Published online Nov 27, 2018. doi: 10.4254/wjh.v10.i11.822
Peer-review started: July 10, 2018
First decision: August 20, 2018
Revised: August 24, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: November 27, 2018
Processing time: 141 Days and 5.6 Hours
End stage liver diseases (ESLD) represent a major, neglected global public health crisis which requires an urgent action towards finding a proper cure. Orthotropic liver transplantation has been the only definitive treatment modality for ESLD. However, shortage of donor organs, timely unavailability, post-surgery related complications and financial burden on the patients limits the number of patients receiving the transplants. Since last two decades cell-based therapies have revolutionized the field of organ/tissue regeneration. However providing an alternative organ source to address the donor liver shortage still poses potential challenges. The developments made in this direction provide useful futuristic approaches, which could be translated into pre-clinical and clinical settings targeting appropriate applications in specific disease conditions. Earlier studies have demonstrated the applicability of this particular approach to generate functional organ in rodent system by connecting them with portal and hepatic circulatory networks. However, such strategy requires very high level of surgical expertise and also poses the technical and financial questions towards its future applicability. Hence, alternative sites for generating secondary organs are being tested in several types of disease conditions. Among different sites, omentum has been proved to be more appropriate site for implanting several kinds of functional tissue constructs without eliciting much immunological response. Hence, omentum may be considered as better site for transplanting humanized bioengineered ex vivo generated livers, thereby creating a secondary organ at intra-omental site. However, the expertise for generating such bioengineered organs are limited and only very few centres are involved for investigating the potential use of such implants in clinical practice due to gap between the clinical transplant surgeons and basic scientists working on the concept evolution. Herein we discuss the recent advances and challenges to create functional secondary organs through intra-omental transplantation of ex vivo generated bioengineered humanized livers and their further application in the management of ESLD as a supportive bridge for organ transplantation.
Core tip: The concept of bioengineering functional humanized neo-organs relies on finding more appropriate immunologically tolerable transplantation site. We have experienced omentum as more appropriate ectopic site with excellent properties of angiogenesis, regeneration, fibrotic reconstruction, and immunological compatibility which together endorse vascularisation, promote tissue healing, and minimize rejection of foreign body. However, regeneration of liver tissue in omentum is still unknown. Despite the amazing breakthroughs in the bioengineered organs, there is much work left to do. The approach described herein harbours enormous potential to overcome the limitations of organ transplantation and may support failing liver through ectopic transplantation as secondary organ.
- Citation: Vishwakarma SK, Lakkireddy C, Bardia A, Paspala SAB, Tripura C, Habeeb MA, Khan AA. Bioengineered functional humanized livers: An emerging supportive modality to bridge the gap of organ transplantation for management of end-stage liver diseases. World J Hepatol 2018; 10(11): 822-836
- URL: https://www.wjgnet.com/1948-5182/full/v10/i11/822.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i11.822
End stage liver diseases (ESLD) have become the major reason for the increasing deaths worldwide. According to the World Health Organisation, the total deaths caused by cirrhosis and liver cancer have increased by 50 million/year since 1990[1]. Liver transplantation is the only standard treatment available so far. However, more than 20% patients die on the waiting list due to a shortage of organ donors[2]. In order to expand the supply of livers available for transplantation, transplant surgeons and physicians have explored several new approaches including split liver transplants, living-related partial donor procedures[3] and the increasing use of “marginal” organs such as older donors, steatotic livers, non-heart-beating donors, donors with viral hepatitis, and donors with non-metastatic malignancy[4]. Despite these medical and surgical developments, it is unlikely that the availability of good liver grafts will ever be sufficient to meet the increasing demand of patients with end stage liver disease.
In order to overcome these limitations, various other treatment options are being explored among which hepatocytes transplantation has been described as the first supportive modality in regenerative medicine. But major challenges with such treatment is its limited availability of therapeutic dose from surgical samples, liver grafts or biopsies and their maintenance in vitro which requires cell-to-cell and cell-to-matrix interactions for proper functioning of anchorage dependent hepatocytes[5]. Usage of hepatocytes from xenogenic sources such as rabbit, porcine or canine, pose the risk of immunogenicity and transmission of zoonosis. This limitation can be addressed to certain extent by the usage of cell lines which can be maintained for longer time with higher growth rates under in vitro culture conditions but modification of gene expression under culture conditions might lead to problems and has issues related to its clinical applicability[6].
The first land mark study to bring hepatocyte transplantation into clinics was by Mito et al[7] in cirrhotic patients. In line with this study, our centre has treated seven acute liver failure patients by intra-peritoneal transplantation of human primary hepatocytes extracted from human foetus’s which showed clinical improvement and support to the failing liver[8]. Following this, various other studies have reported successful transplantation of primary hepatocytes in treating various metabolic diseases[9,10]. Although higher successful rate has been reported using hepatocyte transplantation, yet use of fetal hepatocytes poses major hurdle of ethical issues for its wider clinical applicability. Other potential treatment alternatives discovered in recent years included induced pluripotent stem cells, Mesenchymal stromal cells (MSC) which have the ability to differentiate into hepatocytes but still they couldn’t completely mimic the fully functional hepatocytes pointing towards a need to identify better niche for functional utilization of these cells[11-14].
Other alternative of direct cellular transplantation includes the use of extracorporeal liver support devices which can support a failing liver for a short period of time before organ transplantation[15]. But all these above mentioned treatment strategies may not fulfil the requirements to treat ESLD and may not provide immediate support for a failing liver to maintain normal functions. Hence, there is a need to develop bioengineered transplantable liver grafts which can retain the natural three-dimensional extra cellular matrix (3D-ECM) components and intact vascular networks similar to the native liver with repopulated functional hepatocytes or human hepatic progenitor cells. Rapid progress in the area of stem cell research and organ bioengineering paved a way in generating alternatives to liver transplantation.
After addressing all these limitations next comes the question of choosing an exact transplantable site where in these bioengineered organs can be easily acceptable and can able to perform the function. Recently omentum has been discovered as a wonderful ectopic site for transplantation with excellent properties like remarkable angiogenic[16], stem cell[17,18], fibrotic[19], and immune activities[20], which together endorse vascularization, promote wound healing, and minimize infection. Several studies have already demonstrated the importance of intra-omental transplantation in diabetic animal models[21,22]. However, the regeneration of liver tissue in ectopic sites is still unknown. Few studies have shown the omentum as a reservoir for proliferating renal, pancreatic, splenic[23-25] cells and as a site for hepatocytes engraftment which can be used in tissue engineering[26]. Hence, opting omental transplantation of bioengineered liver may offer development of secondary liver for the treatment of ESLD. This particular approach should offer promising treatment strategy in future and may rule out above mentioned limitations to answer for shortage of organ donors for ESLD.
Since last two decades, significant developments have been made to overcome the limitations of liver transplantation in ESLD. Among these strategies cell transplantation, use of extra-corporeal devices and transplantable bioengineered organs have been explored extensively.
In cell transplantation strategies, hepatocytes transplantation has been the most preferred cell types for infusion into liver due to their ability to perform major liver specific functions. However, getting therapeutic dose of human hepatocytes represents major limitation towards its wider clinical application[27,28]. Although several studies have reported use of 10% liver tissues to isolate enough number of hepatocytes post-in vitro expansion which can provide required clinical response in both animal models and human[5,29]. The in vitro enrichment of hepatocytes is challenging due to their contact-dependent growth, long-term survival and function and maintenance of normal phenotype without de-differentiation[5,30]. Therefore alternative strategies are highly desirable to overcome these limitations.
Recent studies have reported use of embryonic and adult pluripotent stem cells to generate desired number of functional hepatocytes for therapeutic applications. However, use of embryonic stem cells (ESCs) represents ethical hurdles and immune incompatibility for the transplant recipients[31-33]. Moreover, use of induced pluripotent stem cells (iPSCs) has been reported for effective differentiation into functional hepatocytes, however poses potential issues related to genetic instability and lack of functional transplantation studies[11,12]. Mesenchymal stromal cells (MSCs) represents another alternative type of pluripotent cells to generate functional hepatocytes and support liver regeneration[13,14]. However, multi-lineage differentiation of MSCs represents major challenge to control the effective trans-differentiation into desired number of functional hepatocytes while restricting other default lineage cells. Although, stem cell transplantation strategies have showed potential in liver regeneration through various mechanisms, still it has not been considered as durable solution to completely support the lost liver functions[15]. Hence, alternative strategies are highly desirable to generate therapeutic number of functional heaptocytes under controlled conditions.
These are the stem cells that are derived from adult or fetal livers. Adult stem cells are known as oval cells which play an important role in liver regeneration when replication capacity of hepatocytes is impaired[34]. Fetal liver stem cells are known as bipotent hepatoblasts that has ability to differentiate into bile duct cells or hepatocytes[35-37]. Fetal liver stem cells have been used to repopulate liver in animal models[38,39] and cultured hepatoblasts transplanted into immunodeficient mice showed greater in vivo engraftment and differentiation[40]. But the limitation in use of liver derived stem cells is their low number around 0.3% to 0.7% of oval cells in adult liver[41], whereas fetal liver mass comprises only 0.1% of hepatoblasts[42] and has associated ethical issues. Thus isolation and expansion of these cells and usage for transplantation is challenging.
Stem cells derived from bone marrow comprise hematopoietic and MSCs[43]. Among these, mesenchymal stem cells consists greater potential in liver regeneration[44] with immunosuppressive and immunomodulatory properties[45]. But they always pose problem with low rates of liver repopulation[46] and have low trans-differentiation ability to hepatoblasts which limits to restore normal liver function[47].
Stem cells derived from human umbilical cord, human placental tissue, amniotic fluid and human umbilical cord blood constitutes Annex group of stem cells. These are pluripotent stem cells with higher proliferation and differentiation rates than adult stem cells[46,48,49] and are not known to cause teratomas or teratocarcinomas formation in humans. Di Campli et al[50] study on diabetic severe combined immunodeficient mice after acute toxic liver injury when treated with intraperitoneal administration of human umbilical cord stem cells showed rapid liver engraftment, differentiation into hepatocytes, improved liver regeneration, and reduced mortality rates[50].
These are similar to ESCs and the limitation of ethical issue can be overruled by in vitro generation of iPSCs from somatic cells avoiding the usage of embryonic tissue or oocytes[51]. However, the use of these cells in clinical practice is limited due to major hurdles with the genomic instability of these cells.
Extracorporeal liver support devices have been designed with a goal to carry out normal liver function in patients with end-stage chronic, acute-on-chronic and acute liver failure for a short period of time until donor organ gets available. Two types of liver support systems have been designed: (1) Non-biological; and (2) Bio-artificial liver support devices.
Designed to filter and adsorb accumulated toxins that are not cleared by non-functional liver[52]. Three major types of such devices have been explored as follows:
Molecular adsorbent recirculating system: Molecular adsorbent recirculating system (MARS) has been well explored device which is a hollow fiber membrane hemodialyzer which removes soluble and protein-bound substances against albumin-rich dialysate. This device was approved by FDA in 2012 for the treatment of hepatic encephalopathy. However, the major limitation of such devices represents: (1) Short-term detoxification function; (2) Chance of getting sepsis; (3) Cost issues; (4) Can remove only albumin-bound toxins or drugs which are excreted in circulation; (5) Safety and efficacy of MARS has not been demonstrated in controlled, randomized trials; and (6) The effectiveness of MARS in patients that are sedative could not be established in clinical studies and therefore can’t be predicted in sedated patients.
Promethus fractionated plasma separator and adsorption system: Other type of devices includes, promethus fractionated plasma separator and adsorption system (FPSA) which is an artificial device which removes both albumin-bound and water soluble toxins from blood more effectively than MARS. However, its wide applicability has been limited due to following reasons: (1) Direct contact between fractionated plasma and the Prometh anion exchanger causes significant adsorption of procoagulant and anti-coagulant factors, associated with clinically relevant adverse events; (2) Broad disturbances of the coagulation system have been confirmed in FPSA treated liver failure patients; and (3) An ex vivo recirculation model demonstrated nonspecific adsorption of coagulation factors protein S and protein C on the anion exchange cartridge.
Single-pass albumin dialysis: Moreover, to overcome on the limitations of above mentioned extracorporeal liver assist device, single-pass albumin dialysis (SPAD) system was evolved which functions as one-pass dialysis against albumin solution to remove albumin-bound toxins and water-soluble substances. Detoxification system in SPAD is similar to or greater than MARS and is less expensive than MARS and FPSA. However, again the suitability and wide clinical applicability of SPAD is limited due to following limitations: (1) Only albumin bound or water soluble toxins can be removed; (2) Lipid soluble toxins can’t be removed by SPAD; (3) Bleeding risk from acquired coagulopathy; (4) Albumin solution is discarded after a single passage of membrane without being recycled; and (5) Absence of clinical data.
These are the bioreactors containing viable hepatocytes in a 3D network of hollow fibers. These are designed to achieve plasma perfusion and enhance the activities of living liver cells. Conversely, the membranes separating cells from plasma are not capable of achieving enough in vivo perfusion rates, and lack sources of safe, reliable, strongly proliferating and functionally active human cells. Still following major challenges remain to resolve: (1) Bio-artificial livers should be able to provide at least 10% of liver functioning; (2) Very difficult acquiring this many hepatocyte cells; (3) Controversy over the use of porcine cells due to possible transmission of infections; (4) Hepatocytes and plasma have very different physio-chemical properties; (5) Hepatocytes do not perform well when in contact with plasma; (6) Have a very high oxygen uptake rate; (7) Hepatocytes undergo a lot of stress inside of bio-artificial liver; (8) Any stress above 5 dyn/cm2 renders cells useless; (9) Limited volume of the bioreactor; (10) Maximum blood/plasma that can be safely drawn out of liver failure patient is one liter; (11) Difficult to achieve 10% of liver functioning within one liter; and (12) Makes Bio-artificial liver designing very difficult.
Owing to the hurdles in above mentioned devices, there is need to develop transplantable biological systems to provide: (1) Suitable three-dimensional organ architecture; (2) Organ specific intact vasculature for homogeneous supply of oxygen and nutrients; (3) Long-term cell survival and function within the natural organ specific niche; and (4) Metabolic, synthetic and detoxification functions similar to native liver.
Major components of human liver for bioengineering includes (1) Organ specific 3D-bioscaffolds; (2) Organ capsule; (3) Organ vasculature; (4) Cellular distribution in spatial anatomical organization of liver; (5) Biomolecules and growth factors for enhanced survival and function to transplanted cells; (6) Types of cells required for long-term support; and (7) Long-term functional response.
To provide these crucial components recently two major technological advancements have been made: (1) Organ bio-printing; and (2) Humanized neo-organ development.
With the advancements in tissue engineering it is possible to construct complex parenchymal organ structures along with intact vascular network by 3D bio-printing[53]. 3D bio-printing is one of the prevalent examples of bioengineered organs in the science world today, and it is growing and advancing quickly. This jaw dropping technology is one of the hot topics in bioengineering. It still fascinates that we have the potential to build organs from the push of a button. 3D bio-printing is a form of tissue engineering which utilizes inkjet printers and builds the scaffolding of a particular organ, layer by layer[54]. These inkjet printers allow the use of multiple cell types for printing. Robbins et al[55] developed a metabolically active 3D hepatic tissue where they identified increased liver specific function lasting for up to 135 h, and compartment-specific organization, along with a primitive hepatocyte microanatomy of hepatic stellate cells and endothelial cells. Researchers have also build bone repair constructs by coating the 3D printed scaffold with stem cells, which can grow into tissues over time[54]. The mild conditions used for bio-printing and material sintering have allowed viable cells and active therapeutic proteins to be incorporated into the construct production process. Today, this particular technology has been emerged only for ex vivo and its application in vivo has not been experienced which needs to be validated further.
The recent concept of bioengineering functional humanized neo-organs has given a hope towards finding permanent cure as an alternative support to the failing organ. This concept of artificial organs was first originated in the radiation field post-World War II, and was executed in the first bone marrow transplant in the 1970s[56]. According the Llames S tissue engineering has three main constituents: The ex vivo expansion of cells, seeding of these expanded cells in three dimensional structures that mimic physiological conditions and grafting the prototype. The technology relies on the development of whole intact organ scaffolds through whole organ perfusion acellularization procedure which retains extra-cellular matrix and circulatory networks of the native organ post-acellularization[57]. This important phenomenon allows three-dimensional intact acellular organ specific scaffold for efficient repopulation of desired cell population further to generate functional neo-organ system.
With advancement in regenerative medicine it has been possible to create bioengineered functional tissues or organs that can be used clinically[58,59]. So far several successful studies have been published in generating various organs and tissues based on these acellularization and stem cell repopulation[59-61] that can be used for treating patients. Significant progress in generating several types of complex organ biological scaffolds has led to development of an efficient acellularization protocols for whole organs through perfusion based techniques[62-66] (Tables 1 and 2). These acellularized whole organs combined with an efficient recellularization process[67-70] have made it possible to use these bioengineered organs for in vivo preclinical studies in small animal models[71-73].
| Organ | Acellularization agent | Perfusion method | Animal model | Reference |
| Heart | SDS, PEG, Triton X-100, and enzyme-based protocols deoxycholic acid | Antegrade coronary perfusion | Rat | [98] |
| Trypsin, EDTA, NaN3, Triton X-100, and deoxycholic acid | Retrograde aortic perfusion | Pig | [65] | |
| Lung | 0.1% and 0.5% SDS | Antegrade pulmonary arterial perfusion | Rat | [63] |
| CHAPS | Pulmonary artery and tracheal perfusion | Rat | [66] | |
| Triton X-100 and sodium deoxycholate | Right ventricle and tracheal perfusion | Mouse | [99] | |
| Liver | Triton X-100 plus 0.1% SDS | Portal vein perfusion | Rat | [100] |
| SDS | Rat | [70] | ||
| 1% Triton X-100 and 0.1% ammonium hydroxide | Mouse, rat, ferret, rabbit and pig | [69] | ||
| 0.25% and 0.5% SDS | Pig | [101] | ||
| Sodium citrate + SDS + Triton-X-100 | Hepatic artery perfusion | Rat | [74] | |
| Kidney | 0.5, 3, 6, 10% Triton X-100, 5 mM | Renal artery perfusion | Rat | [71] |
| calcium chloride, 5 mM magnesium sulfate, 1 M sodium chloride, DNase, and 4% sodium deoxycholate | Rat | [72] | ||
| 3% Triton X-100, DNase, and 4% SDS | ||||
| 1% SDS and 1% Triton X-100 | ||||
| 1% Triton X-100 and 0.1% ammonium hydroxide | Pig | [68] | ||
| Heparin and antibiotic-containing physiological saline, 0.1-1.0% SDS, 0.1% Triton-X-100 and 0.0025% deoxyribonuclease 1 | Goat | [75] |
| Organ | Acellularization | Study out come | Limitation | References |
| Method | ||||
| Rat liver | Perfusion with detergents (SDS, Triton X-100) | Perfusion with SDS removes most of cells, damages the ECM when treated with Triton X-100 and removes 97 % of DNA | SDS damages the ECM | [69,74] |
| Porcine liver | Mechanical perfusion (electroporation) | Most of the cells are removed, preserves the blood vessels | Disruption of microfilament and microtubule | [102] |
| Mouse heart | Enzymatic, detergents, Acids | Cells are removed | Damages the ECM proteins, poorly maintains the 3D architecture | [103] |
| Porcine trachea | Enzymatic (trypsin) non-enzymatic (EDTA), detergent (Triton X-100) and deionized Water | Cells are removed, clear the cell debris | Disruption of glycosaminoglycan, reduce the laminin and fibronectin | [104] |
| Rat kidney | Perfuse with SDS, deionized water, dTriton X-100 and PBS along with antibiotics | Twice filtration is observed | Loss of cell-mediated functions like transport of solutes | [105] |
| Rat heart | Perfused with detergents | Long-term cell survival, oxygen tension and continuous rhythmic beating | [63,98] | |
| Goat kidney | Perfused with Trypsin- EDTA in PBS, perfuse antibiotics and then with SDS in PBS | Cells are removed, pore to pore interconnection in the scaffold | [75,106] |
Our centre has well expertise in generating various types of acellularized whole organ bioscaffolds including xenogeneic liver through detergent-based perfusion. So far, we have successfully generated acellularized and repopulated humanized whole liver and demonstrated its applicability as better natural 3D-drug testing model system[74]. Apart from liver, we have also generated acellularized kidney[75], heart[76], spleen, meninges, and many more. Still various other studies are in pipeline in generating humanized bioengineered organs from our centre.
Highly specialized thick and complex organs like liver can be subjected to acellularization technology to obtain intact 3D-ECM. Due to delayed co-morbidity beyond marginal criteria or because of delayed ischemic time, in United Kingdom livers offered for transplantation are usually discarded[77]. This act offers a way to use this kind of livers for acellularization. The liver is the largest gland in the body and carries out numerous essential functions such as metabolism, maintaining homeostasis, and the synthesis of amino acids[57]. Therefore, acellularization is extremely beneficial to the liver because it not only maintains the microstructure but also its bio signals such as extracellular matrix proteins and adhesion peptides[57].
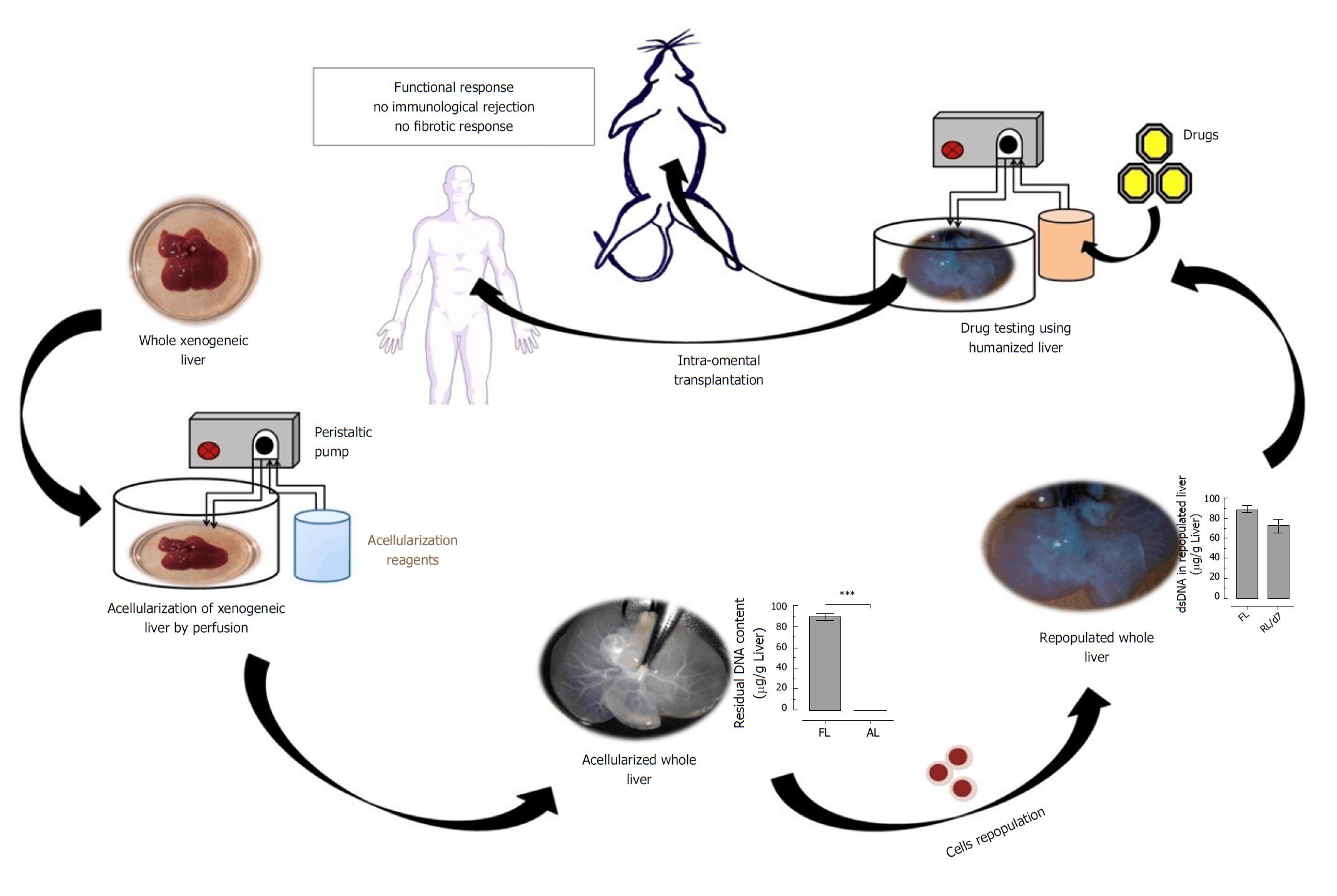
Since extracellular matrices are similar from species to species, whole organ scaffolds have become possible for livers. Several recent studies have been reported for efficient acellularization of livers obtained from various xenogeneic sources[78-81] and the resulting 3D-ECM structure has become an outstanding source for generating highly functionalized liver cells in vitro[82,83]. As these extracellular matrices are conserved between species, the process of recellularization with human cells into an animal scaffold is easier[57] and this kind of approach does not elicit any kind of immune rejection, cross contamination and zoonosis. In our recent study, we have demonstrated development of humanized whole liver using human hepatic progenitor cells repopulation through hepatic artery infusion into acellularized liver scaffolds[74]. These humanized livers perform detoxification and metabolic functions similar to the native liver. However, the complete recellularization of a fully function human liver has not yet been accomplished[57]. Recent advances in isolating and culturing both native cells and stem cells, as well as the development of acellularized organ scaffolds and biocompatible synthetic biomaterials, suggest that we are making rapid progress towards providing new alternatives to donor livers for transplantation[56].
Despite the amazing breakthroughs in the bioengineered organs, there is much work left to do. Simply reconstructing the whole organ will not be sufficient to replace organ transplantation. The approaches described above are fairly new and are still in the developmental stages. There has been only handful of successful transplantation of bioengineered organs into actual humans. Scientists are still working on ways to engineer more complex organs such as the liver. There are also long-term issues to resolve, such as the preservation of the overall function of these bio-engineered organs. However, little is known about the mechanisms by which these grafts may integrate and maintain function. When more complex organs are involved, the scenario is completely different, as investigations are still in very early stages and clinical translation is not foreseeable on the basis of current knowledge and available data[84]. The following major critical issues are yet to be resolved to make these approaches a clinical reality: (1) Liver is a complex organ with various cell types, hence rebuilding liver micro architectures with these cells is yet to be addressed; (2) The optimal cell source that can meet the criteria for recellularization of acellularized liver scaffolds still remains unclear; (3) The first and foremost challenge is the need to address the reconstruction of complete and functional uniform endothelial cell layer throughout acellularized liver scaffolds; (4) It is necessary to reconstruct biliary system which is needed for bile acid excretion to develop a fully functional bioengineered liver; (5) Assessing the functionality of these bioengineered livers after in vivo transplantation for long term needs to be studied clearly; (6) For organ functionality, maintaining its vascular structure is much more important. As hepatocytes require higher amounts of oxygen for their functionality, it is necessary to maintain hierarchical vascular network structure in acellularized liver scaffolds[85]. Critical step in engineering a transplantable liver is the creation of a functional vasculature capable of long-term perfusion following anastomosis. Without an appropriate endothelial lining of the vessels, continuous blood perfusion of the graft in the absence of anticoagulation quickly results in thrombosis; and (7) Finding an appropriate site for providing enough support to the failing liver has been one of the most challenging issue to use the bioengineered organs as secondary liver.
The major question for applying these humanized bioengineered livers relies on finding an exact and more appropriate transplantable site where in these bioengineered organs can be easily acceptable and are able to perform the function. Recently omentum has been discovered as a potential ectopic site for transplantation with excellent properties like remarkable angiogenic[16], stem cell[17,18], fibrotic[19], and immune[20] activities, which together endorse vascularization, promote wound healing, and minimize infection (Table 3). Several studies have already demonstrated the importance of intra-omental transplantation in diabetic animal models[22,86].
| Animal model | Site of transplantation | Mode of graft used | Results | Reference |
| Femoral bone of New Zealand rabbit was | Greater omentum on the left side | Free transplant of the greater omentum | Process of the callus formation and its mineralisation are much quicker and thicker on the defect that was covered with the free transplant of the greater omentum. | [107] |
| Pancreatectomized dogs | Spleen or Omentum | Islet auto-transplantation | Beta cell response to mild non-insulin induced hypoglycemia was normal, whereas the alpha cell response was not. | [108] |
| Murine carotid artery injury model | Omentum was applied to the injured vessel | Omentum + Omental progenitor cells | Omentum can directly contribute reparative progenitor cells to injured tissues upon treatment with Tβ4. | [109] |
| Nondiabetic nude rats | Omentum/kidney capsule | Perinatal porcine islet cell grafts | In both sites, the A-cell volume increased fourfold between weeks 1 and 10 reflecting a rise in A-cell number. In the omental implants, however, the cellular insulin reserves and the percent of proliferating cells were twofold higher than in kidney implants. In parallel, the blood vessel density in omental implants increased twofold, reaching a density comparable with islets in adult pig pancreas. | [110] |
| Diabetic rat and nonhuman primate (NHP) models | Intra-omental | In situ-generated adherent, resorbable plasma thrombin biologic scaffold | Improved metabolic function and preservation of islet cytoarchitecture, with reconstitution of rich intrainsular vascular networks in both species. | [21] |
| Adult male Spraguee Dawley rats | Omental transposition | Hepatic tissue sutured into the omentum mobilization of the omentum and transposition onto the left hepatic lobe | Omental transposition provided adequate microcirculation for proliferation of ectopic hepatic cells after liver resection. | [111] |
The omentum is a visceral adipose tissue derived from mesothelial cells[87] connected to the spleen, stomach, pancreas, and colon[88,89]. Although well known as a visceral fat depot, the role of the omentum in peritoneal immunity was not recognized until the early 1900s, when a British surgeon referred to it as ‘the police man of the abdomen’ due to its ability to attenuate peritonitis and promote surgical wound healing[90]. In fact, omentum was noted to move about the peritoneal cavity and occlude sites of inflammation, such as ruptured ovaries, inflamed appendices, ulcerated intestines, or wounds due to trauma or surgery[90]. Consistent with this observation, the omentum has remarkable angiogenic[16], fibrotic[19], regenerative[17,18] and immune[20] activities, which together promote vascularization, accelerate wound healing, and limit infection. However, these same activities are also likely involved in pathological responses, such as the rapid growth of omental tumour metastases[91].
Once thought of as just a large amount of redundant fat overlying the intestines, surgeons’ attitudes towards the omentum have changed. It is recognized as an organ in its own right, with many diverse functions ranging from its ability to attenuate the spread of sepsis in peritonitis to acting as a source of angiogenic and hemostatic factors involved in tissue healing and repair. The omentum has been identified as a source of adult stem cells which may have future prospects in the fields of tissue engineering and the synthesis of vascular grafts. Its regenerative properties have been exploited in virtually every field of surgery from the reconstruction of complex wounds to the protection of gastrointestinal anastomosis.
The regenerative properties of the omentum have been exploited by surgeons for over a century, ranging from the protection of anastomosis in gastrointestinal surgery, revascularization of arterial ulcers, to the reconstruction of head and neck deformities[92]. The advantage of the omentum is that it is an accessible and versatile source of growth factors, angiogenic factors, and leukocytes. It can be lengthened considerably by careful dissection to produce a mobile organ[93].
The regeneration of liver tissue in ectopic sites is still unknown. It has been discovered that the omnetum is a reservoir for proliferating renal, pancreatic, splenic tissues[23-25] and as a site for hepatocytes engraftment which can be used in tissue engineering[26]. Hepatocyte transplantation has been done in various tissues like spleen, pancreas and omentum[26,72,94-96]. With advancements in tissue engineering hepatocytes seeded onto polymer scaffolds and have been transplanted into omentum wherein engraftment of hepatocytes occurred due to elevated rates of angiogenesis into cell-polymer constructs within the omentum[96].
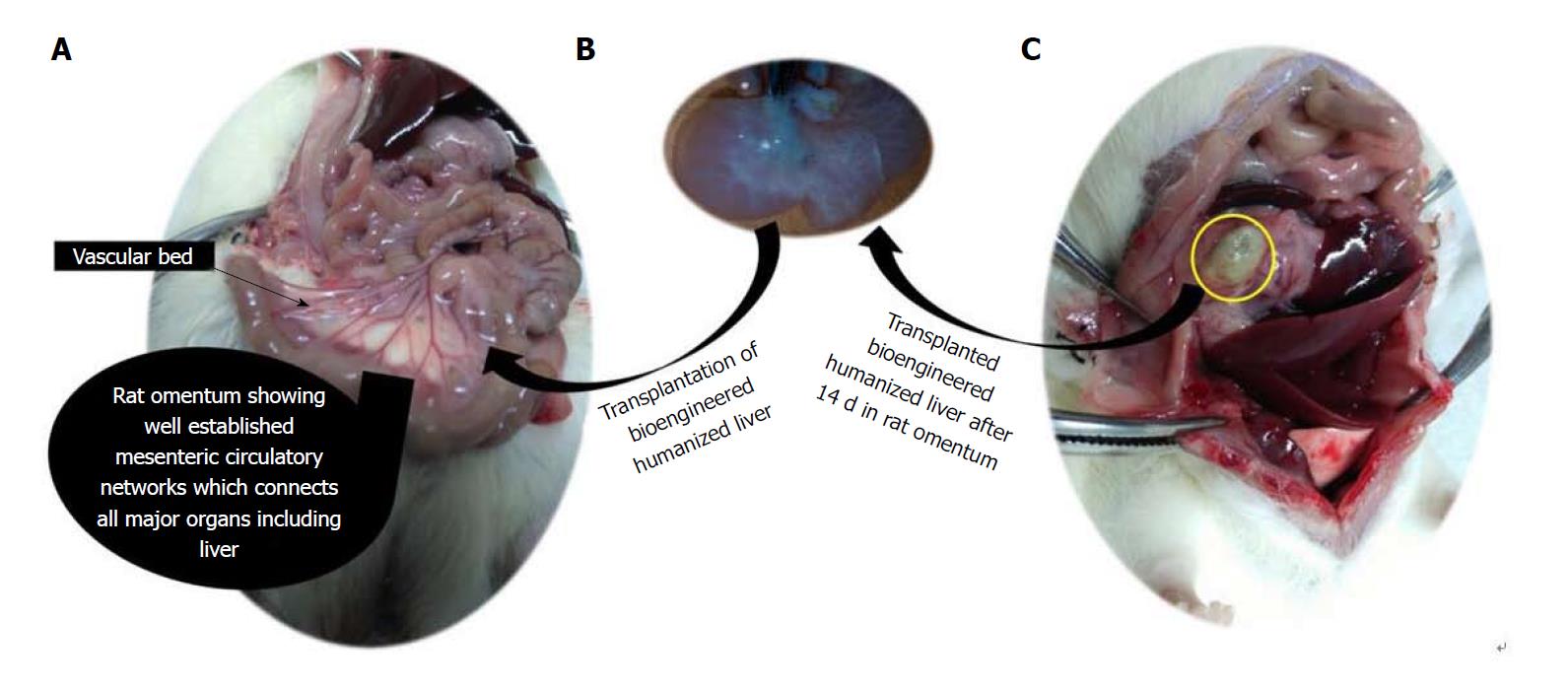
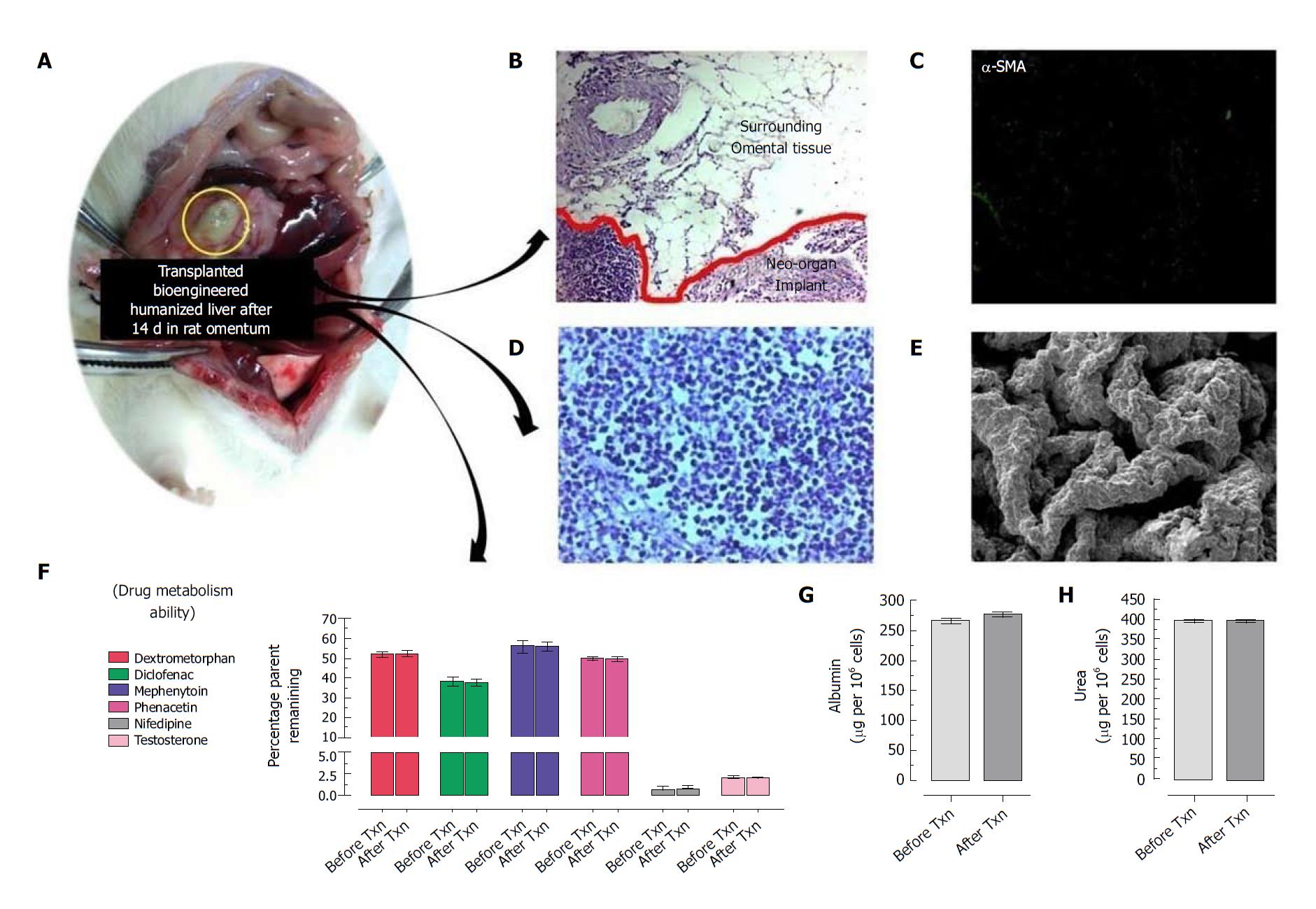
Thus intra-omental transplantation of bioengineered livers may provide adequate microcirculation for proliferation of ectopic hepatic cells repopulated within the bio-artificial liver. It has been observed that portal vein ligation does not affect the ectopic liver regeneration[97]. In our preliminary experiences, we have observed that intra-omental transplantation of bioengineered liver lobes gets easily accommodated into the site without eliciting immunological responses while maintain their biological functions and communicates blood borne growth factors for survival and function of the graft (Figure 1). We also observed that these bioengineered liver grafts survive at omental site in long-term and functions as secondary liver (Figure 2). These findings are well supported by earlier studies wherein other types of grafts have been transplanted into the omentum[21]. Future efforts at understanding mechanisms to regulate ectopic liver regeneration may assist the pursuit for liver tissue/organ bioengineering to support the failing liver functions in long-term.
Engineers and researches have been making monumental breakthroughs in the area of bioengineered organs. These bio-artificial organs may redefine transplants for human applications in future with more critical advancements. The introduction of cells into the human body is designed to stimulate regeneration, promote vascularization and/or supplement the production of hormones and growth factors[56]. Consequently, bioengineered biological substitutes present a new way to restore damaged tissue and maintain their functions. Not only does this provide a new source of organs, but probably even more reliable organs at that. Not only would people not need an organ donation, but their body will more readily accept a bioengineered organ through intra-omental transplantation, most likely reducing recovery time as well (Figure 3). In near future these potential strategies can overcome the limitation of organ donors and these bioengineered organs can even serve as a best natural 3D-drug testing models[74] and investigating precise molecular mechanisms in bio-mimetic natural organ system[112] and could support failing liver through ectopic transplantation as secondary organ in ESLD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bredt LC, Preda C S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
| 1. | Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1209] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 2. | Dutkowski P, Oberkofler CE, Béchir M, Müllhaupt B, Geier A, Raptis DA, Clavien PA. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: a prospective outcome analysis. Liver Transpl. 2011;17:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Brown KA, Moonka DK. Liver transplantation. Curr Opin Gastroenterol. 2001;17:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 4. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 497] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Nagaki M, Miki K, Kim YI, Ishiyama H, Hirahara I, Takahashi H, Sugiyama A, Muto Y, Moriwaki H. Development and characterization of a hybrid bioartificial liver using primary hepatocytes entrapped in a basement membrane matrix. Dig Dis Sci. 2001;46:1046-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Mito M, Kusano M. Hepatocyte transplantation in man. Cell Transplant. 1993;2:65-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 200] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 716] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 10. | Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Roy-Chowdhury N, Wang X, Guha C, Roy-Chowdhury J. Hepatocyte-like cells derived from induced pluripotent stem cells. Hepatol Int. 2017;11:54-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Zeilinger K, Freyer N, Damm G, Seehofer D, Knöspel F. Cell sources for in vitro human liver cell culture models. Exp Biol Med (Maywood). 2016;241:1684-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Zhou X, Cui L, Zhou X, Yang Q, Wang L, Guo G, Hou Y, Cai W, Han Z, Shi Y. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J Cell Mol Med. 2017;21:881-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Meyer U, Wiesmann HP. Tissue engineering: a challenge of today's medicine. Head Face Med. 2005;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | García-Gómez I, Goldsmith HS, Angulo J, Prados A, López-Hervás P, Cuevas B, Dujovny M, Cuevas P. Angiogenic capacity of human omental stem cells. Neurol Res. 2005;27:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Shah S, Lowery E, Braun RK, Martin A, Huang N, Medina M, Sethupathi P, Seki Y, Takami M, Byrne K. Cellular basis of tissue regeneration by omentum. PLoS One. 2012;7:e38368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl Med. 2014;3:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817-2825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 20. | Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C. Bioengineering the Endocrine Pancreas: Intraomental Islet Transplantation Within a Biologic Resorbable Scaffold. Diabetes. 2016;65:1350-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Kim HI, Yu JE, Park CG, Kim SJ. Comparison of four pancreatic islet implantation sites. J Korean Med Sci. 2010;25:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, Hosoya T, Okabe M, Kobayashi E. Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol. 2006;17:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Cuervas-Mons V, Cienfuegos JA, Maganto P, Rodriguez V, Eroles G, Pinedo I, Santamaria L, Ramos J, Ortiz JL, Castillo-Olivares JL. Long-term evaluation of isolated syngeneic hepatocytes transplanted into the normal rat spleen by TC-99M-HIDA scintigraphy. Transplantation. 1985;39:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Mazzoni G, Di Martino C, Scarpelli F, Cristini F, Citarella G, Martini ME. Liver autotransplantation into the pancreas. Transplantation. 1982;34:108-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Lee H, Cusick RA, Utsunomiya H, Ma PX, Langer R, Vacanti JP. Effect of implantation site on hepatocytes heterotopically transplanted on biodegradable polymer scaffolds. Tissue Eng. 2003;9:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 555] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 28. | Sussman NL, Kelly JH. Artificial liver: a forthcoming attraction. Hepatology. 1993;17:1163-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A, Durham J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Treyer A, Müsch A. Hepatocyte polarity. Compr Physiol. 2013;3:243-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 32. | McLaren A. Ethical and social considerations of stem cell research. Nature. 2001;414:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991-12996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 491] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 35. | Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 284] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Lázaro CA, Rhim JA, Yamada Y, Fausto N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998;58:5514-5522. [PubMed] |
| 37. | Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci U S A. 2000;97:12132-12137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Oertel M, Rosencrantz R, Chen YQ, Thota PN, Sandhu JS, Dabeva MD, Pacchia AL, Adelson ME, Dougherty JP, Shafritz DA. Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology. 2003;37:994-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, Narain N, Bock M, Nörder M, Legrand N. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol. 2009;175:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Mahieu-Caputo D, Allain JE, Branger J, Coulomb A, Delgado JP, Andreoletti M, Mainot S, Frydman R, Leboulch P, Di Santo JP. Repopulation of athymic mouse liver by cryopreserved early human fetal hepatoblasts. Hum Gene Ther. 2004;15:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Hayner NT, Braun L, Yaswen P, Brooks M, Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984;44:332-338. [PubMed] |
| 42. | Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 43. | Xu YQ, Liu ZC. Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev. 2008;4:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Cho KA, Ju SY, Cho SJ, Jung YJ, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 46. | Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 950] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 47. | di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 48. | Kestendjieva S, Kyurkchiev D, Tsvetkova G, Mehandjiev T, Dimitrov A, Nikolov A, Kyurkchiev S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int. 2008;32:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 50. | Di Campli C, Piscaglia AC, Pierelli L, Rutella S, Bonanno G, Alison MR, Mariotti A, Vecchio FM, Nestola M, Monego G. A human umbilical cord stem cell rescue therapy in a murine model of toxic liver injury. Dig Liver Dis. 2004;36:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Zacharias DG, Nelson TJ, Mueller PS, Hook CC. The science and ethics of induced pluripotency: what will become of embryonic stem cells? Mayo Clin Proc. 2011;86:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 53. | Guillemot F, Mironov V, Nakamura M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B'09). Biofabrication. 2010;2:010201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Sawkins MJ, Mistry P, Brown BN, Shakesheff KM, Bonassar LJ, Yang J. Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication. 2015;7:035004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Robbins JB, Gorgen V, Min P, Shepherd BR, Presnell SC. A novel in vitro three-dimensional bioprinted liver tissue system for drug development. FASEB J. 2013;27:Abstract 7979. |
| 56. | Murphy SV, Atala A. Organ engineering--combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. Bioessays. 2013;35:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Peloso A, Dhal A, Zambon JP, Li P, Orlando G, Atala A, Soker S. Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Res Ther. 2015;6:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1175] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 59. | Atala A. Engineering tissues, organs and cells. J Tissue Eng Regen Med. 2007;1:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 422] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 61. | L'Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 62. | Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 63. | Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 813] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 64. | Taylor DA. From stem cells and cadaveric matrix to engineered organs. Curr Opin Biotechnol. 2009;20:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 66. | Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1015] [Cited by in RCA: 849] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 67. | Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 68. | Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526-6529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 473] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 70. | Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1162] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 71. | Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 72. | Liu CX, Liu SR, Xu AB, Kang YZ, Zheng SB, Li HL. [Preparation of whole-kidney acellular matrix in rats by perfusion]. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:979-982. [PubMed] |
| 73. | Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16:2207-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 74. | Vishwakarma SK, Bardia A, Lakkireddy C, Nagarapu R, Habeeb MA, Khan AA. Bioengineered humanized livers as better three-dimensional drug testing model system. World J Hepatol. 2018;10:22-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Vishwakarma SK, Bhavani PG, Bardia A, Abkari A, Murthy GS, Venkateshwarulu J, Khan AA. Preparation of natural three-dimensional goat kidney scaffold for the development of bioartificial organ. Indian J Nephrol. 2014;24:372-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Rout S, Vishwakarma SK, Khan AA. Decellularized heart: a step towards creating personalized bioengineered organs. Current Sci. 2014;107:10. |
| 77. | NHS Blood and Transplant. Organ Donation and Transplantation - Activity Report 2013/14. Available from: https://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/activity_report_2013_14.pdf. |
| 78. | Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, Yuan X, Ding Y. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int. 2013;33:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Pan MX, Hu PY, Cheng Y, Cai LQ, Rao XH, Wang Y, Gao Y. An efficient method for decellularization of the rat liver. J Formos Med Assoc. 2014;113:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Nari GA, Cid M, Comín R, Reyna L, Juri G, Taborda R, Salvatierra NA. Preparation of a three-dimensional extracellular matrix by decellularization of rabbit livers. Rev Esp Enferm Dig. 2013;105:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Kajbafzadeh AM, Javan-Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng Part C Methods. 2013;19:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 82. | Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier C. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 83. | Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, Reing J, Gramignoli R, Komori J, Ross M. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 2011;17:677-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 84. | Orlando G, Soker S, Stratta RJ. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann Surg. 2013;258:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 85. | Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y, Okano T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 86. | Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9:91-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 87. | Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 409] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 88. | Williams R, White H. The greater omentum: its applicability to cancer surgery and cancer therapy. Curr Probl Surg. 1986;23:789-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Wilkosz S, Ireland G, Khwaja N, Walker M, Butt R, de Giorgio-Miller A, Herrick SE. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl). 2005;209:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Koppe MJ, Nagtegaal ID, de Wilt JH, Ceelen WP. Recent insights into the pathophysiology of omental metastases. J Surg Oncol. 2014;110:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Losken A, Carlson GW, Culbertson JH, Scott Hultman C, Kumar AV, Jones GE, Bostwick J 3rd, Jurkiewicz MJ. Omental free flap reconstruction in complex head and neck deformities. Head Neck. 2002;24:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Ross WE, Pardo AD. Evaluation of an omental pedicle extension technique in the dog. Vet Surg. 1993;22:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Pancholi N, Patel J, Gudehithlu KP, Kraus MA, Dunea G, Arruda JA, Singh AK. Culture of omentum-induced regenerating liver yielded hepatocyte-committed stem cells. Transl Res. 2010;156:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Panis Y, Puts JP, Ballet F, Penin E, Delelo R, Verthier N, Nordlinger B. The isolated perfused rat spleen. An original method for studying the function of hepatocytes transplanted into the spleen. Transplantation. 1990;49:756-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Dave S, Bal CS, Mathur M, Bhatnagar V. Evaluation of transplanted hepatocytes using HIDA scintigraphy. Indian J Gastroenterol. 2001;20:177-179. [PubMed] |
| 97. | Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011;108:11842-11847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 98. | Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 1824] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 99. | Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 100. | Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis. 2010;6:134-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 101. | Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 102. | Sano MB, Neal RE 2nd, Garcia PA, Gerber D, Robertson J, Davalos RV. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed Eng Online. 2010;9:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 103. | Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 104. | Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 105. | Khan AA, Capoor AK, Parveen N, Naseem S, Venkatesan V 5th, Habibullah CM. In vitro studies on a bioreactor module containing encapsulated goat hepatocytes for the development of bioartificial liver. Indian J Gastroenterol. 2002;21:55-58. [PubMed] |
| 106. | Gupta SK, Dinda AK, Potdar PD, Mishra NC. Modification of decellularized goat-lung scaffold with chitosan/nanohydroxyapatite composite for bone tissue engineering applications. Biomed Res Int. 2013;2013:651945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Josip K, Vjenceslav N, Dubravko H, Irena N, Josip T, Dragutin K, Tomislav A, Tomislav B, Dražen V, Mario Kr, Ozren S. Healing of bone defect by application of free transplant of greater omentum. Veterinarski Arhiv. 2006;76:367-379. |
| 108. | Gustavson SM, Rajotte RV, Hunkeler D, Lakey JR, Edgerton DS, Neal DW, Snead WL, Penaloza AR, Cherrington AD. Islet auto-transplantation into an omental or splenic site results in a normal beta cell but abnormal alpha cell response to mild non-insulin-induced hypoglycemia. Am J Transplant. 2005;5:2368-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Shelton EL, Poole SD, Reese J, Bader DM. Omental grafting: a cell-based therapy for blood vessel repair. J Tissue Eng Regen Med. 2013;7:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Bartholomeus K, Jacobs-Tulleneers-Thevissen D, Shouyue S, Suenens K, In't Veld PA, Pipeleers-Marichal M, Pipeleers DG, Hellemans K. Omentum is better site than kidney capsule for growth, differentiation, and vascularization of immature porcine β-cell implants in immunodeficient rats. Transplantation. 2013;96:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Macedo FI, Eid JJ, Decker M, Herschman B, Negussie E, Mittal VK. Autogenous hepatic tissue transplantation into the omentum in a novel ectopic liver regeneration murine model. J Surg Res. 2018;223:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Vishwakarma SK, Lakkireddy C, Bardia A, Raju N, Paspala SAB, Habeeb MA, Khan AA. Molecular dynamics of pancreatic transcription factors in bioengineered humanized insulin producing neoorgan. Gene. 2018;675:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |