Published online Dec 27, 2020. doi: 10.4254/wjh.v12.i12.1198
Peer-review started: June 15, 2020
First decision: July 30, 2020
Revised: August 26, 2020
Accepted: October 28, 2020
Article in press: October 28, 2020
Published online: December 27, 2020
Processing time: 185 Days and 15.9 Hours
Liver reduction is the main curative treatment for primary liver cancer, but its use remains limited as liver regeneration requires a minimum of 30% functional parenchyma.
To study the dynamics of the liver regeneration process and consequent behavior of cell cycle regulators in rats after extended hepatectomy (90%) and postoperative glucose infusions.
Post-hepatectomy liver failure was triggered in 84 Wistar rats by reducing their liver mass by 90%. The animals received a post-operative glucose infusion and were randomly assigned to two groups: One to investigate the survival rate and the other for biochemical analyses. Animals that underwent laparotomy or 70% hepatectomy were used as controls. Blood and liver samples were collected on postoperative days 1 to 7. Liver morphology, function, and regeneration were studied with histology, immunohistochemistry, and western blotting.
Postoperative mortality after major resection reached 20% and 55% in the first 24 h and 48 h, respectively, with an overall total of 70% 7 d after surgery. No apparent signs of apoptotic cell death were detected in the extended hepatectomy rat livers, but hepatocytes displaying a clear cytoplasm and an accumulation of hyaline material testified to changes affecting their functional activities. Liver regeneration started properly, as early events initiating cell proliferation occurred within the first 3 h, and the G1 to S transition was detected in less than 12 h. However, a rise in p27 (Kip1) followed by p21 (Waf1/Cip1) cell cycle inhibitor levels led to a delayed S phase progression and mitosis. Overall, liver regeneration in rats with a 90% hepatectomy was delayed by 24 h and associated with a delayed onset and lower peak magnitude of hepatocellular deoxyribonucleic acid synthesis.
This work highlights the critical importance of the cyclin/cyclin-dependent kinase inhibitors of the Cip/Kip family in regulating the liver regeneration timeline following extended hepatectomy.
Core Tip: There is a current pandemic of obesity and diabetes and the chronic liver damages they cause, and the outcomes of patients undergoing liver mass reduction for malignant diseases are poor. To design efficient strategies that limit the risk of post-hepatectomy liver failure, we used a rat model to clarify the causes of death after enlarged liver resection. Compared with standard 2/3 hepatectomy, enlarged resection resulted in a loss of hepatocyte functional activities and impaired regenerative capacities, which were associated with an overexpression of p21 and p27 inhibitors. The use of extracorporeal support device with p21 and p27 should be considered for the management of severe liver failure following extended hepatectomy.
- Citation: Moniaux N, Lacaze L, Gothland A, Deshayes A, Samuel D, Faivre J. Cyclin-dependent kinase inhibitors p21 and p27 function as critical regulators of liver regeneration following 90% hepatectomy in the rat. World J Hepatol 2020; 12(12): 1198-1210
- URL: https://www.wjgnet.com/1948-5182/full/v12/i12/1198.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i12.1198
Because the liver is able to regenerate its mass, surgical resection is used routinely as a curative treatment to manage primary liver cancers and hepatic metastases (i.e. those of colorectal and breast cancers[1]). This is a relatively safe procedure for patients and the only efficient treatment for these tumors. The principal challenge faced by surgeons is estimating the volume of liver that can be resected without increasing the risk that the patients will develop post-hepatectomy liver failure (PHLF), a disorder related to the small-for-size syndrome (SFSS) that occurs when too little liver is transplanted. Therefore, to ensure full patient safety and avoid post-resection liver failure, a minimum of 30% functional hepatic parenchyma is required[2].
Hepatocellular carcinoma (HCC), which is the most common primary liver cancer, occurs predominantly in patients with an underlying chronic liver disease. The setting of chronic inflammation involves steatosis, fibrosis, or cirrhosis[3]. Fewer than 30% of HCC patients are eligible for surgery, mainly because of the lesions resulting from chronic inflammation. This situation is becoming even more problematic in the context of the current epidemic of diabetes and obesity that affects 25% to 30% of the global population and is associated with the development of metabolic syndrome and its continuum of chronic liver disorders such as steatosis, fibrosis, non-alcoholic steatohepatitis (NASH) and cirrhosis. When liver reduction is considered as a treatment for liver metastases, the preoperative chemotherapies used to reduce the primary colon or breast tumors and metabolized by the liver can aggravate the clinical picture. All patients should be deemed at risk of developing post-resection liver failure. To prevent or limit fatalities and complications after liver resection, a preoperative evaluation of hepatic functional status is necessary, and the criteria used to define patient eligibility include the Child-Pugh score, the indocyanine green retention rate at 15 min, magnetic resonance imaging[4], and the determination of liver stiffness using FibroScan®[5]. Despite these precautions, it remains difficult to judge recovery after surgery, and the incidence of PHLF still exerts a major impact on 2-year survival following resection[6]. Because PHLF shares a common clinical picture and outcome with SFSS (jaundice, ascites, coagulopathy, encephalopathy, etc.), both syndromes are considered as a single entity.
The cellular and molecular mechanisms giving rise to PHLF remain unclear, but several causal factors are considered to be important. The excessive portal blood inflow and resulting intrahepatic shear stress that occur after the transplantation of a graft that is too small have been shown to play a central role in the development of SFSS[7,8]. For this reason, hemodynamic modulation of the portal vein is proposed to ensure successful adult-to-adult living-donor liver transplantation[9]. As demonstrated by Bucur et al[10] the use of a portal ring to modulate blood inflow can improve liver regeneration after surgical resection in a porcine model[10]. Similar results have been observed using splenectomy to control hemodynamic parameters[11]. An accumulation of liver injuries post-resection has also been suggested as a factor leading to post-operative mortality[12]. Part if not all these injuries are associated with the sustained activation of Küpffer cells because of elevated endotoxin levels in the liver after surgery[13] and the massive oxidative stress that results. Reducing oxidative stress has been shown to enhance markedly the regenerative capacity of the liver in an experimental model of acute liver failure[14-16]. Likewise, preconditioning reduces ischemic reperfusion injuries and improved rat survival after hepatectomy performed on a liver affected by steatosis[17]. However, Lehmann et al[18] showed that failure of regeneration may occur in the absence of serious liver damage affecting the small remnant liver using an improved technique of extended hepatectomy in mice[18]. That study also reported a delay in liver regeneration because of retarded progression through the cell cycle[18]. They showed that extended liver resection positively regulated p21, a cyclin-dependent kinase inhibitor (CKI) at both the G1/S and the G2/M transitions. In addition, p21 deficiency enhances regenerative capacity of multiple tissue types including complete rescue and regeneration of injured liver[18-21]. If confirmed, this finding is important as it will open the way to new therapeutic regimens targeting p21. This is even more important given that patients undergoing liver resection routinely receive intravenous glucose infusions to manage hypoglycemia, and such infusions have been shown to inhibit post-resection liver regeneration in a p21-dependent manner in mice[22].
To create acute liver failure in rats, we performed an extended hepatectomy (eHx) with removal of 90% of the liver mass. We then studied the dynamics of the liver regeneration process and consequent behavior of cell cycle regulators in rats after eHx and post-operative glucose infusions.
All animal procedures were approved by the CE2A-03 CNRS-Orléans Ethics Committee. Male Wistar rats (n = 119) aged 10 wk and weighing 200-230 g were housed at the CNRS-SEAT animal care facility (Université Paris-Sud, Villejuif) and kept on a 12 h day/night cycle with free access to food and water. The number of rats used was in compliance with institutional ethical rules and consistent with common practice in the fields of post-hepatectomy liver regeneration. All the rats were anesthetized by isoflurane inhalation and then underwent a midline incision after sterilization of the area. For a standard 70% hepatectomy, the left lateral and left and rights parts of the median lobes of the liver were resected. For an extensive 90% resection, the left lateral, median, and both right lobes were carefully removed, leaving the two caudate lobes and liver tissues surrounding the vena cava. To prevent hypoglycemia, a subcutaneous injection of 5 mL of 30% glucose solution was administered immediately after liver resection, and then the animals had free access to 20% glucose solution and rat chow ad libitum. Because the administration of glucose might affect the kinetics of liver regeneration, it was injected in all the rats undergoing a 70% or 90% resection. Intraperitoneal injections of bromodeoxyuridine (BrdU) were given to all surviving animals 2 h prior to sacrifice at a dose of 50 mg/kg.
Blood samples were obtained just prior to organ harvest at sacrifice, spun immediately to collect the serum, and frozen until use. Biochemical parameters [aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin] were determined using an Olympus AU400 automat (Centre d’Exploration Fonctionnelles Intégrées, Institut Claude Bernard, Paris, France).
The livers thus collected were fixed overnight at 4 °C in a 4% formalin solution before being embedded in a paraffin block. For histological analysis, liver sections (4 μM) were dewaxed in xylene, rehydrated through graded alcohols, and stained with hematoxylin & eosin. For immunofluorescence, 4 μmol/L liver sections were dewaxed in xylene, rehydrated through graded alcohols, and pressure cooked in a 10 mmoL/L citrate buffer at pH 6 for 10 min. For BrdU staining, the 5-bromo-2′-deoxy-uridine Labelling and Detection Kit I (Roche, Basel, Switzerland) was used according to the supplier’s recommendations. Tissue auto-fluorescence was reduced by applying 10 mmoL/L cupric sulfate in a 50 mmoL/L acetate buffer pH 5 solution for 60 min at room temperature. To visualize nuclei, Hoechst 33342 solution was added to the mounting medium at a concentration of 0.1 mg/mL. For phospho-histone H3 staining, dewaxed rehydrated sections were incubated for 45 min at 37 °C with a primary antibody (Cell Signaling, Danvers, MA, United States), washed in phosphate buffer saline (PBS) (3-times for 5 min), and incubated for 30 min at 37 °C using Alexa fluor® 594 donkey anti rabbit immunoglobulin G (Invitrogen, Carlsbad, CA, United States). The sections were then washed in PBS and mounted using Hoechst 33342 containing mounting medium at a concentration of 0.1 mg/mL. For immunohistochemistry, dewaxed rehydrated sections were blocked for endogenous peroxidase, incubated with primary anti-caspase 3 antibody (Cell Signaling) or anti-signal transducer and activator of transcription 3 (STAT3) antibody (Santa Cruz Biotechnology, Dallas, TX, United States), washed, and incubated with secondary anti-rabbit immunoglobulin G-horseradish peroxidase according to the manufacturer’s instructions (DAKO, Jena, Germany). The sections were counterstained with alcian blue (Sigma, St Louis, MO, United States) before mounting the coverslips. Positively labelled cell counting was performed in 10 random microscopic fields. Cell proliferation, cell death, and cell cycle were assessed by measuring the ratio of the numbers of BrdU-, p-Histone H3-, STAT3-, and caspase 3-positive nuclei to the total nucleus count.
Whole-cell lysates were prepared in ice cold buffer containing 50 mmoL/L Tris-HCl (pH 7.4), 150 mmoL/L NaCl, 1% Nonidet P-40, 0.25% Na-deoxycholate, 1 mmoL/L Na3VO4, 20 mmoL/L NaF, 1 μg/mL aprotinin, 10 μg/mL pepstatin, 10 μg/mL leupeptin, and 1 μM phenylmethylsulfonyl fluoride. Protein concentrations were determined with the Bio-Rad protein assay kit using bovine serum albumin as a standard. Aliquots of 30 μg were denatured by boiling in Tris-Glycine SDS buffer (Invitrogen), separated by 12% SDS and transferred onto nitrocellulose membranes (Whatman, Dominique Dutscher, Brumath Cedex, France) by electroblotting. The membranes were blocked in 5% non-fat dry milk in 0.1% Tween 20 Tris-buffered saline for 1 h and probed with primary antibodies against cyclin E1, cyclin A2, cyclin B1, p27, p21, STAT3, p-STAT3, retinoblastoma protein (Rb), p-Rb, and actin (Santa Cruz Biotechnology).
Normal distribution of the data was analyzed by the Shapiro-wilk test and homogeneity of variances by the Levene test. All groups were normally distributed, and a two-tailed Student’s t-test was used to assess statistical differences between the groups. The statistics were performed with StatView 5.0 freeware (SAS Institute Inc., Cary, NC, United States), and differences with P < 0.05 were considered significant. All data are presented as means over several independent experiments ± standard error of the mean. Survival curve was constructed by the Kaplan-Meier method (log-rank test).
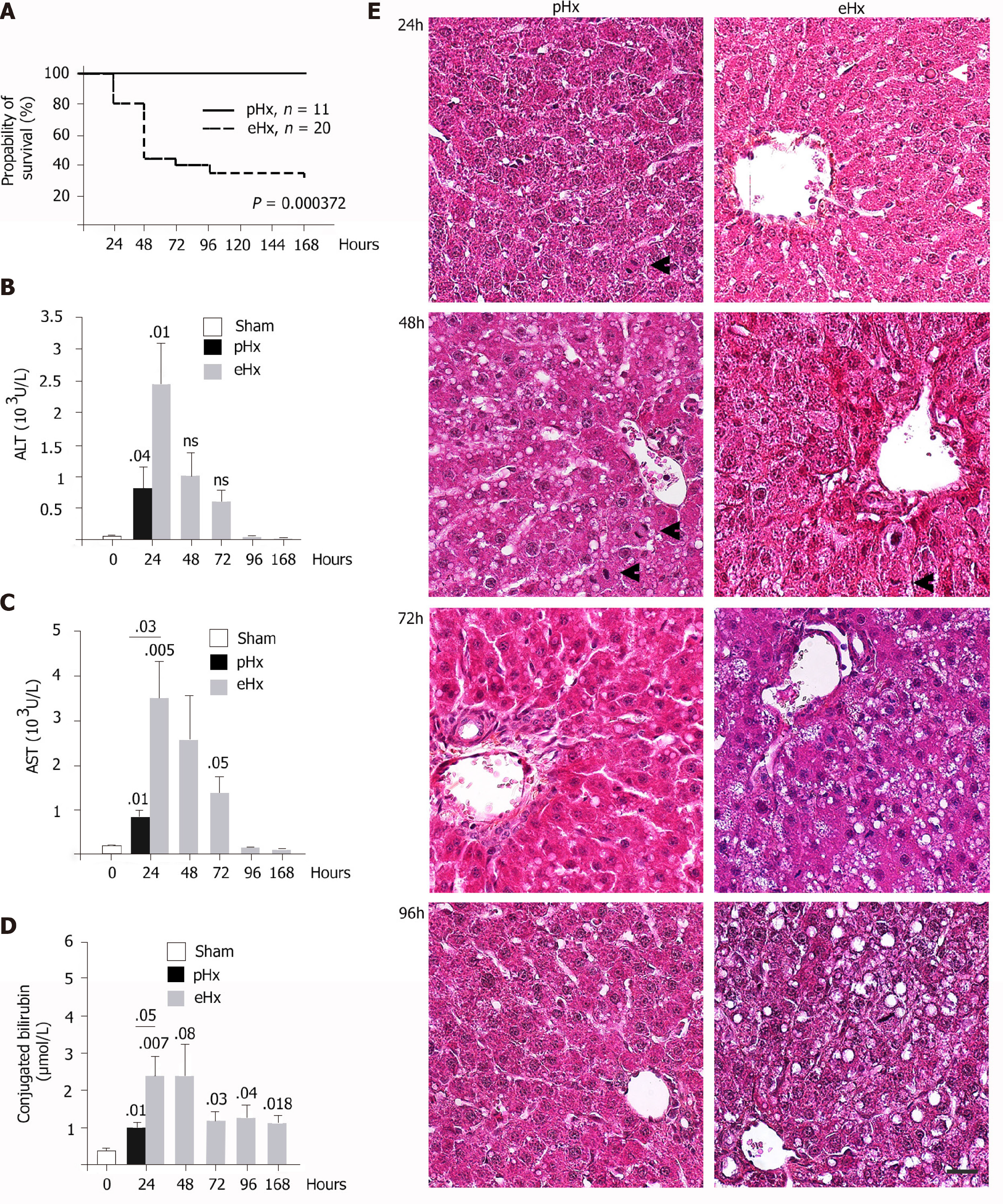
To determine the direct cause of death in liver failure after massive hepatectomy, 84 Wistar rats underwent 90% liver resection (eHx) and were randomly divided into two groups: One (20/84) to analyze rat survival over time and (64/84) another for biochemical analyses. For the second set of experiments, some rats were sacrificed every 24 h for 7 d. To prevent deaths linked to hypoglycemia, the rats were injected post-operatively with glucose solution and subsequently offered free access to a glucose solution. Control groups of either rats that underwent laparotomy (sham) or rats that underwent 70% liver resections (pHx) were treated similarly. As depicted in Figure 1A, the outcomes after liver surgery differed considerably between the groups, with a 30% survival rate after eHx (6/20) compared to 100% (n = 11) after pHx. This rate is consistent with previous studies. Peak mortality was seen within the first 48 h and accounted for 78% (11/14) of all deaths, 28% (4/14) during the first 24 h and a further 50% (7/14) between 24 h and 48 h. Before they died, the animals were hypoactive and displayed signs of liver failure such as jaundice and coma.
Blood was collected from surviving animals at the time they were sacrificed in order to measure markers for liver function and liver damage. Hepatic enzymes (ALT, AST) and bilirubin levels were significantly elevated 24 h after surgery when compared to sham animals (Figure 1B-D). At the same time point, markers levels remained moderate in the pHx rats but were significantly higher in the eHx animals, suggesting an accumulation of parenchymal injuries with an impairment of liver function in this group. The serum levels of both enzymes declined over time to reach a standard level 96 h after surgery, while conjugated bilirubin levels remained elevated in the eHx group even after 7 d indicated that liver functional activities were still impaired at that time (Figure 1D).
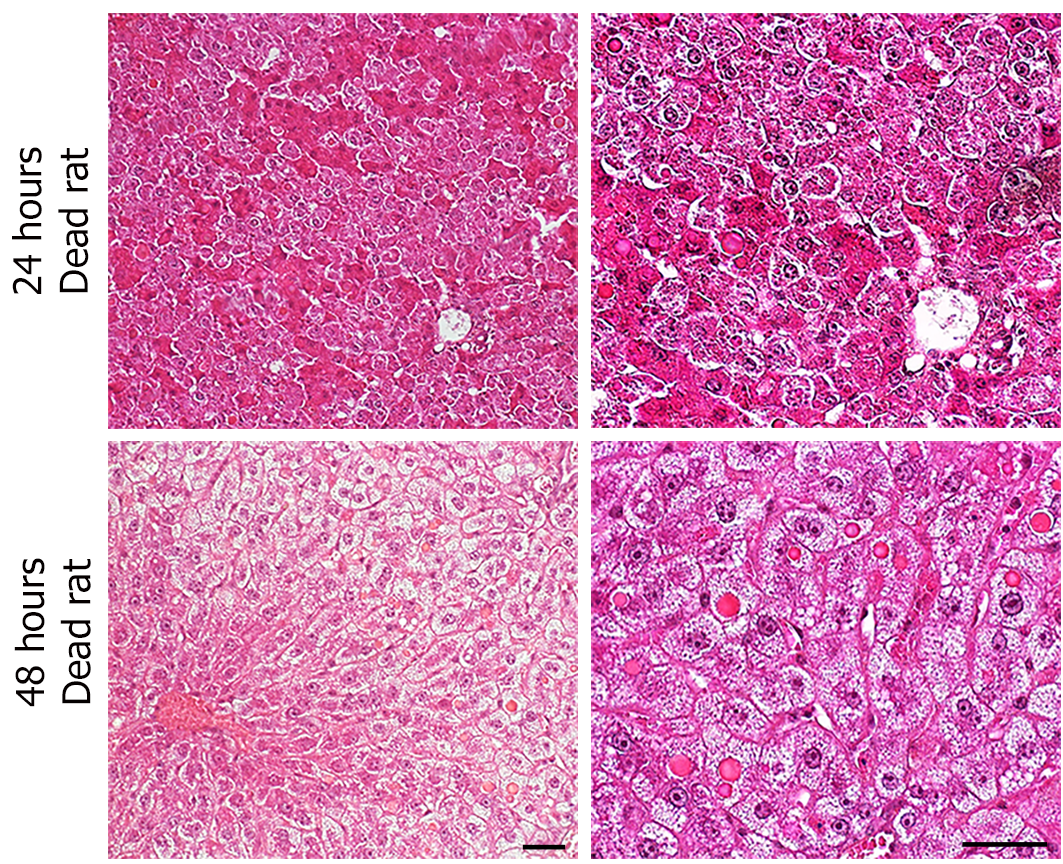
Despite the clinical picture of acute liver failure, the histological analysis of hematoxylin and eosin-stained liver sections did not reveal any signs of extensive apoptotic and necrotic cell death (Figure 1E). This result was confirmed by immunohistochemistry that showed almost no caspase 3-positive cells within the tissue sections (data not shown), indicating that apoptosis was not a major inducer of hepatic failure after excessive hepatectomy in this experimental surgical setting. Nevertheless, a pattern of parenchymal abnormalities was observed over time following resection, and these changes were much more pronounced in the eHx group. Hypertrophic hepatocytes associated with a clear cytoplasm testifying to fluid and lipid infiltration were detected at 24 h and 48 h after eHx. Lipid droplets were visible after 48 h in pHx animals but not until 96 h postoperatively in eHx sections. In addition, globular red hyaline material within hepatocytes was detected in eHx livers, evidencing alterations to protein synthesis and secretion processes (Figure 1, white arrowhead). Post mortem histological analysis of the rat livers that could be collected just after death revealed similar but much more developed changes. As for the rats sacrificed at each time-point, their livers did not display any signs of massive cell death or massive hemorrhagic parenchyma, but hypertrophic hepatocytes associated with a clear cytoplasm corresponding to fluid and lipid infiltration and an accumulation of globular red hyaline material were widely detected in the tissue sections (Figure 2).
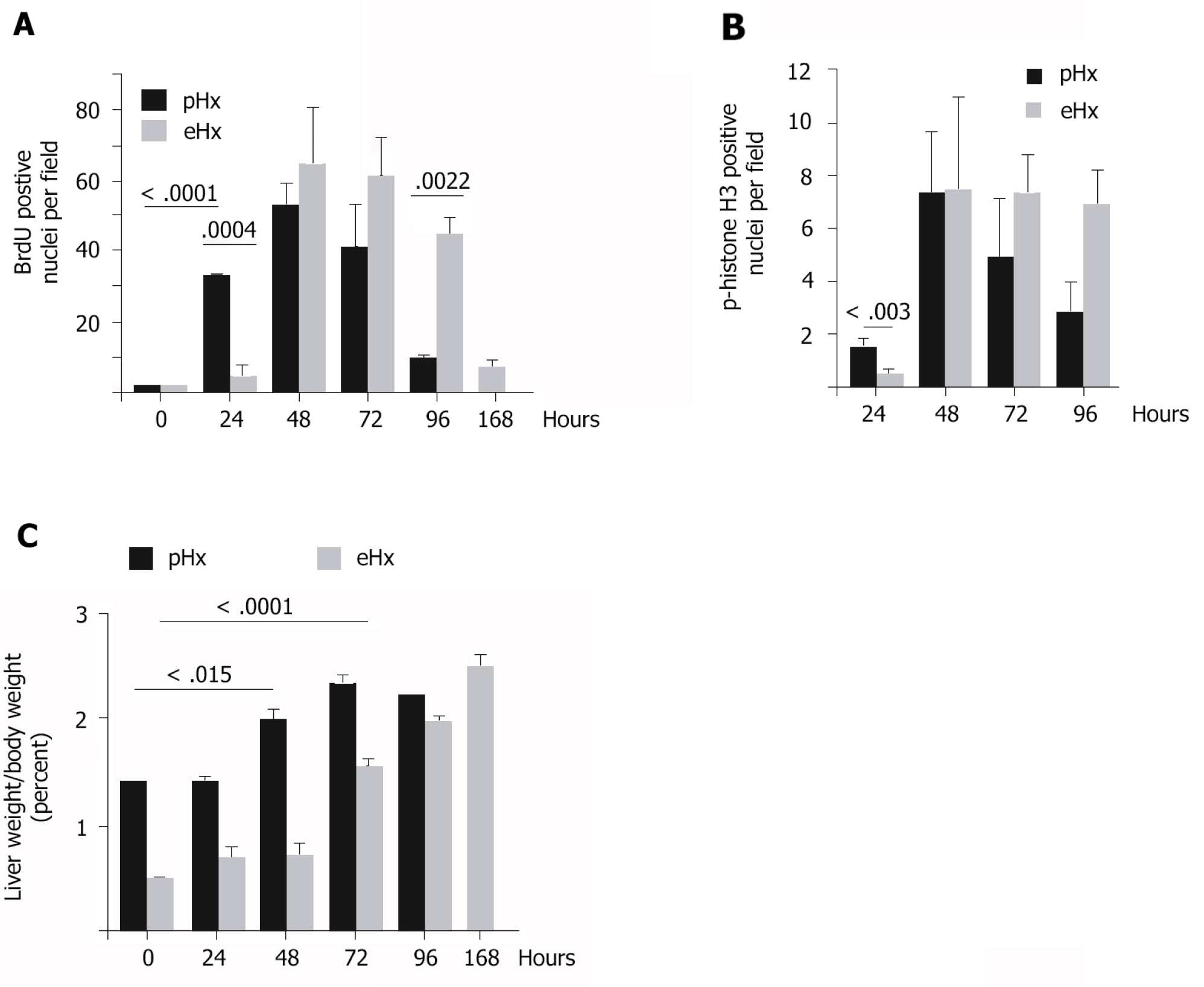
We found numerous mitotic figures histologically in pHx livers as soon as 24 h after surgery (Figure 1E) but not before the 48 h time point in eHx tissues (Figure 1E). Immunohistochemical analyses revealed that although both pHx and eHx rats reached a maximum of BrdU incorporation (Figure 3A) and phospho-histone H3 Labelling (Figure 3B) at 48 h post-surgery, only pHx rats displayed labelled cells at the 24 h time point. Figure 3C shows determinations of the liver weight to body weight (LW/BW) ratio at various post-operative time points. A significant rise in the LW/BW ratio was noted 48 h after surgery in rats that had undergone pHx and at the 72 h point in eHx animals. These findings establish that hepatocyte proliferation and liver mass restoration were delayed in eHx rats.
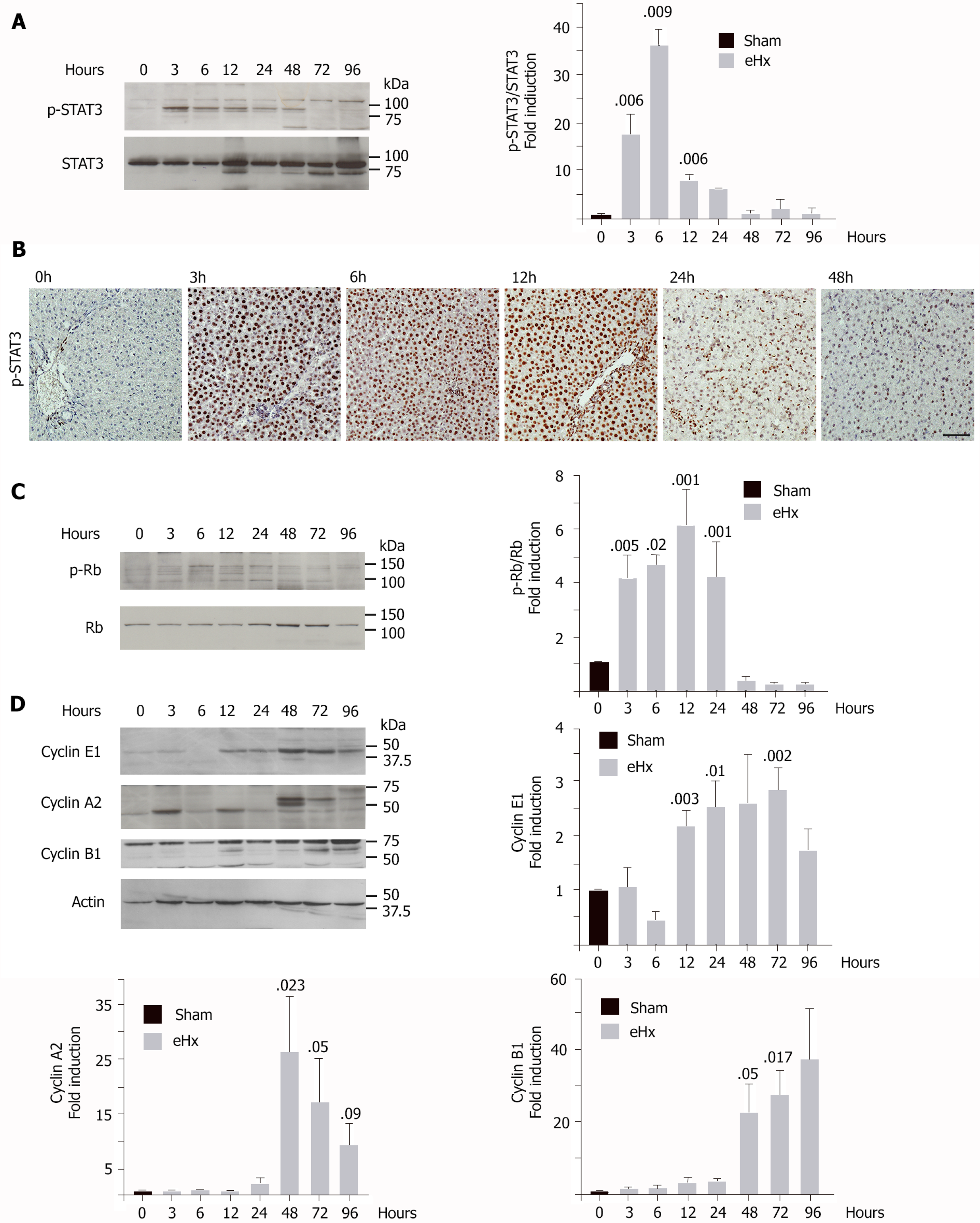
Liver regeneration after pHx is a well-known mechanism that involves the sequential activation of cytokines and growth factor-related pathways. This cascade of events leads to a peak of deoxyribonucleic acid synthesis 24 h after surgery in the rat (for a review, see[23]). To evaluate proper implementation of the mitogenic program, western blot analyses were performed on frozen liver specimens from rats that had undergone enlarged hepatectomy and were sacrificed 3 h, 6 h, 12 h, 24 h, 48 h, 72 h, and 96 h post-surgery. As STAT3 is activated rapidly during liver regeneration in an interleukin 6-dependent manner and drives hepatocytes to switch from a quiescent state into a proliferative wave, STAT3 activation was verified (Figure 4A and B). We found STAT3 activation quickly 3 h post-surgery (Figure 4A and B). Peak STAT3 activation was observed at the 6 h time point and then gradually returned to standard levels 48 h post-resection (Figure 4A and B). These data therefore indicated that the priming of rat hepatocytes had occurred and that they correctly re-entered the cell cycle after eHx, despite the infusion of glucose.
We next studied cell cycle checkpoint proteins p-Rb in G1 (Figure 4C), cyclin E1 for the G1 to S transition (Figure 4D), cyclin A2 for the S to G2 transition (Figure 4D), and cyclin B1 for the G2 to mitosis transition (Figure 4D). Levels of p-Rb were significantly upregulated during the first 3 h after resection, remained high for 24 h, and then normalized by the 48 h time point (Figure 4C). The cyclin E1 level rose significantly after 12 h and remained elevated until the 72 h time point (Figure 4D). Levels of cyclin A2 and B1 remained stable at very low levels for the first 24 h post-surgery, rose markedly after 48 h, and then remained high until the 96 h time point (Figure 4D). Taken together, our results showed that hepatocytes entered the cell cycle correctly, but the absence of any detection of cyclin A2, 24 h after surgery, established a delayed S phase progression.
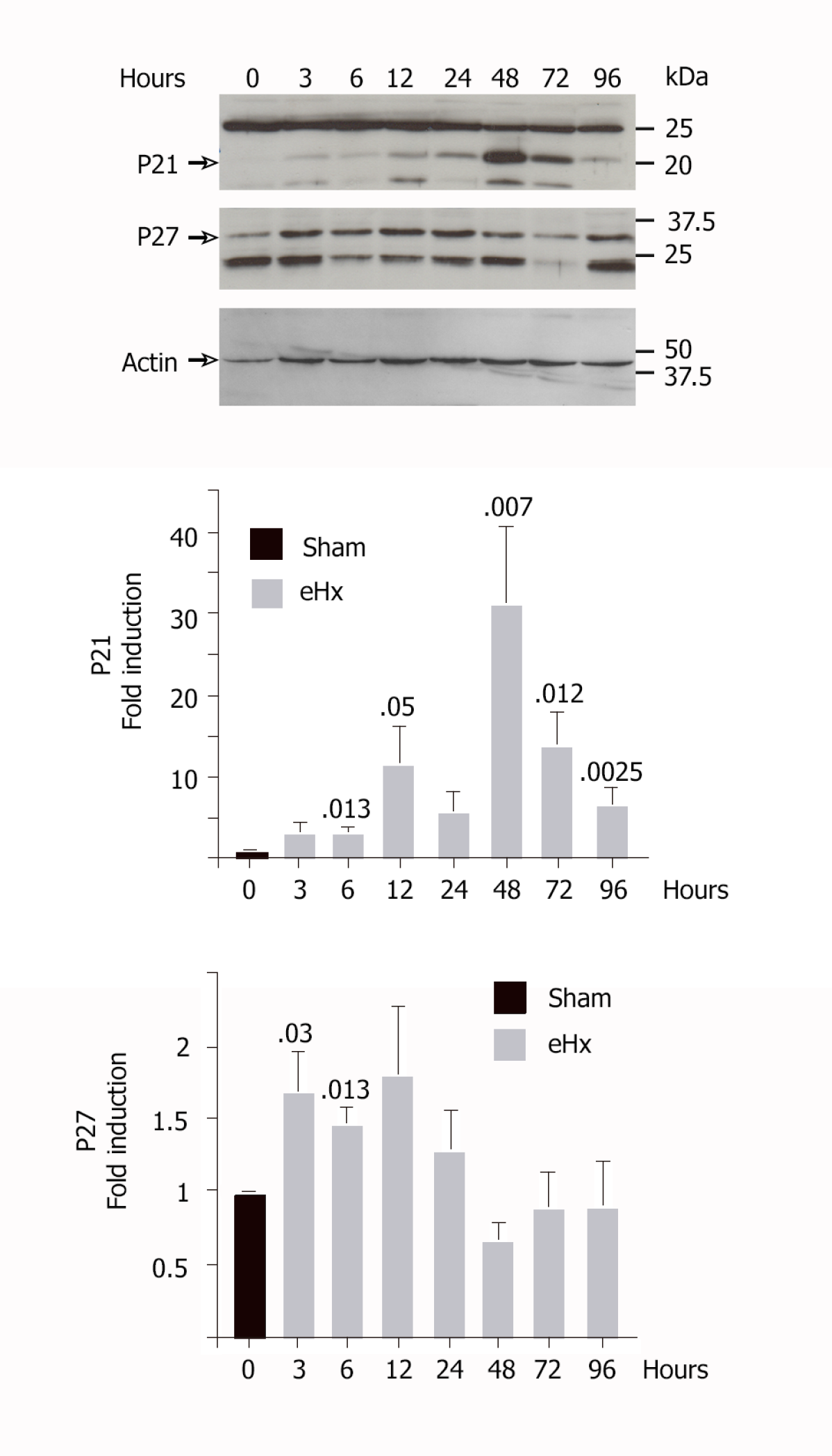
As depicted in Figure 5, our model of eHx rapidly induced an upregulation of the p21 and p27 CKI in hepatocytes, which resulted in decreased regeneration. The p21 protein was undetectable in quiescent rat liver. Its level rose gradually just after surgery to reach an initial peak at 12 h post-resection that corresponded to a 11.6 ± 4.5-fold amplification (P < 0.05). P21 displayed a second peak of expression at 48 h after resection, with a 32 ± 9.3-fold (P = 0.007) amplification vs baseline. Our data also pointed to a prolonged expression of p27 during the first 24 h, the level reaching 1.7 ± 0.25-fold (P = 0.03) as early as 3 h post-eHx, indicating that p27 acts as a rapid brake to S-phase progression. P27 levels then normalized after 24 h. A second wave of p21 expression was detected afterwards.
Liver resection offers a chance of a cure in patients presenting with primary and secondary liver cancers and is currently the gold standard treatment for these malignancies. If performed on appropriate patients, liver reduction is a safe operation. Highly selective criteria based on preoperative assessments of both the extent of the disease and the liver function need to be met in order to minimize post-operative complications. Some surgical strategies have been developed to increase the number of patients who are eligible for resection, such as portal vein embolization, which enables expansion of the portion of healthy liver prior to resection, or two-stage liver resection. However, most patients diagnosed with primary or secondary liver cancer remain ineligible for surgery. The principal challenge for clinicians in the coming years is to find alternative treatments for patients who are denied surgical reduction. This issue is all the more important given the worldwide progression of obesity and diabetes that is causing chronic inflammatory liver disorders, even though the impact of liver resection in obese patients remains controversial[24-26] An increased risk of developing liver failure post-resection was demonstrated when performed in patients and mouse models of steatosis[27], NASH[28] and cirrhosis[29]. To ensure the safety of patients and avoid liver failure post-resection, a minimum of 30% functional hepatic parenchyma is required[2]. However, there is as yet no complete understanding of the mechanistic details of hepatocellular failure below this critical mass.
The development of novel pharmaceutical strategies to help patients recover from extended liver resection requires full identification and characterization of the causes of morbidity-mortality, and thus the reasons why the remnant liver lobes failed to regenerate. A search in the bibliography on this topic generally produces papers that refer to multivariate analyses performed on large cohorts of patients who underwent liver resection and lists the predictive factors that will enable a better stratification of patients prior to surgery[30-32]. These factors include diabetes, steatosis, chemotherapy-associated steatohepatitis, patient age and gender, and, of course, the volume of liver to be removed. However, such a review highlights two principal reasons for post-resection-induced morbidity-mortality: An insufficient number of functioning hepatocytes to achieve proper synthesis, excretion and detoxification, and excessive portal blood inflow that leads to sinusoidal dilatation and necrosis. The use of a portal ring to control portal blood inflow has been shown to improve liver regeneration following surgical resection in a pig mode[10]. Post-operative biochemical parameters were improved in pigs with a portal ring but no significant difference was noted regarding the mortality rate, probably because of the small sample size of eight pigs per condition[10]. Further studies are necessary in both animals and humans to clarify the benefits of this approach. To compensate for the loss of liver function, extracorporeal hepatic support devices have been evaluated in patients presenting with acute post-operative liver failure. These devices, such as MARS®, Prometheus®, and SPAD, are albumin-linked hemodialysis systems that improve the biochemical parameters of patients but fail to improve survival rates[33]. A study combining the use of both liver support and portal ring devices needs to be envisaged in the future so as to determine the effects on perioperative outcomes and long-term survival. However, the results reported at present show that improvements of liver function and portal flow were insufficient to improve survival following major liver resection, suggesting that a different and underestimated mechanism is also responsible for post-resection lethal failure.
This assumption is in line with findings of different studies, including those of Lehmann et al[18], who showed an impairment of the regenerative capacity of the small remnant liver linked to a p21-dependent cell cycle block in a mouse model[18]. Inhibiting p21 in transgenic animals partially restored the regenerative capacity of the liver and improved the survival rate[18], and a treatment with a senescence-inhibiting drug improved liver regeneration after partial hepatectomy by disrupting aberrantly prolonged p21 expression in mice[34].
To investigate further the contribution of CKI in the failed liver regeneration, we examined the earliest events to occur in response to experimentally hepatic insufficiency induced by 90% hepatectomy in the rat. We showed that the delayed liver regeneration of the small remnant liver is associated with altered expression of p27 and p21, being detected as early as 3 h and 12 h post-operatively, respectively. The priming of quiescent hepatocytes occurred correctly, as depicted by STAT3 activation coincident with Rb phosphorylation as early as 3 h post-resection, reflecting entry into the cell cycle. But extended hepatectomy resulted in significant delay in S-phase progression and mitosis, which was compensated in surviving animals by increased deoxyribonucleic acid synthesis at later time points, eventually leading to restored liver mass and functional activity. Our results therefore highlighted the critical importance of the cyclin/cyclin-dependent kinase inhibitors of the Cip/Kip family in regulating the liver regeneration timeline following 90% hepatectomy. To this is added a large number of molecular signals that were switched on or off to guarantee a timely hepatocyte entry and progression into the cell cycle[35,36]. However, the choice of the experimental conditions (hepatectomies ranging from 80% to 95% of total liver weight, glucose supplementation, species-specific features, housing conditions, and diet) affects many of these signaling pathways, accounting for the noticeable differences between the studies.
In our model, peak mortality after eHx was reached within the first 48 h and accounted for 71% of all deaths. Among these, 29% occurred within less than 24 h, and these deaths could not be attributed to cell cycle changes as an increase in liver mass was first detected after 24 h in the control group. Although histological analysis of the liver tissues from dead animals did not reveal any massive liver injuries, hepatocytes displayed a clear cytoplasm with numerous accumulations of globular red hyaline material, testifying to an impairment of liver function that involved the protein excretion process. Our findings were also in accordance with conjugated bilirubin levels that remained elevated in the eHx group (even after 7 d) while ALT and AST returned to normal levels after 96 h.
In conclusion, the loss of hepatocyte functional activities and a hindrance to the regenerative capacities of the remnant lobe both contribute to mortality following major liver resection. The use of extracorporeal support devices along with inhibitors of p21 and p27 now needs to be evaluated in terms of managing liver failure after extended hepatectomy. This combination may facilitate access to curative surgical treatments for primary or secondary cancer for patients who are not eligible according to current standards.
Liver reduction is routinely performed as curative treatment of primary liver cancer and liver metastases, but its use remains limited as liver regeneration requires a minimum of 30% functional parenchyma.
As such, less than 30% of patients with hepatocellular carcinoma are eligible for surgery, and this is connected to the underlying chronic inflammation and the preoperative chemotherapies. Post-surgery accumulation of liver injuries, excessive portal blood inflow, and oxidative stress are the main causal factors suspected to give rise to liver failure, but the molecular mechanisms that block liver regeneration remain unclear.
Our objective was to monitor, step by step, the molecular events in relation to liver regeneration after extended liver resection and so to clearly delineate the blocking points that prevent liver regeneration.
Post-operative liver failure was modelled in the rat by 90% liver resection. Animals undergoing simple laparotomy and 70% hepatectomy were used as control. All animals received glucose infusion to avoid post-operative hypoglycemia. Animals were sacrificed every 3 h for the first 24 h and every 24 h for the following 7 d. Blood and liver samples were collected at the time of sacrifice and used to investigate liver function, morphology, and regeneration by biochemical methods.
Twenty-nine percent of all deaths occurred in the first 24 h in link with massive liver injuries and impaired liver function. For all other deaths, the temporal sequence of events that prime liver regeneration after 90% liver resection occurred properly, but S phase progression and mitosis were delayed by 24 h in conjunction with the rise in p27 (Kip1) and p21 (Waf1/Cip1) cell cycle inhibitor levels.
The cyclin/cyclin-dependent kinase inhibitors of the Cip/Kip family are critical regulators of the liver regeneration following extended hepatectomy.
The use of extracorporeal support devices along with inhibitors of p21 and p27 should be evaluated to manage liver failure after extended hepatectomy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fatkhudinov TK S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Gallinger S, Biagi JJ, Fletcher GG, Nhan C, Ruo L, McLeod RS. Liver resection for colorectal cancer metastases. Curr Oncol. 2013;20:e255-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 3. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Eberhardt C, Wurnig MC, Wirsching A, Rossi C, Feldmane I, Lesurtel M, Boss A. Prediction of small for size syndrome after extended hepatectomy: Tissue characterization by relaxometry, diffusion weighted magnetic resonance imaging and magnetization transfer. PLoS One. 2018;13:e0192847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 6. | Vibert E, Pittau G, Gelli M, Cunha AS, Jamot L, Faivre J, Castro Benitez C, Castaing D, Adam R. Actual incidence and long-term consequences of posthepatectomy liver failure after hepatectomy for colorectal liver metastases. Surgery. 2014;155:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Shimamura T, Taniguchi M, Jin MB, Suzuki T, Matsushita M, Furukawa H, Todo S. Excessive portal venous inflow as a cause of allograft dysfunction in small-for-size living donor liver transplantation. Transplant Proc. 2001;33:1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Vasavada BB, Chen CL, Zakaria M. Portal flow is the main predictor of early graft dysfunction regardless of the GRWR status in living donor liver transplantation - a retrospective analysis of 134 patients. Int J Surg. 2014;12:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M, Voet D, Mortier E, Hesse UJ, de Hemptinne B. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Bucur PO, Bekheit M, Audebert C, Othman A, Hammad S, Sebagh M, Allard MA, Decante B, Friebel A, Miquelestorena-Standley E, Drasdo D, Hengstler JG, Vignon-Clementel IE, Vibert E. Modulating Portal Hemodynamics With Vascular Ring Allows Efficient Regeneration After Partial Hepatectomy in a Porcine Model. Ann Surg. 2018;268:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Athanasiou A, Papalois A, Kontos M, Griniatsos J, Liakopoulos D, Spartalis E, Agrogiannis G, Liakakos T, Pikoulis E. The beneficial role of simultaneous splenectomy after extended hepatectomy: experimental study in pigs. J Surg Res. 2017;208:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 13. | Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Moniaux N, Song H, Darnaud M, Garbin K, Gigou M, Mitchell C, Samuel D, Jamot L, Amouyal P, Amouyal G, Bréchot C, Faivre J. Human hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein cures fas-induced acute liver failure in mice by attenuating free-radical damage in injured livers. Hepatology. 2011;53:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Moniaux N, Darnaud M, Garbin K, Dos Santos A, Guettier C, Samuel D, Amouyal G, Amouyal P, Bréchot C, Faivre J. The Reg3α (HIP/PAP) Lectin Suppresses Extracellular Oxidative Stress in a Murine Model of Acute Liver Failure. PLoS One. 2015;10:e0125584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Senoner T, Schindler S, Stättner S, Öfner D, Troppmair J, Primavesi F. Associations of Oxidative Stress and Postoperative Outcome in Liver Surgery with an Outlook to Future Potential Therapeutic Options. Oxid Med Cell Longev. 2019;2019:3950818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Sikalias N, Karatzas T, Alexiou K, Mountzalia L, Demonakou M, Kostakis ID, Zacharioudaki A, Papalois A, Kouraklis G. Intermittent Ischemic Preconditioning Protects Against Hepatic Ischemia-Reperfusion Injury and Extensive Hepatectomy in Steatotic Rat Liver. J Invest Surg. 2018;31:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Lehmann K, Tschuor C, Rickenbacher A, Jang JH, Oberkofler CE, Tschopp O, Schultze SM, Raptis DA, Weber A, Graf R, Humar B, Clavien PA. Liver failure after extended hepatectomy in mice is mediated by a p21-dependent barrier to liver regeneration. Gastroenterology 2012; 143: 1609-1619. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Albrecht JH, Poon RY, Ahonen CL, Rieland BM, Deng C, Crary GS. Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene. 1998;16:2141-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M, Hui L, Wagner EF. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev. 2006;20:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci USA. 2010;107:5845-5850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Weymann A, Hartman E, Gazit V, Wang C, Glauber M, Turmelle Y, Rudnick DA. p21 is required for dextrose-mediated inhibition of mouse liver regeneration. Hepatology. 2009;50:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 24. | Amptoulach S, Gross G, Kalaitzakis E. Differential impact of obesity and diabetes mellitus on survival after liver resection for colorectal cancer metastases. J Surg Res. 2015;199:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Li Q, Wang Y, Ma T, Lv Y, Wu R. Clinical outcomes of patients with and without diabetes mellitus after hepatectomy: A systematic review and meta-analysis. PLoS One. 2017;12:e0171129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Schmoldt A, Benthe HF, Haberland G. Digitoxin metabolism by rat liver microsomes. Biochem Pharmacol. 1975;24:1639-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JN, Conrad C, Hugh TJ, Garden OJ, Fan ST, Crawford M, Makuuchi M, Yokoyama Y, Büchler M, Padbury R. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford). 2018;20:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Ozawa Y, Tamura T, Owada Y, Shimizu Y, Kemmochi A, Hisakura K, Matsuzaka T, Shimano H, Isoda H, Ohkohchi N. Evaluation of safety for hepatectomy in a novel mouse model with nonalcoholic-steatohepatitis. World J Gastroenterol. 2018;24:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Bachellier P, Rosso E, Pessaux P, Oussoultzoglou E, Nobili C, Panaro F, Jaeck D. Risk factors for liver failure and mortality after hepatectomy associated with portal vein resection. Ann Surg. 2011;253:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Truant S, El Amrani M, Skrzypczyk C, Boleslawski E, Sergent G, Hebbar M, Dharancy S, Pruvot FR. Factors associated with fatal liver failure after extended hepatectomy. HPB (Oxford). 2017;19:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Du ZG, Wei YG, Chen KF, Li B. An accurate predictor of liver failure and death after hepatectomy: a single institution's experience with 478 consecutive cases. World J Gastroenterol. 2014;20:274-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Gilg S, Sparrelid E, Saraste L, Nowak G, Wahlin S, Strömberg C, Lundell L, Isaksson B. The molecular adsorbent recirculating system in posthepatectomy liver failure: Results from a prospective phase I study. Hepatol Commun. 2018;2:445-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Ritschka B, Knauer-Meyer T, Gonçalves DS, Mas A, Plassat JL, Durik M, Jacobs H, Pedone E, Di Vicino U, Cosma MP, Keyes WM. The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev. 2020;34:489-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 35. | Elchaninov A, Fatkhudinov T, Makarov A, Vorobieva I, Lokhonina A, Usman N, Kananykhina E, Vishnyakova P, Nikitina M, Goldshtein D, Bolshakova G, Glinkina V, Sukhikh G. Inherent control of hepatocyte proliferation after subtotal liver resection. Cell Biol Int. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Elchaninov AV, Fatkhudinov TK, Usman NY, Kananykhina EY, Arutyunyan IV, Makarov AV, Lokhonina AV, Eremina IZ, Surovtsev VV, Goldshtein DV, Bolshakova GB, Glinkina VV, Sukhikh GT. Dynamics of macrophage populations of the liver after subtotal hepatectomy in rats. BMC Immunol. 2018;19:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |