Published online Nov 26, 2015. doi: 10.4252/wjsc.v7.i10.1215
Peer-review started: June 10, 2015
First decision: August 4, 2015
Revised: September 30, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: November 26, 2015
Processing time: 172 Days and 4 Hours
AIM: To evaluate adhesion, proliferation and differentiation of human dental pulp stem cells (hDPSCs) on four commercially available scaffold biomaterials.
METHODS: hDPSCs were isolated from human dental pulp tissues of extracted wisdom teeth and established in stem cell growth medium. hDPSCs at passage 3-5 were seeded on four commercially available scaffold biomaterials, SureOss (Allograft), Cerabone (Xenograft), PLLA (Synthetic), and OSTEON II Collagen (Composite), for 7 and 14 d in osteogenic medium. Cell adhesion and morphology to the scaffolds were evaluated by scanning electron microscopy (SEM). Cell proliferation and differentiation into osteogenic lineage were evaluated using DNA counting and alkaline phosphatase (ALP) activity assay, respectively.
RESULTS: All scaffold biomaterials except SureOss (Allograft) supported hDPSC adhesion, proliferation and differentiation. hDPSCs seeded on PLLA (Synthetic) scaffold showed the highest cell proliferation and attachment as indicated with both SEM and DNA counting assay. Evaluating the osteogenic differentiation capability of hDPSCs on different scaffold biomaterials with ALP activity assay showed high level of ALP activity on cells cultured on PLLA (Synthetic) and OSTEON II Collagen (Composite) scaffolds. SEM micrographs also showed that in the presence of Cerabone (Xenograft) and OSTEON II Collagen (Composite) scaffolds, the hDPSCs demonstrated the fibroblastic phenotype with several cytoplasmic extension, while the cells on PLLA scaffold showed the osteoblastic-like morphology, round-like shape.
CONCLUSION: PLLA scaffold supports adhesion, proliferation and osteogenic differentiation of hDPSCs. Hence, it may be useful in combination with hDPSCs for cell-based reconstructive therapy.
Core tip: Recently, the plasticity of postnatal stem cells from dental origin including human dental pulp stem cells (hDPSCs) has been suggested. Their osteogenic potential makes them valuable for craniofacial bone regeneration. hDPSCs can be easily isolated from dental medical wastes, extracted teeth, and expanded ex vivo. Combination of numerous postnatal stem cells and three-dimensional scaffold biomaterials has been used in bone tissue engineering. Selection of an ideal scaffold biomaterial is a challenging part of reconstructive surgeries. Current study aims to evaluate behavior of hDPSCs including adhesion, proliferation, morphology and differentiation on four different scaffold biomaterials. Our finding indicates that PLLA (Synthetic) scaffold supports adhesion, proliferation and osteogenic differentiation of hDPSCs. Therefore, it can be useful for the purpose of craniofacial tissue engineering.
-
Citation: Khojasteh A, Motamedian SR, Rad MR, Shahriari MH, Nadjmi N. Polymeric
vs hydroxyapatite-based scaffolds on dental pulp stem cell proliferation and differentiation. World J Stem Cells 2015; 7(10): 1215-1221 - URL: https://www.wjgnet.com/1948-0210/full/v7/i10/1215.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i10.1215
Non-healing craniofacial defects occur frequently due to various factors such as trauma, infection, tumor, and congenital deformities[1,2]. The treatment of large bone defects has been posed a challenge for reconstructive surgeons. The conventional treatment approach for treatment of craniofacial bone defects is harvesting and transplanting autogenous cancellous bone graft, particularly from ilia crest[3]. However, the surgical procedures are invasive and it usually associated with donor site morbidities and limited availability[4,5]. Another approach is utilization of allogeneic bone which is also limited by the risk of immunogenicity or disease transmission[6]. The concept of harvesting adult stem cells (ASCs) in combination with appropriate three-dimensional (3D) scaffolds have been proposed as a promising alternative approach in reconstructive surgery[7-12].
ASCs have been isolated from various tissues. In dental clinic teeth are often need to be extracted in order to avoid further complications including orthodontic treatments. Human dental pulp has been harvested from extracted teeth and shown to contain multilineage population of progenitor/stem cells, dental pulp stem cells (hDPSCs)[13,14]. The differentiation capability of hDPSCs towards the osteoblast lineage and their accessibility and ease of ex-vivo expansion make them suitable cell source to be considered for repair of skeletal defects[15,16].
Bone formation from hDPSCs is required a 3D structure provided by scaffolds. An ideal scaffold should provide an appropriate environment for cellular attachment, growth, and differentiation. A wide range of scaffold biomaterials have been developed for variety of applications in tissue engineering[17]. Scaffolds can be categorized as following groups: (1) Allograft; (2) Xenograft; (3) Synthetic; and (4) Composite biomaterials. In order to select a suitable scaffold for craniofacial engineering, it is necessary to evaluate the cell-scaffold interactions in vitro. Current study is aimed to investigate hDPSCs behavior including cell adhesion, attachment, and differentiation on four commercially available scaffolds from given groups.
hDPSC cultures are established from dental pulp tissues isolated from extracted teeth of healthy volunteer adults (aged 18-30) as previously described[14]. Briefly, pulp tissues are gently separated from the crown and root and then digested in a solution of 0.075% collagenase type I (Sigma-Aldrich, St. Louis, Missouri, United States) for 1 h at 37 °C. hDPSCs are established by growing the primary cell suspension in stem cell growth medium containing DMEM-HG (Invitrogen, Grand Island, NY, United States), 10% FBS (Invitrogen, Grand Island, NY, United States) and 100 units/mL penicilliny 100 mg/mL streptomycin (Invitrogen, Grand Island, NY, United States) in T-25 flasks overnight at 37 °C and 5% CO2, and non-adherent cells were removed by medium change. The remaining cells were cultured until they reached 80%-90% confluency. Cells were trypsinized using 0.05% Trypsin-EDTA (Invitrogen, Grand Island, NY, United States) and passaged at a ratio of 1:3 until the desired passages were reached. Passage 3-5 were used for subsequent experiments.
Four commercially available bone-graft substitutes were studied. SureOss (HansGBR Biomaterial, Seoul, South Korea) is a freeze dried cortical bone allograft. It is a granular biomaterial with the particle size of 200-850 μm. Cerabone (Biotiss Biomaterial, Zossen, Germany) is a xenograft derived from the mineral phase of bovine bone. It is a granular biomaterial with the particle size of 0.5-1.0 mm. PLLA is a synthetic biomaterial. OSTEON II Collagen (Dentium, Gyeonggi, South Korea) is a composite biomaterials contains HA:β-TCP = 30:70 and natural type I collagen. It has particle size of 0.2-0.5 mm.
Approximately 100 mg of given biomaterial scaffolds were placed into 96-well plates. hDPSCs were seeded onto the scaffolds at a cell density of 104 cells/well in osteogenic medium (Invitrogen, Grand Island, NY, United States). The cells - scaffold constructs were maintained at 37 °C in a humidified 5% CO2 atmosphere for 7 and 14 d.
hDPSCs adhesion on following scaffolds were assessed using scanning electron microscopy (SEM). Seven days after cell seeding, samples were fixed with 2.5% glutaraldehyde (MerckKGaA, Darmstadt, Germany) solution for 30 min. Specimens were then post-fixed with 1% Osmium tetroxide (Sigma-Aldrich, St. Louis, Missouri, United States). After cell fixation, the specimens were subsequently dehydrated by ascending grades of alcohol (25%, 50%, 75%, 96%, and 100% ethanol) for 15 min each step. The specimens were then allowed to dry in air. After complete drying, they were sputtered with gold and analyzed using a SEM imaging (Hitachi, Tokyo, Japan).
To determine the proliferation of hDPSCs on following scaffolds, DNA counting assay were performed using QIAamp® DNA Mini Kit, according to the manufacturer’s description (Qiagen, Valencia, CA, United States). Briefly, cells at days 7 and 14 were harvested using 0.05% Trypsin-EDTA (Invitrogen, Grand Island, NY, United States). Then, cell pellets were collected by centrifugation and re-suspended in phosphate-buffed saline (PBS). DNA was collected with QIAam spin column. The amount of DNA (ng/mL) was measured using Nanodrop (Thermo Scientific, Waltham, MA, United States).
Osteogenic differentiation of hDPSCs on scaffold were evaluated at days 7 and 14 after cell seeding using alkaline phosphatase (ALP) activity assay. The cell-scaffold constructs were rinsed with PBS and homogenized in lysis buffer (pH 7.5, 10 mmol/L Tris-HCl, 1 mmol/L MgCl2, and 0.05% Triton X-100). The resulting mixture was then centrifuged at 12000 rpm for 10 min at 4 °C. The cell lysate was mixed with p nitrophenol phosphate substrate solution (Sigma aldrich, St. Louis, Missouri, United States) and alkaline buffer solution (Sigma aldrich, St. Louis, Missouri, United States). After incubation at 37 °C for 15 min, the above mixture was added to 0.5 N NaOH to stop the reaction and the absorbance at 405 nm was measured using ELIZA reader (BioTek, Winooski, VT, United States).
All quantitative data were expressed as mean ± SE. Analysis of variance and Post hoc tests was conducted for multiple comparisons. A P value less than 0.05 were considered to be significant.
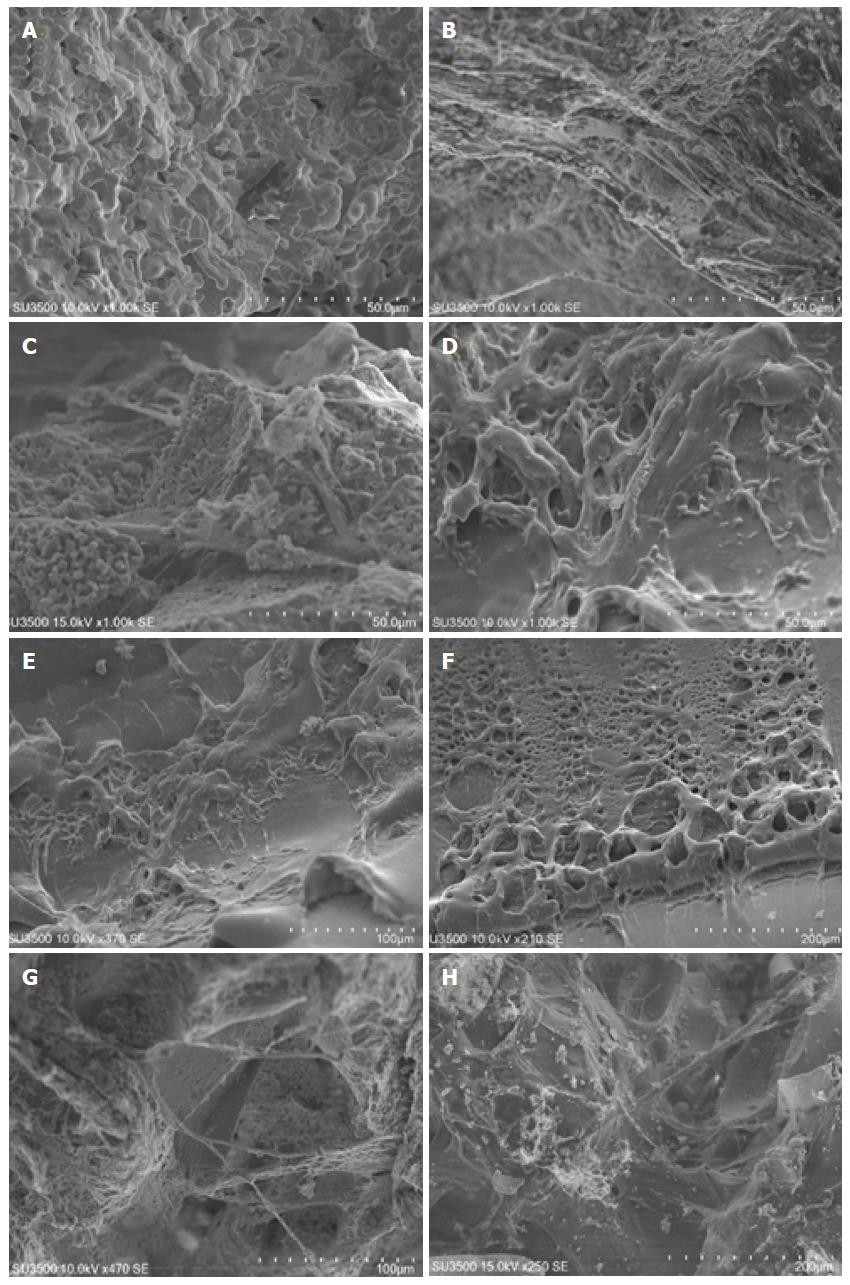
The suitability of the 3D structure of scaffolds for cell seeding was assessed by observing cell morphology and adhesion using SEM imaging. SEM microphotographs after 7 d confirmed the adherence of hDPSCs in all biomaterial scaffolds (Figure 1-D) except SureOss (Allograft) (Figure 1A). Marked cell aggregation was observed on PLLA scaffold (Synthetic) (Figure 1D-F). hDPSCs were covered almost the entire PLLA scaffold surface. In contrast, fewer cells were aggregated on the surface of Cerabone (Xenograft) and OSTEON II Collagen (Composite) scaffolds (Figure 1B and C). The adherent hDPSCs on Cerabone (Xenograft) and OSTEON II Collagen (Composite) scaffolds demonstrated fibroblastic morphology with several cytoplasmic extension (Figure 1-F). The osteoblastic-like morphology, round-like shape, was observed on cells attached on PLLA (Synthetic) scaffold (Figure 1B, C, G and H).
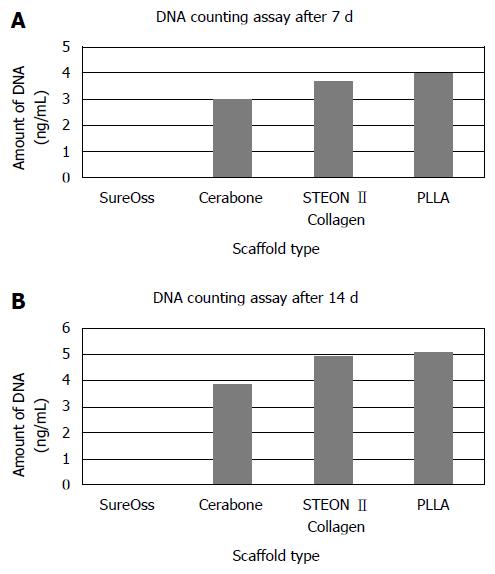
The proliferation of hDPSCs on different scaffold biomaterials were monitored and quantified after 7 and 14 d culturing of the cells by means of DNA counting assay (Figure 2). DNA counting assay confirmed the SEM results. The amount of DNA was highest on PLLA group at both time points (Figure 2A and B).
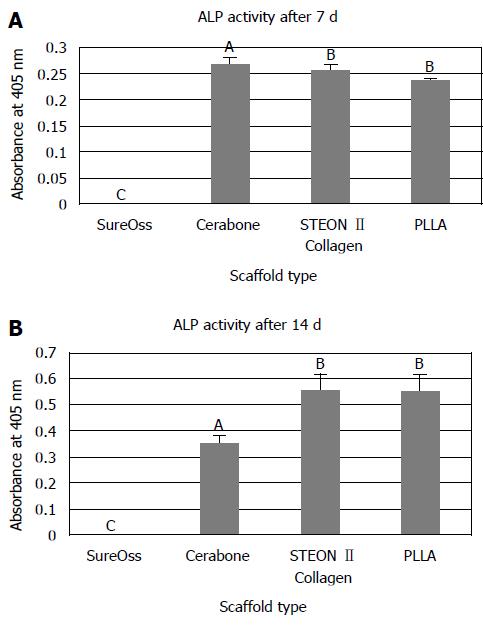
The osteogenic differentiation capability of hDPSCs on different scaffold biomaterials was compared using ALP activity assay. The results are presented in Figure 3A and B. At day 14 both cells cultured on PLLA (Synthetic) and OSTEON II Collagen (Composite) scaffolds showed high level of ALP activity compared to cells on Cerabone (Xenograft). No significant difference observed between osteogenic activity of hDPSCs on OSTEN II Collagen and PLLA group (Figure 3B).
An ideal scaffold should interact with cells, support cell attachment and proliferation, and stimulate tissue regeneration. In current study, we investigated the hDPSC activity and growth behavior on four different 3D scaffold biomaterials. The scaffold biomaterials used in this study are already commercially available for clinical applications. However, comparing their efficiency to provide structural support for hDPSCs is essential. hDPSCs has been selected for our observation, since their clinical application seems feasible. Harvesting and isolating these progenitor population are relatively easy and a sufficient number of the cells can be provided in two weeks[14,15]. In addition, since dental pulp stem cells are derived from neural crest, different origin from mesoderm-derived bone marrow stem cells, they may considered better candidate for repair of damages in neural crest-derived tissues including periodontium and craniofacial defects[15,18].
We have shown that all scaffold biomaterials except SureOss (Allograft) support hDPSC adhesion, proliferation and differentiation. Among them, the highest cell proliferation was observed in the presence of PLLA, as it confirmed with both SEM and DNA counting assay. SEM showed almost all surface of PLLA scaffold was covered by adhering cells. PLLA is one of the few synthetic degradable polymers which approved by the Food and Drug Adminstration for clinical application[19] and has been used extensively in reconstructive surgery in combination with mesenchymal stem cells (MSCs)[20-22]. In our study, we have shown that MSCs derived from dental pulp tissues also are attached, proliferated, within PLLA scaffold.
In addition, SEM examination showed that cell morphology differs clearly between scaffolds. In the presence of Cerabone (Xenograft) and OSTEON II Collagen (Composite) scaffolds, the hDPSCs demonstrated the fibroblastic phenotype, which is the typical MSC morphology[23]. However, the cells on PLLA (Synthetic) scaffold showed the round-like shape which is the typical osteoblastic phenotype[24], indicating that PLLA scaffold not only can improve cell adhesion and growth, but also upregulate the osteoblastic phenotype.
Evaluating hDPSCs adhesion, proliferation and differentiation of HA/β-TCP in composition with collagen, we have shown that despite the increase in ALP activity, the cellular attachment and proliferation was lower compared to PLLA scaffold. Phosphate Ceramics including HA and β-TCP have been described as an osteoinductive materials for many years[25-27]. Despite their similarity to mineral component of human bone, their major drawbacks including their high resorption rate and low mechanical strength[28] hampers their applications in clinics. The combination of these materials and collagen has been reported in various studies to reinforce their mechanical strength and decrease resorption rate[29-31]. Moreover, several studies have been used both collagen and HA in combination with other scaffold biomaterial in order to mimic the natural environment of the bone[32]. Akkouch et al[32], showed increased osteogenic capability of hDPSCs cultured on composite scaffold made of HA, collagen and poly (L-lactide-co-έ-caprolactone) (PLCL). However, there have been controversies among the literature regarding the beneficial effects of these phosphate ceramics on cellular attachment and proliferation. Study of Pereira-Junior et al[33], showed the slow growth of MSCs in the presence of HA granule. Similar study on MSCs seeded on β-TCP has shown the slow bone formation in vivo[34]. In contrast, study of Kasten et al[35], showed that HA ceramics supported the cellular attachment and differentiation. Ling et al[36], compared the attachment, proliferation and osteogenic differentiation of DPSCs on composite scaffold containing HA, collagen and poly (L-lactide) (PLA) with β-TCP. They demonstrated that although DPSCs had more mineralization on β-TCP, cell attachment and proliferation were higher in composite scaffold of HA, collagen and PLA.
Present study was designed to evaluate these bone substitute materials in response to MSCs derived from dental pulp. However, the result of this study needs to be confirmed in vivo, where the interaction of cell-scaffold with host environment is an essential factor.
Our findings indicate that PLLA (Synthetic) scaffold supports adhesion, proliferation and osteogenic differentiation of hDPSCs. Therefore, it can be useful for the purpose of craniofacial tissue engineering.
The treatment of large non-healing craniofacial defects has been posed a challenge for reconstructive surgeries. The conventional treatment approach for treatment of craniofacial bone defects is usually associated with donor site morbidities and limited availability. The concept of harvesting adult stem cells (ASCs) in combination with appropriate three-dimensional (3D) scaffolds have been proposed as a promising alternative approach in reconstructive surgery. Human dental pulp stem cells (hDPSCs) can be easily isolated from dental medical wastes, extracted teeth, and expanded ex vivo. Their osteogenic capability makes them appropriate source of ASCs. Bone formation from hDPSCs is required a 3D structure provided by scaffolds which should provide an appropriate environment for cellular attachment, growth, and differentiation.
A wide range of scaffold biomaterials have been developed for variety of applications in tissue engineering. Scaffolds can be categorized as following groups: (1) Allograft; (2) Xenograft; (3) Synthetic; and (4) Composite biomaterials. In order to select a suitable scaffold for craniofacial engineering, it is necessary to evaluate the cell-scaffold interactions in vitro. Current study is aimed to investigate hDPSCs behavior including cell adhesion, attachment, and differentiation on four commercially available scaffolds from given groups.
The authors’ findings indicate that PLLA (Synthetic) scaffold supports adhesion, proliferation and osteogenic differentiation of hDPSCs.
DPSCs in combination with PLLA scaffold can be useful for the purpose of craniofacial tissue engineering.
A scaffold is an artificial designed biomaterial which mimics extra cellular matrix and it is support cellular attachment, proliferation and differentiation.
The study is well designed and results are clear and support the conclusions.
P- Reviewer: Bugaj AM, Duan SB S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Elsalanty ME, Genecov DG. Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr. 2009;2:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Mitchel D. An introduction to oral and maxillofacial surgery. Oxford: Oxford University Press 2005; . |
| 3. | Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001;12:495-502. [PubMed] |
| 4. | Marx RE, Carlson ER. Tissue banking safety: caveats and precautions for the oral and maxillofacial surgeon. J Oral Maxillofac Surg. 1993;51:1372-1379. [PubMed] |
| 5. | Nkenke E, Radespiel-Tröger M, Wiltfang J, Schultze-Mosgau S, Winkler G, Neukam FW. Morbidity of harvesting of retromolar bone grafts: a prospective study. Clin Oral Implants Res. 2002;13:514-521. [PubMed] |
| 6. | Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Behnia H, Khojasteh A, Kiani MT, Khoshzaban A, Mashhadi Abbas F, Bashtar M, Dashti SG. Bone regeneration with a combination of nanocrystalline hydroxyapatite silica gel, platelet-rich growth factor, and mesenchymal stem cells: a histologic study in rabbit calvaria. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012;40:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Eslaminejad MB, Jafarian M, Khojasteh A, Abbas FM, Dehghan MM. In vivo bone formation by canine mesenchymal stem cells loaded onto HA/TCP scaffolds: qualitative and quantitative analysis. Yakhteh. 2008;10:e205-e212. |
| 10. | Jafarian M, Eslaminejad MB, Khojasteh A, Mashhadi Abbas F, Dehghan MM, Hassanizadeh R, Houshmand B. Marrow-derived mesenchymal stem cells-directed bone regeneration in the dog mandible: a comparison between biphasic calcium phosphate and natural bone mineral. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e14-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Khojasteh A, Behnia H, Hosseini FS, Dehghan MM, Abbasnia P, Abbas FM. The effect of PCL-TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: a preliminary report. J Biomed Mater Res B Appl Biomater. 2013;101:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Rezai-Rad M, Bova JF, Orooji M, Pepping J, Qureshi A, Del Piero F, Hayes D, Yao S. Evaluation of bone regeneration potential of dental follicle stem cells (DFSCs) for treatment of craniofacial defects. Cytotherapy. 2015;In press. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625-13630. [PubMed] |
| 14. | Morad G, Kheiri L, Khojasteh A. Dental pulp stem cells for in vivo bone regeneration: a systematic review of literature. Arch Oral Biol. 2013;58:1818-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 15. | Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2014;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Hilkens P, Meschi N, Lambrechts P, Bronckaers A, Lambrichts I. Dental Stem Cells in Pulp Regeneration: Near Future or Long Road Ahead? Stem Cells Dev. 2015;24:1610-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Tabatabaei FS, Motamedian SR, Gholipour F, Khosraviani K, Khojasteh A. Craniomaxillofacial bone engineering by scaffolds loaded with stem cells: A systematic review. J Dent Sch. 2012;30:e115-e131. |
| 18. | Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99:465-474. [PubMed] |
| 19. | PLLA approval in FDA. [retrived 2015 Sept 29]. Available from: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. |
| 20. | Ardeshirylajimi A, Mossahebi-Mohammadi M, Vakilian S, Langroudi L, Seyedjafari E, Atashi A, Soleimani M. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres. Cell Prolif. 2015;48:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, Hoyland JA. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27:4069-4078. [PubMed] |
| 22. | Xing SC, Liu Y, Feng Y, Jiang C, Hu YQ, Sun W, Wang XH, Wei ZY, Qi M, Liu J. Chondrogenic differentiation of ChM-I gene transfected rat bone marrow-derived mesenchymal stem cells on 3-dimensional poly (L-lactic acid) scaffold for cartilage engineering. Cell Biol Int. 2015;39:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139-2151. [PubMed] |
| 25. | Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407-429; discussion 429. [PubMed] |
| 26. | Bourgeois B, Laboux O, Obadia L, Gauthier O, Betti E, Aguado E, Daculsi G, Bouler JM. Calcium-deficient apatite: a first in vivo study concerning bone ingrowth. J Biomed Mater Res A. 2003;65:402-408. [PubMed] |
| 27. | Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AM, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci USA. 2010;107:13614-13619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 497] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 28. | Tjong SC. Advances in Biomedical Sciences and Engineering. United Arab Emirates: Bentham Science Publishers 2009; . |
| 29. | Araújo MG, Lindhe J. Ridge preservation with the use of Bio‐Oss® collagen: A 6‐month study in the dog. Clin Oral Implan Res. 2009;20:e433-e440. [RCA] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Brkovic BM, Prasad HS, Konandreas G, Milan R, Antunovic D, Sándor GK, Rohrer MD. Simple preservation of a maxillary extraction socket using beta-tricalcium phosphate with type I collagen: preliminary clinical and histomorphometric observations. J Can Dent Assoc. 2008;74:523-528. [PubMed] |
| 31. | Fickl S, Zuhr O, Wachtel H, Stappert CF, Stein JM, Hürzeler MB. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J Clin Periodontol. 2008;35:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Akkouch A, Zhang Z, Rouabhia M. Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly (L-lactide-co-ε-caprolactone) scaffold. J Biomater Appl. 2014;28:922-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Pereira-Junior OC, Rahal SC, Lima-Neto JF, Landim-Alvarenga Fda C, Monteiro FO. In vitro evaluation of three different biomaterials as scaffolds for canine mesenchymal stem cells. Acta Cir Bras. 2013;28:353-360. [PubMed] |
| 34. | Lim JY, Shaughnessy MC, Zhou Z, Noh H, Vogler EA, Donahue HJ. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials. 2008;29:1776-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Kasten P, Luginbühl R, van Griensven M, Barkhausen T, Krettek C, Bohner M, Bosch U. Comparison of human bone marrow stromal cells seeded on calcium-deficient hydroxyapatite, beta-tricalcium phosphate and demineralized bone matrix. Biomaterials. 2003;24:2593-2603. [PubMed] |
| 36. | Ling LE, Feng L, Liu HC, Wang DS, Shi ZP, Wang JC, Luo W, Lv Y. The effect of calcium phosphate composite scaffolds on the osteogenic differentiation of rabbit dental pulp stem cells. J Biomed Mater Res A. 2015;103:1732-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |