.59 | 56.61 ± 81.28 | 57.58 ± 95.50 | 0.141 |
| ALKP in U/L | 89.56 ± 52.69 | 79.77 ± 43.69 | 82.73 ± 38.86 | 0.132 |
| Total bilirubin in mg/dL | 0.74 ± 1.43 | 0.81 ± 1.61 | 0.63 ± 1.08 | 0.227 |
| Direct bilirubin in mg/dL | 0.35 ± 0.60 | 0.40 ± 1.06 | 0.29 ± 0.65 | 0.679 |
| Total cholesterol in mg/dL | 182.07 ± 48.19 | 172.69 ± 49.50 | 175.03 ± 47.37 | 0.222 |
| LDL in mg/dL | 118.84 ± 42.12 | 114.81 ± 42.00 | 115.38 ± 41.05 | 0.022 |
| TG in mg/dL | 118.69 ± 79.73 | 135.74 ± 88.66 | 132.65 ± 88.56 | 0.140 |
| HDL in mg/dL | 52.56 ± 16.27 | 47.30 ± 16.96 | 48.49 ± 16.50 | < 0.001 |
| FibroScan, kPa | 7.43 ± 7.87 | 7.01 ± 8.39 | 8.12 ± 9.49 | 0.174 |
| NFS | -2.74 ± 3.13 | -2.11 ± 2.25 | -1.14 ± 2.13 | 0.290 |
| Variable | Lean | Overweight | Obese | P1 |
| Sex | 0.002 | |||
| Female | 61 (38.4%) | 142 (38.9%) | 359 (48.7%) | |
| Male | 98 (61.6%) | 223 (61.1%) | 378 (51.3%) | |
| Hyperlipidaemia | < 0.001 | |||
| Absent | 76 (47.8%) | 130 (35.6%) | 235 (31.9%) | |
| Present | 76 (47.8%) | 205 (56.2%) | 457 (62.0%) | |
| DM | < 0.001 | |||
| Non-diabetic | 103 (64.8%) | 171 (46.8%) | 294 (39.9%) | |
| Diabetic | 50 (31.4%) | 171 (46.8%) | 405 (55.0%) | |
| HTN | 0.002 | |||
| Normotensive | 103 (64.8%) | 198 (54.2%) | 366 (49.7%) | |
| Hypertensive | 50 (31.4%) | 144 (39.5%) | 333 (45.2%) | |
| NFS reference | < 0.001 | |||
| F0-F2 | 85 (53.5%) | 173 (47.4%) | 256 (34.7%) | |
| F3-F4 | 5 (3.1%) | 16 (4.4%) | 84 (11.4%) | |
| Indeterminate score | 30 (18.9%) | 89 (24.4%) | 237 (32.2%) | |
As shown in Table 1, the lean NAFLD group showed lower serum glycated haemoglobin (i.e. HbA1c) and higher serum high density lipoprotein (i.e. HDL) than either the overweight or obese NAFLD groups. The prevalence of various metabolic diseases differed significantly between the three BMI groups. Hyperlipidaemia was more prevalent in the overweight group (n = 205) and the obese group (n = 457) than in the lean group (n = 76, P < 0.001). Hypertension was also more prevalent in the overweight group (n = 144) and the obese group (n = 333) than in the lean group (n = 50, P = 0.002). Type 2 diabetes mellitus was more prevalent and to a much greater extent in the obese group (n = 405) compared to the overweight group (n = 171, P < 0.001) and lean group (n = 50, P < 0.001).
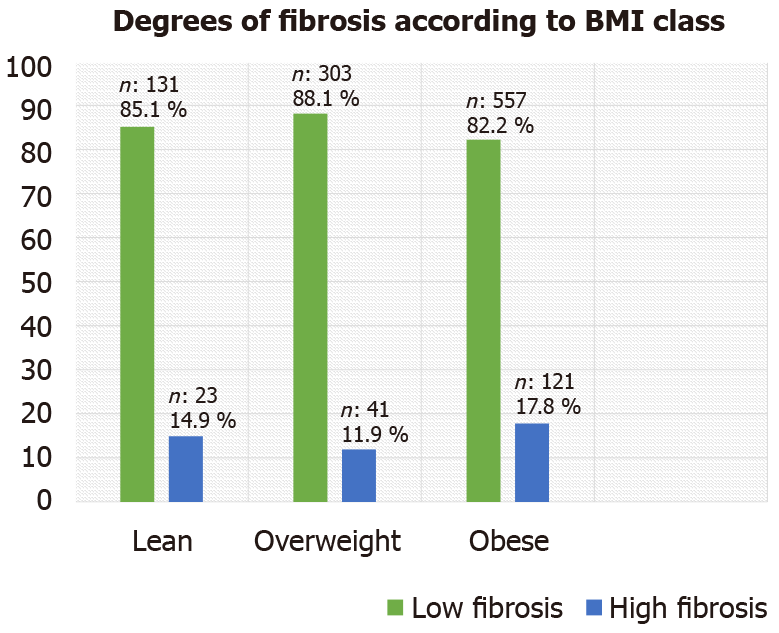
Transient elastography by FibroScan showed the three BMI groups to have a predominance of lower fibrosis measurements (F0-F2, vs higher fibrosis measurements of F3-F4) (Figure 1). In contrast, the NFS showed a significant difference between the three groups, with the lean group showing lower scores for patients in both the lower and higher fibrosis categories compared to that seen in the overweight group (P = 0.041) and the obese group (P < 0.001). Additionally, when the overweight group was compared with the obese group, the NFS was found to be significantly lower for the former (P < 0.001) (Table 2).
Upon evaluation of agreement between the noninvasive measures studied (FibroScan and NFS), the lean and obese groups showed fair agreement and the overweight group showed moderate agreement (Table 3).
| BMI class | Category | NFS < -1.455 | NFS > 0.676 | Agreement, κappa |
| Lean | Low fibrosis | 72 | 1 | 0.37c |
| High fibrosis | 10 | 4 | ||
| Overweight | Low fibrosis | 151 | 8 | 0.43c |
| High fibrosis | 9 | 8 | ||
| Obese | Low fibrosis | 212 | 40 | 0.38c |
| High fibrosis | 30 | 38 |
In order to predict the possible factors that may predict an indeterminate score when NFS is used in patients with NAFLD and to compare them between the different BMI groups, single-predictor binary regression analysis was carried out with age, BMI, sex, HbA1c, AST, ALT, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin, direct bilirubin, total cholesterol, low density lipoprotein, HDL, hyperlipidaemia, diabetes mellitus, and hypertension considered as independent variables (Table 4). Increasing age was found to be a statistically significant predictive factor for obtaining an indeterminate score when the NFS measurement of liver fibrosis was used. Similarly, elevated serum ALT and BMI values were found to be predictive of obtaining an indeterminate score when the NFS was used for overweight and obese groups, respectively.
| Variable | Lean | Overweight | Obese | ||||||
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Age | 1.07 | 1.02, 1.13 | 0.009b | 1.04 | 1.01, 1.08 | 0.016 | 1.03 | 1.02, 1.05 | < 0.001b |
| HbA1c | 1.28 | 0.84, 1.95 | 0.257 | 1.08 | 0.85, 1.36 | 0.541 | |||
| BMI | 1.04 | 1.00, 1.08 | .030 | 1.04 | |||||
| ALT | 0.98 | 0.96, 0.99 | 0.011 | 1.00 | 0.99, 1.00 | 0.169 | |||
| Hyperlipidaemia | 0.75 | 0.31, 1.84 | 0.536 | 1.01 | 0.64, 1.57 | 0.981 | |||
| LDL | 0.99 | 0.98, 1.00 | 0.161 | ||||||
| DM | 0.63 | 0.17, 2.30 | 0.484 | 0.55 | 0.21, 1.39 | 0.204 | 0.99 | 0.65, 1.50 | 0.946 |
| HTN | 0.61 | 0.19, 1.96 | 0.406 | 1.34 | 0.61, 2.91 | 0.464 | 0.77 | 0.51, 1.18 | 0.232 |
The findings from this study reflect real-life data for NAFLD cases of various BMI classes and help to distinguish the distinctive metabolic phenotypes of each, providing particular insight into the lean NAFLD cases that represent a growing cohort worldwide. The lean NAFLD cases in this study were relatively young compared to other BMI groups and their phenotypic profile was closer to that of healthy individuals (in terms of having lower serum HbA1c, higher serum HDL, and less prevalence of type 2 diabetes mellitus, hypertension and hyperlipidaemia). Also, the lean group showed an overall lower fibrosis stage as measured by both FibroScan and NFS. The prevalence of cases yielding an indeterminate score was highest among the obese group (32%), followed by the overweight group (24.4%) and lean group (18.9%). Upon assessment of agreement between these two modalities, the degree of agreement ranged between fair to moderate.
With the increased recognition of the importance of precision medicine in general and increased popular use of treatment algorithms in NAFLD, a proper noninvasive assessment method for liver fibrosis is needed. Indeed, advanced diagnostic methods are emerging. Transient elastography is a bedside test, easily applicable, and cost effective, with the added benefit of patient acceptance. It has been adopted clinically by non-specialist health care providers for initial assessment of liver fibrosis[15,16]. However, the drawbacks and imprecision of this technique include attenuation of the elastic shear waves by visceral obesity and subcutaneous tissues, leading to a failure rate of 3%-16%[17]. Technological enhancement of transient elastography has been made by the use of an XL probe to measure shear waves at a lower degree of fibrosis, yielding negative predictive value of 89% and specificity of 78%; nevertheless, increased BMI still carries the potential for discordance (odds ratio: 9)[14]. Since that advancement, a plethora of other noninvasive tests have been developed to overcome a variety of other obstacles using a combination of blood parameters entered into mathematical models, including direct biological and indirect markers of liver function and fibrosis[6].
Waist circumference and assessment of visceral obesity has been considered as another option to assess the degree of liver fibrosis. It is applied by means of a bedside clinical measurement of the visceral adiposity index (commonly known as the VAI); albeit, that its measurement is reportedly more robust with more advanced stages of fibrosis[18-21]. Using radiological modalities, abdominal ultrasound with assessment of the abdominal wall fat index (commonly known as the AFI)[22], and computed tomography scan with assessments of visceral fat[23], visceral adipose tissue[24] or visceral-to-subcutaneous abdominal fat ratio[25] are able to predict advanced steatohepatitis and liver fibrosis. Moreover, bioelectrical impedance estimated visceral fat (commonly known as BIA)[26] is able to predict histologically advance steatohepatitis and fibrosis.
This study found a combination of transient elastography (FibroScan) and NFS measurements in different BMI classes among individuals with predominantly lower fibrosis degree (accounting for > 80% of each BMI class). The lean NAFLD group of patients, in particular, showed fair agreement of the two tools within a lower category of fibrosis, compared to the moderate agreement shown among the overweight and obese groups. The literature includes reports of different strategies to increase the chance of proper assessment and accuracy. For example, repeat transient elastography is especially useful for when a higher degree of fibrosis is being measured (> 7.9 kPa); as shown by Chow et al[27], this strategy increased accuracy and subsequent normalization of the measurements in up to one-third of the patients examined. Combining FibroScan with other measures has also been shown to further increase accuracy. A novel two-step approach to determine fibrosis in patients with high and indeterminate scores obtained with use of NFS followed by transient elastography measurement as found to minimize the need for liver biopsy compared to the use of either test alone[12]. In a Latin study by Perez-Gutiérrez et al[28] that correlated NFS to biopsy-based grading of liver fibrosis using Brunt criteria, up to 46% of the patients with indeterminate score showed no liver fibrosis; hence, this group would benefit from careful follow-up and possibly repeat liver biopsy.
Factors that affect interpretation of noninvasive assessment data were investigated in this study as well. A German multicentre study (known as the FLAG study) on ultrasound-based diagnosis of NAFLD in conjunction with several noninvasive assessment measures determined differences between the various noninvasive assessments of fibrosis; when groups of no-fibrosis, indeterminate score and advanced fibrosis were compared, the predictive factors were identified as increased age, waist circumference, serum AST, serum gamma-glutamyl transferase, serum ferritin, and type 2 diabetes mellitus[29]. Another study found type 2 diabetes mellitus to adversely affect the accuracy of the noninvasive parameters investigated [i.e. HEPASCORE, AST to platelet ratio index (the APRI) and FIB-4 tests] by down-staging their fibrosis assessment measures[30]. Similar studies have been carried out with real-life situation design. An example of such is a multi-European study that reported indeterminate scores for FIB-4 tests, ranging between 25%-30% among different NAFLD groups at primary care centres[9]. Considering the literature collectively, mitigation of liver fibrosis assessment without resorting to liver biopsy may be achieved by a combination of FibroScan measurement, NFS[12,31], serum M30 (a caspase that is cleaved to form K18 fragments that are released from apoptotic hepatocytes into the blood, where they can be detected by the M30 enzyme linked-immunosorbent assay), and APRI score[32]. Indeed, the increased accuracy achieved with this combination of tests ultimately minimized the need for liver biopsy.
In the study presented herein, patient-related characteristics, serum test results and metabolic diseases were assessed to identify potential predictive factors that may anticipate obtainment of an “indeterminate score” from NFS. Increased age and elevated serum ALT were found to increase the likelihood of need for liver biopsy. Cichoz-Lach et al[33] from Poland reported a similar statistically significant diagnosis of liver fibrosis in patients with indeterminate scores (constituting 30.9% of their cohort) upon analysis of NFS and BARD scores with the predictive factors of increased age, BMI > 30, and high ALT/AST ratio. In the present study, the relatively large study population provided new information of the burden of NAFLD in the region (Saudi Arabia) and the small contribution of lean NAFLD.
Importantly, lean NAFLD has long been considered as more prevalent in Asian countries. In this study, however, upon classifying NAFLD patients by BMI, we see a population prevalence of obesity similar to that in western populations; this also suggests greater generalizability of the region-specific data. Despite the fact that there was a predominantly lower degree of fibrosis in our study population, agreement was found between transient elastography and NFS. It is arguable that lean individuals may have less technical limitation for acquiring transient elastography measurement in their lean body configuration, however they still may score indeterminate score of fibrosis which subsequently impairs a precise estimation and leaves the need for liver biopsy. This limitation related to the low extent of liver fibrosis (and thus availability for the technology to detect) is an issue the merits further study. Additionally, long-term follow-up of patients with indeterminate score by NFS is needed in order to elucidate the prognosis of this measurement.
For lean NAFLD patients, noninvasive tools are valid for assessing liver fibrosis, subject to the same limitations as with obese NAFLD patients. Indeterminate score obtained by NFS is still an issue, with possible need for a subsequent histological-based assessment of liver fibrosis through invasive procedure (i.e. biopsy). Future studies can build upon this knowledge through efforts to determine the best follow-up strategy for such cases.
Nonalcoholic fatty liver disease (NAFLD) is progressively surpassing other aetiologies of chronic liver disease, with its prevalence increasing worldwide. Earlier intervention was advocated to manage cases of less extensive fibrosis before they progress, and this process will involve the conventional invasive detection method of liver biopsy. Due to the increasing emergence of non-obese NAFLD, which is also called lean NAFLD, the need for further study of its phenotype has been recognized and related findings are expected to open new avenues for more accurate detection of fibrosis.
Since lean NAFLD patients are phenotypically healthy, their metabolic syndrome profile is normal. The expected degree of liver fibrosis among these cases is unknown. However, it is well recognized that use of the available noninvasive assessment tools for fibrosis in NAFLD yields a proportion of cases with “indeterminate score” who may require further assessment by liver biopsy.
To identify lean NAFLD characteristics distinguishing from obese NAFLD in terms of the degree of liver fibrosis using noninvasive assessment tools. Additionally, to study predictive factors that may predispose to obtainment of an indeterminate score, which may then be taken into consideration for decision-making on further affirmative evaluation by liver biopsy.
NAFLD patients were categorized based on body mass index into lean, overweight and obese groups. Each group underwent assessment by the noninvasive tools of FibroScan and NAFLD fibrosis score (NFS). Group data based upon the subsequent subcategorizations of fibrosis degree (i.e. low, high and indeterminate) was applied to regression analysis to identify factors predictive of obtaining the indeterminate score.
A total of 1753 patients were recruited and 1262 of these were included in the final analysis. According to body mass index, the patients were grouped as lean (159, 12.6%), overweight (365, 29%) or obese (737, 58.4%). Lower fibrosis score was predominant within all three weight groups. Kappa statistical analysis of the FibroScan and NFS data indicated that lean and obese NAFLD cases had fair agreement between the two tools, while overweight NAFLD cases had moderate agreement. Logistic binary regression analysis performed for predictive factors of the indeterminate score obtained by NFS indicated age as a predictive factor in all three weight groups, and serum alanine aminotransferase and body mass index value as predictive in the overweight and obese groups, respectively.
The lean NAFLD group showed a metabolic profile similar to healthy individuals but having a lower degree of fibrosis than their overweight and obese counterparts. The limitation of indeterminate score by NFS among obese NAFLD patients is similar to that with the lean NAFLD group; unfortunately, this is not explained by the fact that lean body mass index patients receive a more precise measurement of fibrosis than their obese counterparts. Factors that play a role in lean NAFLD patients obtaining an indeterminate score may be applied to overweight and obese counterparts; these being age and serum alanine aminotransferase of the patients.
Considering lean individuals as a latent undiagnosed group among NAFLD cases, efforts to understand and properly evaluate their underlying liver fibrosis still requires systematic consideration. From the perspective of aiming to apply less invasive tools for clinical assessment of liver fibrosis, further data are needed to ascertain the benefits and limitations of the available noninvasive tools, in order to design an approach for accurate assessment of fibrosis in this newly recognized NAFLD group.
The author would like to thank Ms. Malgorzata Jakubowska for assistance with statistical analysis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology, 24030.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tarantino G S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Maheux A, Purcell Y, Harguem S, Vilgrain V, Ronot M. Targeted and non-targeted liver biopsies carry the same risk of complication. Eur Radiol. 2019;29:5772-5783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Mehta R, Neupane A, Wang L, Goodman Z, Baranova A, Younossi ZM. Expression of NALPs in adipose and the fibrotic progression of non-alcoholic fatty liver disease in obese subjects. BMC Gastroenterol. 2014;14:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Yoneda M, Iwasaki T, Fujita K, Kirikoshi H, Inamori M, Nozaki Y, Maeyama S, Wada K, Saito S, Terauchi Y, Nakajima A. Hypoadiponectinemia plays a crucial role in the development of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus independent of visceral adipose tissue. Alcohol Clin Exp Res. 2007;31:S15-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | VanWagner LB, Armstrong MJ. Lean NAFLD: A not so benign condition? Hepatol Commun. 2018;2:5-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 6. | Nallagangula KS, Nagaraj SK, Venkataswamy L, Chandrappa M. Liver fibrosis: a compilation on the biomarkers status and their significance during disease progression. Future Sci OA. 2018;4:FSO250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Broussier T, Lannes A, Zuberbuhler F, Oberti F, Fouchard I, Hunault G, Cales P, Boursier J. Simple blood fibrosis tests reduce unnecessary referrals for specialized evaluations of liver fibrosis in NAFLD and ALD patients. Clin Res Hepatol Gastroenterol. 2020;44:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kamarajah SK, Chan WK, Nik Mustapha NR, Mahadeva S. Repeated liver stiffness measurement compared with paired liver biopsy in patients with non-alcoholic fatty liver disease. Hepatol Int. 2018;12:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Alexander M, Loomis AK, Fairburn-Beech J, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Avillach P, Egger P, Kendrick S, Waterworth DM, Sattar N, Alazawi W. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 10. | Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:6821-6825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 11. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2282] [Article Influence: 126.8] [Reference Citation Analysis (1)] |
| 12. | Chan WK, Nik Mustapha NR, Mahadeva S. A novel 2-step approach combining the NAFLD fibrosis score and liver stiffness measurement for predicting advanced fibrosis. Hepatol Int. 2015;9:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 14. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, Merrouche W, Chu SH, Pesque S, Chan HL, de Lédinghen V. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Tapper EB, Sengupta N, Hunink MG, Afdhal NH, Lai M. Cost-Effective Evaluation of Nonalcoholic Fatty Liver Disease With NAFLD Fibrosis Score and Vibration Controlled Transient Elastography. Am J Gastroenterol. 2015;110:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-Effectiveness Analysis: Risk Stratification of Nonalcoholic Fatty Liver Disease (NAFLD) by the Primary Care Physician Using the NAFLD Fibrosis Score. PLoS One. 2016;11:e0147237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Festi D, Schiumerini R, Marzi L, Di Biase AR, Mandolesi D, Montrone L, Scaioli E, Bonato G, Marchesini-Reggiani G, Colecchia A. Review article: the diagnosis of non-alcoholic fatty liver disease -- availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Ercin CN, Dogru T, Genc H, Celebi G, Aslan F, Gurel H, Kara M, Sertoglu E, Tapan S, Bagci S, Rizzo M, Sonmez A. Insulin Resistance but Not Visceral Adiposity Index Is Associated with Liver Fibrosis in Nondiabetic Subjects with Nonalcoholic Fatty Liver Disease. Metab Syndr Relat Disord. 2015;13:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Díez-Rodríguez R, Ballesteros-Pomar MD, Calleja-Fernández A, González-De-Francisco T, González-Herráez L, Calleja-Antolín S, Cano-Rodríguez I, Olcoz-Goñi JL. Insulin resistance and metabolic syndrome are related to non-alcoholic fatty liver disease, but not visceral adiposity index, in severely obese patients. Rev Esp Enferm Dig. 2014;106:522-528. [PubMed] |
| 20. | Petta S, Amato MC, Di Marco V, Cammà C, Pizzolanti G, Barcellona MR, Cabibi D, Galluzzo A, Sinagra D, Giordano C, Craxì A. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Vongsuvanh R, George J, McLeod D, van der Poorten D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. J Hepatol. 2012;57:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Fukuda K, Seki Y, Ichihi M, Okada T, Hirata A, Kogita S, Sawai Y, Igura T, Tsugawa M, Imai Y. Usefulness of ultrasonographic estimation of preperitoneal and subcutaneous fat thickness in the diagnosis of nonalcoholic fatty liver disease in diabetic patients. J Med Ultrason (2001). 2015;42:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Michielsen P, Van Gaal L. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int J Obes (Lond). 2011;35:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, Cho EJ, Lee JH, Kim HY, Kim YJ, Kim CY. Visceral Obesity Predicts Significant Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Medicine (Baltimore). 2015;94:e2159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Jung CH, Rhee EJ, Kwon H, Chang Y, Ryu S, Lee WY. Visceral-to-Subcutaneous Abdominal Fat Ratio Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis. Endocrinol Metab (Seoul). 2020;35:165-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Hernández-Conde M, Llop E, Carrillo CF, Tormo B, Abad J, Rodriguez L, Perelló C, Gomez ML, Martínez-Porras JL, Puga NF, Trapero-Marugan M, Fraga E, Aracil CF, Panero JLC. Estimation of visceral fat is useful for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2020;26:6658-6668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Chow JC, Wong GL, Chan AW, Shu SS, Chan CK, Leung JK, Choi PC, Chim AM, Chan HL, Wong VW. Repeating measurements by transient elastography in non-alcoholic fatty liver disease patients with high liver stiffness. J Gastroenterol Hepatol. 2019;34:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Pérez-Gutiérrez OZ, Hernández-Rocha C, Candia-Balboa RA, Arrese MA, Benítez C, Brizuela-Alcántara DC, Méndez-Sánchez N, Uribe M, Chávez-Tapia NC. Validation study of systems for noninvasive diagnosis of fibrosis in nonalcoholic fatty liver disease in Latin population. Ann Hepatol. 2013;12:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Hofmann WP, Buggisch P, Schubert L, Dikopoulos N, Schwenzer J, Muche M, Felten G, Heyne R, Ingiliz P, Schmidt A, Stein K, Wedemeyer H, Berg T, Wiegand J, Lammert F, Zeuzem S, Schattenberg JM. The Fatty Liver Assessment in Germany (FLAG) cohort study identifies large heterogeneity in NAFLD care. JHEP Rep. 2020;2:100168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Bertot LC, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Chin J, Huang Y, Adams LA. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018;38:1793-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Drolz A, Wehmeyer M, Diedrich T, Piecha F, Schulze Zur Wiesch J, Kluwe J. [Combination of NAFLD Fibrosis Score and liver stiffness measurement for identification of moderate fibrosis stages (II & III) in non-alcoholic fatty liver disease]. Z Gastroenterol. 2018;56:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Mahadeva S, Mahfudz AS, Vijayanathan A, Goh KL, Kulenthran A, Cheah PL. Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J Dig Dis. 2013;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Cichoż-Lach H, Celiński K, Prozorow-Król B, Swatek J, Słomka M, Lach T. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med Sci Monit. 2012;18:CR735-CR740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |