Published online Mar 27, 2019. doi: 10.4254/wjh.v11.i3.330
Peer-review started: January 14, 2019
First decision: February 22, 2019
Revised: February 27, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: March 27, 2019
Processing time: 75 Days and 1 Hours
Serum levels of cartilage oligomeric matrix protein (COMP) has been presented as a biomarker of liver fibrosis in several cross-sectional studies. COMP is also an essential mediator in carcinoma development and has also been associated with hepatocellular carcinoma. We present a prospective analysis of this biomarker in 38 patients with chronic hepatitis C who were subject to eradication therapy with direct acting antivirals. We confirm previous studies associating COMP elevation with liver cirrhosis. We also show how viral levels are correlated with COMP at baseline. In our prospective analysis, we report that successful eradication of hepatitis C results in improvement in liver stiffness and laboratory liver function tests at 1 year follow-up. In contrast, median COMP-levels remain unchanged during the study period. We conclude that the biomarker potential of COMP in the prospective evaluation of liver diseases, remains to be elucidated.
Core tip: Cartilage oligomeric matrix protein (COMP) is a biomarker of fibrosis that has recently been introduced in the field of hepatology. COMP has also been associated with tumor development. This is the first prospective study of COMP in chronic liver disease. We confirm previous findings, relating S-COMP to liver cirrhosis. Eradication of hepatitis C was associated with improvements of liver function test and liver elasticity. In contrast, and unexpectedly, S-COMP remained unchanged in this cohort. We argue that the biomarker potential of COMP in chronic liver diseases needs further exploration, especially in reference to carcinoma development.
- Citation: Andréasson K, Jönsson G, Hesselstrand R, Norrgren H. Persistent elevation of fibrosis biomarker cartilage oligomeric matrix protein following hepatitis C virus eradication. World J Hepatol 2019; 11(3): 330-334
- URL: https://www.wjgnet.com/1948-5182/full/v11/i3/330.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i3.330
Chronic hepatitis C (CHC) is a widespread disease caused by infection with hepatitis C virus (HCV). It is associated with significant mortality and morbidity due to liver cirrhosis, liver decompensation and hepatocellular carcinoma (HCC). During the last decade, direct acting antiviral agents (DAA) have been introduced for the eradication of HCV infection. These treatments are usually successful in eradication of the virus, resulting in sustained virologic response (SVR) in over 90% of the cases[1]. SVR have been associated with improvements in liver stiffness and biomarkers of CHC, including fibrosis specific biomarkers such as hyaloronic acid and type IV collagen[2], suggesting reversal of CHC induced inflammation and fibrosis.
Cartilage oligomeric matrix protein (COMP) is a pentameric molecule first identified in cartilage and later characterised in fibrotized tissues in tendons, skin and lung. It is readily measurable in serum with a commercially available ELISA. Increased levels have been associated with arthritis and fibrotic states including systemic sclerosis, wound healing, and Crohn’s disease[3,4]. COMP has also been associated with malignancies including breast, prostate and HCC[5-7]. In World Journal of Hepatology, in 2015, Norman et al[5] published the first report suggesting that S-COMP has biomarker potential in chronic liver diseases. S-COMP was associated with both cirrhosis and HCC development. They concluded that “The present exploratory study has provided intriguing results and may assist enhanced management of hepatic fibrosis, in particular the assessment of regression or progression of fibrosis before and after specific therapeutic treatments”. In independent cross-sectional studies, we and others have confirmed that S-COMP is associated with fibrosis stage based on liver biopsy and liver elastography in CHC[8-10]. These findings have been corroborated by experimental studies of liver fibrosis in which COMP originating from hepatocytes was essential for fibrosis development[11].
In order to further explore the biomarker potential of S-COMP in liver fibrosis, we have conducted the first prospective study aimed to investigate the biomarker potential of S-COMP following successful therapy of CHC with DAA.
Consecutive patients with CHC infection eligible for antiviral therapy between August 2015 and April 2017 at the Infectious Clinic, Skåne University Hospital Lund, were included in this study. Patients with concomitant rheumatic disease, organ transplant recipients and patients with documented alcohol abuse were excluded.
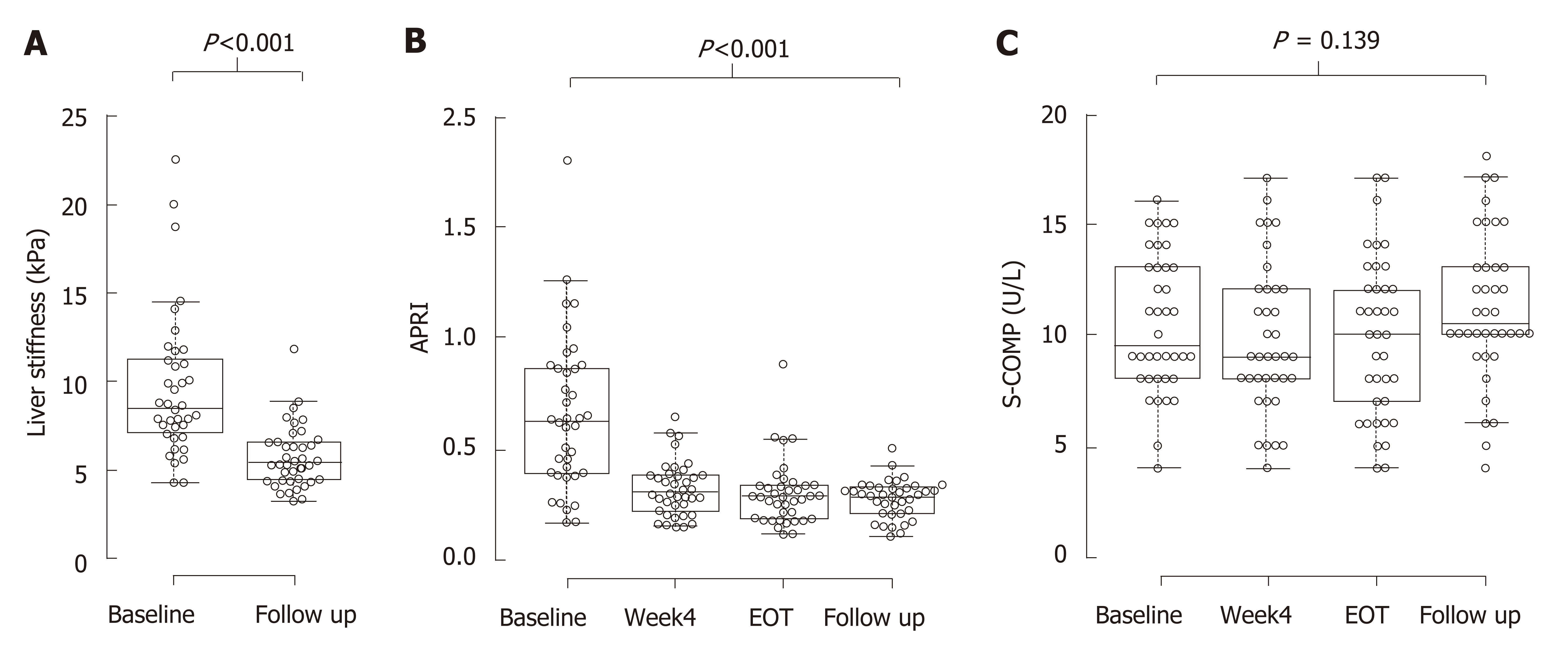
Serum levels of COMP were measured with ELISA (Anamar, United Kingdom), together with aspartate aminotransferase (AST) and platelet levels at baseline, after 4 weeks of treatment, at end of treatment and 1-2 year post-treatment follow-up. Viral genotype and HCV-RNA levels before treatment start were noted. Liver stiffness was measured by transient elastography (TE) at baseline and at 1-2 years post-treatment follow-up. Liver cirrhosis was defined as TE > 12.5 kPa. Non-parametric statistics were consequently used including Wilcoxon signed rank test when comparing repeated measurements within the cohort, and median [interquartile range (IQR)] for descriptive measures. Figures were made with BoxPlotR[12]. The study was approved by the Regional Ethics Board Lund (2017-471) and conducted in accordance with the declaration of Helsinki. All study participants gave informed consent.
This study comprised 38 subjects with CHC who were infected with the following viral subtypes; subtype 1 (n = 24), subtype 2 (n = 3), subtype 3 (n = 11). All received DAA including sofubusvir (n = 37), ledipasvir (n = 25), ribavirin (n = 7), daclatasvir (n = 5), velpatasvir (n = 1), elbasvir (n = 1) and grazoprevir (n = 1). Baseline median (IQR) age, viral levels and liver stiffness were 58 (43–63) years, 2.0 x 106 (9.7 x 105-4.7 x 106) and 8.3 (6.9–11.1) kPa, respectively.
Six patients had a TE measurement indicating liver cirrhosis at baseline. These subjects had higher AST to platelet ratio index (APRI)-scores and S-COMP levels compared to the other patients (0.91 vs 0.55; P = 0.008 and 13 vs 9 U/L; P = 0.036, respectively). Median (IQR) treatment duration was 84 (60-95) days, and all study participants reached SVR. TE was performed at baseline and at follow up, 517 (468-639) days later. Liver stiffness decreased significantly during this period (from 8.3 (6.9–11.1) to 5.4 (4.4–6.6) kPa; P < 0.001; Figure 1A). Also, APRI decreased significantly, from 0.62 to 0.29; P < 0.001, Figure 1B. In contrast, S-COMP levels did not decrease from baseline 9.5 (8.0–13) versus 10.5 (9.8-13) U/L at follow up; P = 0.14. Similar levels of S-COMP were measured at 4 wk of treatment 9.0 (8.0–12) and at end of treatment 10 (6.8–12) U/L (Figure 1C). Furthermore, change in S-COMP over time was not associated with any disease characteristics (data not shown).
There was a significant relationship between baseline S-COMP and viral levels (rs=0.45, P = 0.005) at baseline. In contrast, there was no association between viral levels and liver stiffness or APRI at baseline. Baseline S-COMP was not associated with virus subtype, body mass index, and it was unable to predict change in liver stiffness (data not shown).
One patient developed HCC prior to the inclusion in this study. This patient had an estimated liver elasticity of 6.1 and liver function tests within reference range. This patient exhibited the highest (21 U/L) level of S-COMP in this study.
As previously described in this journal, we show that S-COMP is associated with liver cirrhosis. We also show that S-COMP correlates to viral levels in serum, an established risk factor for HCC development, which to our knowledge have not previously been described. In this prospective study, we also report how DAA induced SVR is associated with improvements in liver function as measured by TE and APRI, which is in agreement with previous studies. In contrast however, S-COMP remained unchanged during the study period.
Magdaleno et al[11] have in detail explored the significance of COMP in the hepatocyte pathology of liver fibrosis, ultimately suggesting that COMP is a mediator of inflammation and fibrosis originating from the hepatocytes. In other diseases, S-COMP has been suggested to reflect a mixture of both synthesis and degradation of extracellular matrix[3,4]. In malignant and premalignant diseases, COMP expression has been associated with tumor invasiveness. We suggest that the origin of elevated S-COMP in CHC to be multifactorial. The results from this study indicate that the biomarker potential of COMP in the longitudinal follow up of chronic liver diseases is yet to be determined. Further studies encompassing a larger number of patients are needed. In such studies, S-COMP should ideally be compared with other fibrotic biomarkers such as hyaluronic acid. Compared to currently available biomarkers of CHC, COMP correlates poorly with other surrogates of disease severity following DAA induced SVR. This finding does however not invalidate COMP’s potential as a biomarker for HCC, as previously suggested in this journal[7].
In conclusion, our study indicates that this biomarker’s dynamics in the context of CHC and possibly also other chronic liver diseases, must be further explored if S-COMP is to be used in a prospective clinical setting[13]. The role of S-COMP in relation to chronic liver disease and the risk of HCC development needs further investigation.
We appreciate the help of head nurse Ann Åkesson in organizing this study. We appreciate advice from professor Tore Saxne when planning and conducting this study. We appreciate the collaboration with Martin Olsson on conducting the COMP-analyses.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Mizuguchi T, Zampino R, Zhu Y S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Vermehren J, Park JS, Jacobson IM, Zeuzem S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J Hepatol. 2018;69:1178-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Miyaki E, Imamura M, Hiraga N, Murakami E, Kawaoka T, Tsuge M, Hiramatsu A, Kawakami Y, Aikata H, Hayes CN, Chayama K. Daclatasvir and asunaprevir treatment improves liver function parameters and reduces liver fibrosis markers in chronic hepatitis C patients. Hepatol Res. 2016;46:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Crnkic M, Månsson B, Larsson L, Geborek P, Heinegård D, Saxne T. Serum cartilage oligomeric matrix protein (COMP) decreases in rheumatoid arthritis patients treated with infliximab or etanercept. Arthritis Res Ther. 2003;5:R181-R185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Li M, Lu S, Liu X, Zhao J, Zhang H, Ling C. [Expression of endoglin in human non-small cell lung cancer and its clinical significance]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Norman GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos GN. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol. 2015;7:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Xiao Y, Kleeff J, Guo J, Gazdhar A, Liao Q, Di Cesare PE, Büchler MW, Friess H. Cartilage oligomeric matrix protein expression in hepatocellular carcinoma and the cirrhotic liver. J Gastroenterol Hepatol. 2004;19:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Englund E, Bartoschek M, Reitsma B, Jacobsson L, Escudero-Esparza A, Orimo A, Leandersson K, Hagerling C, Aspberg A, Storm P, Okroj M, Mulder H, Jirström K, Pietras K, Blom AM. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene. 2016;35:5585-5596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Zachou K, Gabeta S, Shums Z, Gatselis NK, Koukoulis GK, Norman GL, Dalekos GN. COMP serum levels: A new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. Eur J Intern Med. 2017;38:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Andréasson K, Hesselstrand R, Saxne T, Holmberg A, Norrgren H, Jönsson G. Cartilage oligomeric matrix protein: a new promising biomarker of liver fibrosis in chronic hepatitis C. Infect Dis (Lond). 2015;47:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Andréasson K, Waldenström J, Westin J, Norrgren H, Jönsson G, Nyström K, Lagging M. Cartilage oligomeric matrix protein associates with hepatic inflammation and fibrosis in hepatitis C virus infection. J Hepatol. 2017;67:649-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Magdaleno F, Arriazu E, Ruiz de Galarreta M, Chen Y, Ge X, Conde de la Rosa L, Nieto N. Cartilage oligomeric matrix protein participates in the pathogenesis of liver fibrosis. J Hepatol. 2016;65:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Spitzer M, Wildenhain J, Rappsilber J, Tyers M. BoxPlotR: a web tool for generation of box plots. Nat Methods. 2014;11:121-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 552] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 13. | Huang R, Xia J, Yan X, Liu Y, Wu C. Serum cartilage oligomeric matrix protein levels as a novel non-invasive biomarker for liver fibrosis in patients with chronic viral hepatitis? It is too early. Eur J Intern Med. 2017;42:e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |