Published online Feb 27, 2018. doi: 10.4254/wjh.v10.i2.297
Peer-review started: November 25, 2017
First decision: December 18, 2017
Revised: January 13, 2018
Accepted: January 23, 2018
Article in press: January 24, 2018
Published online: February 27, 2018
Processing time: 99 Days and 19.2 Hours
To examine the association between weekend alcohol consumption and the biochemical and histological alterations at two different concentrations of alcohol in both genders in rats.
Wistar rats weighing 170-200 g were divided into groups as follows: (1) Control groups; and (2) weekend alcohol-consumption group: 2 d/weekly per 12 wk, at two different concentrations: (1) Group of males or females with a consumption of a solution of alcohol at 40%; and (2) group of males or females with a consumption of a solution of alcohol at 5%. At the end of the experiment, serum and liver samples were obtained. The following enzymes and metabolites were determined in serum: Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Lactate Dehydrogenase, and Gamma-Glutamyltransferase, and glucose, triglycerides, cholesterol, bilirubin, and albumin. Liver samples from each group were employed to analyze morphological abnormalities by light microscopy.
In all of the weekend alcohol-consumption groups, AST activity presented a significant, 10-fold rise. Regarding ALT activity, the groups with weekend alcohol consumption presented a significant increase that was six times greater. Bilirubin levels increased significantly in both groups of females. We observed a significant increase in the parameters of fatty change and inflammation due to weekend alcohol consumption. Only the group of females that consumed alcohol at 40% presented slight hepatocellular disorganization
The results obtained herein provide solid evidence that weekend alcohol consumption gives rise to liver damage, demonstrated by biochemical and histological alterations, first manifested acutely, and prolonged weekend alcohol consumption can cause greater, irreversible damage.
Core tip: At present, it is considered that the main weekend alcohol consumers comprises the young population, due to the gratifying effect of alcohol, this being a very important social and health problem. Our findings demonstrate an effect of the damage that is caused by weekend alcohol consumption, regardless of gender or the concentration of alcohol. Even more so, greater damage can be observed in females, and the metabolism of ethanol probably participates, specifically due to its first-pass metabolism, which is carried out in the stomach.
- Citation: Morales-González JA, Sernas-Morales ML, Morales-González Á, González-López LL, Madrigal-Santillán EO, Vargas-Mendoza N, Fregoso-Aguilar TA, Anguiano-Robledo L, Madrigal-Bujaidar E, Álvarez-González I, Chamorro-Cevallos G. Morphological and biochemical effects of weekend alcohol consumption in rats: Role of concentration and gender. World J Hepatol 2018; 10(2): 297-307
- URL: https://www.wjgnet.com/1948-5182/full/v10/i2/297.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i2.297
Various reports have demonstrated the adverse effects to health caused by the consumption of alcohol[1-3]. Similarly, it is well known that in Mexico, the main alcoholic beverages consumed are beer and distilled beverages (brandy, tequila, rum, whisky, cognac, vodka, etc.)[3], which contain approximately 5% and 36%-40% of alcohol, respectively[1]. It has been reported that alcohol consumption presents various patterns that are associated with gender, age, socioeconomic situation, consumption type (regular drinkers, intense or weekend drinkers), and, the alcoholic-beverage type (wine, mixed drinks)[4]. On the other hand, according to what has been reported in ENCODAT 2016, young people exhibit the tendency toward alcohol consumption in total population in Mexico (12-65 years of age), with daily alcohol consumption at 2.9% and habitual consumption (weekend) at 8.5%. Likewise, it was found that, although males consume more alcohol, women present an important index of alcohol consumption[3].
Weekend alcohol consumption for young people is becoming an important social and familial problem, but also a considerable health problem[5], and it can be due to the increase of Allopregnanolone (the testosterone metabolite that participates in the gratifying effect of alcohol)[6]. Various reports have associated the harmful effect to health engendered by weekend alcohol consumption, such as the following: reports on and the increase in deaths during Fridays, Saturdays, and Sundays[7]; the greater neurocognitive and neurobehavioral deterioration, which is similar in many aspects to that observed in chronic alcohol drinkers[5]; deaths caused by ischemic heart disease[8,9], or the idiopathic arrhythmias that initiate during weekends, a risk factor for producing visual alterations (dyschromatopsia)[10].
On the other hand, some biochemical alterations have been reported as being caused by weekend alcohol consumption. Stranges et al[11] reported that there are modifications at the level of the enzymes released by the liver into the blood Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Gamma-Glutamyltransferase (GGT), and these authors concluded that these modifications were caused by alcohol consumption, finding that the highest elevated enzyme is GGT, identifying differences by gender: In males, daily alcohol drinkers exhibited highest GGT levels, while in females, highest GGT was observed in weekend alcohol drinkers. The authors concluded that, in addition to the amount of alcohol consumed, the pattern of consumption can affect liver function and that there are gender differences with respect to liver function and possible damage to this organ[11]. Hojnacki et al[12] compared the effect of the daily alcohol-consumption pattern (moderate, 12%) and weekend alcohol consumption (concentrated, 20%) utilizing squirrel monkeys. These authors found that moderate-daily alcohol consumption causes a moderate diminution in Body Weight (BW) and produces increases in the profile of coronary protective lipoproteins [HDL2/HDL3 cholesterol increases and low density lipoprotein (LDL) cholesterol decreases]; in contrast, in concentrated alcohol consumption, unfavorable alterations are produced in the lipoproteins (LDL cholesterol increases and Apolipoprotein B increases), together with weight loss and body fat depletion[12]. Rocha et al[13], on employing a weekend alcohol-consumption model in rat (alcohol in the drinking fountain at 30% during 3 d/wk per 70 d), reported an average alcohol consumption of 4.56 g per day, which gave rise to a dyslipedemic profile, an increase in energy expenditure, and hepatic metabolic changes similar to those associated with chronic ethanol consumption[13]. Finally, Liu et al[14] compared the effect of the damage that two alcohol-consumption patterns cause: Moderate-daily consumption 0.8 g/kg/7 d/wk vs weekend alcohol consumption 2.8 g/kg/2 d/wk, with a weekly consumption in both groups of 5.6 g/kg. The authors found an increase in the levels of total cholesterol and of HDL-C in both groups; on the other hand, a diminution was observed in the LDL-C levels of the moderate-daily consumption pattern, the latter significantly raised in weekend drinkers. Blood concentrations of alcohol as well as BW gain were greater in weekend alcohol consumers. These findings of weekend alcohol consumption (4 wk) favored the development of atherosclerotic plaque (increase in the plaque, diminution in the lux lumen, etc.), while precisely the contrary took place with moderate-daily alcohol consumption[14]. Some reports describe the histologic damage that alcohol causes to the liver[15-17] but, to our knowledge, there are no reports on the histologic changes caused by weekend alcohol consumption to the liver.
There are numerous studies on the effects of chronic weekend alcohol consumption; however, few reports exist, to our knowledge, on the association between weekend alcohol consumption and the damage produced in the liver. The objective of this study was to examine the association between weekend alcohol consumption and the biochemical and histological alterations at two different concentrations of alcohol in both genders in Wistar rats.
All chemical reagents were obtained from Merck (Merck de México, S.A.) and were of the best quality available.
We utilized male Wistar rats with an initial Body Weight (BW) of 170-200 g that were obtained from the Escuela Superior de Medicina (ESM) Bioterium of the Instituto Politécnico Nacional (IPN) in Mexico City. The rats were housed in cages at the ESM Bioterium. They were maintained at a temperature of 22 °C with 12-h/12-h light-dark cycles and received standard rat-pellet food (Purina de México, S.A.) and water ad libitum prior to the treatments. After 14 d of adaptation, the procedure was initiated. The protocol and the experimental procedures were conducted according to the Mexican Official Norm for the Use and Care of Laboratory Animals (NOM-062-ZOO-1999, México)[18]. The protocol was authorized by the Comité Interno del Cuidado y Uso de los Animales de Laboratorio (CICUAL), with registry number ESM.CICUAL-12/23-06-2017, and by the Research Committee with registry number ESM.CI-01/13-06-2017, both committees of the ESM of the IPN.
The animals were randomly divided into six groups in the following manner: (1) Control groups (males and females); and (2) weekend alcohol-consumption group: 2 d/weekly per 12 wk at two different concentrations: (1) group of males with consumption of a solution of alcohol at 40%; (2) Group of females with consumption of a solution of alcohol at 40%; (3) group of males with a consumption of a solution of alcohol at 5%; and (4) group of females with a consumption of a solution of alcohol at 5%. The control groups (females and males) had access to food and water ad libitum at all times. Daily alcohol consumption was quantified, reported in g/kg of weight/day, and BW gain weekly was reported in g.
At the end of the experiment, the animals were sacrificed by decapitation after being previously anesthetized with pentobarbital sodium (at an overdose of 40 mg/kg BW). Blood samples were obtained and centrifuged in a clinical centrifuge to obtain the sera, which were frozen at -70 °C for later use.
Hepatic samples from each group Sere employed for light microscopy. Samples were fixed with formaldehyde (10% in isotonic solution), embedded in paraffin, and stained with Hematoxylin and Eosin. Biopsy specimens were coded and read blindly without knowledge of the other data by independent observers at two different laboratories (MLS-M and JB-R). The criteria utilized to analyze the morphological abnormalities were the same as those reported by Morales-González et al[15] as follows: Fatty infiltration (+, mild; ++, moderate; +++, severe, and ++++, very severe); inflammation (+, zonal localization, focal inflammatory cells; ++, moderate, not restricted to any one zone of the acinus, and +++, diffuse), and hepatocellular disorganization (+, isolated foci in zone 3 of the liver acinus; ++, more widespread, and +++, definitively diffused in the hepatic acini); apoptosis (+, mild; ++ moderate; +++ severe, and ++++ very severe), and mitosis(+ mild; ++ moderate; +++ severe, and ++++ very severe).
The activities of serum Alanine Aminotransferase [ALT; Expansion Coefficient (EC) 2.6.1.2], Aspartate Aminotransferase (AST, EC 2.6.1.1), Lactate Dehydrogenase (LDH, EC 1.1.1.27), and Gamma-Glutamyltransferase (GGT, EC 2.3.2.2) were measured colorimetrically utilizing diagnostic kits (Spinreact de México, SA de CV), following the manufacturer’s instructions; the results are reported in units/L.
Serum concentrations of glucose, triglycerides, cholesterol, bilirubin, and albumin were determined by spectrophotometric techniques using diagnostic kits (Spinreact de México, SA de CV) following the instructions provided by the manufacturer; the results are reported in mg/dL, except for albumin, which is reported in g/dL.
The results were analyzed using the SigmaPlot ver. 12.3 statistical software program. The results are expressed as the mean ± SE of the mean (SEM), as required. We carried out a statistical analysis using Student t test and/or Analysis of Variance (ANOVA) and the Student-Newman-Keuls method as post-hoc evaluation for multiple comparisons. We considered differences among the groups to be statistically significant when P < 0.05.
Average alcohol consumption per group was as follows: In females, at 5% of 0.83 g/kg per day; in the male group at 5% of 1.63 g/kg per day, and, in groups of females and males, at 40%, this was 5.52 and 2.26 g/kg per day, respectively (Table 1).
| Group | Initial body weight (g) | Final body weight (g) | Body weight gain (g) (%) | Average alcohol consumption (g/kg/d) |
| Control | 176.50 ± 3.3 | 255.00 ± 23 | 78.5 ± 22 (43) | 0 |
| Females 5% | 171.00 ± 10.57 | 269.00 ± 15.16 | 98.0 ± 20.71a (57) | 0.83 ± 0.04 |
| Females 40% | 172.92 ± 4.61 | 294.25 ± 13.39 | 121.3 ± 13.91a (70) | 5.52 ± 1.85 |
| Males 5% | 198.00 ± 3.21 | 412.00 ± 8.74 | 214.0 ± 11.53a (108) | 1.63 ± 0.01 |
| Males 40% | 172.80 ± 9.17 | 300.00 ± 85.43 | 127.8 ± 41.29a (73) | 2.26 ± 0.61 |
All of the weekend alcohol-consumption groups presented a significant weight gain in comparison with the control group. The group of females as well as in that of males with 40% alcohol consumption presented an increase in weight gain similar to that of 121.3 and 127.8 g, respectively. Surprisingly, the group of males at 5% exhibited a very important weight gain of 214 g; however, this was not so in the group of females with 5% consumption (98 g) (Table 1).
The effect of weekend alcohol consumption was evaluated by determining the activity of ALT, AST, LDH, and GGT, because these enzymes classically reflect liver function, which is dependent on morphofunctional integrity.
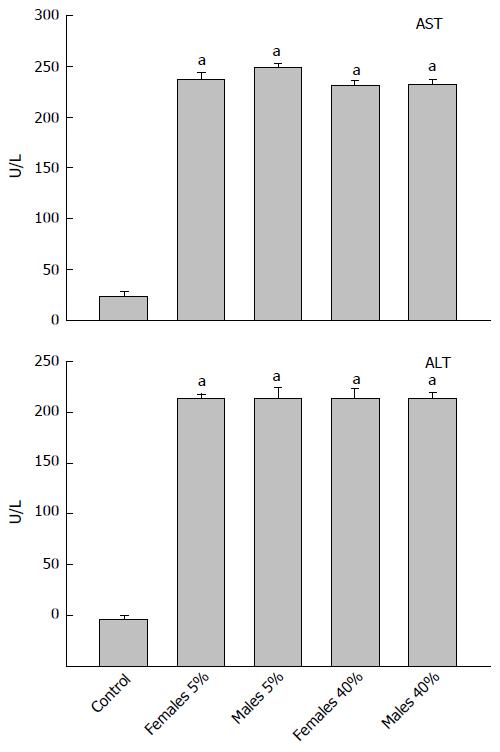
In Figure 1, we observe the AST activity (upper panel) in the diverse experimental groups. In all of the weekend alcohol-consumption groups, AST activity presented a significant rise of 10 times in comparison with the control (23.8 U/L). Regarding ALT activity, the control presented ALT activity of 36.5 U/L, and the remaining groups with weekend alcohol consumption presented a significant increase that was 6-fold greater, independently of the alcohol concentration (Figure 1, lower panel).
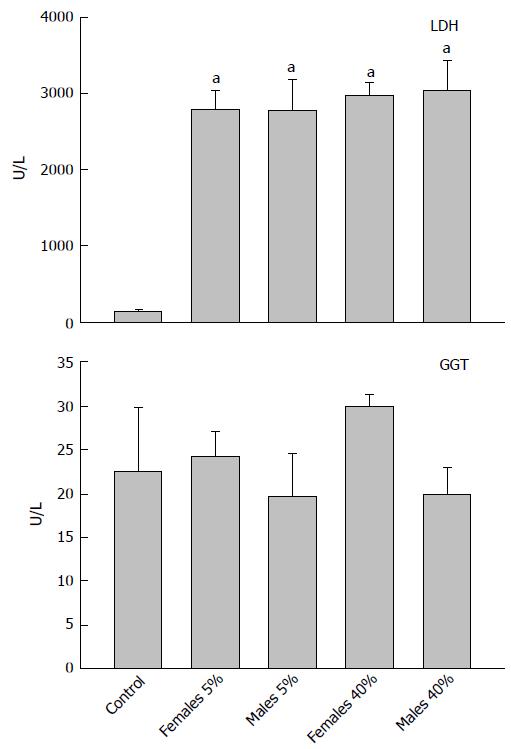
The activity of the LDH enzymes (upper panel) and of the GGT enzymes (lower panel) can be observed in Figure 2. LDH activity in the control group was 147 U/L, noting that weekend alcohol consumption favored an increase in all groups of between 18 and 20 times greater in comparison with the control group, not finding differences among these in terms of the weekend alcohol-consumption groups with regard to of the activity of this (LDH) enzyme. On the other hand, with respect to the activity of the GGT enzyme, differences were not found in any weekend alcohol-consumption group in comparison with the control (22.6 U/L), or among the alcohol-treated groups.
In the weekend alcohol-consumption groups (Table 2), we quantified serum metabolite modifications, which depend on the hepatic metabolism, such as glucose, cholesterol, and triglycerides. Regarding glucose levels, it may be observed in Table 2 that weekend alcohol consumption in all groups favored the increase of the serum levels of this metabolite significantly with a P < 0.05 vs control. The serum concentration of cholesterol in the control group was 58 mg/dL, while in the groups with weekend alcohol consumption, the following was found: in the group of females at 5% (71.83 mg/dL); males at 5% (70.67 mg/dL), and in females at 40% (68.33 mg/dL), this was statistically significant (P < 0.05) in all alcohol-consumption groups vs control. The serum levels of triglycerides were raised due to alcohol consumption (Table 2).
The results of the metabolic integrity of the liver are presented in Table 3. Bilirubin levels increased significantly in both groups of females (0.26 mg/dL), in comparison with the control group (0.15 mg/dL; P < 0.05). Regarding the groups of males with weekend alcohol consumption at 5% and 40%, their bilirubin levels were found to be 0.15 and 0.18 mg/dL, respectively, the latter not significant in comparison with the control group (0.15 mg/dL). Surprisingly, the levels of albumin in serum were not affected in any weekend alcohol-consumption group in comparison with the control group (Table 3).
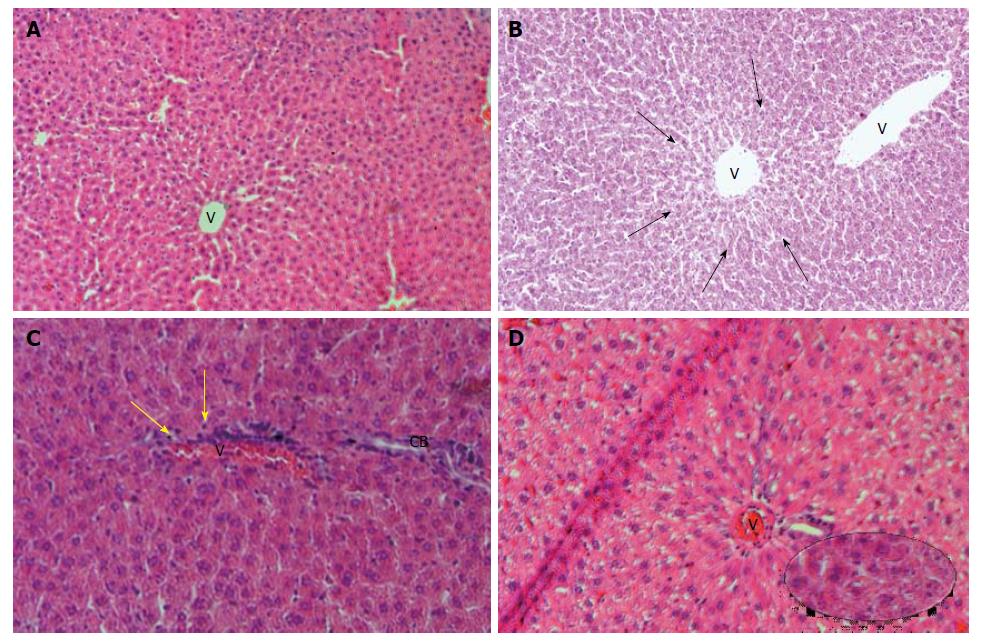
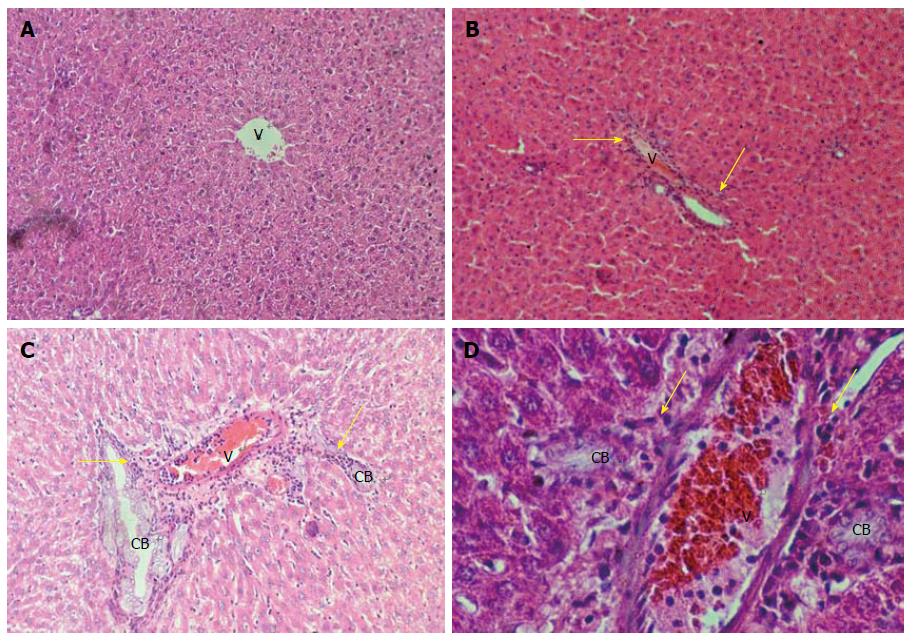
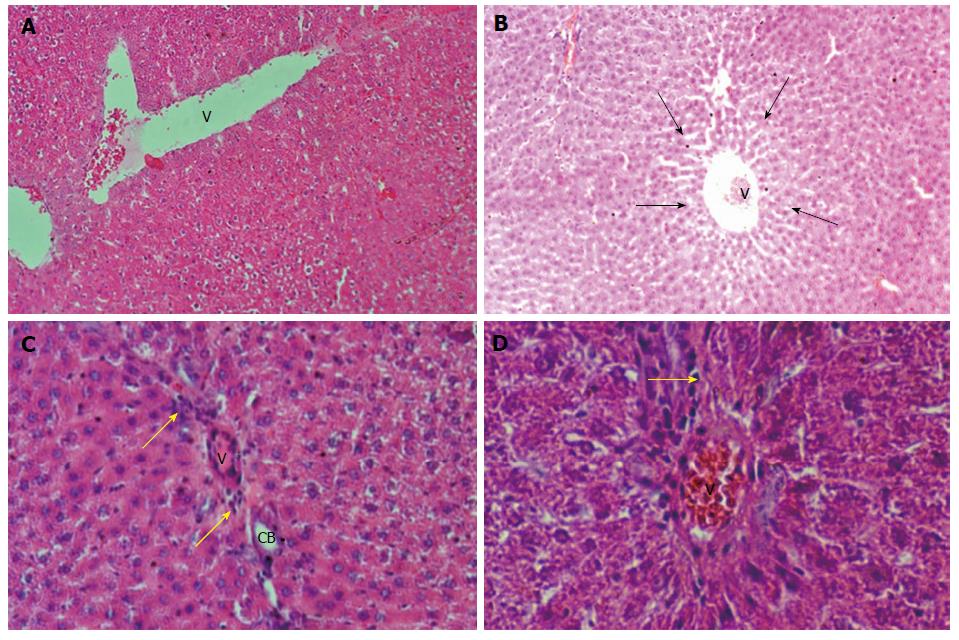
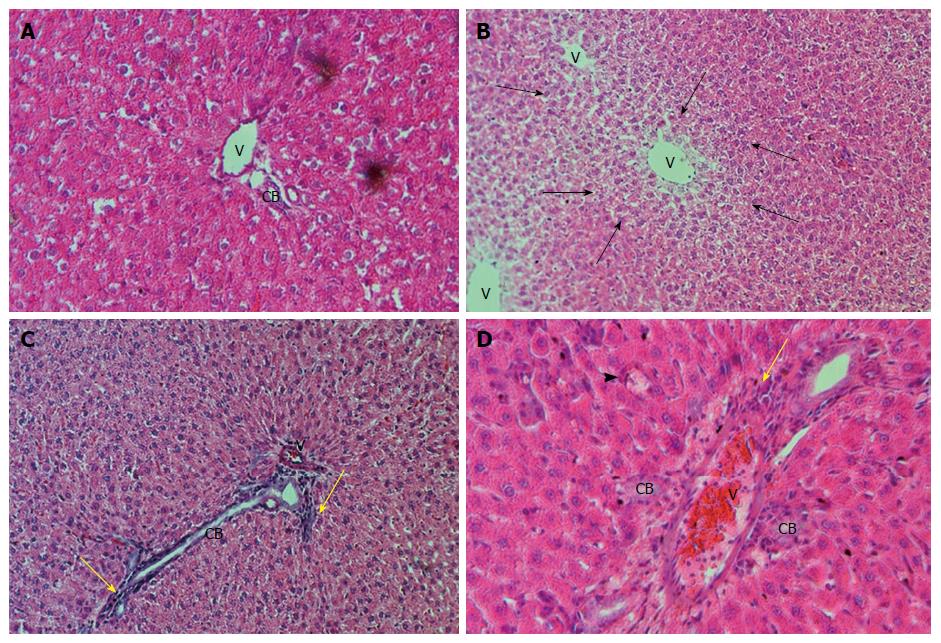
In Table 4, we can observe the effect exerted by weekend alcohol consumption on histological changes. We noted a significant increase in the parameters of fatty change and inflammation due to weekend alcohol consumption compared with the control group (Table 4). In Figure 3, we are able to observe representative images of the female group with alcohol consumption at 5%, where inflammation and steatosis are observed, as well as slight periportal fibrosis. In Figure 4, we may observe slight inflammation, steatosis, and slight periportal fibrosis in the group of males with alcohol consumption at 5%, while the groups of males and females with alcohol consumption at 40% were those with greatest histological changes in terms of the parameters of fatty change and inflammation, respectively (Table 4). Only the group of females that consumed alcohol at 40% presented slight hepatocellular disorganization (Table 4). Figure 5 presents the images of the group of females with alcohol consumption of 40%, where steatosis is observed, and inflammation, although this was more marked in comparison with the group of females with alcohol consumption at 5%. The latter can be noted, because it surpasses the limiting plaque and the periportal fibrosis is more evident (which can be observed as the loss of the amount of the periportal cell, replaced by fibrotic tissue). In Figure 6, moderate inflammation with leukocytes is observed that surpasses the limiting plaque, and also, the degree of periportal fibrosis is very important; likewise, apoptosis was only present in the group of males with alcohol consumption at 40% (Table 4). Last, the necrosis parameter is present in all of the groups that consumed alcohol on weekends, this being greater in the groups that consumed alcohol on weekends at 40% (females and males) (Table 4).
| Group | Fatty change | Inflammation | Hepatocellular disorganization | Necrosis | Apoptosis |
| Control | 0 | 0 | 0 | 0 | 0 |
| Females 5% | +/++ | +/++ | 0 | 0/+ | 0 |
| Males 5% | +/++ | 0/++ | 0 | 0/+ | 0 |
| Females 40% | +/+++ | + | 0/+ | 0/++ | 0 |
| Males 40% | +/++ | +/+++ | 0 | 0/++ | 0/++ |
It is known that chronic alcohol consumption is a risk factor for various diseases, such as diabetes, cancer, gastritis, and gastric ulcers, and that it is especially related to liver damage[1]. In recent years, attention has been focused on weekend alcohol consumption as a cause of cognitive-intellectual alterations[5], a risk factor for homicides[7], cardiovascular diseases such as arrythmias or infarct[8,9], and even of visual alterations[10]. At present, it is considered that main weekend alcohol consumers comprise the young population, due to the gratifying effect of alcohol[6], this being a very important social and health problem.
Rocha et al[13], on utilizing the weekend alcohol consumption model for 10 wk in male rats and an alcohol concentration in the drinking fountain of 30%, reported an average alcohol consumption of 4.56 g/d, and that alcohol favors the increase of BW by approximately 30%, diminution in glucose levels, the rise of triglycerides and of ALT, and normal levels of total protein and cholesterol. In our study, alcohol consumption at a concentration similar to that reported by Rocha was 40%, finding similarity in average daily consumption of alcohol, in addition to an increase in BW in females (70%) as well as in males (73%) in comparison with the control (43%) (Table 1), the latter analogous to that reported previously by Rocha. In the same manner, we, like Rocha, found neither changes in albumin levels nor in those of total proteins, respectively (Table 3), which is probably due to that the damage is not yet chronic and severe. The weight gained is probably due to the increase in lipids, which may be observed in their elevation in blood (Table 2), as well as in hepatic tissue (Table 4) (Figures 3-6), caused by weekend alcohol consumption. Other reports demonstrate that weekend alcohol consumption gives rise to alterations in BW that are greater than those compared with moderate-daily alcohol consumption, and that this moderate consumption produces an increase in the protective lipoprotein profile. In contrast, concentrated alcohol consumption produces unfavorable alterations in the lipoproteins[12,14].
Surprisingly, alcohol consumption at 5% caused the greatest BW increase (108%) (Table 1). This can be explained by the metabolism of ethanol, in that, at different concentrations, the metabolism of the first pass of ethanol is modified, which is predominantly gastric[19] and is supplied mainly by the activity of the gastric ADH[20]. Previously, Roine et al[21] demonstrated that consumption of a concentrated solution of alcohol (40%) results in low levels of alcohol in blood in comparison with a diluted solution (4%), and that this effect is associated with first-pass metabolism and with lesser bioavailability with high concentrations of ethanol. Dohmen et al[22] demonstrated that, on administering a solution of alcohol at 5%, first-pass metabolism is low, while with a 40% ethanol solution, first-pass metabolism is high and is furnished by the activity of the gastric dehydrogenase alcohol. It is probable that, in the groups of weekend alcohol consumption with a concentration of 40%, the subjects had initial alcohol metabolism in the stomach, and low ethanol concentration. In contrast, the group of males with a consumption of 5% had highest ethanol concentration, which probably modified the metabolites involved in the accumulation of body fat, such as glucose, triglycerides, and cholesterol, as may be observed in Table 2, where these metabolites exhibit a slight increase in comparison with the remaining groups. Our data are in agreement with those reported by Rocha et al[13] where, after 10 wk of alcohol consumption (3 d per week at 30% ad libitum), the authors found that food consumption was very similar to that exhibited between the control group and the group with alcohol consumption. Nonetheless, the group that consumed alcohol had a greater weight gain than the control. Thus, we think that it is the alcohol and its metabolism that is the cause of the weight gain, because it alters the fat level in the liver (Table 4), which we have previously reported[2,15,16]. Baraona et al[23] found that the first metabolic pass of the alcohol is found to be diminished in women due to low activity of gastric ADH; the authors concluded that the latter can increase the vulnerability of women to the effects of ethanol. In our study, we found that the levels of bilirubin were higher in both groups of females, regardless of the concentration of alcohol administered (Table 3). Likewise, greater histological changes in fatty change and inflammation (Table 4) and in alcohol consumption levels were lower in the group of females at 5% (0.83 ± 0.04) (Table 1).
On the other hand, the transaminase enzymes are indicators for the diagnosis of liver diseases. For example, ALT and AST are released into the bloodstream at high concentrations, in which there is a membrane alteration in the hepatocyte; however, hepatocellular necrosis is not a requirement for the release of these enzymes, which gives rise to low correlation between the level of aminotransferases and liver damage[15]. This could be explained by the early release of hepatocytes, which initiate a proliferative process such as a sign, due to that it has been reported that liver regeneration is linked by selective enzyme release[15]. We previously reported that these comprise an alteration in the levels of transaminases depending on time of exposure to alcohol. Parra-Vizuet et al[24] reported a diminution in the serum levels of AST and ALT 24 h after the administration of a unique dose of alcohol of 1.5 g/kg. On the other hand, Morales-González et al[15] and Ramírez-Farías et al[25] reported an increase in the serum levels of the transaminases (ALT, AST, LDH, GDH and OTC) 7 d after the administration of ethanol. Similarly, Morales-González et al[26] reported a diminution in the activity of transaminase enzymes in hepatic tissue 24 h after ethanol administration (5 g/kg). Rocha et al[13] reported a rise in ALT during weekend alcohol consumption (4.5 g/d). Our results demonstrate that weekend alcohol consumption produces an increase in the serum of transaminases (AST, ALT and LDH) regardless of gender or of the concentration of alcohol (Figures 1 and 2). Nonetheless, surprisingly, alcohol consumption does not affect the serum levels of GGT (Figure 2). On the other hand, Stranges et al[11] reported that the principal transaminase affected by alcohol consumption is GGT. We consider that, in our study, there was no elevation of GGT, due to that weekend alcohol consumption was of 12 wk, and probably, sufficiently severe damage did not exist for it to be reflected by alteration of GGT, or by the levels of albumin (Table 3). This coincides with that reported by Rocha et al[13] in terms of protein levels in blood not being affected on consuming alcohol.
It has been reported that ethanol favors hepatic steatosis, probably because of the increase of lipogenesis, diminution of lipid transport of the liver, alteration of oxygenation of fatty acids[27], and even infiltration of monocytes, macrophages, etc., which are fundamental for pathogenic activity after acute or chronic hepatic injury[27]. The latter can explain in part the histological findings encountered in our study, where fatty change as well as inflammation comprised the most important changes in weekend alcohol consumption in all of the groups (Table 4), probably due to that they are related with the acute damage caused by ethanol to the liver[16-24]. Likewise, it was reported that acute treatment with ethanol in rats induced hepatic steatosis accompanied by an increase in the production of neutral fats (triglycerides). Thus, the serum levels of the triglycerides may not only reflect the production of the liver, but also the equilibrium between the production and utilization of neutral fats[28]. Our data reveal that weekend alcohol consumption produces fatty liver (Table 4) and the mobilization of neutral fats (Table 2), which is more evident when alcohol is consumed at 40%. Notwithstanding this, the group of males that consumed alcohol at 5%, a lesser amount than the groups at 40%, exhibited a pattern that was very similar in terms of levels of triglycerides and steatosis.
In conclusions, our findings demonstrate an effect of the damage that is caused by weekend alcohol consumption, regardless of gender or the concentration of the alcohol. Even more so, greater damage can be observed in females, and the metabolism of ethanol probably participates, specifically due to its first-pass metabolism, which is carried out in the stomach. Finally, the results obtained herein provide solid evidence that weekend alcohol consumption gives rise to liver damage, demonstrated by the biochemical and histological alterations, first manifested acutely, and their prolonged consumption can cause greater, irreversible damage.
It is known that alcohol consumption is a risk factor for various diseases, specifically liver diseases. Due to that alcohol consumption, especially weekend alcohol consumption, has Increased, in recent years the effect has been studied of the damage that this pattern of alcohol consumption can cause to various organs, for example, to the heart and the liver. This study contributes, to our knowledge for the first time, solid evidences of the damage caused by weekend alcohol consumption to the liver, as well as of the relationship that gender and the concentration of alcohol maintain in generating liver damage.
The consumption of alcohol is a problem of prime magnitude at the worldwide level that gives rise to a great number of medical (gastritis, cirrhosis, cancer, infarcts, etc.), as well as non-medical problems (automobile accidents, homicides, absenteeism from work, etc.). Therefore, carrying out investigations to understand the mechanisms of cellular damage and identifying patterns of alcohol consumption that are on the rise, such as weekend alcohol consumption, and how these patterns come to damage the liver at different magnitudes, are of utmost importance.
The majority of studies that investigate alcohol-associated liver damage address chronic or acute alcohol consumption. Few experimental studies inquire into the liver damage that is caused by weekend alcohol consumption. To our knowledge, this is the first report that describes the histological alterations caused by weekend alcohol consumption. It is of utmost importance to ascertain the mechanisms of liver damage given rise to by weekend alcohol consumption, due to the growing number of young people who acquire this consumption pattern, and to find a therapeutic window.
While the histological study is well known worldwide and has been employed in an infinite number of investigations, it can newly be a very important tool to find mechanisms of cellular damage caused by alcohol that are complemented with biochemical assays, in this manner taking the first steps in utilizing other investigative tools, such as electronic microscopy, molecular biology assays, etc. The most important and novel in this is the experimental design.
Histological and biochemical findings demonstrate that weekend alcohol consumption causes liver damage, irrespective of gender or the concentration of the alcohol. The contribution of this work resides in that a probable mechanism of damage to the liver due to weekend alcohol consumption comprises the metabolism of the first pass of the alcohol, which is carried out in the stomach. Lacking is the study in this model of variables such as age, food consumption, doses of alcohol, the consumption of antioxidants, etc.
As conclusions of the investigation, this is the first report, to our knowledge, that describes the histological alterations caused by weekend alcohol consumption that, in addition to the biochemical assays, provides solid evidence on the damage caused by weekend alcohol consumption, which initially can be acute and reversible, but that probably can become irreversible. We employed a known technique, histology, but with the experimental design being novel. This type of study, in which the mechanisms of damage are investigated, can open a therapeutic window in future clinical practice.
These perspectives include, in the first place, considering weekend alcohol consumption as a health problem of utmost importance, and even the same as chronic alcohol consumption. In second place, we conducted the investigation of the mechanisms of liver damage in terms of weekend alcohol consumption, and third, we found that this would be novel in the design of experimental models, in this manner utilizing techniques such as histology and biochemical assays, which comprise the first step in terms of orientation to the mechanisms of damage caused by alcohol, and these can be confirmed with molecular biology techniques. The questions to solve include knowing the following in terms of this model: How is the activity in the hepatic and gastric dehydrogenase alcohol enzyme found? Does the Nrf2 factor participate as cytoprotector?, and can the consumption of antioxidants prevent the alterations that weekend alcohol consumption cause?
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hamaguchi M, Tziomalos K S- Editor: Wang JL L- Editor: A E- Editor: Li D
| 1. | Morales González JA. Alcohol, Alcoholismo y Cirrosis. Un Enfoque Multidisciplinario. Pachuca: Universidad Autónoma del Estado de Hidalgo 2007; 240. |
| 2. | Piña Garza E, Gutiérrez Salinas J, Morales González JA, Zentella de Piña M. ¿Es tóxico el alcohol? Temas Bioquímicos de vanguardia. México: Facultad de Medicina UNAM 2003; 121-146. |
| 3. | Encuesta Nacional de Consumo de Drogas, Alcohol y Tabaco (ENCODAT). Consumo de alcohol: prevalencias globales, patrones de consumo y variaciones estatales. Comisión Nacional contra las Adicciones, México. Available from: https://www.gob.mx/cms/uploads/attachment/file/246052/hojasresumen_Alcohol-V3.pdf. |
| 4. | Berrios X, Jadue L, Pantoja T, Poblete JA, Moraga V, Pierotic M. [Alcohol consumption in the adult population from the metropolitan region: prevalence and consumption modalities]. Rev Med Chil. 1991;119:833-840. [PubMed] |
| 5. | García-Moreno LM, Expósito J, Sanhueza C, Angulo MT. [Prefrontal activity and weekend alcoholism in the young]. Adicciones. 2008;20:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Sánchez P, Castro B, Torres JM, Ortega E. Effects of different ethanol-administration regimes on mRNA and protein levels of steroid 5α-reductase isozymes in prefrontal cortex of adolescent male rats. Psychopharmacology (Berl). 2014;231:3273-3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Pridemore WA. Weekend effects on binge drinking and homicide: the social connection between alcohol and violence in Russia. Addiction. 2004;99:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Chenet L, Britton A, Kalediene R, Petrauskiene J. Daily variations in deaths in Lithuania: the possible contribution of binge drinking. Int J Epidemiol. 2001;30:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Kupari M, Koskinen P. Time of onset of supraventricular tachyarrhythmia in relation to alcohol consumption. Am J Cardiol. 1991;67:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Mergler D, Blain L, Lemaire J, Lalande F. Colour vision impairment and alcohol consumption. Neurotoxicol Teratol. 1988;10:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, Trevisan M. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol Clin Exp Res. 2004;28:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hojnacki JL, Deschenes RN, Cluette-Brown JE, Mulligan JJ, Osmolski TV, Rencricca NJ, Barboriak JJ. Effect of drinking pattern on plasma lipoproteins and body weight. Atherosclerosis. 1991;88:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Rocha KK, Souza GA, Seiva FR, Ebaid GX, Novelli EL. Weekend ethanol consumption and high-sucrose diet: resveratrol effects on energy expenditure, substrate oxidation, lipid profile, oxidative stress and hepatic energy metabolism. Alcohol Alcohol. 2011;46:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Liu W, Redmond EM, Morrow D, Cullen JP. Differential effects of daily-moderate versus weekend-binge alcohol consumption on atherosclerotic plaque development in mice. Atherosclerosis. 2011;219:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Morales-González JA, Gutiérrez-Salinas J, Yáñez L, Villagómez-Rico C, Badillo-Romero J, Hernández-Muñoz R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: role of route and timing of administration. Dig Dis Sci. 1999;44:1963-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Morales-González JA, Jiménez-García LF, Guitérrez-Salinas J, Sepúlveda J, Leija-Salas A, Hernández-Muñoz R. Effects of ethanol administration on hepatocellular ultrastructure of regenerating liver induced by partial hepatectomy. Dig Dis Sci. 2001;46:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Madrigal-Santillán E, Bautista M, Gayosso-De-Lucio JA, Reyes-Rosales Y, Posadas-Mondragón A, Morales-González Á, Soriano-Ursúa MA, García-Machorro J, Madrigal-Bujaidar E, Álvarez-González I, Morales-González JA. Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J Gastroenterol. 2015;21:7718-7729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Available from: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF. |
| 19. | Lim RT Jr, Gentry RT, Ito D, Yokoyama H, Baraona E, Lieber CS. First-pass metabolism of ethanol is predominantly gastric. Alcohol Clin Exp Res. 1993;17:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Baraona E, Abittan CS, Lieber CS. Contribution of gastric oxidation to ethanol first-pass metabolism in baboons. Alcohol Clin Exp Res. 2000;24:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Roine RP, Gentry RT, Lim RT Jr, Baraona E, Lieber CS. Effect of concentration of ingested ethanol on blood alcohol levels. Alcohol Clin Exp Res. 1991;15:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Dohmen K, Baraona E, Ishibashi H, Pozzato G, Moretti M, Matsunaga C, Fujimoto K, Lieber CS. Ethnic differences in gastric sigma-alcohol dehydrogenase activity and ethanol first-pass metabolism. Alcohol Clin Exp Res. 1996;20:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 357] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Parra-Vizuet J, Camacho LA, Madrigal-Santillán E, Bautista-Ávila M, Esquivel-Soto J, Esquivel-Chirino C, García-Luna-y- González-Rubio M, Mendoza-Pérez JA, Chanona-Pérez J and Morales-González JA. Hepatoprotective effects of glycine and vitamin E, during the early phase of liver regeneration in the rat. Afr J Pharm Pharmacol. 2009;3:384-390. |
| 25. | Ramírez-Farías C, Madrigal-Santillán E, Gutiérrez-Salinas J, Rodríguez-Sánchez N, Martínez-Cruz M, Valle-Jones I, Gramlich-Martínez I, Hernández-Ceruelos A, Morales-Gonzaléz JA. Protective effect of some vitamins against the toxic action of ethanol on liver regeneration induced by partial hepatectomy in rats. World J Gastroenterol. 2008;14:899-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Morales-González JA, Gutiérrez-Salinas J, Hernández-Muñoz R. Pharmacokinetics of the ethanol bioavailability in the regenerating rat liver induced by partial hepatectomy. Alcohol Clin Exp Res. 1998;22:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 27. | Bhopale KK, Amer SM, Kaphalia L, Soman KV, Wiktorowicz JE, Shakeel Ansari GA, Kaphalia BS. Proteomic Profiling of Liver and Plasma in Chronic Ethanol Feeding Model of Hepatic Alcohol Dehydrogenase-Deficient Deer Mice. Alcohol Clin Exp Res. 2017;41:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Hernández-Muñoz R, Santamaría A, García-Sáinz JA, Piña E, Chagoya de Sánchez V. On the mechanism of ethanol-induced fatty liver and its reversibility by adenosine. Arch Biochem Biophys. 1978;190:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |