Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1215
Peer-review started: March 14, 2021
First decision: June 14, 2021
Revised: June 25, 2021
Accepted: September 6, 2021
Article in press: September 6, 2021
Published online: October 27, 2021
Processing time: 221 Days and 19.7 Hours
Emerging worldwide data have been suggesting that coronavirus disease 2019 (COVID-19) pandemic consequences are not limited to the respiratory and cardiovascular systems but encompass adverse gastrointestinal manifestations including acute liver injury as well. Severe cases of liver injury associated with higher fatality rates were observed in critically ill patients with COVID-19. Intensive care units (ICU) have been the center of disposition of severe cases of COVID-19. This review discusses the pathogenesis of acute liver injury in ICU patients with COVID-19, and analyzes its prevalence, consequences, possible drug-induced liver injury, and the impact of the pandemic on liver diseases and transplantation programs.
Core Tip: In this manuscript, liver dysfunction is seen more in patients with more severe disease upon presentation. It is difficult to separate the independent effect of viral infection from various treatment modalities, including antibiotics and experimental antiviral drugs that are used in these patients.
- Citation: Omar AS, Kaddoura R, Orabi B, Hanoura S. Impact of COVID-19 pandemic on liver, liver diseases, and liver transplantation programs in intensive care units. World J Hepatol 2021; 13(10): 1215-1233
- URL: https://www.wjgnet.com/1948-5182/full/v13/i10/1215.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i10.1215
More than a year ago, the global pandemic started from its epicentre in Wuhan. In coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the lung is the main organ targeted by the virus[1]. The organism exhibits a wide range of severity and a diverse disruption of extra-pulmonary systems, including gastrointestinal, renal, cardiac[2,3], hepatic[4], and even multi-organ damage[2,5]. Moderate or severe symptoms have been reported in almost 20% of all COVID-19 patients, while 5% progress into critical stages of the disease[6].
The rate of intensive care unit (ICU) admission due to COVID-19 is quite variable, ranging from 3% to 100% in literature[7]. The liver could be affected in COVID-19 through several mechanisms, including virus-related liver cell injury, disorganized immune response, drug-induced liver injury (DILI) and ischemic liver dysfunction in the settings of multisystem organ failure[8]. The reported rate of COVID-19-induced liver injury ranged from 14.8% in one study[9] and up to 74% in another[10]. In a case series of critically ill patients with COVID-19, liver injury was frequent but transient and non-severe[11]. Patients may not be equally affected by the pandemic, certain patient populations are potentially more vulnerable. Immunocompromised patients and patients with cirrhosis are probably more susceptible to worse outcomes after SARS-CoV-2 infection[5]. The data in literature on how chronic immunosuppression can influence COVID-19 outcomes is scarce[6]. This minireview will discuss the pathogenesis of acute liver injury in ICU patients with COVID-19, focusing on its prevalence, consequences, DILI, and its impact on existing liver diseases and liver transplantation programs.
Liver injury in COVID-19 can be related to the direct cytopathic effect of the virus, DILI, uncontrolled immune reaction, or sepsis[12]. SARS-CoV-2 ribonucleic acid has been detected in blood and stool samples of COVID-19 patients who presented with diarrhoea, indicating the liver's probable involvement in the disease pathogenesis[13,14]. It has been suggested that there is a considerable expression of angiotensin-converting enzyme 2 (ACE2) receptors in cholangiocytes, where SARS-CoV-2 binding may adversely affect liver function. Moreover, COVID-19 may worsen the underlying chronic liver disease(s) (CLD), leading to hepatic decompensation or acute-on-chronic liver failure and increasing the risk of mortality, particularly in critically ill patients[12,15-17]. However, in severe COVID-19, liver damage is more likely due to the inflammatory cytokine storm[12,18] rather than the direct cytopathic effects of the virus[12].
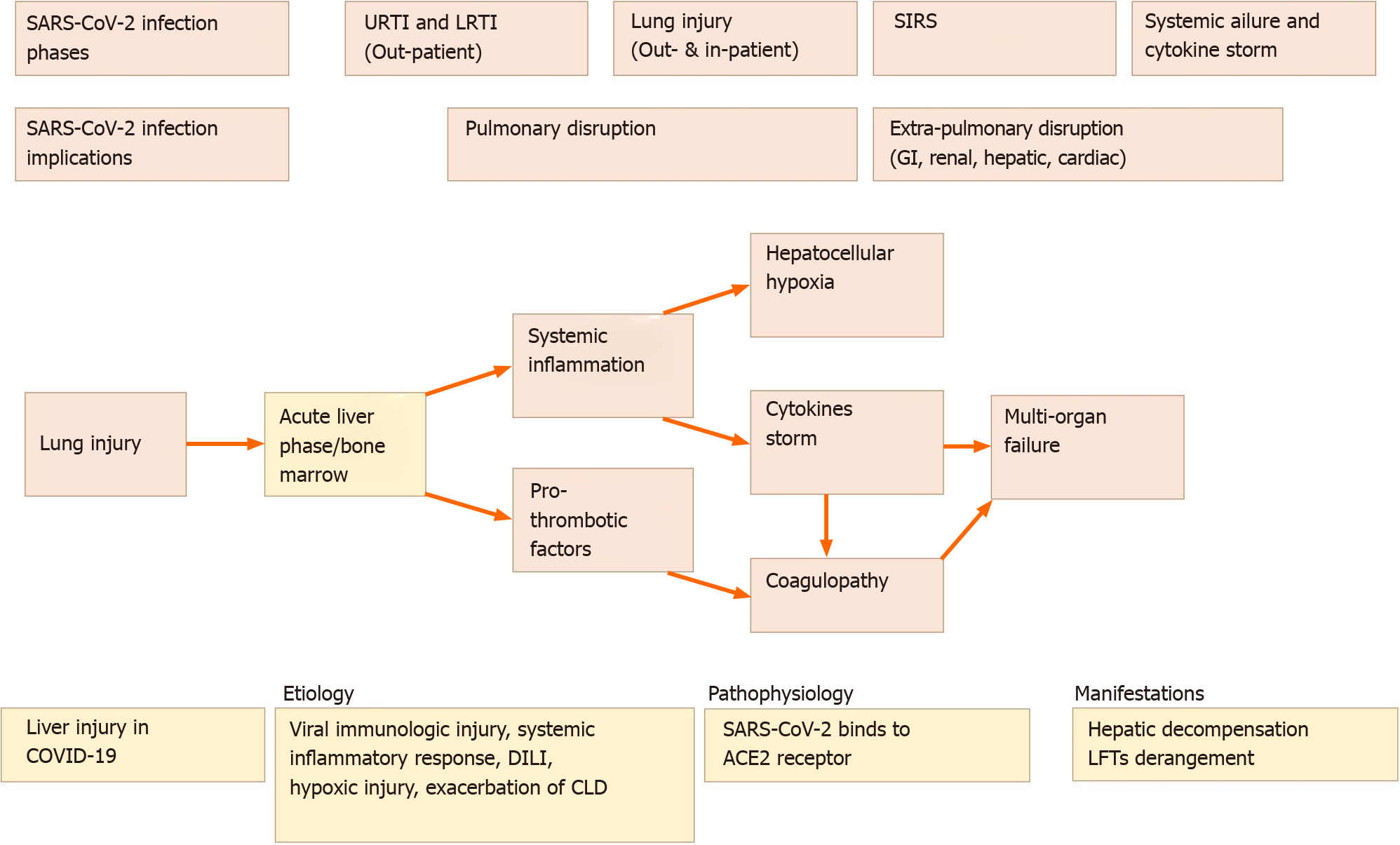
The progression of SARS-CoV-2 infection has been divided into four phases: Upper and lower respiratory tract infection, usually treated as outpatients, COVID-19 associated lung injury, usually treated as inpatients, systemic inflammatory response syndrome (SIRS), and systemic failure. Liver involvement is often observed in the latter phases but can also occur in the earlier ones. In SIRS, pro-thrombotic factors accumulate due to bone marrow and liver acute phase response causing thrombosis, whereas in the last phase, multi-organ vascular dysfunction and cytokine storm occur in view of the ongoing interaction between the lung and systemic inflammation[19].
Hypoxic hepatitis (HH), known as shock liver or ischemic hepatitis, is an acute liver injury resulting from liver hypoxia[20]. The extensive complex vascular supply together with high metabolic efficacy results in a liver vulnerable to circulatory disturbances. Critically ill patients with circulatory or respiratory manifestations which may influence liver perfusion are at higher risk of HH[3,21,22]. The mechanism by which SARS-CoV-2 infection leads to HH is not fully understood. Multiple theories have been postulated, including hypoxemia developed due to COVID-19 pneumonia[2] and systemic stress caused by SIRS[19]. Both may provide a route to a compen
The liver injury induced by COVID-19, including its pattern and severity, has not been uniformly defined or well characterized[25,26]. Some definitions reported in the literature, including DILI, are presented in Table 1. Secondary liver injury was the most common, being the first occurrence[3]. Liver injury has been reported as the elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels[25,26]. Thus, the liver injury appears to be of a hepatocellular (56%) rather than cholestatic (24%) or mixed (19%) pattern[3,25-28], while jaundice is uncommon[3]. Alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) as markers of bile duct injury have not increased significantly in the respective studies[3,25]. However, not all liver function tests (LFTs) have been strictly reported[29].
| Term | Definition(s) |
| Liver disorder | Serum ALT or AST > 2 × ULN, TB > 2 × ULN, ALP ≥ 2 ULN[75] |
| Liver injury or acute liver injury | ALT and/or AST above 3 × ULN, ALP, GGT, and/or TB above 2 × ULN[9,34] |
| ALT and/or AST ≥ 2 × ULN, with TB ≥ 2 × ULN and/or INR ≥ 1.7[70] | |
| ALT levels above 3 × the ULN[28] | |
| Mild liver injury | ALT above the ULN and below 2 × the ULN[25] |
| Moderate liver injury | ALT between 2-5 × the ULN[25] |
| Severe liver injury | ALT above 5 × the ULN[25] |
| Any elevation of enzymes above 3 × the ULN and bilirubin above 2 × the ULN[5] | |
| Liver test abnormalities | Elevation of the following serum liver enzymes: ALT > 40 U/L, AST > 40 U/L, GGT > 49 U/L, ALP > 135 U/L, and TB > 17.1 μmol/L[34] |
| De novo LFTs abnormality | The occurrence of abnormal LFTs in patients with normal LFTs at admission[27] |
| LFTs elevation | Increase in serum liver enzyme levels above the ULN[27,28] |
| Mild LFTs elevations | Elevation 1-2 times above the ULN[25,34] |
| Hepatocellular or hepatocyte type | The pattern of abnormal LFTs with predominantly elevated ALT and AST[27] |
| Patients with raised ALT and/or AST more than 3 × the ULN[34] | |
| AST/ALT activity is higher than the ALP/GGT activity, with liver enzyme activities calculated by multiples of their ULN[34] | |
| Cholestatic or cholangiocyte type | Pattern of abnormal LFTs with predominantly elevated ALP and GGT[27] |
| Patients with raised ALP or GGT 2 × the ULN[34] | |
| ALP/GGT activity was higher than the AST/ALT activity, with the liver enzyme activities calculated by multiples of their ULN[34] | |
| Mixed type | Mixed pattern when the extents of AST/ALT and ALP/GGT are similar[27] |
| A combination of both ALT/AST elevated more than 3 × the ULN and ALP/GGT twice the ULN[34] | |
| Drug-induced liver injury | Any elevation in liver enzymes or TB after the initiation of the drug in the absence of identified common causes of liver disease[5] |
Liver injury in COVID-19, manifested as changes in LFTs, is usually mild and transient[26,30-33] and does not require treatment[30,32]. However, severe cases have been reported[26,30,32]. Mild, moderate, and severe injuries were reported in 45%-65%, 21%, and 6.4% of the cases, respectively[25-27]. In one study, mild elevation in LFTs levels was reported in 90% of patients admitted to hospital with marked elevation during hospital stay[34]. Elevation in AST levels is more common than ALT and other LFTs[29,30]. In one report, the acute liver injury occurred on day 17 after the onset of symptoms[28]. In patients with severe COVID-19, the elevation in the transaminases and bilirubin levels was at least double that in patients with mild and moderate disease[33]. Elevation in GGT levels was more noticeable in severe cases, while ALP levels usually remained normal in both mild and severe cases[35]. Variable and inconsistent degrees of LFTs abnormalities, ranging from 3.75% to more than 50% of all patients, have been described[5,25,33,36]. A meta-analysis found a pooled incidence of elevated liver enzymes by 23.1%[37]. Although some studies did not show a statistical difference in abnormal LFTs between patients with severe and non-severe disease[37,38] or between survivors and non-survivors[39], many other studies have consistently shown elevated LFTs to be more prevalent in fatal or severe disease[1,2,14,28,34,40-43] in up to 58%-78% of cases[40,44,45].
Patients with LFTs abnormalities had a more severe inflammation[25-27] and degree of organ dysfunction[27]. At least two meta-analyses have confirmed the association between liver injury and the severity of COVID-19[46,47]. Liver injury had prognostic implications in patients with COVID-19. Liver injury or abnormal LFTs were associated with increased risk of ICU admission[25,27,48,49], intubation[25,49], mechanical ventilation need[27], acute renal injury, vasopressor use[25,27], long hospital stays[27], mortality[25,27,28,37,48,49], and composite of ICU admission and mortality[27,50]. Tables 2 and 3 present selected liver injury-related markers and clinical outcomes of non-survivors[39,43,44,51-55], or patients with severe disease[1,2,9,28,34,40,42,56-63], including those admitted to ICU due to COVID-19[1,2,57].
| Ref. | N (all) n (non-survivors) | Age (year) | Male | Pre-existing CLD | Type of liver disease | Elevated LFTs on admission (%) | LFTs levels on admission. ALT/AST/ALP/GGT (U/L)/TB (μmoL) | Selected complications or clinical outcomes |
| Cao et al[51]. China | N = 102 (n = 17) | 53 vs 72 | 47.1% vs 76.5% | 2.4% vs 5.9% | - | ALT: NR vs 41.1% | ALT: NR vs 40 | ALI: 24.7% vs 76.5%; ARDS: 5.9% vs 88.2%; Shock: 3.5% vs 41.1%; MV: 2.4% vs 70.6% |
| Chen et al[52]. China | N = 274 (n = 113) | 51 vs 68 | 55% vs 73% | - | HBV surface antigen positivity | ALT: 19% vs 27%; AST: 16% vs 52% | ALT: 20 vs 28; AST: 25 vs 45; ALP: 64 vs 76; GGT: 28 vs 42; TB: 8.4 vs 12.6 | ALI: 2% vs 9%; ARDS: 52% vs 100%; Shock: 0% vs 41%; MV: 82% vs 16% |
| Chen et al[53]. China | N = 55 (n = 19)1 | 72 vs 77 | 50% vs 84.2% | 2.8% vs 5.3% | - | ALT: 19.4% vs 31.6%; AST: 50% vs 73.7% | ALT: 40 vs 44;AST: 55 vs 78 | MV: 30.6% vs 68.4% |
| Du et al[54]. China | N = 852 | 65.8 | 72.9% | 5.9% | - | ALT: 16.5%; AST: 32.9%; TB: 35.3% | ALT: 72.9; AST: 94.4; TB: 18.4 | ALI: 35.3%; ARDS: 74.1%; Shock: 81.2%; MV: 93%3 |
| Wu et al[42]. China | N = 84 (n = 44)4 | 50 vs 68.5 | 77.5% vs 65.9% | 3.5%5 | - | - | ALT: 35 vs 39; AST: 38.5 vs 37; TB: 11.6 vs 14.5 | MV: 57.5% vs 97.8%3; Others reported as association |
| Yang et al[55]. China | N = 922 | 69.8 | 53.3% | 3.3% | - | - | ALT: 27; AST: 31; TB: 13.6 | ALI: 16.5%; ARDS: 80.2%; MODS: 15.4% |
| Yang et al[39]. China | N = 52 (n = 32) | 51.9 vs 64.6 | 70% vs 66% | - | - | - | TB: 13.1 vs 19.5 | ALI: 30% vs 28%; ARDS: 45% vs 81%; MV: 35% vs 94% |
| Zhang et al[44]. China | N = 822 | 72.5 | 65.9% | 2.4% | - | ALT: 30.6%; AST: 61.1%; TB: 30.6% | ALT: 26; AST: 72; TB: 13.6 | Hepatic damage: 78%; Liver-associated death: 1.2%; MV: 40.2% |
| Zhou et al[43]. China | N = 191 (n = 54) | 52 vs 69 | 59% vs 70% | - | - | ALT: 24% vs 48% | ALT: 27 vs 40 | ARDS: 7% vs 93%; Shock: 0% vs 70%; MV: 2% vs 100%3 |
| Ref. | N (all), n (severe disease). Patient population | Age (year) | Male | Pre-existing CLD | Type of pre-existing CLD | Elevated LFTs on admission (%) | LFTs levels on admission. ALT/AST/ALP/GGT (U/L)/TB (μmoL) | Selected complications or clinical outcomes |
| Arentz et al[56]. United States | N = 21. Critically ill | 70 | 52% | 4.8% | Cirrhosis | - | ALT: 108; AST: 273; ALP: 80; TB: 0.6 mg/dL | ALI: 14.3%; Severe ARDS: 57.1%; MV:71%; Death: 52.4% |
| Cai et al[34]. China | N = 318 (n = 85)1. Non-severe vs severe | 47. All patients | 47.5%. All patients | 5%. All patients | ALD, NAFLD, HVB | ALT: 6.4% vs 21.1%; AST: 0.68% vs 18.8%; GGT: 5.1% vs 29.4%; TB: 1.2% vs 7% | - | MOF: 0% vs 11.7% |
| Cai et al[9]. China | N = 298 (n = 58). Non-severe vs severe | 41 vs 62.5 | 44.1% vs 67.2% | 8.3% vs 13.7% | NAFLD: 3.3% vs 10.3%; ALD: 3.3% vs 1.7%; HBV: 1.7% vs 1.7% | - | ALT: 20 vs 26.8; AST: 26 vs 36; ALP: 61 vs 58; GGT: 21 vs 35.2; TB: 10.9 vs 11.2 | ALI: 9.6% vs 36.2%; Discharge: 93.3% vs 75.9%; Hospital-stay: 19 d vs 27 d; Death: 0% vs 5.2% |
| Du et al[57]. China | N = 109. Non-ICU vs ICU | 72.7 vs 68.4 | 65.5% vs 70.6% | 3.4% vs 0% | - | ALT: 13.8% vs 19.6%; AST: 49% vs 43.1% | ALT: 21.6 vs 27; AST: 32 vs 40 | Invasive MV: 0% vs 64.7%; Hospital-stay: 12.5 d vs 15.9 d |
| Guan et al[40]. China | N = 1099 (n = 173). Non-severe vs severe | 45 vs 52 | 58.2% vs 57.8% | 2.4% vs 0.6% | HBV | ALT: 19.8% vs 28.1%; AST: 18.2% vs 39.4%; TB: 9.9% vs 13.3% | - | ARDS: 1.1% vs 15.6%; MV: 0% vs 38.7%; Discharge: 5.4% vs 2.9%; Hospital-stay: 11 d vs 13 d; Death: 0.1% vs 8.1% |
| Huang et al[2]. China | N = 41 (n = 13). Non-ICU vs ICU | 49 vs 49 | 68% vs 85% | 4% vs 0% | - | AST: 25% vs 62% | ALT: 27 vs 49; AST: 34 vs 44; TB: 10.8 vs 14 | ARDS: 4% vs 85%; Shock: 0% vs 23%; Invasive MV: 0% vs 15%; Discharge: 75% vs 54%; Death: 4% vs 38% |
| Lei et al[28]. China | N = 5771 (n = 1186). Non-severe vs severe | 55 vs 59 | 45.1% vs 55.3% | 1.2% vs 2.1% | Viral hepatitis Cirrhosis | - | ALT: 23 vs 26; AST: 22 vs 31; ALP: 65 vs 63; TB: 10.3 vs 10.6 | Reported as association not absolute values |
| Li et al[58]. China | N = 548 (n = 269). Non-severe vs severe | 56 vs 65 | 45.2% vs 56.9% | 1.1% vs 0.7% | HBV | ALT: 22.3% vs 24.1%; AST: 23.3% vs 43.4%; TB: 2.3% vs 6.4% | - | ALI: 15.8% vs 23%; ARDS: 9.7% vs 68%; MV: 4% vs 34.2%2; Discharge: 72.9% vs 31.7%; Death: 1.1% vs 32.5% |
| Mo et al[59]. China | N = 155 (n = 85)3. General vs refractory | 47 vs 61 | 44.3% vs 64.7% | 2.9% vs 5.9% | - | - | ALT: 20 vs 28; AST: 32 vs 37 | Critical case: 4.3% vs 40%; MV: 0% vs 41.2%; Others reported as association |
| Wan et al[60]. China | N = 135 (n =40). Mild vs severe | 44 vs 56 | 54.7% vs 52.5% | 1% vs 2.5% | - | AST: 16% vs 37.5% | ALT: 21.7 vs 26.6; AST: 22.4 vs 33.6; TB: 8.6 vs 9.8 | ARDS: 1.1% vs 50%; Shock: 0% vs 2.5%; Discharge: 10.5% vs 12.5%; Death: 0% vs 2.5% |
| Wang et al[1]. China | N = 138 (n = 36). Non-ICU vs ICU | 51 vs 66 | 52% vs 61.1% | 3.9% vs 0% | - | - | ALT: 23 vs 35; AST: 29 vs 52; TB: 9.3 vs 11.5 | ARDS: 4.9% vs 61.1%; Shock: 1% vs 30.6%; Invasive MV: 0% vs 47.2% |
| Wu et al[42]. China | N = 201 (n = 84)4. Non-ARDS vs ARDS | 48 vs 58.5 | 58.1% vs 71.4% | 3.5%5 | - | - | ALT: 27 vs 35; AST: 30 vs 38; TB: 10.5 vs 12.9 | MV: 0% vs 78.6%2; Others reported as association |
| Zhang et al[61]. China | N = 221 (n = 55). Non-severe vs severe | 51 vs 62 | 44% vs 63.6% | 1.8% vs 7.3% | - | - | ALT: 22 vs 32; AST: 27 vs 51; TB: 9.6 vs 11.4 | ARDS: 0% vs 87.3%; Shock: 0% vs 27.3%; MV: 1.2% vs 74.6%2; Discharge: 21.1% vs 12.7%; Death: 0% vs 21.8% |
| Zhang et al[62]. China | N = 140 (n = 58). Non-severe vs severe | 51.1 vs 64 | 46.3% vs 56.9% | 5% vs 6.9% | Fatty liver and abnormal liver function | - | - | - |
| Zheng et al[63]. China | N = 161 (n = 30). Non-severe vs severe | 40 vs 57 | 50.4% vs 46.7% | 3.1% vs 0% | - | ALT: 6.1% vs 16.7%; AST: 7.6% vs 40%; TB: 4.6% vs 10% | ALT: 19.3 vs 23.9; AST: 23.4 vs 31.6; TB: 10.7 vs 12.7 | - |
Underlying CLD in patients with COVID-19 have been reported in several studies and ranged from 2% to 11%[30,36,64], up to 19% in one study[65]. Pooled prevalence of pre-existing CLD in one meta-analysis was 3%[66], which was comparable to that of another meta-analysis (3.6%)[5]. The latter reported pooled prevalence of CLD of 3.9% and 4.7% among severely infected patients and the non-survivors, respectively[5]. Compared with patients without underlying liver diseases, the odds ratio (OR) of developing severe disease was 0.81 [95% confidence interval (CI): 0.31-2.09, P = 0.67][67]. The presence of underlying liver disease was associated with increased the risk of mortality and hospitalization, before {[risk ratio (RR): 2.8, 95%CI: 1.9–4.0, P < 0.001]; (RR: 1.7, 95%CI: 1.2-2.0, P < 0.001)} and after propensity matching [(RR: 3.0, 95%CI: 1.5-6.0, P = 0.001); (RR: 1.3, 95%CI: 1.1-1.6, P = 0.006)], when compared to those without liver diseases, respectively[68].
The presence of CLD was also found to be an independent predictor for ICU admission (adjusted OR 1.77, 95%CI: 1.03-3.04, P = 0.04) and mechanical ventilation need (adjusted OR 2.08, 95%CI: 1.20-3.60, P = 0.0092)[65]. The reported etiologies of the pre-existing liver diseases before COVID-19 included chronic viral hepatitis B and C, alcoholic and metabolic liver disease, cirrhosis of any cause, and others[5,26,31]. Liver cirrhosis is the end-stage of these liver-related diseases[31]. In one study (n = 363), 19% of patients had a pre-existing liver disease with the predominance of non-alcoholic fatty liver disease (NAFLD) (79.7%). Compensated cirrhosis, decompensated cirrhosis, and viral hepatitis B and C accounted for 8.7%, 4.3%, 2.9%, and 8.7% of all patients, respectively[65]. In contrast, the reported rates in one meta-analysis of 107 studies (n = 20874) were, CLD/cirrhosis in 61.1%, NAFLD in 19.5%, hepatitis B in 17.8%, and hepatitis C in 0.73% of patients[5].
Hepatitis B virus co-infection may subject COVID-19 patients to an exacerbated liver injury[30] and a more severe disease[69]. Acute liver injury in COVID-19 patients with hepatitis was significantly higher than that in patients without chronic hepatitis (15.0% vs 7.0%, P < 0.001)[70]. Patients with NAFLD, renamed as metabolic-associated fatty liver disease[26,31], had a significantly higher likelihood of abnormal LFTs, longer viral shedding time, and higher rate of COVID-19 progression (OR: 6.4, 95%CI: 1.5-31.2), compared to those without NAFLD[71]. NAFLD was significantly associated with ICU admissions (adjusted OR: 2.30, 95%CI: 1.27-4.17, P = 0.03) and mechanical ventilation need, (adjusted OR: 2.15, 95%CI: 1.18-3.91, P = 0.02) but not with mortality[65]. Furthermore, NAFLD in younger patients (< 60 years) was associated with the prevalence of severe COVID-19 (adjusted OR: 2.67, 95%CI: 1.13–6.34, P = 0.03)[72]. COVID-19 patients with liver cirrhosis were found to be at increased risk of mortality compared with those without the disease (RR: 4.6, 95%CI: 2.6–8.3, P < 0.0001)[68,71]. Multivariate analysis showed that liver cirrhosis was an independent predictor for mortality (adjusted OR: 12.5, 95%CI: 2.16-72.5, P = 0.009) but not for ICU admission or mechanical ventilation need[65].
Numerous medications that are currently used to treat SARS-CoV-2 infection carry the risk of hepatoxicity. Given that many medications are being used in combination, the interpretation of the commonly seen raised liver transaminases in patients with COVID-19 can be biased. While the efficacy of these medications towards improving COVID-19’s morbidity and mortality is still to be proven, their safety should be monitored closely[73]. A retrospective study aimed to investigate adverse drug reactions (ADRs) in 217 COVID-19 patients using a hospital pharmacovigilance system in China found that 82 patients experienced 94 ADRs, with 13.8% of them were categorized as liver disorders. A multivariate analysis showed that the occurrence of ADRs has been associated with the length of stay (OR: 2.02, 95%CI: 1.03–3.96, P = 0.04), number of drugs used in hospital (OR: 3.12, 95%CI: 1.60–6.27, P = 0.001) and underlying diseases (OR: 2.07, 95%CI: 1.02–4.23, P = 0.045)[73]. In a prospective study using pharmacovigilance system in Spain, patients with COVID-19 had a higher incidence of hepatitis as a serious ADR than that in non-COVID-19 patients (45.1% vs 23.7%)[74]. In a meta-analysis of 10 studies, DILI in COVID-19 was reported in 25.4% of the total patients[5]. Therapies that have been implicated in hepatotoxicity included remdesivir, lopinavir/ritonavir, oseltamivir, hydroxychloroquine, paracetamol[5], tocilizumab[74], in addition to antibiotics, non-steroidal anti-inflammatory drugs, herbal medications, and interferon[34]. In a retrospective, observational cohort study (n = 1827), the use of lopinavir/ritonavir, hydroxychloroquine, remdesivir, and tocilizumab was associated with statistically significant abnormal ALT and AST levels (i.e., > 5 × upper limit of normal)[75].
Data on DILI’s clinical significance have not been consistent. Sun et al[73] reported 18.1% of ADRs to be of serious severity, with 82.3% of them related to liver injury[73]. Ramírez et al[74] reported a mortality rate of 30.5% in COVID-19 patients with serious ADRs compared with 3.9% in non-COVID-19 patients with serious ADRs[74]. However, Kulkarni et al[5] concluded that remdesivir and lopinavir/ritonavir DILI was not life-threatening[5]. A systematic review and network meta-analysis of 110 studies reported no association between a regimen or an agent with non-cardiac severe adverse events[76]. In a multicenter and retrospective study (n = 565) on hospitalized COVID-19 patients, de novo LFTs abnormality was noted with tocilizumab (82% vs 52%; P = 0.009) and lopinavir/ritonavir (64% vs 48%; P = 0.045). Moreover, there was a trend towards an increased composite endpoint of death or transfer to ICU associated with de novo LFTs abnormality with an incidence of 14% vs 5% (P = 0.069)[27]. Although published data regarding the incidence, severity and clinical significance of DILI have not been consistent, it warrants close monitoring of LFTs. Table 4 summarizes the reported DILI of selected therapies against COVID-19[5,27,34,74,75,77-95].
| Medication (class) | Pattern of liver injury | Evidence |
| Corticosteroids (Anti-inflammatory agent) | Acute liver injury[77] | Multicenter cohort study (n = 774); COVID-19 with ARDS: Incidence of ALI versus control (18.3% vs 9.9%; P = 0.001)[77] |
| Meta-analysis; critically ill COVID-19 patients: No association with serious adverse effects[78] | ||
| RECOVERY trial: No reported serious ADRs or DILI[79] | ||
| Favipiravir (RdRp inhibitor) | Abnormal LFTs[80] | RCT (n = 150); mild-to-moderate COVID-19: Abnormal LFTs versus control 6.8% vs 2.7%)[80] |
| Elevation of transaminases levels[81] | RCT; moderate COVID-19: Elevated ALT and AST were reported[81] | |
| Hydroxychloroquine (Antimalarial agent) | Liver toxicity is not common[82]. Elevation of transaminases levels[74,75,82-84] | Retrospective study (n = 153): Elevation in AST (11%) and ALT (9%)[82] |
| RCT (n = 504); mild-to-moderate COVID-19: Elevation in ALT or AST elevation 10.6% in HCQ plus azithromycin, 9% in HCQ, and 3.5% in control arm (P = 0.008)[83] | ||
| Systematic review: Elevations of LFTs was transient[84] | ||
| Recovery trial: No reported DILI[85] | ||
| Interferon | - | Data on safety in COVID-19 patients is scarce |
| Lopinavir/ritonavir (Protease inhibitor) | Rise in liver function parameters[5,27,34,74,86] | RCT (n = 199): Elevated AST versus control (2.1% vs 5.1%), elevated ALT (1.1% vs 1 %), elevated TB (3.2% vs 3 %)[86] |
| Hyperbilirubinemia[5,34] | Meta-analysis: DILI in 37.2% of patients (as hyperbilirubinemia followed by elevation of transaminases)[5] | |
| Remdesivir (RdRp inhibitor) | Not well established. Elevation of transaminases levels[5,75,87-89]. Elevation of TB levels[88]. Hypoalbuminemia[88] | Case series: Elevated aminotransferases in 23 % discontinuation in 4% of patients[87] |
| RCT (n = 237) in severe COVID-19: Elevated TB versus placebo (10% vs 9%) and AST (5% vs 12%), hypoalbuminemia (13% vs 15%). Discontinuation in 1% of patients[88] | ||
| Open-label, phase 3 trial: Elevated ALT (5%-6%) and AST (7%-8%)[89] | ||
| Meta-analysis: Pooled incidence of DILI of 15.2%[5] | ||
| Meta-analysis: No difference as compared to placebo in liver enzymes elevation[90] | ||
| Tocilizumab (Humanized recombinant monoclonal antibody) | Elevation of transaminases levels[27,75,91-94]. Liver injury as early as 24 h with a 40-fold increase in transaminases that normalized in 10 d[91] | Case series; 7 severe COVID-19 patients: Up to 4.5 folds elevated baseline ALT and AST. Transaminases normalized in 3 wk[92] |
| Retrospective study (n = 1827): AST > 5 × ULN in 69.1%, and ALT > 5 × ULN in 72.1% of patients[75] | ||
| Observational study (n = 104): Minor increase of AST, ALT (P < 0.001) and GGT (P = 0.003; no safety concerns on follow up[93] | ||
| RCT (n = 243): ALT elevation versus placebo (5% vs 4.9%), AST elevation in 3.7%[94] | ||
| RCT (n = 130); moderate or severe COVID-19: No increase in hepatitis risk[95] |
Calcineurin inhibitors (CNIs), such as cyclosporine and FK506 (tacrolimus), antimetabolite drugs, such as mycophenolate mofetil (MMF), mycophenolic acid, and corticosteroids are commonly used for immunosuppression after liver transplantation (LTX)[96]. Some centres adopted dose modifications based on expert opinion with many uncertainties regarding the best approach for combination therapies and immunosuppressive agents against COVID-19. In two large academic centers in New York City including 90 patients with solid organ transplant, antimetabolite drugs doses were reduced or held in 88% of patients, steroids in 7%, and CNIs in 18%, with no reported acute rejection cases at 20-day follow-up[6]. In a prospective European study of 57 liver transplant patients with COVID-19, immunosuppression therapy doses were reduced in 39% of patients and discontinued in 7%. Reduction or continuation of therapy did not affect mortality, while the discontinuation effect was not assessed[97]. Drug interactions between COVID-19 medications and immunosuppression therapy were also considered. For instance, lopinavir-ritonavir combination interacts with CNIs and MMF, it is not recommended to be used with steroids[98] and has been reported to interact with mechanistic target of rapamycin (mTOR) as well[18]. Moreover, tocilizumab may decrease CNIs plasma concentration, unlike remdesivir which does not interact with the immunosuppressive drugs[98]. Hydroxychloroquine has been reported to interact with CNIs and mTOR[18]. Relevant recommendations included checking for drug interactions[18,98], dose reduction of steroids, CNIs and MMF[98,99], switching mTOR to CNIs[18], switching MMF and CNIs to steroids, and withdrawal of agents such as CNIs and MMF in severe COVID-19[99]. Monitoring of immunosuppressive drug levels should be warranted when possible[98,99]. The European Society of Clinical Microbiology and Infectious Diseases advised not to reduce the doses of immunosuppressive drugs in liver transplanted patients and raised the importance of considering vaccination with Streptococcus pneumonia and influenza vaccines[100].
The unprecedented disturbance created by the COVID-19 pandemic has impacted different sectors of health care systems worldwide. For instance, elective services were cancelled or postponed while lifesaving transplant programs, including those for a liver transplant, have been continued. However, the non-lifesaving transplant services were frequently delayed, exposing patients to emergency situations[101]. LTX is the most common solid organ transplantation procedure after the kidney, with a global rate of 3.7 per million population[102]. The indication of LTX in the acute phases of liver diseases includes acute liver cell failure, metabolic liver diseases, advanced complicated cirrhosis, and CLD associated with systemic complications[103]. Elective LTX indications include advanced cirrhosis associated with deteriorating synthetic function, renal function, and the related complications[104]. The general precautions before LTX currently comprise a COVID-19 testing for both donors and recipients awaiting transplant and consenting for the possible hazard of acquiring nosocomial COVID-19[100]. The standard method of COVID-19 testing is through a naso
Strict infection control measures are required in the post-operative care of LTX patients to prevent nosocomial infections that include COVID-19[107]. During the admission of LTX patients, they should be directed to separate rooms away from the general wards, and strict disinfection and isolation practices should be in place. Medical and surgical rounds should be minimized, and laboratory testing and radiological studies should be reduced to the least required[108]. Acquiring symptoms suggestive of COVID-19 in a LTX patient should prompt urgent evaluation with the relevant investigations[105]. Other challenges prompted by the COVID-19 pandemic include the increased demand for ICU beds, requiring health care practitioners to work in a dynamic way to maximize ICU bed utilization[109]. During the pandemic, the settings required for ICU should include separate units equipped with high-efficiency particulate air filters[110]. The goals of ICU disposition for LTX patients comprise neurological monitoring, hemodynamic monitoring and support, early weaning from the mechanical ventilator, preventing nosocomial infections and graft-related complications and enhancing early graft recovery[111]. Some institutes screen for COVID-19 in LTX recipients[112]. Simple and effective measures could be implemented to shorten the ICU stay for LTX patients through fast-track procedures, including operating room early extubation, reduction of ventilation time, and direct transfer from the recovery room to surgical wards[113,114]. Transplant services constantly demand resources, which have become extremely limited with the emergence of the COVID-19 pandemic due to staff shortage, saturation of the ICU, and drainage of supplies. Exceptional scarcity of donors and demand for organs also aggravate this problem[115,116].
Reports regarding the outcomes of LTX patients have been inconsistent. Although early reports have not found more severe or worse outcomes among immunosuppressed patients[117], subsequent data showed that solid organ transplant recipients diagnosed with COVID-19, including LTX, seemed to be at increased risk of severe disease, morbidity, and poor outcomes[6,118], such as high mortality with an in-hospital mortality rate of 29%[119]. Bossini et al[120] reported a higher rate of acute respiratory distress syndrome (ARDS) and death among patients who received solid organs[120], while others reported similar outcomes in COVID-19 patients with and without solid organ transplant[121]. In a multi-centre study of ICU patients after solid organ transplant, the rate of ARDS, duration of mechanical ventilation, vasopressors requirements, and death were similar between groups[122].
It was reported that in liver transplant recipients, COVID-19 infection was not associated with increased mortality. However, these patients are subjected to severe disease, as evidenced by a higher rate of both ICU admission and mechanical ventilation use[123]. The European Association for the Study of the Liver (EASL) suggested a particular form of judging the vaccination decision based on patient’s morbidities[124]. The immune response to COVID-19 vaccination could be lower in LTX patients when compared with healthy subjects. Poor response to vaccination is affected by age, renal function, and enhanced immune suppression[125].
The COVID-19 pandemic has been presented as an unprecedented global health care crisis, causing significant setbacks among various health care services including the management of CLD. Besur et al[35] reported that screening for CLD, its complications and regular follow up visits were deferred which affected slowing or reversing the progression of CLD and worsened the prognosis of patients with CLD. Late identification of CLD complications such as hepatocellular carcinoma (HCC) could also affect the clinical outcomes in these patients. Social distancing measures have put CLD patients at risk of malnutrition, reduced mental health capacity, and decompensation[35]. The evidence associating acute liver injury with poor patients’ outcomes and increased severity of COVID-19 is growing, and more research is necessary to further explain the relation between liver biomarkers changes and patients’ outcomes in COVID-19[126]. Various factors influence the course of COVID-19, and there is a need for international collaborative registries to clarify the full spectrum of the disease. The registries, Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion (SECURE-Cirrhosis) and Coronavirus (COVID-19) in liver disease reporting registry (COVID-HEP) were established to report data on patients with liver disease. The last report published in August 2020 by SECURE-Cirrhosis and the EASL supported COVID-Hep, reported 158 deaths (31%) among patients who had cirrhosis and developed SARS-CoV-2 infections[127]. When this article was written, the latest update from both COVID-Hep and SECURE-Cirrhosis registries reported 1341 cases that included 645 cases with cirrhosis, 205 liver transplant recipients and 270 deaths as of February 12, 2021[128].
COVID-19 pandemic has disrupted various healthcare services worldwide, limiting the services offered to urgent and emergent cases. These changes in services, clinician behaviour and re-organization of hospital activities can indirectly affect morbidity and mortality[129]. delaying or halting diagnostic and therapeutic services for diseases with a high global burden such as cardiovascular diseases can contribute to long-term and indirect adverse health outcomes. For example, cardiac diagnostics procedures, stress tests[129], emergency department (ED) and hospital admissions, procedures and treatments were markedly declined during the pandemic year as compared with that of the previous years[130]. In 909 inpatient and outpatient centres from 108 countries, the rate of cardiac diagnostic procedures decreased by 42% and 64% as of March and April 2020, respectively, with the highest reduction of 78% observed for the stress tests, as compared with March 2019.[130]. A further 22% reduction was noted in low and low-middle income countries, which might be attributed to inaccessible personal protective equipment and telehealth services[130].
In United Kingdom, a cross-sectional study conducted in nine hospitals compared the hospitals’ cardiovascular activity data between October 2019 and May 2020 with the respective weeks in 2018 and 2019. There was a marked decline in ED attendances, admissions and hospital procedures and treatments[129]. Patients with other chronic diseases which require close follow up have been negatively affected as well. A cross-sectional study of six referral centres in France showed that in 2020 significantly fewer patients with HCC were referred to the multidisciplinary tumour board (P = 0.034) and fewer received the first diagnosis of HCC (P = 0.083) compared with 2019[131]. Therapy optimization and frequency of follow-up visits were also affected by the global pandemic in response to social distancing and re-allocation of services towards fighting COVID-19. A delay in therapy modification for more than one month was noted in 21.5% vs 9.5% of patients during 2020 compared with 2019 (P < 0.001), respectively[131]. In patients with hepatitis C virus who were following up for HCC, there was significant reduction in their scheduled visits, i.e., by before 75%, 63.0%, and 49.1% in March to May 2020, respectively, compared with 97% before February 2020[132]. Surgical interventions for HCC have significantly declined or stopped across many centers in the world due to increased risk of blood transfusion, ICU stay, prolonged hospitalization and developing COVID-19 after surgery[133]. In a national survey by the Italian Association for the Study of the Liver, HCC treatment was affected; where surgical treatment was reduced in 44% and suspended in 44% of the participating centres, while the loco-regional treatment was reduced in 34% and suspended in 8% of the centres[134].
The pathogenesis and characteristics of COVID-19-related multifactorial liver injury can be explained by multiple mechanisms. The knowledge about the full spectrum of SARS-CoV-2 infection is being accumulated, given the novelty of the disease and the constantly reported new data. Liver dysfunction is commonly seen in patients presenting with the severe form of COVID-19. Various therapeutic options used for COVID-19 can lead to DILI and contribute to the exacerbation of the existing liver injury. It is challenging to identify the causal factor in the settings of infection, sepsis, and/or hypoxia, especially when the liver enzymes abnormalities are non-specific. The underlying liver disease has not been linked with poor outcomes. Hospitalized patients or those with liver comorbidities should be monitored closely. Patients with COVID-19 and LTX must maintain strict infection control and monitor drug interactions while maintaining immunosuppressive therapy at regular doses. Future research would help explain liver injury associated with SARS-CoV-2 infection and design specific guidelines for the management of COVID-19 in these patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Qatar
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Islam MM, Naganuma H, Şehirli AÖ S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14766] [Article Influence: 2953.2] [Reference Citation Analysis (0)] |
| 2. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30114] [Article Influence: 6022.8] [Reference Citation Analysis (3)] |
| 3. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (2)] |
| 4. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5781] [Article Influence: 1156.2] [Reference Citation Analysis (2)] |
| 5. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 6. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 665] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 7. | Tuleutaeva GA. [Dynamics of clinico-immunological indicators in children with neurodermatitis during climatotherapy]. Vopr Kurortol Fizioter Lech Fiz Kult. 1988;29-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 8. | Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, Zhang T, Chen XM, Lu FM. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 9. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 10. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 11. | Cardoso FS, Pereira R, Germano N. Liver injury in critically ill patients with COVID-19: a case series. Crit Care. 2020;24:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 13. | Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 14. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 15. | Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci. 2004;61:2738-2743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 17. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281-292.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6155] [Article Influence: 1231.0] [Reference Citation Analysis (0)] |
| 18. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 19. | Polidoro RB, Hagan RS, de Santis Santiago R, Schmidt NW. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front Immunol. 2020;11:1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 20. | Henrion J. Hypoxic hepatitis: the point of view of the clinician. Acta Gastroenterol Belg. 2007;70:214-216. [PubMed] |
| 21. | Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 23. | Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265:174-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 147] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 26. | El Ouali S, Romero-Marrero C, Regueiro M. Hepatic manifestations of COVID-19. Cleve Clin J Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, Framba V, Cerruti L, Mantovani A, Martini A, Luchetti MM, Serra R, Cattelan A, Vettor R, Angeli P; COVID-LIVER study group. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40:2394-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 29. | Labenz C, Toenges G, Wörns MA, Sprinzl MF, Galle PR, Schattenberg JM. Liver injury in patients with severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Su TH, Kao JH. The clinical manifestations and management of COVID-19-related liver injury. J Formos Med Assoc. 2020;119:1016-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Konturek PC, Harsch IA, Neurath MF, Zopf Y. COVID-19 - more than respiratory disease: a gastroenterologist's perspective. J Physiol Pharmacol. 2020;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 32. | Cheong J, Bartell N, Peeraphatdit T, Mosli M, Al-Judaibi B. Gastrointestinal and liver manifestations of COVID-19. Saudi J Gastroenterol. 2020;26:226-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 34. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 35. | Besur S, Begum R, Gunabushanam V, Bonkovsky HL. Liver disease in the Era of Coronavirus Disease 19 (COVID-19) pandemic. Ann Clin Gastroenterol Hepatol. 2020;4:052-057. |
| 36. | Papadopoulos N, Vasileiadi S, Deutsch M. COVID-19 and liver injury: where do we stand? Ann Gastroenterol. 2020;33:459-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, Wang D, Liu C, Meng Y, Cui L, Yu J, Cao H, Li L. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020;288:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 38. | Cao W, Shi L, Chen L, Xu X, Wu Z. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. 2020 Preprint. Available from: medRxiv:20026963. [DOI] [Full Text] |
| 39. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6659] [Article Influence: 1331.8] [Reference Citation Analysis (0)] |
| 40. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18875] [Article Influence: 3775.0] [Reference Citation Analysis (7)] |
| 41. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 42. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5517] [Article Influence: 1103.4] [Reference Citation Analysis (1)] |
| 43. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18198] [Article Influence: 3639.6] [Reference Citation Analysis (0)] |
| 44. | Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 45. | Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. 2020 Preprint. Available from: MedRxiv:20029009. [DOI] [Full Text] |
| 46. | Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 47. | Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, S Farhoud A, Elnahla A, Shihabi A, Toraih EA, S Fawzy M, Kandil E. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Ponziani FR, Del Zompo F, Nesci A, Santopaolo F, Ianiro G, Pompili M, Gasbarrini A; “Gemelli against COVID-19” group. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 49. | Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, Cooley V, Hussain S, Kim SH. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin Gastroenterol Hepatol. 2020;18:2378-2379.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 50. | Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ; WCM-GI research group•. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159:1137-1140.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 51. | Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, Liu Q. Clinical Features and Short-term Outcomes of 102 Patients with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 52. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2550] [Article Influence: 510.0] [Reference Citation Analysis (2)] |
| 53. | Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, Chen X, Luo M, Liang K, Gao S, Zhang Y, Deng L, Xiong Y. Clinical Characteristics and Outcomes of Older Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Single-Centered, Retrospective Study. J Gerontol A Biol Sci Med Sci. 2020;75:1788-1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 54. | Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am J Respir Crit Care Med. 2020;201:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 628] [Cited by in RCA: 628] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 55. | Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020;92:2511-2515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 56. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1624] [Article Influence: 324.8] [Reference Citation Analysis (0)] |
| 57. | Du RH, Liu LM, Yin W, Wang W, Guan LL, Yuan ML, Li YL, Hu Y, Li XY, Sun B, Peng P, Shi HZ. Hospitalization and Critical Care of 109 Decedents with COVID-19 Pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17:839-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 58. | Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1438] [Article Influence: 287.6] [Reference Citation Analysis (0)] |
| 59. | Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 601] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 60. | Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 61. | Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, Peng Z, Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 441] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 62. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (0)] |
| 63. | Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404-3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 109] [Reference Citation Analysis (0)] |
| 64. | Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020;83:521-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (2)] |
| 65. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 66. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 67. | Pongpirul WA, Mott JA, Woodring JV, Uyeki TM, MacArthur JR, Vachiraphan A, Suwanvattana P, Uttayamakul S, Chunsuttiwat S, Chotpitayasunondh T, Pongpirul K, Prasithsirikul W. Clinical Characteristics of Patients Hospitalized with Coronavirus Disease, Thailand. Emerg Infect Dis. 2020;26:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 69. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 70. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 71. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 72. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 73. | Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, Feng J, Liu J, He G. Incidence of Adverse Drug Reactions in COVID-19 Patients in China: An Active Monitoring Study by Hospital Pharmacovigilance System. Clin Pharmacol Ther. 2020;108:791-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 74. | Ramírez E, Urroz M, Rodríguez A, González-Muñoz M, Martín-Vega A, Villán Y, Seco E, Monserrat J, Frías J, Carcas AJ, Borobia AM. Incidence of Suspected Serious Adverse Drug Reactions in Corona Virus Disease-19 Patients Detected by a Pharmacovigilance Program by Laboratory Signals in a Tertiary Hospital in Spain: Cautionary Data. Front Pharmacol. 2020;11:602841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 76. | Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020;17:e1003501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 77. | Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, Cai J, Huang S, Guo J, Zhang L, Chen Y, Zhu W, Du H, Liu Y, Wang T, Chen L, Wen Z, Annane D, Qu J, Chen D. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417-6428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 78. | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1679] [Article Influence: 335.8] [Reference Citation Analysis (0)] |
| 79. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7383] [Article Influence: 1845.8] [Reference Citation Analysis (1)] |
| 80. | Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, Kadam J, Wu W, Caracta CF, Tandon M. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 81. | Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, Gordeev IG, Ilin AP, Karapetian RN, Kravchenko DV, Lomakin NV, Merkulova EA, Papazova NA, Pavlikova EP, Savchuk NP, Simakina EN, Sitdekov TA, Smolyarchuk EA, Tikhomolova EG, Yakubova EV, Ivachtchenko AV. AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin Infect Dis. 2021;73:531-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 82. | Satlin MJ, Goyal P, Magleby R, Maldarelli GA, Pham K, Kondo M, Schenck EJ, Rennert H, Westblade LF, Choi JJ, Safford MM, Gulick RM. Safety, tolerability, and clinical outcomes of hydroxychloroquine for hospitalized patients with coronavirus 2019 disease. PLoS One. 2020;15:e0236778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020;383:2041-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 767] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 84. | Das S, Bhowmick S, Tiwari S, Sen S. An Updated Systematic Review of the Therapeutic Role of Hydroxychloroquine in Coronavirus Disease-19 (COVID-19). Clin Drug Investig. 2020;40:591-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 85. | RECOVERY Collaborative Group; Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383:2030-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 853] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 86. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3627] [Article Influence: 725.4] [Reference Citation Analysis (0)] |
| 87. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1884] [Article Influence: 376.8] [Reference Citation Analysis (0)] |
| 88. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2487] [Article Influence: 497.4] [Reference Citation Analysis (0)] |
| 89. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 987] [Article Influence: 197.4] [Reference Citation Analysis (0)] |
| 90. | Piscoya A, Ng-Sueng LF, Parra Del Riego A, Cerna-Viacava R, Pasupuleti V, Roman YM, Thota P, White CM, Hernandez AV. Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. PLoS One. 2020;15:e0243705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 91. | Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 92. | Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Corominas H, Castellví I, Pomar V, Antonijoan R, Mur I, Matas L, Gich I, de Benito N, Laiz A, Castillo D, Villamarin L, Filella D, Millán AM, Quijada MÁ, Puig M, Casademont J, Domingo P. Effectiveness and safety of intravenous tocilizumab to treat COVID-19-associated hyperinflammatory syndrome: Covizumab-6 observational cohort. Clin Immunol. 2021;223:108631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 998] [Cited by in RCA: 1014] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 95. | Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P; CORIMUNO-19 Collaborative Group. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 559] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 96. | Parente A, Manzia TM, Angelico R, Tirotta F, Muiesan P, Tisone G, Framarino Dei Malatesta M. COVID-19, liver transplant, and immunosuppression: Allies or foes? Transpl Infect Dis. 2021;23:e13417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 98. | El Kassas M, Alboraie M, Al Balakosy A, Abdeen N, Afify S, Abdalgaber M, Sherief AF, Madkour A, Abdellah Ahmed M, Eltabbakh M, Salaheldin M, Wifi MN. Liver transplantation in the era of COVID-19. Arab J Gastroenterol. 2020;21:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Forns X, Navasa M. Liver transplant immunosuppression during the covid-19 pandemic. Gastroenterol Hepatol. 2020;43:457-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 101. | Spoletini G, Bianco G, Graceffa D, Lai Q. Transplantation during the COVID-19 pandemic: nothing noble is accomplished without danger. BMC Gastroenterol. 2020;20:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Carmona M, Álvarez M, Marco J, Mahíllo B, Domínguez-Gil B, Núñez JR, Matesanz R. Global organ transplant activities in 2015. Data from the Global Observatory on Donation and Transplantation (GODT). Transplantation. 2017;101:S29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 700] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 104. | Yu L, Ioannou GN. Survival of liver transplant recipients with hemochromatosis in the United States. Gastroenterology. 2007;133:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 105. | Hollander JE, Carr BG. Virtually Perfect? N Engl J Med. 2020;382:1679-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1849] [Cited by in RCA: 1892] [Article Influence: 378.4] [Reference Citation Analysis (0)] |
| 106. | Hong Z, Li N, Li D, Li J, Li B, Xiong W, Lu L, Li W, Zhou D. Telemedicine During the COVID-19 Pandemic: Experiences From Western China. J Med Internet Res. 2020;22:e19577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 107. | Kumar D, Manuel O, Natori Y, Egawa H, Grossi P, Han SH, Fernández-Ruiz M, Humar A. COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 108. | American Association for the Study of Liver Diseases. AASLD clinical insights for hepatology and liver transplant providers during the COVID-19 pandemic [online]. [cited 31 January 2021]. Available from: https://www.aasld.org/sites/default/files/2020-04/AASLD-COVID19-ClinicalInsights-4.07.2020-Final.pdf. |
| 109. | Dwosh HA, Hong HH, Austgarden D, Herman S, Schabas R. Identification and containment of an outbreak of SARS in a community hospital. CMAJ. 2003;168:1415-1420. [PubMed] |
| 110. | Al-Dorzi HM, Aldawood AS, Khan R, Baharoon S, Alchin JD, Matroud AA, Al Johany SM, Balkhy HH, Arabi YM. The critical care response to a hospital outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: an observational study. Ann Intensive Care. 2016;6:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Feltracco P, Barbieri S, Galligioni H, Michieletto E, Carollo C, Ori C. Intensive care management of liver transplanted patients. World J Hepatol. 2011;3:61-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Cardoso FS. Liver Transplantation in an ICU Dominated by COVID-19. Liver Transpl. 2020;26:1064-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Mandell MS, Stoner TJ, Barnett R, Shaked A, Bellamy M, Biancofiore G, Niemann C, Walia A, Vater Y, Tran ZV, Kam I. A multicenter evaluation of safety of early extubation in liver transplant recipients. Liver Transpl. 2007;13:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 114. | Glanemann M, Busch T, Neuhaus P, Kaisers U. Fast tracking in liver transplantation. Immediate postoperative tracheal extubation: feasibility and clinical impact. Swiss Med Wkly. 2007;137:187-191. [PubMed] |
| 115. | Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 116. | Vitale A, Volk ML, De Feo TM, Burra P, Frigo AC, Ramirez Morales R, De Carlis L, Belli L, Colledan M, Fagiuoli S, Rossi G, Andorno E, Baccarani U, Regalia E, Vivarelli M, Donataccio M, Cillo U; Liver Transplantation North Italy Transplant program (NITp) working group. A method for establishing allocation equity among patients with and without hepatocellular carcinoma on a common liver transplant waiting list. J Hepatol. 2014;60:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 117. | D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 492] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 118. | Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, Redondo-Pachón MD, Murphy B, Florman S, Cyrino LG, Grafals M, Venkataraman S, Cheng XS, Wang AX, Zaza G, Ranghino A, Furian L, Manrique J, Maggiore U, Gandolfini I, Agrawal N, Patel H, Akalin E, Riella LV. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140-3148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 119. | Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD. COVID-19 in Liver Transplant Recipients: An Initial Experience from the U.S. Epicenter. Gastroenterology. 2020;S0016:5085(20)34703-X. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 120. | Bossini N, Alberici F, Delbarba E, Valerio F, Manenti C, Possenti S, Econimo L, Maffei C, Pola A, Terlizzi V, Salviani C, Moscato M, Pasquali S, Zambetti N, Tonoli M, Affatato S, Pecchini P, Viola FB, Malberti F, Depetri G, Gaggiotti M, Scolari F; Brescia Renal COVID task force. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am J Transplant. 2020;20:3019-3029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 121. | Favà A, Cucchiari D, Montero N, Toapanta N, Centellas FJ, Vila-Santandreu A, Coloma A, Meneghini M, Manonelles A, Sellarés J, Torres I, Gelpi R, Lorenzo I, Ventura-Aguiar P, Cofan F, Torregrosa JV, Perelló M, Facundo C, Seron D, Oppenheimer F, Bestard O, Cruzado JM, Moreso F, Melilli E. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am J Transplant. 2020;20:3030-3041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 122. | Molnar MZ, Bhalla A, Azhar A, Tsujita M, Talwar M, Balaraman V, Sodhi A, Kadaria D, Eason JD, Hayek SS, Coca SG, Shaefi S, Neyra JA, Gupta S, Leaf DE, Kovesdy CP; STOP-COVID Investigators. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am J Transplant. 2020;20:3061-3071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 123. | Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 124. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 125. | Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O, Katchman H. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 126. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 127. | Combined Update. (2020, August 25). Retrieved March 5, 2021, from SECURE-Cirrhosis and EASL supported COVID-Hep. [cited 4 April 2021]. Available from: https://www.covid-hep.net/img/update_20200825.pdf. |
| 128. | COVID-Hep. net. Updates. [cited 5 March 2021]. Available from: https://www.covid-hep.net/updates.html. |
| 129. | Ball S, Banerjee A, Berry C, Boyle JR, Bray B, Bradlow W, Chaudhry A, Crawley R, Danesh J, Denniston A, Falter F, Figueroa JD, Hall C, Hemingway H, Jefferson E, Johnson T, King G, Lee KK, McKean P, Mason S, Mills NL, Pearson E, Pirmohamed M, Poon MTC, Priedon R, Shah A, Sofat R, Sterne JAC, Strachan FE, Sudlow CLM, Szarka Z, Whiteley W, Wyatt M; CVD-COVID-UK Consortium. Monitoring indirect impact of COVID-19 pandemic on services for cardiovascular diseases in the UK. Heart. 2020;106:1890-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 130. | Einstein AJ, Shaw LJ, Hirschfeld C, Williams MC, Villines TC, Better N, Vitola JV, Cerci R, Dorbala S, Raggi P, Choi AD, Lu B, Sinitsyn V, Sergienko V, Kudo T, Nørgaard BL, Maurovich-Horvat P, Campisi R, Milan E, Louw L, Allam AH, Bhatia M, Malkovskiy E, Goebel B, Cohen Y, Randazzo M, Narula J, Pascual TNB, Pynda Y, Dondi M, Paez D; the; INCAPS COVID Investigators Group. International Impact of COVID-19 on the Diagnosis of Heart Disease. J Am Coll Cardiol. 2021;77:173-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 131. | Amaddeo G, Brustia R, Allaire M, Lequoy M, Hollande C, Regnault H, Blaise L, Ganne-Carrié N, Séror O, Larrey E, Lim C, Scatton O, El Mouhadi S, Ozenne V, Paye F, Balladur P, Dohan A, Massault PP, Pol S, Dioguardi Burgio M, Vilgrain V, Sepulveda A, Cauchy F, Luciani A, Sommacale D, Leroy V, Roudot-Thoraval F, Bouattour M, Nault JC; Paris Liver Cancer Group. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021;3:100199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 132. | Toyoda H, Yasuda S, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yamamoto S, Shiota S, Furoi M, Koyabu T, Furukawa D, Kumada T, Sumida Y. Impact of COVID-19 pandemic on surveillance of hepatocellular carcinoma: a study in patients with chronic hepatitis C after sustained virologic response. GastroHep. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Fancellu A, Sanna V, Scognamillo F, Feo CF, Vidili G, Nigri G, Porcu A. Surgical treatment of hepatocellular carcinoma in the era of COVID-19 pandemic: A comprehensive review of current recommendations. World J Clin Cases. 2021;9:3517-3530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Aghemo A, Masarone M, Montagnese S, Petta S, Ponziani FR, Russo FP; Associazione Italiana Studio Fegato (AISF). Assessing the impact of COVID-19 on the management of patients with liver diseases: A national survey by the Italian association for the study of the Liver. Dig Liver Dis. 2020;52:937-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |