Published online Oct 26, 2016. doi: 10.4252/wjsc.v8.i10.342
Peer-review started: June 21, 2016
First decision: July 4, 2016
Revised: July 23, 2016
Accepted: September 7, 2016
Article in press: September 8, 2016
Published online: October 26, 2016
Processing time: 124 Days and 2.2 Hours
To show the existence of a structural formative role of magnetic fields (MFs) with respect to biological objects by using our proposed model of an acupoint.
We introduced a magnetised 10-100 μT metal rod (needle) into culture dishes with a negatively charged working surface and observed during 24 h how cells were arranged by MFs and by electrical fields (EFs) when attached. Rat and human bone marrow-derived stromal stem cells (rBMSCs and hBMSCs), human nonadherent mononuclear blood cells, NCTCs and A172 cells, and Escherichia coli (E. coli) were evaluated. The dish containing BMSCs was defined as the model of an acupoint. rBMSCs proliferative activity affected by the needle was investigated. For investigating electromagnetic field structures, we used the gas discharge visualisation (GDV) method.
During 24 h of incubation in 50-mm culture dishes, BMSCs or the nonadherent cells accumulated into a central heap in each dish. BMSCs formed a torus (central ring) with an inner diameter of approximately 10 mm only upon the introduction of the needle in the centre of the dish. The cells did not show these effects in 35- or 90-mm culture dishes or hydrophobic dishes or rectangular cuvettes. NCTCs and A172 cells showed unstable the effects and only up to two weeks after thawing. Moreover, we observed that the appearance of these effects depended on the season. In winter, BMSCs showed no the effects. GDV experiments revealed that the resonant annular illumination gradually formed from 10 to 18-20 s in polar solutions with and without cell suspension of BMSCs, NCTCs and E. coli when using circular 50-mm dishes, stimulation at 115 V and switching of the electrode poles at 1 kHz. All these data demonstrate the resonant nature of the central ring. Significant influence of MFs on the rBMSC proliferation rate was not observed.
BMSCs can be moved by MFs when in the presence of a constant EF and MF, when the cells are in the responsive functional state, and when there is a resonant relationship between them.
Core tip: On the basis of the literature, we propose the simplest possible model of an acupoint. This model that allowed us to move bone marrow-derived stromal stem cells (BMSCs) using magnetic fields (MFs) without any magnetised nanoparticles. This is a newly identified property of BMSCs, which may be involved in the formation, maintenance and regeneration of tissues and organs. The associated movements of BMSCs may occur via acupoints, and the meridian system may thus control the processes of structural regeneration and be the most ancient regulatory system. Not until the cells become MF amplifiers (resonators) can MFs move the cells. That is possible within our acupoint model.
- Citation: Emelyanov AN, Borisova MV, Kiryanova VV. Model acupuncture point: Bone marrow-derived stromal stem cells are moved by a weak electromagnetic field. World J Stem Cells 2016; 8(10): 342-354
- URL: https://www.wjgnet.com/1948-0210/full/v8/i10/342.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i10.342
At present, various types of stem cells (SCs) have been successfully used to rescue acquired or congenital defects in human tissues. Tissue grafts (dermal equivalents, biodegradable polymer-based grafts, etc.) are used for large tissue defects or for correcting the disordered structures of hollow viscera. However, reproducing the structure of parenchymatous organs (e.g., liver, kidney) has not yet been successful[1].
It has been suggested that the spatial structure of the body and its organs, in their phylogenesis and ontogenesis, were formed not only under the influence of various internal and external physiological factors but also under the influence of certain non-physiological laws of morphogenesis[2]. Influencing the shape during organogenesis was shown in heterotopic transplantation experiments when grafting under the kidney capsule of a Millipore membrane filter folded with mouse bone marrow stem cells on its inner surface. Not until the filter was present and was sufficiently folded did bone structures form[3]. Biological fields are believed to play a significant role in these patterns that influence morphology[4,5]. The nature of these formative fields has been defined in the modern experimental studies[6] and theoretical constructs[7] as an electromagnetic. Influences of electric, magnetic and electromagnetic forces in cells, tissues and the whole organism have been addressed in many papers[8,9]. In particular, there are a number of studies that investigated the effects of electromagnetic fields in the visible and infrared ranges on various types of SCs[10]. However, the influence of the physical forces stated above at physiological doses on the morphogenesis of biological tissues has not been shown.
Cells somehow know how to line up in space, e.g., during in vivo regeneration of surface defects. Their ability to regenerate the external body shape seems amazing, for example, in coelenterates and some vertebrates. That is possible only if there is an EMF mould within at least a very small distance over the surface of the wound, which would determine the directional synthesis of extracellular matrix by the surface cells and then the directional cell movement upon that matrix. It has to be the same in embryo morphogenesis. To date, no structure responsible for establishment of shape-supporting EMFs has been detected in living organisms. The only system that could be qualified for this role is the acupoint and acupuncture channel system. There are many theories about this system, none of which has been completely proven. Moreover, the system has not been proven to exist in the body[11]. However, acupoints are known to have certain anatomical and physiological characteristics[12,13]. In particular, relative to surrounding tissues, acupoints appear to have an elevated electrical potential and a reduced electrical resistance[14,15]. Areas with reduced impedances and higher electric potentials have also been found in plants[16].
In this study, we sought to show that existing EM forces in the body not only can influence the intracellular, interstitial and intra-organ physiological processes but also can significantly affect the structures of organs and tissues. We primarily used SCs in our work because the processes of shaping in the body connect with its regeneration system. As the electrical matrix, we used culture dishes with a negatively charged working surface because acupoints have heightened electrical potentials. As a magnetic component, we used a magnetised metal rod. From our perspective, such a culture dish with cells placed in it can be considered the simplest model of an acupoint. This model represents a cross-section of an acupoint. The negatively charged surface of the culture dish models the interior of the acupoint. The hydrophobic walls of the dishes model the transition from the inner space of the acupoint to the surrounding tissues.
Thus, the aim of this work was to show the possible structural formative role of EMFs with respect to biological objects. The main objective was to show that EM forces, which are probably present in acupoints, can greatly affect the spatial arrangement of cells that are in the scope of action of the forces.
In total, 8 outbred white rats at 2-6 mo of age were used for all of the experiments (2 years, one rat per season). The rats were maintained from birth at 23 °C, 50% humidity, with ad libitum access to food and water, and with artificial light from 8:00 to 16:00 and with natural light provided by large windows. Rat bone marrow-derived stromal stem cells (rBMSCs) were isolated as described by Javazon et al[17] with some modifications. Briefly, rats were anesthetised with ethoxyethane and then sacrificed by cervical dislocation in accordance with the guidelines approved by the Institutional Animal Care and Utilisation Committee. BM was collected from the femurs and tibias by inserting a 22-gauge needle into the shaft of the bone and flushing it with phosphate-buffered saline (PBS). Clots of cells were broken by syringing. Next, the cells were loaded onto Histopaque-1077 (Sigma, United States) for density gradient centrifugation (500 × g, 20 min). The cells were collected from the interface, resuspended in PBS and centrifuged at 370 × g for 10 min. After centrifugation, the residual cells were resuspended in Eagle’s minimum essential medium, alpha modification (α-MEM, Sigma, United States) containing 2 mmol/L L-glutamine, 100 U/mL antibiotic-antimycotic (PenStrep, GIBCO, Canada) and 100 mL/L foetal bovine serum (FBS, HyClone, United States) and seeded at 5-7 × 106 cells per 50-mm culture dish. The cells were cultured in a humidified atmosphere of 50 mL/L CO2 at 37 °C. After cell colonies arose, nonadherent cells were discarded, and adherent cells were grown to 90% confluence in fresh medium. The cells were detached by trypsinisation, harvested by centrifugation at 500 × g for 10 min, counted using a counting chamber, seeded at 5-10 × 103 cell/cm2 into flasks or dishes (passage 1) and placed in a humidified atmosphere of 50 mL/L CO2 at 37 °C for amplification. After cells were grown to 70%-80% confluence, they were reseeded.
The procedures were carried out as described by Wolfe et al[18] with some modifications. BM aspirate obtained from 2 healthy adult donors with informed consent was used in this research. Briefly, after the approval of the local Ethics Committee, human BMSCs (hBMSCs) were obtained from a patient selected for the study protocol: 6 mL of BM was aspirated from the posterior iliac crest and supplemented with 100 U/mL heparin for transportation. Within 1 h after aspiration, the extracted BM suspended in 30 mL PBS and centrifuged at 200 × g for 5 min. The residual cells were resuspended in 10 mL PBS and loaded onto Histopaque-1077 (Sigma, United States) for density gradient centrifugation (500 × g, 20 min). The subsequent procedures were the same as for rBMSCs. Unlike rBMSCs, the culture medium was changed once every three days, and after the first medium change, the nonadherent mononuclear cells (mostly leukocytes) were not discarded. They were cultivated in the same fresh medium separately from the BMSCs.
The two stable cell lines used in the experiments were provided by the Russian Cell Culture Collection of Institute of Cytology of the Russian Academy of Sciences (St. Petersburg). One was NCTC clone 929 of the cell line L (NCTCs, murine fibroblast-like cells from subcutaneous connective tissue), and the other was A-172 cells (human fibroblast-like glioblastoma cells). The cells were recovered from frozen vials and resuspended in Dulbecco’s modified Eagle’s medium (Biolot, Russia) supplemented with 100 mL/L FBS, 2 mmol/L L-glutamine and 100 U/mL antibiotic-antimycotic. The cells were grown in a humidified atmosphere of 50 mL/L CO2 at 37 °C. After cells were grown to 70%-80 % confluence, they were reseeded.
Escherichia coli (E. coli) was cultured at 37 °C in Luria-Bertani culture medium: 10 g/L bactotryptone and 5 g/L yeast extract in 10 g/L aqueous solution of NaCl.
For the experiments, cells at 70%-80% confluence were detached by trypsinisation (2.5 g/L trypsin, 0.2 g/L EDTA: GIBCO, United States) for 2-5 min at 37 °C, flushed with PBS, harvested by centrifugation at 500 × g for 10 min, resuspended in the appropriate fresh culture medium, and plated at 10 × 103 cell/cm2 in 50-mm culture dishes for the proliferation assay and at 20 × 103 cell/cm2 in culture dishes (diameter, 35 mm, 50 mm, 90 mm) to observe the sites of cell attachment to the bottoms of the culture dishes. The volumes of culture medium per dish were as follows: 2 mL per 35-mm diameter dish, 4 mL per 50-mm dish, 10 mL per 90-mm dish. Those volumes were selected to obtain a 3-mm height of liquid in each type of culture dish. The lids of the culture dishes were replaced with lids with openings for the needles, and magnetised needles were inserted into those openings. The cells were incubated in a humidified atmosphere of 50 mL/L CO2 at 37 °C for 1, 3 or 6 d.
The metal rods were acupuncture needles of surgical steel (Kangnian, China) with a diameter of 0.3 mm and a length of 75 mm (working portion, 35 mm long). Needles of surgical steel are highly resistant to corrosion and do not leave visible traces of metal when introduced into human tissue and left in for at least several days (normal exposure of “buttons” is 3-4 d). Needles were magnetised by a permanent magnet to a residual magnetisation of 10-100 μT.
We also used NunclonTMΔ Surface (NUNC, England) culture dishes. These are ordinary dishes used for culturing cells and possess a hydrophilic bottom surface that has negative carboxyl groups (COO-) chemically attached to the plastic in a uniform distribution. Meanwhile, the vertical, non-working walls of the dish remain hydrophobic. In addition, 14-сm culture dishes were used as “shirts”. Before the experiments, holes the diameter of the needle trough covers of both the culture dishes and the “shirts” were made as follows: In the centre of the “shirt” covers and in the centre or at a certain distance from the centre of the culture dish covers. Before use, the covers and the needles were treated with 700 mL/L ethanol and dried under ultraviolet radiation for at least 10 min. The needle was introduced immediately after cell transfer into the culture dishes. The needles were introduced vertically into the culture dishes, reaching the bottom of the dish. A general view of the culture dish within the “shirt” and with the inserted needle is shown in Figure 1.
Cells were stained as described previously[19] with some modifications. Cells were fixed by the addition of 110 g/L glutaraldehyde solution up to a concentration of 10 g/L glutaraldehyde within the culture medium. After gentle shaking for 15 min at room temperature, culture dishes were washed with deionised water and dried. Cells were stained by the addition of a 1 g/L solution of crystal violet dissolved in 200 mmol/L MES buffer, pH 6. After shaking for 20 min at room temperature, excess dye was removed by extensive washing with deionised water. The culture dishes with stained cells were dried, and then their vertical walls were removed using special cutting pliers. The bottoms of the dishes were scanned using an HP LaserJet 3392 scanner.
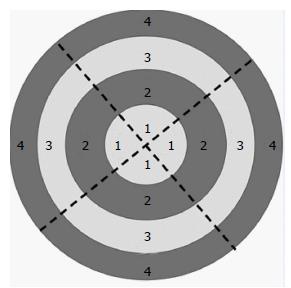
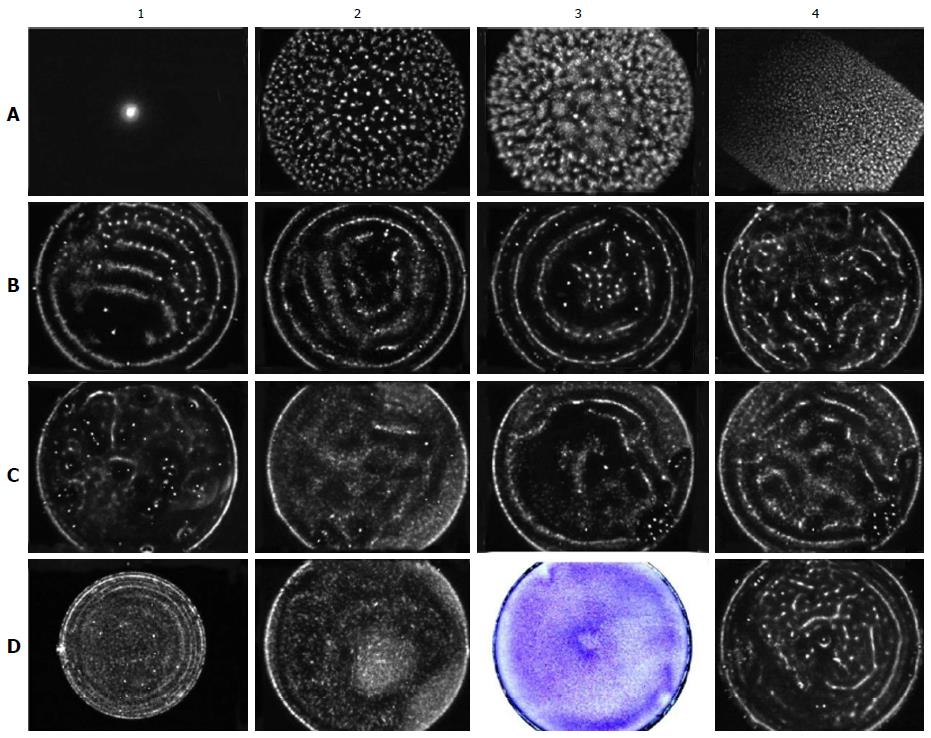
In addition, live cells were filmed within culture dishes. We used lenses of 4× and 10× magnification and an ocular magnification of 10×. For more accurately estimating cell attachments, the culture dish bottoms were divided into four concentric regions of equal width and four equal sectors. Images were collected from all concentric areas of each of the four sectors (a total of 16 fields). The photographed fields are shown in Figure 2.
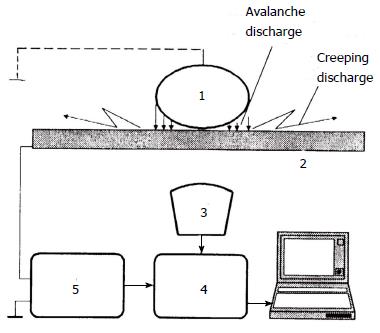
The gas discharge visualisation (GDV) method was formulated by KG Korotkov in the book “The foundations of GDV-bioelectrography”, which was published in 2001[20]. The GDV method is based on the visualisation of gas discharge-induced photoelectron emission from the surface of an object placed in a high-tension electric field (Kirlian effect). We used a hardware-software complex called the “GDV Pro Camera” (Biotechprogress, Russia, ktispb.eng). A schematic of this device is shown in Figure 3.
A culture dish with either adherent cells or suspended cells at 10 × 103-20 × 103 cell/cm2 in 4 mL of culture medium or PBS was placed onto the transparent electrode of the GDV camera. The ground electrode was then placed vertically in the culture dish to the depth of contact with liquid. The voltage mode was fixed at 90, 115 or 125 V. The frequency at which the poles of the electrodes changed was 1 kHz. The exposure time was varied from 0.6 to 32 s. Photography was carried out in the dark. As controls, GDV was performed using culture dishes of the different diameters (35 and 50 mm) or with square cuvettes, with culture medium or PBS without cells, with distilled water, and with suspensions of E. coli or nonpolar particles of chalky powder. A general view of the experimental setup is shown in Figure 4. In these GDV experiments, the glow appears to be largely due to photoelectron emission from charged particles within the solutions and to avalanche gas discharges from the bottom of the culture dish.
Statistical comparisons of rBMSC proliferation were based on the results of three separate experiments with the same incubation time. Cells were detached and counted using a counting chamber. The result is expressed as the mean value ± standard error. Statistical significance of differences between the needle-exposed group and the needle-free control group was performed using Student’s t test and the Wilcoxon-Mann-Whitney U test, P = 0.05.
First, we investigated the effects of the magnetised rod (needle) on the proliferation of rBMSCs. rBMSCs, 10000 cells/cm2 (passages 2-3) were placed in culture dishes of 20 cm2 (50 mm diameter). Needles were introduced into the centre of each dish, and the dishes were incubated for 1, 3 or 6 d. Then, the cells were photographed and counted. As a control, rBMSCs were grown without the needles. We did not observe any significant differences between the experimental and control cells (Table 1).
| Exposure time (d) | The average of three independent replicates of the experiment (the number of cells is × 103/cm2± SE) | Experimental t statistic | Standard t statistic (tst) | Experimental U statistic | Standard U statistic (Ust) | |
| Experiment | Control | |||||
| 1 | 10.77 ± 3.31 | 10.67 ± 1.28 | 0.06 | tst = 2.78 when the number of degrees of freedom κ = 4 and P = 0.05 | 3 | Ust = 0 when the number of observations n1 = n2 = 3, P = 0.05 |
| 3 | 20.33 ± 2.36 | 21.67 ± 0.47 | 1.78 | 2 | ||
| 6 | 31.77 ± 2.29 | 30 | 2.76 | 3 | ||
| from 3 to 6 | 11.44 ± 3.24 | 8.33 ± 0.47 | 1.12 | |||
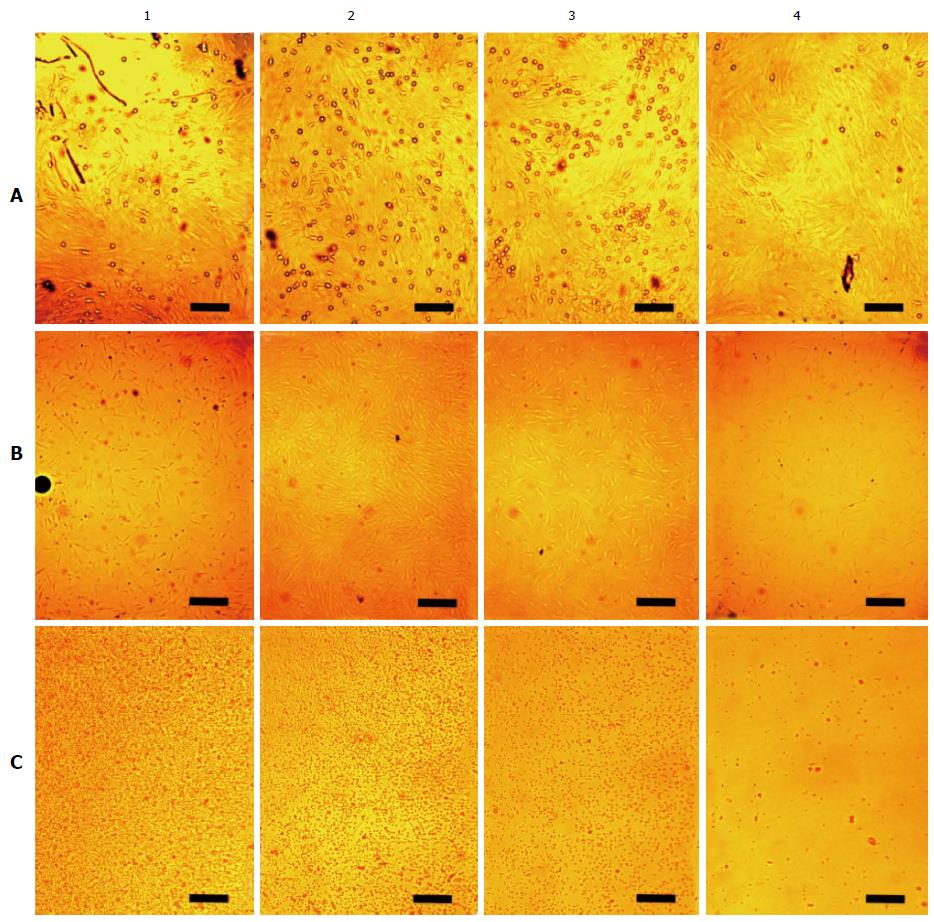
The cells were directly photographed in culture dishes after their incubation. When rBMSCs were cultured for 6 d, they formed a monolayer over which small, rounded cells (approximately 1 μm diameter) were clearly visible. Figure 5A shows that these cells are unevenly arranged on the dish bottom. There are few cells near the needle in region 1. There are more cells in areas 2 and 3, and the number of cells decreases again in region 4, along the outer edge of the dish. After incubating the cells with a needle for 3 d, a similar pattern emerged. However, the small cells were almost entirely eliminated after the third passage. When using hBMSCs, similar results were obtained. On cultivating BMSCs for a day with the needle spread BMSCs were obtained unevenly distributed too. Because the cells do not have time to reproduce any during a day we increased the concentration of spread BMSCs up to 20 thousands per square centimetre. Figure 5B shows hBMSCs exposed with the needle during a day. There is most cell density in the region 2.
Figure 5C shows the distribution of suspended human cells without exposure to the magnetised rod. Mononuclear leukocytes made up the majority of the cells, and the cells appear as a heap in the centre of the dish. There are many cells in region 1, fewer in areas 2 and 3, and practically no cells in region 4. This effect arose during a single day of incubation. We gently redistributed the cells uniformly within the dish. However, the next day, they had again piled up in the centre. We introduced the needle into the cell heap. The next day, the cells had redistributed over the dish bottom more or less uniformly. No heaping was observed for cells cultured in dishes with uncharged, hydrophobic bottoms. Square and rectangular flasks with negatively charged bottoms showed faint clustering of the cells.
Photographing living cells allows the cells to be used in experiments again. However, it is obvious that this method is not ideal for establishing exactly how the cells are distributed along the dish bottom. Therefore, to clarify the cell distribution, the cells were fixed in the dishes and then stained, and the entire dish bottoms were scanned. Over the 2 years of experiments, approximately 160 dishes were stained.
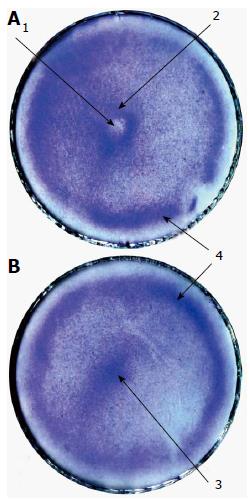
In using rBMSCs, positive results were obtained from the first to 11th-12th passages. Figure 6 shows rBMSCs exposed and unexposed (control) to the needle. The needled cells show a rarefaction in the centre of the dish where the end of the needle was (the central depression). Annular crowding of the cells in a 3-5 mm width is observed at approximately 5 mm from the centre of the dish (the central ring). In some dishes, there is another ring-shaped cluster of cells at approximately 5 mm from the edge of the dish (the peripheral ring). The controls may include two clusters of cells: A cell accumulation in the centre of the dish (the central heap) and the peripheral ring. There was only small, inconstant effect involved when using 35- or 90-mm culture dishes. Therefore, only the 50-mm dishes were utilised in the subsequent experiments.
To eliminate the possible influence of metal ions diffusing from the needle on the formation of the central depression, the effects of the non-magnetised needles and comparable plastic rods on the arrangement of rBMSCs were evaluated. Non-magnetised needles did not cause the formation of the central depression and the central ring, and neither did thin plastic rods. When the non-magnetised needles or plastic rods were inserted away from the centres of the dishes, rarefactions around them remained absent. Further, magnetised needles inserted away from the centres of the dishes also did not form rarefactions (Figure 7B, C and D). However, the central heap seemed to change its shape, becoming irregular and elongated, when magnetised needles were applied in different positions. We next tested the influence of the needles’ magnetic polarisation on the arrangement of the rBMSCs, and we did not observe significant differences in these influences. The influence of the north pole seemed to produce a somewhat more vivid picture.
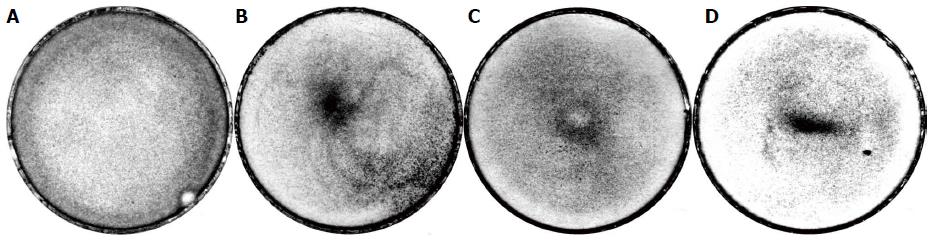
In addition, we observed that the generation of these effects depended on the season. The most pronounced response of BMSCs to the magnetised rods occurred in spring. However, there were always a number of unresponsive cells. In the images, this is reflected by the presence of the background (Figure 6). There were more unresponsive cells in summer and autumn. This was expressed both as a more intense background and as the disappearance of the peripheral ring (Figure 7B, C and D). In winter, from mid-November to mid-January, BMSCs showed no responses to the magnetised needle (Figure 7A). There was always the strongest effect in spring, some effect in summer and autumn, and no effect in winter. Approximately 40 dishes were tested for each season. The 40 dishes for winter (from mid-November to mid-January) showed no effect. The 40 dishes for spring (from March to April) all showed the effect. Consequently, seasonality is present at P < 0.05.
In winter, studies were performed to determine the effects on the rBMSCs distribution of both the method of plating the cells and the method of setting the needles. The dishes with just the cells placed in them were moved horizontally on the bench surface back and forth in the 8 cardinal directions, for three times per direction. Thereafter, some of the dishes were moved horizontally in a circle one or three times, creating a torque in the culture medium. Needles were not inserted in these dishes. Notably, these dishes showed no central heaps. In some instances, a formation resembling the peripheral ring appeared. However, in these cases, this “ring” was not uniform and complete, and it was located closer to the edge of the dish than the peripheral ring. To test the method of setting the needles in the culture dishes, needles were introduced perpendicularly into the dishes, heavily or slightly rest against the bottom, and rotated clockwise or anticlockwise. In these cases, we found no central depressions or central rings.
In addition to human and rat BMSCs, in the spring, we used two stable cell lines: Mouse fibroblast NCTC L929 cells and human glioblastoma A172 fibroblasts. NCTCs, which proliferate rapidly, were used in our experiments beginning at passage 2 (i.e., 4 d after thawing). These cells showed a certain unstable positive result for two weeks after thawing both in the control and in the experiment. However, after that time period, the cells showed no effects of the magnetised needles and distributed uniformly across the dish bottoms both in the control and in the experiment (as in Figure 7A). Similar results were obtained for the A172 cells.
We investigated the gas discharge glow (GDG) from circular culture NUNC dishes of diameters of 35 (9.6 cm2) and 50 mm (20 cm2) and from rectangular cuvettes with an area of 20 cm2. The emissions at voltages of 90, 115 and 125 V were investigated. The same volumes of water, PBS or culture medium with or without cells were placed in the dishes as in the experiments with the needles. Annular GDG gradually forming from 10 to 18-20 s was observed when using circular 50-mm dishes and a voltage of 115 V (Figure 8C3 and C4). Three peripheral rings were always clearly visible when the location of the ground electrode was in the centre of the dish. The central ring was less pronounced. The average distances between the rings were 5-7 mm (Figure 8B1 and C4). Annular illumination was not observed without the presence of liquid in the dish (Figure 8A1), when the liquid was water (uncharged liquid, Figure 8A2), or from hydrophobic dishes or rectangular cuvettes (Figure 8A3 and A4). The dishes filled with PBS produced annular illuminations more clearly than those filled with culture medium and/or various cells. Three positions of the grounding electrode were evaluated: In the centre, next to the edge and equidistant from the centre and the edge of the dish (Figure 8B1, B2 and B3). Two outer rings were recorded in all cases. The central ring appeared only when the ground electrode was located in the centre of the dish. With a voltage of 90 V, the rings were not formed (Figure 8C2). When using 35-mm dishes, some faint crowded rings around the periphery of the dish were formed at approximately 30 s with a voltage of 125 V (Figure 8D1). The rings were not formed in 50-mm dishes at a voltage of 125 V. Thus, EM resonances existed in the system.
Patterns regarding the shape and size of the dishes, the location of the grounding electrode and the voltage were detected in both the GDV study of culture medium or PBS and the GDV study of suspended rBMSCs, NCTCs or E. coli cells. The rings were the most prominent and stable when using NCTCs. The rings could be obtained by placing these cells in the high-voltage field for 32 s for many consecutive applications. Although the suspension of rBMSCs provided a relatively clear image at approximately 18-20 s of exposure, the glow disappeared within 4-5 s after that and never reappeared. The glow also did not appear in repeated attempts after a while, whether in fresh culture medium or PBS. After a single treatment with the high-voltage field during 32 s, NCTCs and E. coli stopped proliferating for several days but then continued to multiply actively. Rat and human BMSCs treated thus ceased proliferating permanently, but when attached to the bottom of the dish, they remained viable for 2-3 wk. A few dishes filled with NCTC suspension after GDV were placed carefully without shaking in an incubator for one day and then fixed and stained. These dishes did not present cell formations similar to the light patterns identified by GDV. When using chalky powder of various concentrations (uncharged suspension), no annular illumination was obtained. In addition, after a day of using a needle to spread the cells into the central depression and ring, we observed GDV. After GDV, the cells were fixed and stained. In these cases, we obtained no annular illumination (Figure 8D2 and D3). In addition, no annular emission was obtained from dishes filled with culture medium and preincubated for a day (Figure 8C1). In most cases, E. coli produced GDG that was close to annular (Figure 8D4).
Previously, other authors observed that MF increased, decreased or did not affect the proliferation of various types of cells[21,22]. No clear response patterns of biological systems to the influences of MF were observed. We did not observe significant influence of MFs on the rBMSC proliferation rate. This may indicate that the influence of MFs on cell proliferation is indirect. It may be that the direct effect of MFs is related to morphogenesis. It is logical that structure maintenance of tissues and organs and cell renewal are regulated by different mechanisms.
If fibroblasts are spread on plastic or glass, their attachment occurs only after 30-40 min from placing them into a dish; their spreading continues during the following day[23]. At 5-10 min after placing cells into a dish, most of the cells have reached the bottom. Upon reaching the bottom of the dish, for approximately half an hour, fibroblasts are in contact with the dish but still not attached to it. At that point, the fibroblast performs a type of “rolling” movement and “releases” and “inhales” its filopodia. Thus, there is a gap between touching and attachment of a fibroblast during which it can be moved by an EF.
We have used culture dishes coated with a uniform negative electric charge on the bottom surface. In accordance with Ostrogradsky’s-Gauss’s divergence theorem, the electric field strength in the plane of the charge, along the dish bottom, decreases from the periphery to the centre of the bottom, where it reaches zero. Thus, the charged particles (cells) situated in the plane of the dish, when of appropriate size and weight, will move to the periphery of the dish (opposite charge, “+”) or to its centre (same charge, “-”). Because culture media are ionic solutions, these electrical forces affect only cells in contact with the bottom of the dish. The forces are absent in the medium column because of an electrical double layer. The participation of the charge of the dish bottom in the cell distribution across the dish has been confirmed by the absence of movement of suspended cells towards the bottom centres when using dishes with hydrophobic bottoms and by the absence of GDV rings in the dishes with culture medium without cells after a day of incubation (Figure 8C1).
It is widely believed that cells can carry one type of electrical charge or another and can be moved by microelectrophoresis[24]. In our experiment without the use of the magnetised needles, some BMSCs accumulated in the dish centre (central heap), some accumulated in the dish periphery (peripheral ring), and some did not respond to the EF. Thus, cells within the same population may apparently have either positive or negative charges on their surfaces or may be electrically neutral. This may depend on the availability and structure of the glycocalyx. The glycocalyx, due to its sulfate groups, creates a negative charge on the cell surface, whereas the outer surface of the cell membrane has a positive charge due to ion pumps. The fact that the glycocalyx plays a pivotal role in the cell movement in EFs along the dish bottoms is demonstrated by the accumulation of suspended cells (leukocytes) in the dish centres through one day of incubation. These cells, as they were not subjected to trypsinisation, retained an intact glycocalyx on their surfaces. In addition, these cells cannot crawl from place to place like fibroblasts. BMSCs do not appear to crawl across the dish to produce the effects induced by EFs. Further, one day is insufficient time for BMSCs to crawl the distances involved. That the BMSCs are not crawling is partly illustrated by the positions of their “tails” in all directions after one day of incubation with the needle (Figure 5B). To remove the cells from the substrate, we applied trypsin for 5 min. With this treatment, a large part of the glycocalyx is retained. It has been shown that the glycocalyx is completely removed from the cell surface only after 15 min of incubation in 2.5 g/L trypsin solution[25]. After trypsinisation during cell isolation from tissue, the complete restoration of the glycocalyx occurred in 7-10 d[26]. Therefore, by the time the cells were subjected to EMFs (4-7 d after passaging), the cells appeared to have a complete layer of glycocalyx. In spring, rBMSCs trypsinised for 5 or 10 min showed the effect involved. In contrast, rBMSCs during winter and NCTCs and A172 cells at two weeks after thawing showed no such effect. Based on these data, one can conclude that the molecular structure of the glycocalyx is more important than its thickness. In spring, NCTC and A172 cells showed the effect before 2 wk after thawing, but later, they showed no such effect. The behaviour of NCTCs and A172 cells led to the conclusion that the glycocalyx charge depends on protein synthesis. Those proteins are related to some proteins of transformed cells that are not synthesised until the cell’s DNA is restored.
Thus, upon reaching the bottom of the dish by gravity, BMSCs begin to move, perhaps by rolling, in response to the EF to the centre or the periphery of the dish, in accordance with the glycocalyx charge, whereby they form the central heap and the peripheral ring.
With the introduction of the magnetised needle, magnetic forces are established, and the resultant vector is directed from the centre to the periphery of the dish (note the sparseness around the needle). It is likely that because BMSCs bear negative charges on their surfaces, they move towards the dish centre as long as the magnetic force directed from the centre equals the electric force directed towards the dish centre. It is similar for moving the cells away from the dish centre. Thus, a “donut” (torus) of cells (the central ring) might arise.
Firmly established mechanisms of primary reception of MFs and EMFs in the range of non-thermal effects have not yet been identified. Cytoskeletal rearrangements and intracellular signalling as a result of exposure to EMF on cell surface membrane are assumed to be among the primary magnetoreceptive mechanisms[8]. However, these mechanisms cannot explain the effects involved here. To date, no one has shown that MFs can move SCs. Currently, to move cells, magnetic nanoparticles are introduced into them[27]. In our experiments, the cells were moved through MFs over distances of up to 5 mm (the radius of the central ring). As seen from experiments with leukocyte suspensions (Figure 5C), this distance can probably be extended. It is obvious that EFs and MFs affect different cellular mechanisms. However, these mechanisms are somehow associated with each other, given that the cells were not moved in the dishes with the hydrophobic bottoms. Magnetic and electrical mechanisms appear to be nonlinearly related. Therefore, the electrical effects detectable in the controls showed significant variation between experiments. This was manifested in the presence or the absence of the peripheral ring and in the different forms of the central heap. Meanwhile, the magnetic effects, specifically the central depression and the central ring, did not show changeable shapes.
For a MF to move a cell, it must have a directed ion stream. Although there are transmembrane ion currents, these currents are balanced and therefore cannot serve as mechanisms by which MFs can induce cells to move. Protozoan (e.g., the nutrition of Paramecium caudatum) and plant cells (e.g., cytoplasmic current in the cambium layer) possess cytoplasmic currents. It is likely that the slow transport in the axons of nerve cells and the circulation of the outer membrane by ruffling are accompanied by directional circular currents of cytoplasm. It is likely that cells can also have circular cytoplasmic currents when detached. If this is actually the case, there is also circular movement of cytoplasm ions, which would produce MFs. Then, the MF of the magnetised needle would affect the cytoplasm, moving it and moving the entire cell together with it. In addition, cells would move in opposite directions in accordance with the direction of their cytoplasmic motion. Our finding that both the N and S needle poles could cause the central depression supports this model. In 1999, Makarevich[28] used yeast cultures to show that different permanent magnetic poles produced only quantitative differences in cellular responses. We have also shown that not all cells respond to EM exposure. Thus, there are three types of cells in the same population: Cells with cytoplasm circulating clockwise, cells with cytoplasm circulating anticlockwise, and those with non-circulating cytoplasm. This suggestion accounts for the observation of the same type of central depression in response to either magnetic pole of the needle. The fact that cells of the same population can react differently to the same MF has been shown previously[29].
The above model cannot explain all of the observed effects. The formation of the peripheral ring remains unclear. Based on the above assumptions, some of the cells should accumulate next to the dish wall and others near the needle. Furthermore, in this model, the phenomena causing the needle MF should be observed when setting the needle outside the centre of the dish. All these reasons led us to explore in more detail the EMFs formed in the culture dishes.
The movement of the cytoplasm create a MF in the range of nT or less. This is likely insufficient to move the cells. Therefore, an amplifier of the MF is needed. In the model system some resonances appear to exist which function as such an amplifier. This assumption is confirmed by findings of the GDV study as follows: The presence of glowing rings; the importance of the shape and size of the dish; the importance of the electric voltage magnitude; and the fundamentally unchanged ring-shaped glow upon setting the ground electrode off to the side from the dish centre (Figure 8). This effect in particular may indicate that it is not necessary to introduce the needle to the acupoint centre only to achieve a therapeutic effect. All of the acupuncture practice has noted this; the exact anatomical centre has not been established for any acupoint. The resonant nature of the field created by the magnetised needle is also demonstrated by the absence of sparseness around the needles set eccentrically and by the poor results when using culture dishes with diameters of 35 and 90 mm. The same findings also demonstrate that there is little or no effect of metal ion diffusion from the needle on the investigated phenomenon. The absence of cell sparseness around the plastic hydrophobic rod demonstrates a very small value of hydrophobic forces produced by the dish wall. Thus, magnetic resonance phenomena are present in the system of the culture dish, the BMSCs, and the needle. There are obvious similarities between the rings obtained by cell staining and observed in GDV (Figure 8C4 and D3). Furthermore, GDV (the same as the cell cultivation in the dishes with hydrophobic bottoms) shows the necessity of a polar liquid or suspension for the effect to exist, with annular GDG being absent from water and from chalky suspensions. We believe that the observed resonance phenomena explain the sparseness around the needle only in the dish centre, the absence of cell accumulation around the needle and forming the peripheral ring. The resonant nature of the meridian system (and, consequently, acupoints) has been proposed previously[30]. However, experimental verification of this phenomenon has so far been absent.
In a series of studies, we have shown the fundamental insignificance of the methods of cell application and distribution across the dish bottom and the mode of needle administration for the generation of the central heap, the central depression and the central ring. We have shown that if the cells do not form these structures themselves, we cannot form them by neither some special twisting the needle nor by some rocking or spins the dish as a whole. Clearly, the uniform distribution of cells across the dish bottom may be broken in those cases. Additionally, as shown by GDV, the dish charge sometimes appears to be unevenly distributed. For example, in the bottom left sector, a bit below the centre of Figure 8B1, a gap is clearly visible, distorting the overall picture of the glow. These irregularities in charge also seem to affect the movement of the cells.
In addition, we observed a seasonal pattern in the effects involved. Seasonal phenomena have already been described in the literature in studying whole organisms and their foetuses. Temporary physiological “windows” from minutes and hours to seasons have been reported[31,32]. Blank et al[33] showed Na-K-ATPase in various enzyme activity to respond or to non-respond to the same EMF. Laboratories have long empirically known that cell cultures can behave unconventionally during the summer, from mid-July until the end of August, and during winter, from mid-November to mid-January. However, no one has shown the seasonal effect in cell cultures. It is likely that there are transition states from autumn to winter and from winter to spring, but examining those states was beyond the scope of this study.
In conclusion, in this study, we first showed that a magnetic field of 10-100 μT can move human and rat BMSCs over a distance visible to the naked eye without the introduction of magnetic nanoparticles inside the cells. We have shown that achieving this effect requires a constant EF from one source, a constant MF from another source, and cells in the responsive functional state. We have demonstrated that EM resonances exist in this system. We propose a model to explain the results. We hypothesise that the electrical phenomena depend on the glycocalyx and that the magnetic phenomena may depend on movements of the cytoplasm. Thus, we propose an experimental model of acupoint that may provide the basis for an explanation of EM phenomena in acupoints as well as some features of acupoint treatments (acupoints as a biological MF amplifier). In this study, we also showed that the responses of human and rat BMSCs to EFs and MFs in this system depend on the season. This result is consistent with known features of acupoint function.
From our perspective, aside from the roles of vascular nerve sensors[13] and connective tissue connectors[34], acupoints act as MF amplifiers for maintaining the structure of organs and tissues of the body. Based on our present findings, this idea seems less outlandish. Obviously, there may be opportunities for the conditions in acupoints for MFs structural formative action on cells, tissues, and organs to exist. This possibility needs now to be explored in a whole organism.
Further studies may be advanced on several fronts. If using this model of acupoint one may investigate the influence of various physical and chemical factors such as visible, infrared and ultraviolet light. There is the nature of the resonances observed to be resolved. Solving this problem would allow cells to be moved much further than 5 mm. Verification of the movable cytoplasm hypothesis is also important. If the cytoplasm of the cell genuinely somehow moves directionally, it may be another cellular mechanism for the regulation of cellular functional activity. For example, it has long been known that rearrangement of the actin cytoskeleton first requires its complete disassembly. That cytoskeletal peculiarity may be directly linked to the movement of the cytoplasm. An interesting question is the nature of the glycocalyx charge. The glycocalyx can reach a thickness of 20 nm and thus create tissue electrical potential gradients. Therefore, the model proposed here may prompt various studies in cell biology and physiology.
The authors are heartily grateful to Georgy Petrovich Pinaev, the head of the Department of Cell Cultures of Institute of Cytology of Russian Academy of Sciences. Unfortunately, he passed away. The study was carried out solely thanks to him. He participated in all the phases of the current experimental research. Also we express gratitude to Svetlana A Alexandrova, Irina A Chistyakova and Danila E Bobkov for valuable advices in carrying out the experiments.
Regeneration occurs via two types of processes: (1) forms and structures are reproduced; and (2) the cellular-intercellular mass is reproduced. Stem cells reproduce the cellular mass. It is clear that there must be some mechanism for controlling the reproduction of the form and structure of tissues and organs. This mechanism may be related to the acupuncture meridian system.
At present, various types of stem cells have been successfully used to treat acquired or congenital defects in human tissues. Tissue grafts are used for correcting large tissue defects or the disordered structure of hollow viscera. However, reproducing the structures of solid organs such as the liver, kidney or heart has not yet been successful. Although the morphological and electrical features of acupoints are known, the nature of the meridian system has yet to be determined.
The authors first showed experimentally that a magnetic field may be involved in the mechanism of structural formative regeneration and the acupuncture meridian system. The authors first showed that when using a magnetic field of 10-100 μT, human and rat bone marrow derived stromal stem cells can be moved without the introduction of magnetic nanoparticles inside the cells. The authors have shown that achieving this effect requires a constant electrical fields from one source, a constant magnetic fields from another source, and cells in the responsive functional state. The authors have demonstrated that EM resonances exist in this system.
To verify the discovery, the authors proposed the acupuncture point as a model. This model allows the investigation of the many cell properties involved in the cell moving by a magnetic field. Of course, it may take considerable effort to develop methods of managing cell movement in the body by means of a magnetic field without magnetic nanoparticles. However, the authors are now confident that these methods exist. Furthermore, the same methods will able to affect the existing stem cells of a patient to induce the recreation and maintenance of the correct structures of the patient’s organs and tissues.
BMSCs: Bone marrow derived stromal stem cells. These multipotent cells serve as source for other types of stem cells and regenerative processes. GDV: Gas discharge visualisation is a method based on the visualisation of gas discharge induced photoelectron emission from the surface of an object placed in a high-tension electric field (Kirlian effect). Ultimately, the authors see a glow from the object. This method uses an electromagnetic field of 1 kHz frequency. Thus, the glow is caused by both electrical and magnetic fields.
This is an important research describing about the relationship between BMSCs and electromagnetic field. The manuscript could be of interest in its field due to the novelty of use of acupuncture needles as source of a low magnetic field.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Scarfì S, Tanabe S, Zou TM S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Atala A, Lanza R, Thomson JA, Nerem RM. Principles of regenerative medicine. Burlington: Academic Press Elsevier Inc 2010; 733-1116. |
| 2. | Lubischew AA. Philosophical aspects of taxonomy. Annu Rev Entomol. 1969;14:19-38. [DOI] [Full Text] |
| 3. | Friedenstein AJ, Luria EA. Cellular bases of hemopoietic microenvironment [in Russian]. Moscow: Meditsina 1980; 49-75. |
| 4. | Gurwitsch AG. Biological field theory [in Russian]. Moscow: Sovetscaja Nauka 1944; 1-156. |
| 5. | Beloussov LV, Opitz JM, Gilbert SF. Life of Alexander G. Gurwitsch and his relevant contribution to the theory of morphogenetic fields. Int J Dev Biol. 1997;41:771-777; comment 778-779. [PubMed] |
| 6. | Kaznacheev VP, Mikhailova LP, Kartashov NB. Distant intercellular electro-magnetic interaction between two tissue cultures. Bull Exp Biol Med. 1980;89:345-348. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Yung KT. A birdcage model for the Chinese Meridian System: part I. A channel as a transmission line. Am J Chin Med. 2004;32:815-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Binhi VN. Magnetobiology: Underlying physical problems. New York: Academic Press 2002; 39-208. |
| 9. | Adey WR. Biological effects of electromagnetic fields. J Cell Biochem. 1993;51:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 168] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Emelyanov AN, Kiryanova VV. Photomodulation of proliferation and differentiation of stem cells by the visible and infrared light. Photomed Laser Surg. 2015;33:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Chang S. The meridian system and mechanism of acupuncture-a comparative review. Part 1: the meridian system. Taiwan J Obstet Gynecol. 2012;51:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Dung HC. Anatomical features contributing to the formation of acupuncture points. Am J Acupunct. 1984;12:139-143. |
| 13. | Lou XF, Jiang SH. Anatomical characters and classification of acupoint. Zhongguo Zhenjiu. 2012;32:319-323. [PubMed] |
| 14. | Ahn AC, Wu J, Badger GJ, Hammerschlag R, Langevin HM. Electrical impedance along connective tissue planes associated with acupuncture meridians. BMC Complement Altern Med. 2005;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Shenberger R. Acupuncture meridians retain identity after death. Am J Acu-punct. 1977;5:357-361. |
| 16. | Hou TZ, Dawitof M, Wang JY, Li MD. Experimental evidence of a plant meridian system: I. Bioelectricity and acupuncture effects on electrical resistance of the soybean (Glycine max). Am J Chin Med. 1994;22:1-10. [PubMed] |
| 17. | Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Wolfe M, Pochampally R, Swaney W, Reger RL. Isolation and culture of bone marrow-derived human multipotent stromal cells (hMSCs). In: Prockop DJ, Phinney DG, Bunnell B. Mesenchymal Stem Cells: Methods and Protocols. Methods Mol Biol. 2008;449:3-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;182:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 498] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Korotkov KG. The foundations of GDV-bioelectrography. St. Petersburg: SPbGITMO (TU) 2001; 24-78. |
| 21. | Ross SM. Combined DC and ELF magnetic fields can alter cell proliferation. Bioelectromagnetics. 1990;11:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Malko JA, Constantinidis I, Dillehay D, Fajman WA. Search for influence of 1.5 Tesla magnetic field on growth of yeast cells. Bioelectromagnetics. 1994;15:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1602] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 24. | Jiang D, Sims CE, Allbritton NL. Microelectrophoresis platform for fast serial analysis of single cells. Electrophoresis. 2010;31:2558-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Hamouda H, Kaup M, Ullah M, Berger M, Sandig V, Tauber R, Blanchard V. Rapid analysis of cell surface N-glycosylation from living cells using mass spectrometry. J Proteome Res. 2014;13:6144-6151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Lee YS, Hsu TS. Relationship between reestablishment of sarcolemma-glycocalyx ultrastructures and restoration of transmembrane potentials in cultured rat heart cells. J Electrocardiol. 1987;20:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Cores J, Caranasos TG, Cheng K. Magnetically Targeted Stem Cell Delivery for Regenerative Medicine. J Funct Biomater. 2015;6:526-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Makarevich AV. Cell biophysics - effect of magnetic fields of magnetoplastics on the growth of microorganisms. Biophysics. 1999;44:65-69. |
| 29. | Semm P, Beason RC. Responses to small magnetic variations by the trigeminal system of the bobolink. Brain Res Bull. 1990;25:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Yung KT. A birdcage model for the Chinese meridian system: part II. The meridian system as a birdcage resonator. Am J Chin Med. 2004;32:985-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Ho MW, Stone TA, Jerman I, Bolton J, Bolton H, Goodwin BC, Saunders PT, Robertson F. Brief exposures to weak static magnetic field during early embryogenesis cause cuticular pattern abnormalities in Drosophila larvae. Phys Med Biol. 1992;37:1171-1179. [PubMed] |
| 32. | Ružič R, Jerman I, Gogala N. Water stress reveals effects of Elf magnetic fields on the growth of seedlings. Electromag Biol Med. 1998;17:17-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Blank M, Soo L. The threshold for Na-K-ATPase stimulation by electromagnetic fields. Bioelectrochem Bioenerg. 1996;40:63-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Langevin HM. Connective tissue: a body-wide signaling network? Med Hypotheses. 2006;66:1074-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |