Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1727
Peer-review started: June 11, 2021
First decision: July 6, 2021
Revised: July 19, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: November 27, 2021
Processing time: 166 Days and 0.8 Hours
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CF transmembrane conductance regulator gene. CF liver disease develops in 5%-10% of patients with CF and is the third leading cause of death among patients with CF after pulmonary disease or lung transplant complications. We review the pathogenesis, clinical presentations, complications, diagnostic evaluation, effect of medical therapies especially CF transmembrane conductance regulator modu
Core Tip: Cystic fibrosis(CF) liver disease is caused by abnormal cholangiocyte function, altered biliary secretion and abnormal innate immune response with abnormal response to endotoxins. CF liver disease can present with a wide variety of clinical features from a heterogenous liver on ultrasound, to life threatening gastrointestinal bleeds secondary to portal hypertension. Novel treatment strategies directly targeting the ion channel abnormality-cystic fibrosis transmembrane conductance regulator modulators are available and has significantly improved the clinical status and life expectancy of the cystic fibrosis patients.
- Citation: Valamparampil JJ, Gupte GL. Cystic fibrosis associated liver disease in children. World J Hepatol 2021; 13(11): 1727-1742
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1727.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1727
Cystic fibrosis (CF) the most frequent fatal autosomal recessive disorder in Caucasians, is caused by autosomal recessive disease caused by mutations in the CF transmem
CF liver disease (CFLD) usually develops within the first 20 years of life and has a stable non-progressive or mildly progressive course in later life[3,4]. Most children with CF will have some degree of steatosis but clinically significant liver disease develops in < 10% of pediatric CF patients usually by 10 years of age. CF related cirrhosis is a disease of the childhood and adolescence while predominant biliary involvement mimicking sclerosing cholangitis mostly occurs in adulthood[5]. The diagnosis of liver disease has profound implications in short and long term prognosis in CF patients and is the third leading cause of mortality in CF. Analysis of a large cohort of patients from the CF Foundation Patient Registry database showed that in CF patients with liver disease, the estimated 10-year cumulative rate of any adverse liver-related outcomes was approximately 20%[2]. Liver disease with cirrhosis and or portal hypertension has been classified as severe CFLD.
CFLD is a genetic disorder of cholangiocyte transport protein defect, resulting in chronic cholangiopathy caused by reduced ductal bile flow generation and reduction in biliary chloride and bicarbonate secretion caused by the dysfunction of CFTR[6,7]. But this mechanism alone cannot explain CFLD, because CFTR deficiency is present in all patients while CFLD occurs only in a small population of CF patients and has varying clinical manifestations and severity. As described below, a combination of factors including CFTR genotype, non-CFTR genetic variability, abnormal intracellular interactions, abnormal cholangiocyte function, altered biliary secretion, pathologic stimulation of innate immune response with abnormal response to endotoxins lead to CFLD.
Abnormal CFTR results in inhibition of cyclic adenosine monophosphate dependent chloride and bicarbonate secretion. This reduces the bile flow and alkalinity resulting in the biliary epithelial damages deriving from the retention of cytotoxic bile acids and xenobiotics and from the reduction in natural defenses against microbiologic pathogens. The response to chronic epithelial damage and the progression in the liver damage depends on the immunogenetic response of the individual and on other modifier genes.
CFTR mediated liver injury is also postulated to be caused by ability to regulate the function of other proteins by physically associating in macromolecular complexes at the membrane (protein-protein interaction)[8,9]. CFTR interacting proteins are located not only in the plasma membrane but also in nucleus, endoplasmic reticulum, Golgi apparatus, trafficking vesicles, proteasomes and cytoskeleton[9]. For example, the interaction of CFTR with proteins regulating the function of non-receptor tyrosine kinase Rous sarcoma oncogene cellular homologue can modulate innate immune responses in cholangiocytes[8]. Dysfunction of interactions can have systemic consequences resulting from the perturbation of the interconnected cellular networks accounting for some of the phenotypic variation in CF[8].
The conventional theory of CFLD postulates that biliary epithelial CFTR dysfunction causes alterations in the volume and composition of bile, resulting in loss of protective effect of biliary bicarbonate and mucus and an accumulation of toxic bile acids causing damage to the epithelium by initiating an inflammatory response[8]. But it is now postulated that the abnormal inflammatory response is due to lack of tolerance in the innate immune system[7]. CFTR is a now thought as a regulator of cholangiocyte innate immune responses and defective CFTR results in aberrant activation of Src tyrosine kinase causing upregulation of innate inflammatory responses via the Toll-like receptor 4/NF-κB axis[7,10]. This results in lack of tolerance of biliary epithelium to endotoxin (e.g. pathogen-associated molecular patterns) from bile and intestine, leading to a para-inflammatory process in the biliary epithelium with the release of cyto/chemokines and the infiltration of the portal spaces with inflammatory cells[7,10].
There is a substantial reduction in the richness and diversity of gut bacteria in patients with CF from early childhood until late adolescence and the changes deviate progressively farther from the path of healthy controls with increasing age[11]. Gut dysbiosis results in reduction in anti-inflammatory short-chain fatty acids, altered ratios of arachidonic acid/Linoleic acid and arachidonic acid/docosahexaenoic acid leading to increased gut inflammation[8,12]. This causes increased permeability of intestinal epithelia, increasing the exposure of biliary epithelial cholangiocytes to endotoxins, perpetuating the inflammatory cascade[8,12]. But it is not certain if intestinal inflammation is caused by the altered microbiota in CF or is the consequence of an altered environment[8,12].
There is massive heterogenicity in CFTR phenotype among patients with CFLD and CFTR genotype-phenotype correlations are generally weak. The functional conse
The prevalence of CFLD varies widely in children and adolescents, based upon the diagnostic criteria used ranging from < 5% to 68%[17,18]. CFLD is more common and the median age of diagnosis is earlier in males[19]. Liver involvement in CF may be subclinical until diffuse liver damage occurs. Liver involvement can vary from mild elevation of aminotransferases to cirrhosis with synthetic failure and portal hypertension. The degree of liver involvement and the rate of progression of liver disease varies significantly among individuals. The awareness of CFLD and its clinical implications has increased as evidenced by an early diagnosis and a drop in the median time at diagnosis from adolescence to < 3 years of age[17,18].
Risk factors for CFLD include male sex, presence of severe mutations, presence of SERPINA 1Z allele, history of meconium ileus, exocrine pancreatic insufficiency and CF-related diabetes[20]. The most common clinical feature is asymptomatic hepatomegaly detected by clinical examination or ultrasonography[18]. Pancreatic insufficiency occurs in 99% of patients with CFLD[19]. Liver involvement in CF can be classified into two broad categories based on the presence of cirrhosis/portal hypertension (Table 1).
| Spectrum of cystic fibrosis liver disease in children |
| Liver |
| Neonatal cholestasis |
| Pre-clinical |
| Elevated aminotransferases |
| Increased GGT |
| Steatosis |
| Portal hypertension including non-cirrhotic portal hypertension |
| Cirrhosis |
| Focal biliary |
| Multi-lobular |
| Gallbladder and biliary system |
| Cholelithiasis |
| Abnormal size/function |
| Intra and extrahepatic biliary strictures (sclerosing cholangitis) |
Cholestasis: Neonatal/infantile cholestasis is the earliest manifestation of liver involvement in CF, but is very rare (< 2%). It is important to exclude other common causes of neonatal cholestasis like biliary atresia and also to consider the diagnosis of CF in infants who present with cholestasis[21].
Abnormal liver enzymes: The commonly noticed abnormalities include intermittent rise in serum transaminases (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) and/or increased serum levels of alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT). Elevated liver enzymes can precede clinical and radiological abnormalities by several years. Bile duct damages can be demonstrated even in asymptomatic cases[22]. About 53%–93% of patients with CF have at least one abnormal value of AST/ALT, while over one-third have abnormal levels of GGT by 21 years of age[23]. CFLD patients with cirrhosis with portal hypertension can have normal liver biochemistry and synthetic function. Fluctuations in liver biochemistry is common and can be due to medications, infection or malnutrition.
Steatosis: Steatosis is common in CF patients, seen in upto 70% children undergoing liver biopsies[24]. The etiology is uncertain, but postulated to be due to malnutrition, deficiencies of essential fatty acid, carnitine and choline[24,25]. Steatosis in CF patients can also be caused by impaired glucose tolerance, diabetes mellites, hypertriglyceridemia and obesity[23]. Significant steatosis has become uncommon due to earlier diagnosis of CFLD and appropriate nutritional management. Alcohol consumption should be considered in adolescent CF patients with steatosis. Steatosis in CF was previously thought to be a benign condition, but with the emergence of nonalcoholic steatohepatitis as a leading cause of cirrhosis and understanding of the pathology, this might no longer be the case. Other signs of chronic liver disease or portal hypertension are usually not present.
Gallbladder and biliary tract involvement: Abnormalities of gallbladder (GB) can be present in children with CF. Micro-GB has been described in up to 33% of patients and GB might even be absent in CF patients[26]. Abnormal function of gallbladder and gallstones can also present. Black pigmented stones are more commonly found in patients with CF compared to cholesterol gallstones which are common in general population[26]. Symptomatic GB disease (4%) and need for cholecystectomy is common in adults[26].
Intra- or extrahepatic biliary strictures and segmental dilation has been reported in children with CF. Bile duct strictures and associated complications frequently occur even in patients with mild variants of CF. Magnetic resonance (MR) cholangiography data has shown that up to 70% of patients can have abnormalities of biliary tree regardless of biochemical or clinical evidence of liver disease and can mimic primary sclerosing cholangitis[24,26]. There is no correlation between severity of liver disease, abnormal liver tests and the presence of biliary strictures[24,26].
Portal hypertension: Variceal bleed can occur with or without cirrhosis and frequently occurs in the context of preserved hepatic synthetic function. Varices can be seen in 10- 70% with CFLD and may be present at diagnosis of CFLD in 25%[19,27,28]. Isolated gastric varices may be seen in 15%[19]. Variceal bleed can be the sentinel event in CFLD leading to the diagnosis of portal hypertension/cirrhosis in up to 50% and may also be fatal, either from bleed itself or by precipitating liver failure. The age at first bleed can range from 10-30 years and recurrent bleeds can also occur[4]. Variceal bleed is associated with 5 fold risk of liver transplantation (LT)[2]. Thrombocytopenia has been postulated as a marker of severe CFLD with portal hypertension, so decreasing or persistently low platelet counts should prompt evaluation for portal hypertension[19]. Non cirrhotic portal hypertension can also occur in CFLD[28,29]. This has been postulated to be due to perisinusoidal portal venopathy caused by inflammation and fibrosis[24,29].
Focal biliary cirrhosis: Focal biliary cirrhosis is characterized by focal areas of scarring and furrowing in the liver with large areas of normal preserved hepatic architecture in between. Histologically, it is characterized by cholestasis, significant focal fibrosis, plugging of bile ducts with eosinophilic material, bile duct proliferation and expansion of portal tract leading to the postulation that bile duct plugging is the causative factor.
Focal biliary cirrhosis is clinically silent without any abnormalities on physical examination and normal liver biochemistry. Radiological imaging is also frequently noncontributory. Postmortem studies have shown that the incidence of focal biliary cirrhosis increases with advanced age- 11% in infants, 27% at 1 year and 25%–70% of adults[24]. Only a small subset of patients will progress to more severe liver disease and eventually multilobular cirrhosis, but the factors causing this is not known.
Multilobular cirrhosis: Biliary cirrhosis with portal hypertension is the most severe clinical manifestation of CFLD. Clinically, liver is multilobulated and firm- extensive lobulation is characteristic of CF cirrhosis. Signs of chronic liver disease such as clubbing, spider angioma, and palmar erythema may be present but is uncommon and often occurs late in the disease course. There are no clinical or biochemical abnormalities or radiological features that consistently predict the presence of cirrhosis or risk of development of portal hypertension[28]. Majority of the morbidity due cirrhosis is caused by complications arising from portal hypertension. Hepatic encephalopathy is rare complication of cirrhosis per se in CFLD and mostly has occurred after therapeutic portosystemic shunting for management of portal hypertension[24]. Hepatic decompensation as evidenced by progressive decrease in albumin levels and development of ascites represents poor prognosis and necessitates LT evaluation.
Patients with cirrhosis are at risk of significant malnutrition as compared to CF patients without liver disease. This is due to anorexia, micronutrient deficiency, early satiety due to organomegaly and increased catabolism. In a study comparing CFLD patients with CF patients without liver disease, body fat measurements, including triceps, subscapular, and supra-iliac skinfold measures, were significantly less in the CFLD patients[27]. However, weight, height and mid upper arm circumference were not different between the two groups[27].
Liver enzymes (AST, ALT, GGT) are poor predictors or indicators of cirrhosis or the risk of development of cirrhosis or CFLD and are neither sensitive or specific. There is poor correlation of liver enzymes with histologic findings, with 25% of CFLD patients with biopsy proven severe liver fibrosis having normal ALT levels[28]. But patients presenting with significant or persistently elevated liver biochemistries warrant further investigation for evidence of CFLD and other etiologies (Table 2). Persistently elevated GGT might be a pointer to biliary disease (e.g., sclerosing cholangitis). Thrombocytopenia with splenomegaly is suggestive of development of portal hypertension. The synthetic function of liver (clotting, albumin) should be checked in all patients with suspected CFLD. If deranged after correcting nutritional (poor diet, vitamin deficiency) defects, should be thoroughly investigated.
| Condition | Investigation |
| Acute/chronic viral hepatitis | Serology for HAV, HBV, HCV, EBV, CMV, adenovirus, HHV 6, parvovirus |
| α1 antitrypsin deficiency | Serum α1 antitrypsin level, including phenotype |
| Autoimmune hepatitis | Non-organ specific autoantibodies (SMA, anti-LKM1, LC1) |
| Celiac disease | Total IgA, IgA anti-tissue transglutaminase |
| Wilson disease | Ceruloplasmin, serum copper, 24 h urinary copper |
| Drug induced liver injury | Antibiotics (cyclines, macrolides, amoxicillin-based, and cephalosporins) & antifungals (azoles and polyenes) |
| Genetic hemochromatosis (adults) | Iron, Ferritin, Transferrin binding capacity |
| Other causes of steatosis | Malnutrition, diabetes, obesity |
Ultrasound (US) of the hepatobiliary system with Doppler measurements of hepatic vasculature is non-invasive and may be a valuable marker of early CFLD[30]. Partial or complete hyper echogenicity liver, suggestive of steatosis is the most common US finding in CF[31]. Another fatty infiltration pattern, pseudomasses, seen as lobulated fatty structures of 1–2cm causing heterogeneity in the liver parenchyma is typical of CF[31]. Focal biliary cirrhosis appears sonographically as regions of increased echogenicity in periportal areas[31,32]. Cirrhotic liver has a nodular appearance with a coarsened echotexture[32]. Right hepatic lobe atrophy and hypertrophy of the caudate and lateral segments of the left lobe may be seen[32]. Splenomegaly, portosystemic shunts, hepatofugal flow in portal vein, and ascites can be seen with portal hypertension.
Abnormal echogenicity frequently precedes biochemical/clinical evidence of liver disease, with one study showing that two thirds of the children with abnormal liver echotexture and 50% with portal hypertension had no biochemical/clinical evidence of CFLD at the time when US changes were first noted[30]. Heterogeneous pattern of liver has been shown to be associated with higher risk of development of advanced liver disease in CF patients[30,33]. However, there is significant intra/ interobserver variability in US imaging and children with normal hepatic US can have advanced fibrosis, so a normal US does not exclude significant liver fibrosis or CFLD[3].
Assessment of the intra and extrahepatic biliary tree is better with MR cholangiography. The typical appearances include strictures, beading, narrowing, or dilatation of the intrahepatic ducts; diffuse narrowing or focal stricture of the common bile duct; and calculi[32].
Liver biopsy (LB) the gold standard in diagnosing fibrosis and cirrhosis, but is difficult to perform in CF patients because of the invasive nature and presence of associated comorbidities. Also because of the patchy distribution of lesions in CFLD, LB may underestimate the severity of lesions[25]. LB should be reserved for evaluation for other potential causes of fibrosis (autoimmune hepatitis, Wilson’s disease, hepatotrophic infections) or drug-induced liver injury.
The early detection and monitoring of fibrosis, assessment of stage of fibrosis and progression to CFLD is challenging because routinely available tests to measure liver damage can often be normal even in advanced cirrhosis and liver biopsy is invasive with potential risk of complications. Non-invasive tests are divided into direct and indirect markers of liver fibrosis and imaging modalities as outlined in Table 3.
| Non-invasive marker | Ref. | Outcome measured | AUC | Sensitivity | Specificity | Comments |
| Indirect markers of liver fibrosis | ||||||
| APRI | Leung et al[37] | CFLD diagnosis and severe CFLD | 0.81 | 73% | 70% | APRI score cut-off > 0.264; Predict CFLD and significant fibrosis in CFLD with a high degree of accuracy |
| FIB-4 | Leung et al[37] | Portal hypertension | 0.91 | 78% | 93% | FIB-4 cutoff 0.358 |
| Direct markers of liver fibrosis | ||||||
| TIMP-1 | Pereira et al[38] | CFLD diagnosis | 0.76 | 64% | 83% | Significantly increased in CFLD vs no-CFLD |
| Prolyl hydroxylase | Pereira et al[38] | CFLD | 60% | 91% | Negative correlation between serum TIMP-1 levels and the stage of histological fibrosis; Prolyl hydroxylase useful in distinguishing CFLD patients with early fibrogenesis vs extensive fibrosis; Not able to differentiate CFLD versus no-CFLD | |
| diagnosis | ||||||
| TIMP-2 | Rath et al[38] | CFLD diagnosis | 0.69 | - | - | |
| m-RNA’s | Cook et al[39] | CFLD diagnosis | 0.78 | 47% | 94% | Able to differentiate between CFLD versus no-CFLD but quantify not fibrosis stage; Pathological significance not yet certain, more studies needed |
| Imaging methods | ||||||
| Transient elastography | Witters et al[40] | Liver stiffness | 0.86 | 63% | 87% | Less inter and intra-observer variability; Easy to learn and perform; Regular measurements for serial follow-up feasible |
| Rath et al[34] | Liver stiffness | 0.68 | - | - | Few centres have access to technology | |
| MR elastography | Palermo et al[41] | Liver stiffness | - | 100% | 100% | Small study, paucity of data; Shear stiffness significantly elevated in CF patients with cirrhosis; Costly with limited availability |
Direct markers are components of extracellular matrix degradation or fibrogenesis in serum include Matrix Metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and 2, procollagen III peptide, collagen type-IV, hyaluronic acid, laminin, prolyl hydroxylase and YKL-40. These are not readily available in the routine clinical setting, are costly and are not validated in large scale studies. Indirect markers are serum-based tests and consist of readily available biochemical surrogates and clinical risk factors (AST, ALT, platelet count, age) for liver fibrosis. These include aspartate aminotransferase to platelet ratio index (APRI) and Fibrosis-4 (Fib-4). Stonebraker et al[19] demonstrated in a large pediatric cohort (n = 497) with CFLD and portal hypertension that APRI and Fib-4 values could differentiate patients who developed complications of portal hypertension and were significantly different in CFLD patients with and without oesophageal varices.
Advanced imaging modalities which quantify liver stiffness as a marker of fibrosis such as transient elastography (TE, Fibroscan®), acoustic radiation force impulse and MR elastography have been shown to accurately reflect advanced liver disease/end-stage fibrosis in CF. Liver stiffness as measured by TE had high diagnostic accuracy and was increased in CFLD compared to CF patients without liver disease[34]. Serial monitoring using TE is more useful as progressive enhancement of liver stiffness as this might reflect progression of liver disease thereby facilitating early detection[34,35]. MR elastography is currently the most accurate noninvasive method across the spectrum of liver fibrosis and offers promise in the assessment of response to antifibrotic drugs but is not well studied in the context of CF liver disease[36].
Noninvasive methods are valuable for excluding advanced fibrosis or cirrhosis, but are not sufficiently predictive when used in isolation and have not yet been demonstrated to accurately reflect fibrosis change in response to treatment, limiting their role in disease monitoring[36]. Combination of serum markers with liver stiffness analysis might improve the sensitivity and negative predictive value without altering the specificity[34]. The negative predictive value of noninvasive tests is generally very high, allowing the clinician to be confident that advanced fibrosis or cirrhosis has been excluded.
The wide spectrum, variability of presentation at different age groups, presence of confounding factors and the absence of specific markers or tests makes it difficult to diagnose CFLD. The common differential diagnosis to be considered in CFLD are listed in Table 2.
The commonly used diagnostic criteria are described in Table 4.
| Debray et al[25] | CF foundation classification[24] |
| Hepatomegaly and/or splenomegaly- increased liver span at midclavicular line and spleen size in longitudinal coronal plane for age and sex, confirmed by ultrasonography | CF related liver disease with cirrhosis/portal hypertension (based on clinical exam/imaging, histology, laparoscopy) |
| Abnormalities of liver function tests-elevated AST and ALT and GGT levels above the upper limit of normal with at least at 3 consecutive determinations over 12 months after excluding other causes of liver diseases | Liver involvement without cirrhosis/portal hypertension consisting of at least one of the following: (1) Persistent AST, ALT, GGT > 2 times upper limit of normal; (2) Intermittent elevations of the above laboratory values; (3) Steatosis (histologic determination); (4) Fibrosis (histologic determination); (5) Cholangiopathy (based on ultrasound, MRI, CT, ERCP); and (6) Ultrasound abnormalities not consistent with cirrhosis |
| Ultrasonographic evidence of coarseness, nodularity, increased echogenicity, or portal hypertension | Preclinical: No evidence of liver disease on clinical examination, imaging or laboratory values |
| Liver biopsy showing cirrhosis |
Management of CFLD should be done by a multi-disciplinary team and is mainly supportive since there is no effective therapy to treat or prevent progression of fibrosis, portal hypertension, or cirrhosis in CFLD. The CF foundation guidelines recommends annual screening for CFLD in children with examination of abdomen (hepatosplenomegaly), biochemical evaluation (bilirubin, AST, ALT, GGT, ALP, albumin, prothrombin time, platelet count), abdominal US and pulse oximetry (screening for hepatopulmonary syndrome)[25]. Salicylic acid and non-steroid anti-inflammatory drugs are contraindicated once CFLD is diagnosed and vaccination against hepatitis A and B should be done.
Ursodeoxycholic acid (UDCA) is recommended for all children diagnosed with CFLD at 20 mg/kg/d divided twice daily initially and increased up to 30 mg/kg/d[25]. A Cochrane review[42] had shown that there were only few trials assessing the effectiveness of UDCA with poor quality of evidence and there was no data on the effect of UDCA on long term outcomes including need for LT or mortality. Hence, the long term continuation of UDCA should be individualized.
Optimal nutrition is the cornerstone of CFLD management. Malnutrition in CF is multifactorial including malabsorption due to pancreatic insufficiency, recurrent infections, chronic inflammation, chronic liver disease and anorexia. Nutrition should be managed by experienced CF dietetic team. It is recommended that CFLD patients increase energy intake to 150% of Recommended Daily Allowance preferably by increasing proportion of fat to 40%–50% of the energy content of the feed or diet, with supplementation in medium chain triglycerides and special attention to polyunsaturated fatty acids[25].
About 3 g/kg/d of protein and sufficient pancreatic enzymes to allow optimal absorption of long-chain triglycerides and essential fatty acids is also recommended. High dose oral fat soluble vitamin supplements is recommended- vitamin A (5000–15000 international units daily), vitamin E (alpha tocopherol 100–500 mg daily), vitamin D (alphacalcidiol 50 ng/kg to maximum of 1 μg) and vitamin K (1–10 mg daily)[25]. Plasma levels of vitamins (A, D and E) and prothrombin time needs to be closely monitored to prevent toxicity or deficiencies.
Salt supplementation should be avoided in CF patients with cirrhosis and portal hypertension due to the risk of development of ascites. If adequate caloric intake cannot be achieved orally, nasogastric feeding may be required to ensure adequate caloric intake. CFTR modulator therapy has resulted in less pulmonary exacerbations, decrease in levels of inflammatory makers, better body mass index and pancreatic function resulting in better overall nutritional status[14].
Management of varices in CFLD is complicated by the fact that non-selective beta-blocker (propranolol or carvedilol) might be contraindicated due to the associated lung disease and repeated general anesthesia required for screening of therapeutic endoscopic procedures may also reduce lung function and predispose to infections. Primary variceal prophylaxis in CFLD most commonly involves endoscopic variceal band ligation, but there is lack of quality evidence in children[24].
Variceal bleeding in the absence of decompensated cirrhosis in CFLD is most commonly managed by therapeutic endoscopy (band ligation +/- sclerotherapy)[4]. Sclerotherapy is useful if variceal band ligation is unsuccessful or gastric varices are present. Patients with refractory life threatening bleeds might require transjugular intrahepatic portosystemic shunt (TIPSS) or in rare circumstances surgical portosystemic shunting as an lifesaving procedure. Careful evaluation of liver disease and lung disease is necessary before proceeding with an elective TIPSS procedure. In a study[4] specifically analyzing outcomes of variceal bleeds in CFLD, out of 35 bleeding episodes, 30 were controlled by endoscopic procedures, while 11% (4 episodes) required either TIPSS, surgical shunts procedures.
LT evaluation should be offered for CFLD patients with intractable complications of portal hypertension and/or end stage liver disease since LT confers significant survival advantage[43]. The main indications of isolated LT in CFLD is listed in Table 5. Poor growth and nutrition as an indication remains controversial because studies have not shown consistent improvement after LT[43]. LT should be considered when nutritional deficiencies are believed to be sequelae of advanced liver disease and portal hypertensive enteropathy impacting clinical outcomes[43]. Lung function may improve, remain stable or deteriorate after LT and any short term advantage with improvement of lung function is lost within 3 years of LT[44,45]. So, rapidly deteriorating lung function alone should not be an indication for isolated LT in stable CFLD[46].
| Indications and contraindications | |
| Indications | |
| Strong | (1) Progressive hepatic dysfunction with hypoalbuminemia and coagulopathy (Coagulopathy not corrected by vitamin K, cholestasis not attributed to other causes); (2) Complications of portal hypertension (Intractable/recurrent variceal bleeding which is not controlled by medical or endoscopic management); (3) Hepatopulmonary and porto-pulmonary syndrome; (4) Overt hepatic encephalopathy; and (5) Hepatorenal syndrome |
| Controversial | (1) Deteriorating pulmonary function (FEV1/FVC <50%) with increased frequency and severity of pulmonary infective episodes requiring hospitalization; and (2) Severe malnutrition, unresponsive to intensive nutritional support |
| Contraindications | |
| Absolute | (1) Extrahepatic malignancies not amenable to curative therapy; (2) Multiorgan disease for which transplant would not be considered life-sustaining; (3) Uncontrolled systemic or pulmonary infection, active exacerbation, or veno-arterial extracorporeal membrane oxygenation; and (4) Severe porto-pulmonary hypertension nonresponsive to medical management |
| Relative | (1) Hepatocellular carcinoma; (2) Noncompliance or psychosocial concerns unamenable to transplant; (3) Uncontrollable CF-related diabetes; (4) Substance abuse; (5) Severe cardiopulmonary disease; and (6) Infection/colonization with multi-resistant organism (e.g., Burkholderia cenocepacia and Mycobacterium abscessus) |
Long term outcomes after LT are lower in children with CFLD as compared to other etiologies[44]. Table 6 illustrates details of few published series on LT in CFLD in children. For those patients with end-stage liver disease and significant pulmonary complications, combined liver-lung or liver-heart-lung transplantation may be considered, but outcomes are worse compared to isolated LT[45,46].
| Ref. | Type | Number of pediatric recipients | Type of transplants | Males | Mean age at isolated liver transplantation (yr) | Lung function after Liver transplantation | 5-year survival |
| Milkiewicz et al[45], 2002 | Single center | 9 | Liver; Liver- lung -heart | Not available | 15 | Improved | Not available |
| Fridell et al[21], 2003 | Single center | 12 | Liver | 83% | 10 ± 4.5 | Improved or remained unchanged | 75% |
| Molmenti et al[47], 2003 | Single center | 10 | Liver | 90% | 9.7 (1.23–19) | Not available | 60% |
| Mendizabal et al[44], 2011 | Analysis of United Network for Organ Sharing database | 148 | Liver; Liver- lung (3.4%) | 62% | 11 ± 4.7 | Not available | 86% |
| Miguel et al[48], 2011 | Single center | 11 | Liver | 67% | 12 (5.4–17) | Worsened or remained unchanged | > 85% |
| Dowman et al[49], 2012 | Single center | 19 | Liver | Not available | 11.8 (9.5–16.5) | Stable/improved initially, deteriorated > 5 years after transplant | > 60% |
Careful assessment of liver disease, pulmonary function, nutritional status and type of transplant to be performed should be done by an experienced multidisciplinary team. Concomitant causes or other etiologies of liver injury as listed in Table 3 should be ruled out before LT is considered. Alpha-1-antitrypsin level and genotype, screening for autoimmune hepatitis and Wilson’s disease should be done as a part of the workup especially if the child is seen for the first time in a LT center. CFLD patients being considered for LT should have endoscopic variceal surveillance and possibly coordinated with bronchoscopy and dental procedures as part of the LT evaluation to minimize the number anaesthetic procedures[43]. Careful evaluation of cardiac function should be done since patients with cardiomyopathy or severe pulmonary hypertension may require combined heart, lung, and liver transplantation.
A thorough evaluation by a pediatric pulmonologist with CF and lung transplantation expertise should be a part of the LT assessment, irrespective of the forced expiratory volume in one second (FEV1). Analysis of United Network for Organ Sharing data from 1987 through 2009 suggested that patients with a predicted forced vital capacity (FVC) > 75% and FEV1 > 60% (possibly even ≥ 40%) may be safely offered isolated LT[50]. The possibility of progressive deterioration in lung function after LT should be communicated to the family. The most difficult group to decide is patients who require LT but present with borderline (FEV1 40%-60% predicted) and/or rapidly declining (10% FEV1 predicted/year) pulmonary function[43].
Microbial considerations, such as multidrug resistant bacterial infections and history of recurrent/ invasive fungal infections are critical since post-transplant sepsis is a leading cause of mortality[43,50]. Flexible bronchoscopy with bronchioalveolar lavage with cultures for mycobacteria, fungus, and quantitative bacterial analysis from at least 2 locations within each lung is recommended[43]. The presence of multidrug resistant Mycobacterium abscessus in the lungs, even with well-preserved pulmonary function, carries a high risk of mortality in the first year after transplant and needs to be considered carefully before recommendation for LT[43].
Patients should be evaluated for nasal polyps and chronic sinusitis and treated immediately if identified[43]. CF–related diabetes should be evaluated and well controlled prior to LT. Dietetic and nutritional assessment is an integral part of the evaluation.
Immunosuppression after LT in patients with CF will vary from center to center but typically consists of triple drug therapy with tacrolimus, steroids and mycophenolate mofetil/azathioprine. Close collaboration between the CF, transplant and infectious diseases teams is crucial because of the increased risk of mortality from infections. Early mortality (< 6 mo) post-LT is due to disseminated aspergillosis/candidiasis, and sepsis with gram-negative enteric bacteria and staphylococcus aureus while later deaths are a result of progressive pulmonary disease[43]. Post-transplant antibiotic prophylaxis in our unit consists of fluconazole for candida species, acyclovir for herpes simplex virus, valganciclovir for cytomegalovirus and trimethoprim-sulfamethoxazole for Pneumocystis jiroveci. Distal intestinal obstructive syndrome (DIOS) causing acute potentially life-threatening intestinal obstruction can develop post- transplant in >20% of pediatric patients[49]. In the pre-transplant period, DIOS occurs typically in older CF patients in adolescence and adulthood, in those with advanced liver disease, severe CFTR mutations, pancreatic insufficiency and diabetes mellitus. In our unit, patients are categorized into low risk (no episodes of DIOS in previous 5 years) and high risk (episodes of DIOS in previous 5 years and previous abdominal surgery) before LT. Our pre and post-LT protocol for prevention and treatment of DIOS is given in Table 7. High risk patients should be counselled for loop ileostomy formation at transplant assessment.
| Pre and post-transplant protocol | |
| Low risk | (1) 600 mg N-acetyl-cysteine in 120 mL water orally/nasogastric tube twice/day. Senna twice daily; (2) 2 liters of Klean prep per day post-transplant; (3) Consider early nasogastric tube in patients with delayed gastric emptying studies pre-operatively; (4) All patients in intensive care unit should only receive only elemental feed via nasogastric tube as this does not require pancreatic enzyme replacement. Once transferred to ward, can be restarted on regular feeding and pancreatic enzyme supplements; (5) Try and reduce opiates early during hospital stay; and (6) Treat all patients with proton pump inhibitors. |
| High risk | (1) As per low risk management; and (2) High risk of developing DIOS and subsequent surgical gut decompression is associated with a high mortality. So these patients should receive a prophylactic loop ileostomy. |
| Treatment of DIOS | (1) Stop feeding, nasogastric tube on free drainage and intravenous fluids; (2) 100 mL gastrografin in 400 mL water enterally and repeat after 6 h; (3) Subsequent management is with Klean prep in 1 L water over 1 h via oral/nasogastric tube and can be repeated up to 4 times every 24 h until bowel movement is achieved; and (4) If no improvement after 48 h, then it is unlikely to resolve without surgery to decompress the gut and also consider total parenteral nutrition. |
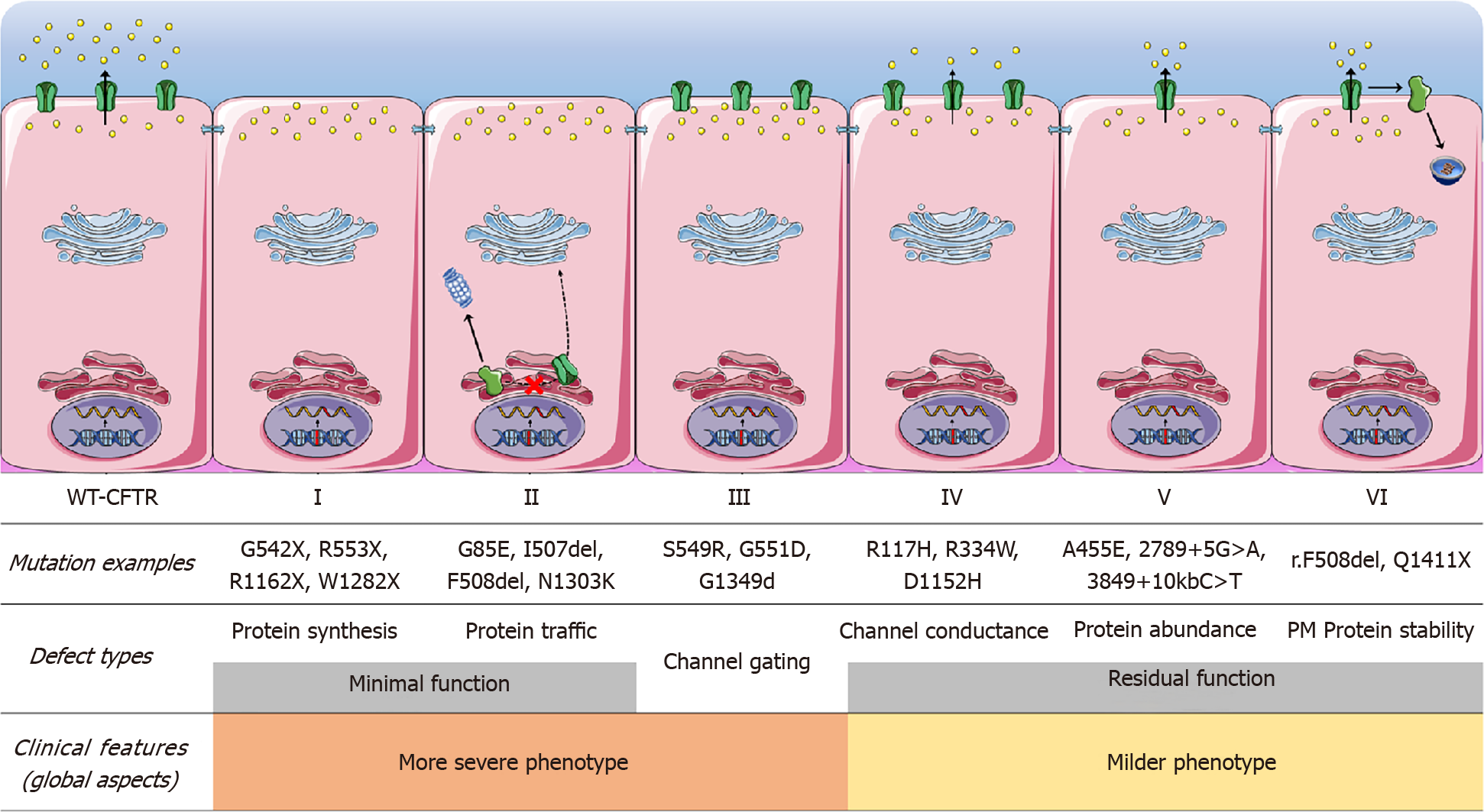
CFTR modulator drugs enhance or even restore the expression, function, and stability of a defective CFTR by different mechanisms[14,51] (Table 8). These treatments target the underlying cause of CF and is classified into five main groups depending on their effects on CFTR mutations[14,51] (Table 8). Different CFTR genetic variants can benefit from the same type of modulator and this is the base of a new system recently introduced to classify and group common and rare CFTR variants based on their response to modulators called ‘theratyping’.
| Type of modulator | Mechanism of action | Mutation class in which drug is effective | Example | Clinical effects/present status of modulator |
| Potentiators | Restore or even enhance the channel open probability, thus allowing for CFTR-dependent anion conductance | Classes III and IV | Ivacaftor | Improvement in lung function, pancreatic function and body mass index |
| Correctors | Rescue folding, processing and trafficking to the plasma membrane of a CFTR mutant. Enhance protein conformational stability during the endoplasmic reticulum folding process | Class II | Lumacaftor; Tezacaftor; Posenacaftor; Elexacaftor | Significant improvement in lung function when used with Ivacaftor |
| Stabilizers | Anchor CFTR at the plasma membrane, thus preventing its removal and degradation by lysosomes | Class VI | Cavosonstat | First CFTR stabilizer studied in clinical trials- studies terminated because of lack of clinical efficacy |
| Read-through agents | Induce ribosomal over-reading of premature termination codon, enabling the incorporation of a foreign amino acid in place and continued translation to the normal end of the transcript | Class I | Ataluren (PTC124) | Clinical trials terminated |
| Amplifiers | Increase expression of CFTR mRNA and thus biosynthesis of the CFTR protein | Class V | Nesolicaftor (PTI-428) | Clinical trial planned |
The first United States Food and Drug Administration (FDA) approved drug was ivacaftor (Kalydeco, Vertex Pharmaceuticals)[14,51]. Other FDA approved CF modulators combinations are lumacaftor/ivacaftor (Orkambi®, Vertex Pharmaceuticals), tezacaftor/ivacaftor (Symdeko® or Symkevi®, Vertex Pharmaceuticals) for patients aged ≥ 12 years who are F508del-homozygous or F508del-heterozygous with a residual function mutation[14,20]. Lumacaftor/ivacaftor has been approved for F508del homozygous patients aged ≥ 2 years[14]. The triple combination elexacaftor/ ivacaftor/tezacaftor (Trikafta™, Vertex Pharmaceuticals) has been by the FDA for the treatment of CF patients aged ≥ 12 years with F508del mutation in at least one allele, benefiting 90% of CF population[14,51].
Abnormal elevation aminotransaminases (> 8 times upper limit of normal, more commonly in pediatric patients) and bilirubin (> 3 times upper limit of normal) has been reported 3%-15% of patients on CFTR modulators[52-54]. Lumacaftor/ivacaftor was shown to have less hepatic steatosis as assessed by MR imaging proton density fat fraction in a small cohort[55]. In a study[56] of 117 patients with CFTR gating mutations (partially F508 del heterozygous) treatment with Ivacaftor partially restored disrupted FGF19-regulated bile acid homeostasis. Worsening of liver function and liver failure leading to death has been reported in CF patients with pre-existing cirrhosis and portal hypertension receiving lumacaftor/ivacaftor.
Recommendations for dose adjustment are based on Child Pugh classification: no dose adjustment for Child-Pugh Class A but dose reduction is recommended for Child-Pugh Class B and C. This is applicable to adults and no specific recommendations exist in the literature for children with CFLD. Lumacaftor/ivacaftor should be used with caution in patients with advanced liver disease and only if the benefits are expected to outweigh the risks.
Because an association with liver injury cannot be excluded, assessments of liver function tests (ALT, AST and bilirubin) are recommended before initiation, a month after starting the treatment and every 3 mo during the first year of treatment, and annually thereafter. For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered in collaboration with a pediatric hepatology centre. In the event of significant elevation of ALT or AST, with or without elevated bilirubin [either ALT or AST > 5× the upper limit of normal (ULN), or ALT or AST > 3× ULN with bilirubin > 2× ULN and/or clinical jaundice], dosing with CFTR modulators should be discontinued and closely followed up until the abnormalities resolve. A thorough investigation of potential causes should be conducted and patients should be followed closely for clinical progression. Following resolution of transaminase elevations, the benefits and risks of resuming CFTR modulators should be considered.
Metabolism of CFTR inhibitors is by the CYP450 enzyme pathway. Hence concomitant use of lumacaftor/ivacaftor with these immunosuppressants is not recommended at present as they may reduce efficacy of immunosuppressants by induction of the CYP3A pathway. Given the fact that respiratory function may eventually worsen after LT, CFTR modulators might need to be initiated post-transplant due to significant beneficial effects on lung function, nutritional status and decreased pulmonary exacerbations[43].
CFLD is the most important non-pulmonary cause of death in CF. CFLD is has a wide spectrum from asymptomatic elevation of liver enzymes to severe disease with portal hypertension and cirrhosis with synthetic failure. The degree of liver involvement and the rate of progression of liver disease varies significantly among individuals. There are no specific clinical features or tests for prediction or early detection of CFLD, so regular screening is essential for CF patients. Currently, there is no medical therapy to prevent or treat or CFLD. With the advent of CFTR modulators, improvement in medical management has resulted in significantly improved life expectancy in patients with CF and this will have implications in the management of CFLD in future. The long term effects of CFTR modulators on CFLD and liver function is not known, but will hopefully have a beneficial effect. LT is indicated in patients with CFLD with severe portal hypertension or impaired synthetic function of liver either alone or in combination with lung transplantation.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Pediatrics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ayoub F, Costache RS S-Editor: Wang JL L-Editor: A P-Editor: Guo X
| 1. | Cutting GR, Engelhardt J, Zeitlin PL. Genetics and pathophysiology of Cystic Fibrosis. In: Wilmott R, Bush A, Deterding R, Ratjen F, Sly P, Zar H, Li AP, editors. Kendig’s Disorders of the Respiratory Tract in Children. Philadelphia: Elsevier, 2019: 757-768. |
| 2. | Ye W, Narkewicz MR, Leung DH, Karnsakul W, Murray KF, Alonso EM, Magee JC, Schwarzenberg SJ, Weymann A, Molleston JP; CFLDnet research group. Variceal Hemorrhage and Adverse Liver Outcomes in Patients With Cystic Fibrosis Cirrhosis. J Pediatr Gastroenterol Nutr. 2018;66:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology. 1999;30:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 213] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Gooding I, Dondos V, Gyi KM, Hodson M, Westaby D. Variceal hemorrhage and cystic fibrosis: outcomes and implications for liver transplantation. Liver Transpl. 2005;11:1522-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Leung DH, Narkewicz MR. Cystic Fibrosis-related cirrhosis. J Cyst Fibros. 2017;16 Suppl 2:S50-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Staufer K, Halilbasic E, Trauner M, Kazemi-Shirazi L. Cystic fibrosis related liver disease--another black box in hepatology. Int J Mol Sci. 2014;15:13529-13549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Strazzabosco M, Fiorotto R, Cadamuro M, Spirli C, Mariotti V, Kaffe E, Scirpo R, Fabris L. Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1374-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Fiorotto R, Strazzabosco M. Pathophysiology of Cystic Fibrosis Liver Disease: A Channelopathy Leading to Alterations in Innate Immunity and in Microbiota. Cell Mol Gastroenterol Hepatol. 2019;8:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Lim SH, Legere EA, Snider J, Stagljar I. Recent Progress in CFTR Interactome Mapping and Its Importance for Cystic Fibrosis. Front Pharmacol. 2017;8:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, Strazzabosco M. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498-1508, 1508.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Nielsen S, Needham B, Leach ST, Day AS, Jaffe A, Thomas T, Ooi CY. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci Rep. 2016;6:24857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Garg M, Ooi CY. The Enigmatic Gut in Cystic Fibrosis: Linking Inflammation, Dysbiosis, and the Increased Risk of Malignancy. Curr Gastroenterol Rep. 2017;19:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 14. | Lopes-Pacheco M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front Pharmacol. 2019;10:1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 15. | Debray D, Corvol H, Housset C. Modifier genes in cystic fibrosis-related liver disease. Curr Opin Gastroenterol. 2019;35:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, Castaldo G, Castellani C, Cipolli M, Colombo C, Colombo JL, Debray D, Fernandez A, Lacaille F, Macek M Jr, Rowland M, Salvatore F, Taylor CJ, Wainwright C, Wilschanski M, Zemková D, Hannah WB, Phillips MJ, Corey M, Zielenski J, Dorfman R, Wang Y, Zou F, Silverman LM, Drumm ML, Wright FA, Lange EM, Durie PR, Knowles MR; Gene Modifier Study Group. Genetic modifiers of liver disease in cystic fibrosis. JAMA. 2009;302:1076-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol. 2004;41:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Dos Santos ALM, de Melo Santos H, Nogueira MB, Távora HTO, de Lourdes Jaborandy Paim da Cunha M, de Melo Seixas RBP, de Freitas Velloso Monte L, de Carvalho E. Cystic Fibrosis: Clinical Phenotypes in Children and Adolescents. Pediatr Gastroenterol Hepatol Nutr. 2018;21:306-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Stonebraker JR, Ooi CY, Pace RG, Corvol H, Knowles MR, Durie PR, Ling SC. Features of Severe Liver Disease With Portal Hypertension in Patients With Cystic Fibrosis. Clin Gastroenterol Hepatol. 2016;14:1207-1215.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Staufer K. Current Treatment Options for Cystic Fibrosis-Related Liver Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Fridell JA, Bond GJ, Mazariegos GV, Orenstein DM, Jain A, Sindhi R, Finder JD, Molmenti E, Reyes J. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center's experience. J Pediatr Surg. 2003;38:1152-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90-S102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Sakiani S, Kleiner DE, Heller T, Koh C. Hepatic Manifestations of Cystic Fibrosis. Clin Liver Dis. 2019;23:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Flass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. J Cyst Fibros. 2013;12:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10 Suppl 2:S29-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 26. | Assis DN, Debray D. Gallbladder and bile duct disease in Cystic Fibrosis. J Cyst Fibros. 2017;16 Suppl 2:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Rowland M, Gallagher CG, O'Laoide R, Canny G, Broderick A, Hayes R, Greally P, Slattery D, Daly L, Durie P, Bourke B. Outcome in cystic fibrosis liver disease. Am J Gastroenterol. 2011;106:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Lewindon PJ, Shepherd RW, Walsh MJ, Greer RM, Williamson R, Pereira TN, Frawley K, Bell SC, Smith JL, Ramm GA. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology. 2011;53:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Witters P, Libbrecht L, Roskams T, Boeck KD, Dupont L, Proesmans M, Vermeulen F, Strandvik B, Lindblad A, Stéphenne X, Sokal E, Gosseye S, Heye S, Maleux G, Aerts R, Monbaliu D, Pirenne J, Hoffman I, Nevens F, Cassiman D. Noncirrhotic presinusoidal portal hypertension is common in cystic fibrosis-associated liver disease. Hepatology. 2011;53:1064-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Lenaerts C, Lapierre C, Patriquin H, Bureau N, Lepage G, Harel F, Marcotte J, Roy CC. Surveillance for cystic fibrosis-associated hepatobiliary disease: early ultrasound changes and predisposing factors. J Pediatr. 2003;143:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Akata D, Akhan O. Liver manifestations of cystic fibrosis. Eur J Radiol. 2007;61:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Fields TM, Michel SJ, Butler CL, Kriss VM, Albers SL. Abdominal manifestations of cystic fibrosis in older children and adults. AJR Am J Roentgenol. 2006;187:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Siegel MJ, Freeman AJ, Ye W, Palermo JJ, Molleston JP, Paranjape SM, Stoll J, Leung DH, Masand P, Karmazyn B, Harned R, Ling SC, Navarro OM, Karnsakul W, Alazraki A, Schwarzenberg SJ, Seidel FG, Towbin A, Alonso EM, Nicholas JL, Murray KF, Otto RK, Sherker AH, Magee JC, Narkewicz MR; CFLD Network. Heterogeneous Liver on Research Ultrasound Identifies Children with Cystic Fibrosis at High Risk of Advanced Liver Disease: Interim Results of a Prospective Observational Case-Controlled Study. J Pediatr. 2020;219:62-69.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Rath T, Menendez KM, Kügler M, Hage L, Wenzel C, Schulz R, Graf J, Nährlich L, Roeb E, Roderfeld M. TIMP-1/-2 and transient elastography allow non invasive diagnosis of cystic fibrosis associated liver disease. Dig Liver Dis. 2012;44:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Gominon AL, Frison E, Hiriart JB, Vergniol J, Clouzeau H, Enaud R, Bui S, Fayon M, de Ledinghen V, Lamireau T. Assessment of Liver Disease Progression in Cystic Fibrosis Using Transient Elastography. J Pediatr Gastroenterol Nutr. 2018;66:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 37. | Leung DH, Khan M, Minard CG, Guffey D, Ramm LE, Clouston AD, Miller G, Lewindon PJ, Shepherd RW, Ramm GA. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology. 2015;62:1576-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Pereira TN, Lewindon PJ, Smith JL, Murphy TL, Lincoln DJ, Shepherd RW, Ramm GA. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol. 2004;41:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Cook NL, Pereira TN, Lewindon PJ, Shepherd RW, Ramm GA. Circulating microRNAs as noninvasive diagnostic biomarkers of liver disease in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2015;60:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Witters P, De Boeck K, Dupont L, Proesmans M, Vermeulen F, Servaes R, Verslype C, Laleman W, Nevens F, Hoffman I, Cassiman D. Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. J Cyst Fibros. 2009;8:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Palermo J, Kotyk JJ, Siegel MJ. Magnetic resonance elastography for assessment of cystic fibrosis liver disease. Gastroenterology. 2011;140:S-686. |
| 42. | Cheng K, Ashby D, Smyth RL. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst Rev. 2014;CD000222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Freeman AJ, Sellers ZM, Mazariegos G, Kelly A, Saiman L, Mallory G, Ling SC, Narkewicz MR, Leung DH. A Multidisciplinary Approach to Pretransplant and Posttransplant Management of Cystic Fibrosis-Associated Liver Disease. Liver Transpl. 2019;25:640-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Mendizabal M, Reddy KR, Cassuto J, Olthoff KM, Faust TW, Makar GA, Rand EB, Shaked A, Abt PL. Liver transplantation in patients with cystic fibrosis: analysis of United Network for Organ Sharing data. Liver Transpl. 2011;17:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Milkiewicz P, Skiba G, Kelly D, Weller P, Bonser R, Gur U, Mirza D, Buckels J, Stableforth D, Elias E. Transplantation for cystic fibrosis: outcome following early liver transplantation. J Gastroenterol Hepatol. 2002;17:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Morrell MR, Kiel SC, Pilewski JM. Organ Transplantation for Cystic Fibrosis. Semin Respir Crit Care Med. 2019;40:842-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Molmenti EP, Squires RH, Nagata D, Roden JS, Molmenti H, Fasola CG, Prestidge C, D'Amico L, Casey D, Sanchez EQ, Goldstein RM, Levy MF, Benser M, McPhail W, Andrews W, Andersen JA, Klintmalm GB. Liver transplantation for cholestasis associated with cystic fibrosis in the pediatric population. Pediatr Transplant. 2003;7:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Miguel M, Andres AM, Lopez-Santamaria M, Barrena S, Hierro L, Hernandez F, Ramírez M, Frauca E, Encinas JL, Lopez-Fernandez S, Jara P, Tovar JA. Liver transplantation in children with cystic fibrosis: experience in our centre and preliminary results with a combined en bloc liver-pancreas graft. Eur J Pediatr Surg. 2012;22:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Dowman JK, Watson D, Loganathan S, Gunson BK, Hodson J, Mirza DF, Clarke J, Lloyd C, Honeybourne D, Whitehouse JL, Nash EF, Kelly D, van Mourik I, Newsome PN. Long-term impact of liver transplantation on respiratory function and nutritional status in children and adults with cystic fibrosis. Am J Transplant. 2012;12:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Arnon R, Annunziato RA, Miloh T, Padilla M, Sogawa H, Batemarco L, Willis A, Suchy F, Kerkar N. Liver and combined lung and liver transplantation for cystic fibrosis: analysis of the UNOS database. Pediatr Transplant. 2011;15:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Cuevas-Ocaña S, Laselva O, Avolio J, Nenna R. The era of CFTR modulators: improvements made and remaining challenges. Breathe (Sheff). 2020;16:200016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, Southern KW, Robertson S, Green Y, Cooke J, Rosenfeld M; KIWI Study Group. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 53. | Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL; VX16-445-001 Study Group. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N Engl J Med. 2018;379:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 539] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 54. | Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, Milla CE, Robinson PD, Waltz D, Davies JC; VX14-809-109 investigator group. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 55. | Kutney K, Donnola SB, Flask CA, Gubitosi-Klug R, O'Riordan M, McBennett K, Sferra TJ, Kaminski B. Lumacaftor/ivacaftor therapy is associated with reduced hepatic steatosis in cystic fibrosis patients. World J Hepatol. 2019;11:761-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 56. | van de Peppel IP, Doktorova M, Berkers G, de Jonge HR, Houwen RHJ, Verkade HJ, Jonker JW, Bodewes FAJA. IVACAFTOR restores FGF19 regulated bile acid homeostasis in cystic fibrosis patients with an S1251N or a G551D gating mutation. J Cyst Fibros. 2019;18:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |