Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.657
Peer-review started: July 29, 2014
First decision: September 28, 2014
Revised: December 10, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: April 26, 2015
Processing time: 268 Days and 9 Hours
AIM: To improve osteogenic differentiation and attachment of cells.
METHODS: An electronic search was conducted in PubMed from January 2004 to December 2013. Studies which performed smart modifications on conventional bone scaffold materials were included. Scaffolds with controlled release or encapsulation of bioactive molecules were not included. Experiments which did not investigate response of cells toward the scaffold (cell attachment, proliferation or osteoblastic differentiation) were excluded.
RESULTS: Among 1458 studies, 38 met the inclusion and exclusion criteria. The main scaffold varied extensively among the included studies. Smart modifications included addition of growth factors (group I-11 studies), extracellular matrix-like molecules (group II-13 studies) and nanoparticles (nano-HA) (group III-17 studies). In all groups, surface coating was the most commonly applied approach for smart modification of scaffolds. In group I, bone morphogenetic proteins were mainly used as growth factor stabilized on polycaprolactone (PCL). In group II, collagen 1 in combination with PCL, hydroxyapatite (HA) and tricalcium phosphate were the most frequent scaffolds used. In the third group, nano-HA with PCL and chitosan were used the most. As variable methods were used, a thorough and comprehensible compare between the results and approaches was unattainable.
CONCLUSION: Regarding the variability in methodology of these in vitro studies it was demonstrated that smart modification of scaffolds can improve tissue properties.
Core tip: Currently, special attention has been directed to the design of new scaffolds by adding bioactive molecules and nanoparticles. “Smart scaffolds” in bone tissue engineering not only act as cell delivery materials, but they are also responsive to their environment and therefore stem cells are more likely to attach, proliferate and differentiate on them. These scaffolds can be fabricated by adding either of growth factors, extracellular matrix proteins or nanoparticles to the bone substitutes using various techniques. These modifications can enhance the in vitro response of bone scaffolds toward cells.
- Citation: Motamedian SR, Hosseinpour S, Ahsaie MG, Khojasteh A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J Stem Cells 2015; 7(3): 657-668
- URL: https://www.wjgnet.com/1948-0210/full/v7/i3/657.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i3.657
Tissue engineering is a helpful alternative strategy for conventional treatments in medicine. It was defined by Langer and Vacant[1] as “an interdisciplinary field of research that applies the principals of engineering and life sciences towards the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ”. Bone tissue engineering uses life sciences and engineering to aid the function of injured bone tissue via a triad of artificial extracellular matrix (ECM) scaffold, stem cells that can become osteoblasts and growth factors[2,3]. The efficacy of this combination has been studied in animals[4-6] and humans[7-9]. Mesenchymal stem cells, the most common source of osteoprogenitor cells, can be derived from bone marrow[10-13], adipose tissue[14-16], and dental and periodontal tissues[17-19]. Growth factors, such as bone morphogenic proteins (BMPs), have the capability to modulate stem cell activity towards bone regeneration[20-24].
Scaffolds play a key role in bone tissue engineering providing a 3-dimensional environment for cell seeding and proliferation as well as filling bone defects while providing mechanical competence during bone regeneration[25-27]. Osteoconductivity, porosity and biodegradation are the required properties for a scaffold to be successful in bone tissue engineering, enhancing bone formation and angiogenesis and supporting attachment and proliferation of osteoblasts[28]. In this context, various scaffolds such as hydroxyapatite HA, tricalcium phosphate (TCP), collagen, chitosan, polycaprolactone (PCL), and poly(lactic-co-glycolic acid) (PLGA), have been used[2,3].
Recently, tissue engineers have focused on structural design and surface properties of scaffolds. Various modifications such as addition of bioactive molecules or nanoparticles can enhance attachment and proliferation of stem cells on the scaffold[29-31]. These “smart scaffolds” improve osteogenic differentiation of stem cells leading to a more responsive reaction to changes in their surrounding environment[32]. The current study reviews newly developed scaffolds and their smart modifications.
Papers relevant to in-vitro experiments on newly fabricated smart scaffolds were reviewed. Three modifications were considered as smart namely: (1) addition of nanoparticles; (2) addition of ECM-like molecules; and (3) addition of growth factors. Studies which performed these smart modifications on conventional bone scaffold materials were included. Scaffolds with controlled release or encapsulation of bioactive molecules were not included. Experiments which did not investigate response of cells toward the scaffold (cell attachment, proliferation or osteoblastic differentiation) were also excluded.
An electronic search was conducted in PubMed limited to English language, relevant publications from January 2004 to December 2013 with available full texts. Published papers on smart scaffolds were found using the following keywords alone or in combination: bone tissue engineering, smart scaffold, xenograft, xenogenous bone, allograft, allogenous bone, bone substitute, bovine bone matrix, allogenic bone, xenogenic bone, coat*, surface modification, surface enhancement, biofunctional*, bioact*, biomimet*, nano*, extracellular matrix, collagen and growth factor.
Initial paper selection was done by reviewing titles and abstracts of all selected papers. The full texts of potentially suitable articles were obtained for final assessment according to the exclusion and inclusion criteria. The reports of the most relevant data were included and analyzed in a qualitative manner.
This study was a literature review and no statistical method was used in this study.
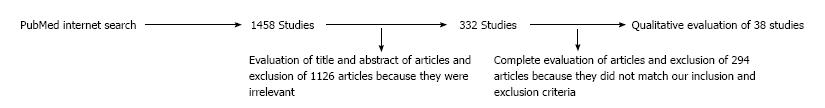
Figure 1 demonstrates the design of the current review. The initial search resulted in 1458 articles. Following the screening of titles, abstracts and full texts, 38 papers had the criteria for review.
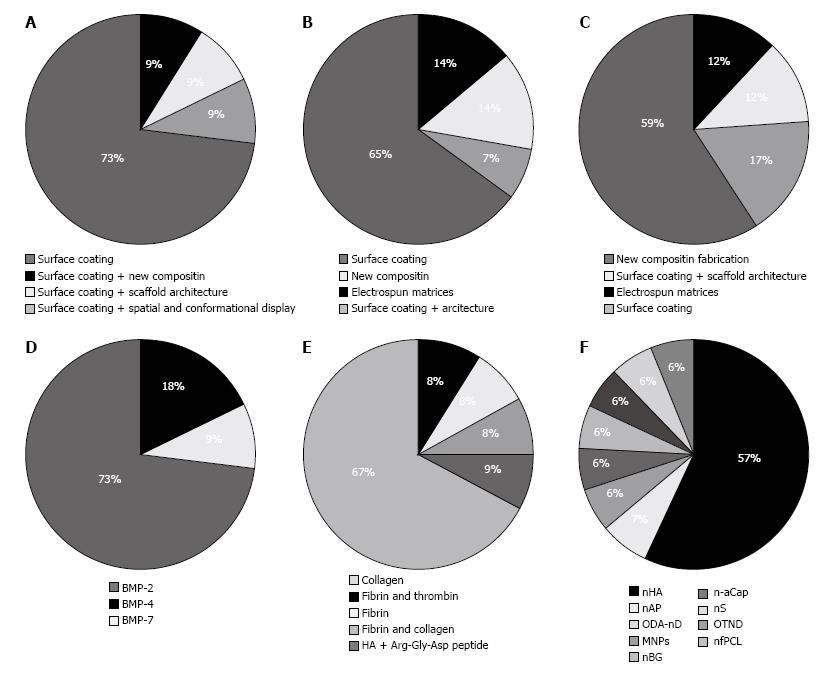
Eleven experiments were studied (Table 1) for scaffold modification; in all these studies, surface coating via growth factors to increase scaffold activity was used (Figure 2A). In addition, modification of architecture and spatial conformation of the main scaffold was performed[38]. The main scaffold differed in each study. The most commonly used scaffold was PCL, followed by TCP, PLGA, Bio-Oss, Calcium/magnesium-doped mesoporous silica, calcium phosphate cement, collagen, carbon nanotubes, TolAIII fusion-osteopontin-switch tag and glutaraldehyde cross-linked gelatin (GTG). The most commonly applied growth factor was BMPs including BMP-2, BMP-4 and BMP-7 (Figure 2D). Different methods were used to add BMPs to the main scaffold: attachment of BMP-4 to scaffolds by cross linking conjugating techniques, deep and dry methods for addition of BMP-2 to Bio-Oss and using a set of nanoparticles providing the release of BMP-2. Scaffold fabrication methods varied vastly among studies.
| Ref. | Scaffold | Fabrication | Type of modification | Cell type | Tests and results |
| Yang et al[33], 2005 | GTG + BMP-4 | - | Surface modification | NRCOCs | ALP activity: ALP levels in GTG + BMP-4 samples higher than GTG samples H and E staining: Greater numbers of attached cells and richer matrix deposits in the GTG + BMP-4 samples VK staining: Larger mineralizing nodules, in greater numbers |
| Turhani et al[34], 2007 | TCP + rhBMP-2 | - | Surface coating | SaOS-2Cs | Cell viability and ALP activity: TCP + BMP-2 > TCP (control) OC secretion: TCP + BMP-2 = TCP |
| Abarrategi et al[29], 2008 | β-TCP + rhBMP-2 | Homogenized + demineralized + heterogeneous deacetylation | Surface coating | C2C12Cs | No alteration in biocompatibility In vivo: New bone formation 3 wk after surgery, much shorter time than control β-TCP ceramics |
| Fei et al[35], 2008 | PLGA/CPC + rhBMP-2 | Solvent-extraction technique | Surface coating | BMMSCs | ELISA: OC: PLGA/CPC + rhBMP-2: 1.2 ng/mL rhBMP-2/CPC: 0.4 ng/mL ALP activity: PLGA/CPC + rhBMP-2: 0.12 μg/h rhBMP-2/CPC: 0.06 μg/h |
| Yilgor et al[36], 2010 | PCL + BMP-2/BMP-7 | Plotting procedure Bioplotter’s CAD/CAM software + wet spinning | Scaffold architecture + surface coating | BMMSCs | ALP activity: BMP-2/BMP-7 + PCL: 1.2 nmol/min |
| Zhang et al[37], 2010 | PCL + BMP-2 | Crosslinking conjugation method | Surface coating | BMMSCs | RT-PCR: (relative) p-smad: PCL + BMP-2 conjugated five times higher than adsorption and control Col 1: PCL + BMP-2 conjugated: 3 PCL + BMP-2 adsorption: 1.5 Control (PCL): 1 ALP activity: 2 times higher than adsorption and control |
| Mitchell et al[38], 2010 | Tol-OPN-ST+BMP2 | - | Spatial and conformational display + surface coating | BMMSCs | Cell attachment: Tol-OPN-ST + BMP2: 175 mm3 Tol-OPN-ST: 60 mm3 Luciferase activity: Tol-OPN-ST + BMP2: 8000 ability unit Tol-BMP-ST: 6000 ability unit |
| Huh et al[39], 2011 | Bio-Oss® + rhBMP-2 + iH | Deep and dry methods | Surface coating | MG63Cs | Cytotoxicity and proliferation: No difference compared to control (Bio-Oss®) ALP activity: 0.2 μmol/min per μg higher than control |
| Dai et al[40], 2011 | CMMS + rhBMP-2 | Polymeric sponge method | New composition + surface coating | BMMSCs | MTT assay: More viable cells on CMMS + rhBMP-2 compared to CMMS RT-PCR: (relative) RunX2: CMMS + rhBMP-2: 32 Control (CMMS): 4 OPN: CMMS + rhBMP-2: 38 Control: 3 In vivo: Induced the ectopic bone formation in the thigh muscle pouches of mice |
| Lu et al[41], 2012 | Col/PLGA + CBD-BMP4 | Forming collagen microsponges | Surface coating | BMMSCs | ALP activity: No differences compared to control group Scaffold supports cell adhesion and proliferation |
| Li et al[42], 2013 | SWNTs-COOH/SWNTs-CH3 + BMP-2 | Organic phase/aqueous phase replacement approach + sonication | Surface coating | C2C12Cs | ALP activity: (relative) SWNTs-ch3 + BMP-2: 150% SWNTs-cooh + BMP-2: 120% |
Regarding the study methods, bone marrow mesenchymal stem cells (BMMSCs) were used in 6 experiments, while different type of cells such as neonatal rat calvarial osteoblast cells, Human osteosarcoma cells (MG63Cs), sarcoma osteogenic cells (SaOS-2Cs) and immortalized mouse myoblast cells (C2C12Cs) were used in other studies. In almost all studies, cell viability was assessed with alkaline phosphatase (ALP) activity assay and the best results were obtained using new compositions compared to control groups. In just one study, no meaningful changes were observed[41]. One study used luciferase activity as their major assay[38]. In 2 studies, real time polymerase reaction assay was used with different biomarkers such as: BMP-2, Runt-related transcription factor 2 (Runx-2), Osteopontin, P-smad and collagen 1.
Among 67 articles, which used ECM-like molecules to enhance scaffold properties, 13 experiments were studied (Table 2). Type of scaffold modification in 10 studies was surface coating. In 1 study, architectural and spatial conformation was performed on the scaffolds[48], and in 2 experiments collagen fibers were elecrospun in conjugation with polymer and ceramic components to form a fibrous scaffold (Figure 2B)[45,52]. Xu et al[30] produced a new composite scaffold by combining collagen, bioglass, hyaluronic acid and phosphatidylserine. In a study by Lechner et al[44], fibrinogen was added to bone tissue core. In their study, CD31 was assessed before and after treatment with newly developed scaffold by fluorescent-activated cell scan techniques and their results showed 100-fold increase in expression of CD31 marker[44]. The most commonly used ECM-like molecule was collagen 1. Other molecules were fibrin, collagen IV, and hyaluronic acid (Figure 2E). The main scaffolds used were TCP, HA, PCL, bioactive glass nanofiber, octacalcium phosphate, Bio-Oss and Bioglass. Scaffold fabrication methods varied vastly among included studies.
| Ref. | Scaffold | Fabrication | Type ofmodification | Cell type | Tests and results |
| Kim et al[43], 2006 | BGNF + Col 1 | Electrospinning process | Surface coating | MG63Cs | ALP activity: BGNF + Col 1 > Col 1 |
| Lechner et al[44], 2006 | β-TCP + F + T | - | New composition | BMMSCs | ALP activity: Increased during 28 d of culture FACS: CD 31: Increased expression by 100 folds |
| Turhani et al[34], 2007 | HA + Col 1 + rhBMP-2 | - | Surface coating | SaOS-2Cs | XTT proliferation assay: (relative) HA + col 1 + rhBMP-2: 1.5 Control: 0.5 ALP activity: HA + col 1 + rhBMP-2: 50 U/μg Control: 25 U/μg |
| Srouji et al[45], 2008 | PCL + Col | Electrospun meshed scaffold | Electrospun nanofiber membrane | BMMSCs | Alamar Blue assay: PCL + Col = PCL [Data as mean ± SD (P < 0.05)] In vivo: Good integration after subcutaneous implantation |
| Hao et al[46], 2010 | A: ADSCs-Col/PLGA-β-TCP B: Acellular Col/PLGA-β-TCP | Low- temperature deposition manufacturing (LDM) based on the layer-by-layer manufacturing principle of solid free-form fabrication | Surface coating | ADSCs | ALP activity: ECM mineralization in group A > group B Evident calcification level in group A, no apparent calcification in group B In vivo: Woven bone with a trabecular structure in group A No bone formation in group B |
| Xu et al[30], 2009 | BG + Col-HYA-PS | Sol–gel method | New composite fabrication | BMMSCs | Cell attachment: Number of attached cells on BG + Col - HYA - PS was the highest Cell proliferation: BG + Col - HYA - PS > BG + Col and BG + Col - HYA > BG ALP activity: BG + Col - HYA - PS > BG + Col and BG + Col - HYA > BG |
| Kawai et al[47], 2009 | OCP + Col | Mixing + lyophilization | Surface coating | MSST-2 Cs | Proliferation and attachment : OCP + Col > OCP (control) In vivo: OCP + Col: Enhanced bone regeneration ratio 83:17 generated maximum repair level of approximately 64% of the defect at 12 wk |
| Zhang et al[48], 2010 | β-TCP + Col | Electrospun + impregnating methods | Architecture + surface coating | MG63Cs | MTT assay: (relative) β-TCP + Col: 0.30 Control (β-TCP): 0.25 |
| Qu et al[49], 2010 | C + HA + RGD peptide | In situ compositing hybridization + lyophilization | Surface coating | BMMSCs | Fluorescence microscopy: Cell adhesion rate: CS + HA: 54.7% C + HA + RGD peptide: 71.6% and 80.7% ALP activity: C + HA + RGD peptide: 0.00596 ± 0.00081 U/L per ng C + HA: 0.00283 ± 0.00025U/L per ng |
| Kang et al[50], 2011 | Fi + HAH | The multi-head deposition system | Surface coating | ADSCs | ALP activity: Single day treatment: 0.5 mmol/mg No treatment: 0.5 mmol/mg Daily treatment: 3 mmol/mg |
| Marelli et al[51], 2011 | nBG + DC | Plastic compression technique | Surface coating | MC3T3-E1 CS | Confocal microscopy of fluorescently: Attachment no difference ALP activity: nBG + DC: 3.5 × 105 Control (nBG): 2.5 × 105 Alamar blue assay: nBG + DC: 6.5 × 105 Control: 8 × 105 |
| Phipps et al[52], 2011 | PCL + Col I + nHA | Electrospun | Bone-mimetic electrospun matrices | BMMSCs | Activation of focal adhesion kinase: Cells seeded onto PCL/Col/nHA scaffolds were better spread, and exhibited greater amounts MTS assay: (relative) PCL + Col + nHA: 5 PCL + nHA: 2.5 PCL: 1 |
| Weeks et al[53], 2012 | PLLA + CXCL12, 13 + F + Col IV | - | Surface coating | BMMSCs | Antibody-blocking studies: Anti-α5β1 inhibits MSC attachment: PLLA + CXCL12, 13 + F + Col IV: 500 cells/mm2 PLLA + F + Col IV: 400 cells/mm2 |
Regarding the study method, in the majority of studies cell differentiation was assessed with ALP activity assay and higher activity was reported for experimental scaffolds compared to control groups. In addition, various methods were applied to assess cell attachment, proliferation and differentiation. In 6 studies, the cell type was BMMSCs; whereas in other studies MG63Cs, SaOS-2Cs, osteoblast precursor cells (MC3T3-E1Cs), adipose-derived stem cells (ADSCs), and mouse bone marrow cells (MSST-2 Cs) were used.
Among 85 articles, which used nano particles to enhance scaffold properties, 17 were studied (Table 3). Type of modification was surface modification and scaffold architecture in 5 studies; in another 6 experiments, spatial conformation was performed. Others fabricated new scaffolds by adding nanoparticles to the scaffold composition (Figure 2C). The main scaffold on which modifications were made varied in the studies. These scaffolds included PCL, chitosan, PLGA, gelatin, TCP, poly l-lactic acid (PLLA), single-walled carbon nanotubes, polyamide and HA. Nanoparticles of HA were the most commonly added particles followed by nano fibrous PCL, nano diamond, amorphous calcium phosphate nanoparticles, nano silica, nano apatite, magnetic nanoparticles and nano-sized bioactive glass (Figure 2F). Different fabrication techniques were applied: flame spray pyrolysis, sonication, thermally induced phase, electrospun, blunt-end needle tip and gamma high voltage. Scaffold fabrication method varied vastly among the included studies.
| Ref. | Scaffold | Fabrication | Type of modification | Cell type | Tests and results |
| Kim et al[54], 2006 | PLGA + nHA | SC/PL and GF/PL | New composition fabrication | RCOCs | Average cell density: GF/PL = 2.4 × 106 cells/scaffold (86.5% increase) SC/PL = 2.1 × 106 cells/scaffold (69.7% increase) ALP activity: GF/PL= 0.6 mol/min per 106 SC/PL= 0.5 mol/min per 106 |
| Wang et al[55], 2007 | PA + nHA | Thermally induced phase inversion | Surface coating | BMMSCs | MTT assay and ALP activity: No negative effects on the BMMSCs in vitroIn vivo: Good biocompatibility and extensive osteoconductivity with host bone in vivo |
| Lv et al[56], 2009 | PLGA + nHA | Microsphere sintering method (modification of the emulsion and solvent evaporation method) | New composition fabrication | BMMSCs | MTS assay: (cell number) PLGA + nHA : 1.2 million/mm2 PLAGA: 0.06 million/mm2 ALP activity: PLGA + nHA: 0.10 mL/μg PLAGA: 0 mL/μg |
| Roohani- Esfahani et al[57], 2010 | BCP/PCL + nHA | Sonication | Surface coating | PHOLCs | ALP activity: BCP/PCL + nHA: 2 mmol/h per mg BCP: 0.5 mmol/h per mg RT-PCR: (relative) BSP: BCP/PCL + nHA: 0.4, PCL: 0 Runx2: BCP/PCL + nHA: 8, PCL: 5 OCN: BCP/PCL + nHA: 1.2, PCL: 0.8 Col I: BCP/PCL + nHA: 4.5, PCL: 2.5 |
| Ye et al[58], 2010 | PCL + nAP | Freeze-dried | Scaffold architecture | MG63Cs | Cell proliferation on composite scaffolds porosity of 76% > porosity of 53% |
| Phipps et al[52], 2011 | PCL+ Col I + nHA | Electrospun | Bone-mimetic electrospun matrices | BMMSCs | Activation of focal adhesion kinase: Cells seeded onto PCL + col I + nHA scaffolds were better spread, and exhibited greater amounts MTS assay: (relative) PCL + Col + nHA: 5 PCL + nHA: 2.5 PCL: 1 |
| Zhang et al[59], 2011 | PLLA + ODA-nD | Sonication | Surface coating | 7F2CS | Alamar Blue assay: A slight reduction in cell viability compared to control RT-PCR: (relative) ALP: PLLA + ND-ODA: 1, PLLA: 1 OCN: PLLA + ND-ODA: 3, PLLA: 2.8 |
| Zeng et al[31], 2012 | HA + MNPs | Tuning | New composition fabrication | MC3T3-E1Cs ROS 17/1.8Cs | MTT assay: HA + MNPs: MC3T3-E1: 0.2 OD value, ROS 17/1.8: 1.8 OD value HA: MC3T3-E1: 0.9, ROS 17/1.8: 0.25 ALP activity: HA + MNPs: MC3T3-E1: 0.75 U/mg, ROS 17/1.8: 4.5 U/mg HA: MC3T3-E1: 0.5 U/mg, ROS 17/1.8: 3.5 U/mg BGP activity: HA + MNPs: MC3T3-E1: 350 ng/L, ROS 17/1.8: 4000 ng/L HA: MC3T3-E1: 300 ng/L, ROS 17/1.8: 3500 ng/L |
| Hafezi et al[60], 2012 | G + nBG | Homogenization through stirring | New composition fabrication | hAFCs | MTT assay: No difference compared to control In vivo: Radiographic evaluation: Improved the speed of the bone healing process |
| Buschmann et al[61], 2012 | PLGA + n-aCaP | Electrospun | Electrospun PLGA/a-CaP scaffold architecture | ADSCs | MGTS: Extracellular matrix production was significantly higher FACS: CD13, CD29, CD44 and CD105 were expressed on PLGA + n-aCaP |
| Ganesh et al[62], 2012 | nfPCL + nS | Electrospun | New composition fabrication | BMMSCs | FACS: CD29 = 3.3%, CD44 = 77.1%, CD73 = 94%, CD31, 34, 45 = 0% Cell viability: No difference compared to control BCA assay: NS + PCLN: 250 Ug/mg, PCLN: 100 |
| Im et al[63], 2012 | SWCNT + C + nHA | Lyophilization procedure | Scaffold architecture + new composition | BMMSCs | Cell adhesion and proliferation: SWCNT + C + nHA > SWCNT + C |
| Rodrigues et al[64], 2012 | TCP + nfPCL | Electrospun + dynamic culturing environment | Scaffold architecture + new composition | BMMSCs | ELISA: TCP + nfPCL: 0.8 μg/mL TCP: 0.2 μg/mL ALP assay: TCP + nfPCL: 100 mol/h TCP: 20 mol/h |
| Panzavolta et al[65], 2013 | G + nHA | Foaming + freeze-drying method | Surface coating+ scaffold architecture | BMMSCs | ALP activity: No difference compared to control RT-PCR: (Relative) ALP: G + nHA: 3.2, G: 1.8 Col I: G + nHA: 0.25, G: 0.25 Runx2: G + nHA: 0.5, G: 0.4 TGF-b1: G + nHA: 0.6, G: 0.55 |
| Xing et al[66], 2013 | PLLA + OTND | Manual perfusion technique under pressure | New composition fabrication | BMMSCs | BCA assay: No difference compared to control RT-PCR: (relative) OPN: PLLA + OTND: 3.5, PLL: 1 BSP: PLLA + OTND: 3, PLL: 1 BMP-2: PLLA + OTND: 4, PLL: 1 In vivo: New bone formation: PLLA + OTND: 50%, PLL: 10% |
| Liu et al[67], 2013 | C + nHA | Electrospun | New composition fabrication | BMMSCs | Cell attachment: C + nHA: 1100 μm2 C: 250 RT-PCR: (Relative) BMP-2: C + nHA: 1.5, C: 1 BMP-4: C + nHA: 25, C: 0 Smad1: C + nHA: 9, C: 1 ALP: C + nHA: 1.2, C: 1 Runx2: C + nHA: 22, C: 1 Itga1: C + nHA: 17, C: 1 Itgb1: C + nHA: 7, C: 1 Itgb3: C + nHA: 8, C: 1 Myh9: C + nHA: 7, C: 1 Myh10: C + nHA: 3.5, C: 1 Col 1: C + nHA: 4.5, C: 1 In vivo: Superior ability of bone reconstruction |
| Wang et al[68], 2014 | C + nHA | Lyophilization procedure + cold atmospheric plasma (CAP) treatment | Scaffold architecture + surface coating | BMMSCs | SEM: MSCs adhesion and infiltration were enhanced ELISA: (Relative) Fibronectin: C + nHA 0.8 compared to control (C) Vitronectin: C + nHA 1.1 compared to control |
Regarding the study method, various tests were performed to assess cell viability, attachment, proliferation and differentiation. ALP activity assay, 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay, 5-[3-(carboxymethoxy)phenyl]-3-(4,5-dimethyl-2-thiazolyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt assay, bicinchoninic acid assay, bone Gla protein activity and Alamar blue assay were among these tests. In 5 studies, PCR test was used to assess level of gene expression. Various biomarkers were used in each study such as ALP, OCN, Runx-2, bone sialoprotein, TGF-β1, BMP-2, collagen 1 and integrin subunits together with myosins. In 10 cases, cell type used in the study was BMMSCs. Other cell types used in the studies were: Mouse bone marrow cells (7F2Cs), MC3T3-E1Cs, osteoblastic cells (ROS 17/1.8 Cs), human airway fibroblast cells, ADSCs, MG63Cs, primary human osteoblasts-like cells and rat calvarial osteoblast cells.
This study reviewed current trends in smart scaffolds for bone regeneration. The most relevant applied methods to design smart scaffolds are surface modification by adding nano particles such as nHA and adding an ECM-like molecule such as collagen or growth factor like BMP-2 to the scaffold. Factors such as scaffold material, fabrication method and type of modification defining the physical, chemical and mechanical properties of scaffolds need assessment. Due to the variability of influencing factors including methodology and scaffold properties, the results were not comparable to determine the most successful design. In vitro tests for analyzing the behavior of the fabricated scaffolds vary among the studies. The source of cells also influences the results, and qualitative report of cell migration, attachment, infiltration and differentiation must be similar to compare the results. The results of included in vitro experiments demonstrated that smart design of scaffolds supported cell attachment, proliferation and differentiation.
Surface modification of scaffold materials was the most commonly used design. However, when implanting the graft materials, their surfaces are covered with ECM proteins and molecules and thermodynamic forces cause surface absorption. This issue is commonly overlooked. Alteration of surface properties influences cell adhesion. In addition, surface plays a role in cell migration towards the scaffold and stem cell differentiation. Addition of either HA or collagen to the outer surface of polyethylene terephthalate (PET) caused greater attachment of rat bone marrow cells to the surface and deposition of an HA layer on the surface[69]. The morphology of attached cells mimics that of more differentiated cells. Although chemical modification of scaffold surface affects cell to scaffold response, effect of material design on cell behavior should also be considered[70]. Hatano et al[71] demonstrated that cell differentiation is affected by surface roughness. In the cited study, rougher surfaces caused expression of osteoblastic markers.
Understanding the physiological processes of bone regeneration and the involved regulating molecules is necessary to enhance bone repair. Several attempts at adding these molecules to scaffolds were demonstrated (Tables 1 and 2). Regulating molecules include growth factors and other proteins found in bone ECM.
In the reviewed studies, BMPs seemed to be more frequently used in the scaffolds compared to other growth factors. BMPs induce osteogenic differentiation of stem cells and osteoprogenitor cells, and their efficient and safe use in human was licensed in 2001. In addition, growth factors like BMPs and vascular endothelial growth factors can accelerate healing processes[72,73]. Chemically conjugated BMP-2 on PCL scaffolds caused significantly greater ALP gene expression compared to un-treated PCL scaffolds[37]. In a study by Lu et al[41], results indicated that spatial immobilization of BMP-4 in a collagen-PLGA hybrid scaffold caused high expression of osteogenic genes and supported cell adhesion and proliferation[41]. In 2005, Yang et al[33] evaluated the effect of BMP-4 immobilization on GTG scaffolds on NRCOC activity. A significant effect of BMP-4 was reported after 4 wk. The cited study also showed greater cell attachment and osteoblast differentiation, higher Gla-type osteocalcin (Gla-OC) activity and larger mineralizing nodules following co-culture of osteoblasts with BMP-4 immobilized scaffold. The same results were achieved using BMP-2. Dai et al[40] reported that rhBMP-2 significantly promoted the osteogenic differentiation of BMMSCs, by enhanced expression of Runx-2, osteopontin, osteocalcin and bone sialoprotein. On the other hand, combination of BMP-4 and PLGA scaffold resulted in a reduction in ALP activity[41]. Although BMPs seem to have positive effects on the function of scaffolds and cells, their regularity and efficacy constraints limit their clinical application.
Surface of scaffolds can be modified by proteins and plasma treatment. Immobilization of proteins like integrin and laminin on the scaffold surface not only facilitates cell adhesion but also increases the surface wettability[74]. To add a mixture of fibrin and hyaluronic acid, multihead deposition system and fibrin-thrombin shell formation were used[50]. Using immobilized RGD peptide (Arg-Gly-Asp) improved cell adhesion from 71.6% to 80.7% and increased ALP activity[49]. Similarly, high cell proliferation, differentiation and viability were reported following addition of other ECM molecules to scaffold surfaces[34,47].
On the other hand, although biomolecules like growth factors and ECM proteins can improve scaffold properties, these molecules do not tolerate severe chemical conditions or high temperature and only soft fabrication methods like sol-gel, which does not allow interconnected porous scaffold synthesis, are applicable[75].
Nano particle addition to the scaffold is another way to improve scaffold properties. The most commonly applied nanoparticle was nano-HA, which provides osteoconductivity while the main scaffold provides the porosity. This combination resembles organic/inorganic nature of the bone matrix and mimics the nano-sized characteristics of natural bone[26]. Addition of nano-HA to scaffold doubled the expression of osteogenic genes[67] and increased osteoblastic attachment[63]; whereas osteocalcin gene expression level in PLLA-nano diamond composites was 3 times less than in the PLLA scaffold[59]. Ye et al[58] produced a composite scaffold containing nano non-stoichiometric apatite and poly-epsilon-caprolactone, which had well-interconnected pores encouraging cell proliferation, migration and stimulation.
In vitro studies are generally considered as primary steps for evaluating newly developed materials. These experiments evaluate cell viability, attachment, proliferation and differentiation on new scaffolds by various methods. Although some factors like the effect of recipient bed, body fluids and interaction between different cell types cannot be evaluated in in-vitro experiments, they are useful to analyze the behavior of materials toward cells omitting individual characteristics of animal or human models. However, animal and clinical studies are necessary to assess the regenerative ability of materials in-vivo. A systematic review on in-vivo bone tissue engineering studies by Khojasteh et al[2] demonstrated that only few animal experiments used smart modification of scaffolds; most of which, were addition of BMP-2. It seemed that the application of BMP-2 with stem cells such as ADSCs and BMSC in TCP scaffolds significantly enhanced osteogenesis[2]. Use of growth factors such as BMP-2 in combination with scaffold and stem cells could complete the classic tissue engineering triangle. However, the results of in vitro studies showed that addition of ECM-like molecules and nano-particles also induced osteoblastic differentiation. The most common composite used in bone tissue engineering was found to be polymer-ceramic and use of smartly modified scaffolds in animal models has been relatively fewer[76]. Compared to the large number of in-vitro studies assessing the properties of newly developed materials, in-vivo application of these smart scaffolds and the effect of structural features and surface modification on in-vivo bone regeneration have yet to be studied.
In conclusion, comparing smart scaffolds to other presently available conventional materials shows great advantages, as they have the ability of in-situ osteoblastic differentiation induction and interesting biological functions. The more we understand the fundamentals of cell proliferation, differentiation and mechanisms of osteoblast adhesion, the better we can design smart materials. The researches now aim to design and synthesize composite materials made from fusion of different kinds of synthetic and natural materials. Surface treatment for facilitating cell attachment and differentiation and fabrication of strong scaffolds that can tolerate physiological forces are the main challenges in this regard. In-vitro studies should be performed primarily using standard methods followed by in-vivo investigations. Combination of stem cells and smart scaffolds with a modulus of elasticity of the same magnitude as that of natural bone, and incorporating growth factors may complete the bone tissue engineering triad. Several factors including material, fabrication and modification methods as well as physical, chemical and mechanical properties may determine bone scaffold features. Considering the variability of in-vitro methodology of researches and variety of scaffolds, a thorough and comprehensible comparison between the results and approaches of these in vitro experiments was unattainable. However, the results of the included in-vitro studies indicate that incorporation of growth factors and other bioactive molecules as well as nano-particles as smart modifications can enhance cell differentiation, proliferation and attachment and therefore may improve new bone formation. The current study presented various types of smart modifications, which may provide an acceptable design to approach in-vivo and in-vitro hard tissue engineering.
Scaffold, cells and growth factors are the three main parts of bone tissue engineering. Currently, special attention has been directed to the design of new scaffolds by adding bioactive molecules and nanoparticles. Hydroxyapatite-based scaffolds carry cells to bone defects and provide an extracellular matrix (ECM). By changing the physical and chemical properties of the bone substitutes, smart interaction with seeded cells such as acceleration of differentiation, increasing in proliferation and attachment of the cells has been reported. It seems that these “smart modifications”, can improve osteogenic differentiation and attachment of cells; therefore, they can better respond to their surrounding environment.
Surface modification of scaffold materials was the most commonly used design. However, it is not clear how surface would influence bone regeneration in vivo. This study reviewed current trends in smart scaffolds for bone regeneration. Adding nano particles such as nHA and adding an ECM-like molecule such as collagen or growth factor like bone morphogenic protein (BMP)-2 to the scaffold increase bioactivity of synthetic materials.
Several factors such as scaffold material, fabrication method and type of modification defining the physical, chemical and mechanical properties could affect in vitro experiments. Due to the variability of influencing factors including methodology and scaffold properties, the results were not comparable to determine the most successful design. Overall conclusion shows that in most studies smart design of scaffolds supported more cell attachment, proliferation and differentiation.
Although use of growth factors such as BMP-2 in combination with scaffold and stem cells could complete the classic tissue engineering triangle and result in bone regeneration in vivo, in vitro studies suggest that addition of ECM-like molecules and nano-particles also might have same results as BMP-2. The efficacy of these modifications should be assessed in vivo.
The matter of the review is interesting.
P- Reviewer: Chan JKY, Dorozhkin SV, Vilaboa N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8352] [Cited by in RCA: 6644] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 2. | Khojasteh A, Behnia H, Dashti SG, Stevens M. Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg. 2012;70:972-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Tabatabaei FS, Motamedian SR, Gholipour F, Khosraviani K, Khojasteh A. Craniomaxillofacial Bone Engineering by Scaffolds Loaded with Stem Cells: A Systematic Review. J Den Sch. 2012;30:113-130. |
| 4. | Behnia H, Khoshzaban A, Zarinfar M, Mashhadi Abbas f, bahraminasab H, Khojasteh A. Histological Evaluation of Regeneration in Rabbit Calvarial Bone Defects Using Demineralized Bone Matrix, Mesenchymal Stem Cells and Platelet Rich in Growth Factors. J Dent Sch. 2012;30:143-154. |
| 5. | Behnia H, Khojasteh A, Kiani MT, Khoshzaban A, Mashhadi Abbas F, Bashtar M, Dashti SG. Bone regeneration with a combination of nanocrystalline hydroxyapatite silica gel, platelet-rich growth factor, and mesenchymal stem cells: a histologic study in rabbit calvaria. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Khojasteh A, Eslaminejad MB, Nazarian H. Mesenchymal stem cells enhance bone regeneration in rat calvarial critical size defects more than platelete-rich plasma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:356-362; discussion 363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Khoshzaban A, Keshel SH, Atashi R. Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012;40:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Jafarian M, Eslaminejad MB, Khojasteh A, Mashhadi Abbas F, Dehghan MM, Hassanizadeh R, Houshmand B. Marrow-derived mesenchymal stem cells-directed bone regeneration in the dog mandible: a comparison between biphasic calcium phosphate and natural bone mineral. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e14-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Khojasteh A, Behnia H, Hosseini FS, Dehghan MM, Abbasnia P, Abbas FM. The effect of PCL-TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: a preliminary report. J Biomed Mater Res B Appl Biomater. 2013;101:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Khojasteh A, Eslaminejad MB, Nazarian H, Morad G, Dashti SG, Behnia H, Stevens M. Vertical bone augmentation with simultaneous implant placement using particulate mineralized bone and mesenchymal stem cells: a preliminary study in rabbit. J Oral Implantol. 2013;39:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Khojasteh A, Dashti SG, Dehghan MM, Behnia H, Abbasnia P, Morad G. The osteoregenerative effects of platelet-derived growth factor BB cotransplanted with mesenchymal stem cells, loaded on freeze-dried mineral bone block: a pilot study in dog mandible. J Biomed Mater Res B Appl Biomater. 2014;102:1771-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Yang M, Ma QJ, Dang GT, Ma Kt, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005;7:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Dragoo JL, Lieberman JR, Lee RS, Deugarte DA, Lee Y, Zuk PA, Hedrick MH, Benhaim P. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1322] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 18. | Morad G, Kheiri L, Khojasteh A. Dental pulp stem cells for in vivo bone regeneration: a systematic review of literature. Arch Oral Biol. 2013;58:1818-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Houshmand B, Behnia H, Khoshzaban A, Morad G, Behrouzi G, Dashti SG, Khojasteh A. Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int J Oral Maxillofac Implants. 2013;28:e440-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Mortazavi SH, Khojasteh A, Vaziri H, Khoshzaban A, Roudsari MV, Razavi SH. The effect of fluoxetine on bone regeneration in rat calvarial bone defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Khojasteh A, Behnia H, Naghdi N, Esmaeelinejad M, Alikhassy Z, Stevens M. Effects of different growth factors and carriers on bone regeneration: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e405-e423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008;2:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 417] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 23. | Lo KW, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012;64:1277-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Cui Q, Dighe AS, Irvine JN. Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Curr Pharm Des. 2013;19:3374-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Ma PX, Langer R. Fabrication of biodegradable polymer foams for cell transplantation and tissue engineering. Methods Mol Med. 1999;18:47-56. [PubMed] |
| 26. | Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1131] [Cited by in RCA: 831] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 27. | Fröhlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;3:254-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Zhang X, Chang W, Lee P, Wang Y, Yang M, Li J, Kumbar SG, Yu X. Polymer-ceramic spiral structured scaffolds for bone tissue engineering: effect of hydroxyapatite composition on human fetal osteoblasts. PLoS One. 2014;9:e85871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Abarrategi A, Moreno-Vicente C, Ramos V, Aranaz I, Sanz Casado JV, López-Lacomba JL. Improvement of porous beta-TCP scaffolds with rhBMP-2 chitosan carrier film for bone tissue application. Tissue Eng Part A. 2008;14:1305-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Xu C, Wang Y, Yu X, Chen X, Li X, Yang X, Li S, Zhang X, Xiang AP. Evaluation of human mesenchymal stem cells response to biomimetic bioglass-collagen-hyaluronic acid-phosphatidylserine composite scaffolds for bone tissue engineering. J Biomed Mater Res A. 2009;88:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Zeng XB, Hu H, Xie LQ, Lan F, Jiang W, Wu Y, Gu ZW. Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. Int J Nanomedicine. 2012;7:3365-3378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 923] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 33. | Yang SH, Hsu CK, Wang KC, Hou SM, Lin FH. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein--a viable scaffold for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Turhani D, Weissenböck M, Stein E, Wanschitz F, Ewers R. Exogenous recombinant human BMP-2 has little initial effects on human osteoblastic cells cultured on collagen type I coated/noncoated hydroxyapatite ceramic granules. J Oral Maxillofac Surg. 2007;65:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Fei Z, Hu Y, Wu D, Wu H, Lu R, Bai J, Song H. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med. 2008;19:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Yilgor P, Sousa RA, Reis RL, Hasirci N, Hasirci V. Effect of scaffold architecture and BMP-2/BMP-7 delivery on in vitro bone regeneration. J Mater Sci Mater Med. 2010;21:2999-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Zhang H, Migneco F, Lin CY, Hollister SJ. Chemically-conjugated bone morphogenetic protein-2 on three-dimensional polycaprolactone scaffolds stimulates osteogenic activity in bone marrow stromal cells. Tissue Eng Part A. 2010;16:3441-3448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Mitchell EA, Chaffey BT, McCaskie AW, Lakey JH, Birch MA. Controlled spatial and conformational display of immobilised bone morphogenetic protein-2 and osteopontin signalling motifs regulates osteoblast adhesion and differentiation in vitro. BMC Biol. 2010;8:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Huh JB, Kim SE, Song SK, Yun MJ, Shim JS, Lee JY, Shin SW. The effect of immobilization of heparin and bone morphogenic protein-2 to bovine bone substitute on osteoblast-like cell’s function. J Adv Prosthodont. 2011;3:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Dai C, Guo H, Lu J, Shi J, Wei J, Liu C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based μCT. Biomaterials. 2011;32:8506-8517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Lu H, Kawazoe N, Kitajima T, Myoken Y, Tomita M, Umezawa A, Chen G, Ito Y. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials. 2012;33:6140-6146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Li Z, Gan Q, Zhang W, Zhang J, Yuan Y, Liu C. Surface-induced conformational and functional changes of bone morphogenetic protein-2 adsorbed onto single-walled carbon nanotubes. Biochem Biophys Res Commun. 2013;440:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Kim HW, Song JH, Kim HE. Bioactive glass nanofiber-collagen nanocomposite as a novel bone regeneration matrix. J Biomed Mater Res A. 2006;79:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Lechner S, Huss R. Bone engineering: combining smart biomaterials and the application of stem cells. Artif Organs. 2006;30:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Srouji S, Kizhner T, Suss-Tobi E, Livne E, Zussman E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J Mater Sci Mater Med. 2008;19:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Hao W, Dong J, Jiang M, Wu J, Cui F, Zhou D. Enhanced bone formation in large segmental radial defects by combining adipose-derived stem cells expressing bone morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop. 2010;34:1341-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Kawai T, Anada T, Honda Y, Kamakura S, Matsui K, Matsui A, Sasaki K, Morimoto S, Echigo S, Suzuki O. Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Eng Part A. 2009;15:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Zhang S, Zhang X, Cai Q, Wang B, Deng X, Yang X. Microfibrous β-TCP/collagen scaffolds mimic woven bone in structure and composition. Biomed Mater. 2010;5:065005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Qu Z, Yan J, Li B, Zhuang J, Huang Y. Improving bone marrow stromal cell attachment on chitosan/hydroxyapatite scaffolds by an immobilized RGD peptide. Biomed Mater. 2010;5:065001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Kang SW, Kim JS, Park KS, Cha BH, Shim JH, Kim JY, Cho DW, Rhie JW, Lee SH. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR, Nazhat SN. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915-8926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 52. | Phipps MC, Clem WC, Catledge SA, Xu Y, Hennessy KM, Thomas V, Jablonsky MJ, Chowdhury S, Stanishevsky AV, Vohra YK. Mesenchymal stem cell responses to bone-mimetic electrospun matrices composed of polycaprolactone, collagen I and nanoparticulate hydroxyapatite. PLoS One. 2011;6:e16813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Weeks S, Kulkarni A, Smith H, Whittall C, Yang Y, Middleton J. The effects of chemokine, adhesion and extracellular matrix molecules on binding of mesenchymal stromal cells to poly(l-lactic acid). Cytotherapy. 2012;14:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 495] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 55. | Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 490] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 56. | Lv Q, Nair L, Laurencin CT. Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. J Biomed Mater Res A. 2009;91:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Roohani-Esfahani SI, Nouri-Khorasani S, Lu Z, Appleyard R, Zreiqat H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials. 2010;31:5498-5509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 58. | Ye L, Zeng X, Li H, Ai Y. Fabrication and biocompatibility of nano non-stoichiometric apatite and poly(epsilon-caprolactone) composite scaffold by using prototyping controlled process. J Mater Sci Mater Med. 2010;21:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Zhang Q, Mochalin VN, Neitzel I, Knoke IY, Han J, Klug CA, Zhou JG, Lelkes PI, Gogotsi Y. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials. 2011;32:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 60. | Hafezi F, Hosseinnejad F, Fooladi AA, Mafi SM, Amiri A, Nourani MR. Transplantation of nano-bioglass/gelatin scaffold in a non-autogenous setting for bone regeneration in a rabbit ulna. J Mater Sci Mater Med. 2012;23:2783-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Buschmann J, Härter L, Gao S, Hemmi S, Welti M, Hild N, Schneider OD, Stark WJ, Lindenblatt N, Werner CM. Tissue engineered bone grafts based on biomimetic nanocomposite PLGA/amorphous calcium phosphate scaffold and human adipose-derived stem cells. Injury. 2012;43:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Ganesh N, Jayakumar R, Koyakutty M, Mony U, Nair SV. Embedded silica nanoparticles in poly(caprolactone) nanofibrous scaffolds enhanced osteogenic potential for bone tissue engineering. Tissue Eng Part A. 2012;18:1867-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Im O, Li J, Wang M, Zhang LG, Keidar M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomedicine. 2012;7:2087-2099. [PubMed] |
| 64. | Rodrigues MT, Martins A, Dias IR, Viegas CA, Neves NM, Gomes ME, Reis RL. Synergistic effect of scaffold composition and dynamic culturing environment in multilayered systems for bone tissue engineering. J Tissue Eng Regen Med. 2012;6:e24-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Panzavolta S, Torricelli P, Amadori S, Parrilli A, Rubini K, della Bella E, Fini M, Bigi A. 3D interconnected porous biomimetic scaffolds: In vitro cell response. J Biomed Mater Res A. 2013;101:3560-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Xing Z, Pedersen TO, Wu X, Xue Y, Sun Y, Finne-Wistrand A, Kloss FR, Waag T, Krueger A, Steinmüller-Nethl D. Biological effects of functionalizing copolymer scaffolds with nanodiamond particles. Tissue Eng Part A. 2013;19:1783-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Liu H, Peng H, Wu Y, Zhang C, Cai Y, Xu G, Li Q, Chen X, Ji J, Zhang Y. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials. 2013;34:4404-4417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 68. | Wang M, Cheng X, Zhu W, Holmes B, Keidar M, Zhang LG. Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2014;20:1060-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Yamamoto M, Kato K, Ikada Y. Ultrastructure of the interface between cultured osteoblasts and surface-modified polymer substrates. J Biomed Mater Res. 1997;37:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Seal B, Otero T, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater SciEng R. 2001;34:147-230. [DOI] [Full Text] |
| 71. | Hatano K, Inoue H, Kojo T, Matsunaga T, Tsujisawa T, Uchiyama C, Uchida Y. Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene. Bone. 1999;25:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 72. | Jansen JA, Vehof JW, Ruhé PQ, Kroeze-Deutman H, Kuboki Y, Takita H, Hedberg EL, Mikos AG. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 73. | Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991-5998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 75. | Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413-3431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2914] [Cited by in RCA: 2183] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 76. | Liu Y, Lim J, Teoh SH. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv. 2013;31:688-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |