Published online Apr 26, 2024. doi: 10.4252/wjsc.v16.i4.434
Peer-review started: December 30, 2023
First decision: January 23, 2024
Revised: February 5, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 26, 2024
Processing time: 116 Days and 8 Hours
Mesenchymal stem cells (MSCs) have been extensively studied for therapeutic potential, due to their regenerative and immunomodulatory properties. Serial passage and stress factors may affect the biological characteristics of MSCs, but the details of these effects have not been recognized yet.
To investigate the effects of stress factors (high glucose and severe hypoxia) on the biological characteristics of MSCs at different passages, in order to optimize the therapeutic applications of MSCs.
In this study, we investigated the impact of two stress conditions; severe hypoxia and high glucose on human adipose-tissue derived MSCs (hAD-MSCs) at passa
Cells at P6 showed decreased proliferation and increased apoptosis under conditions of high glucose and hypoxia compared to control, while the extent of senescence did not change significantly under stress conditions. At P8 hAD-MSCs cultured in stress conditions had a significant decrease in proliferation and apoptosis and a significant increase in senescence compared to counterpart cells at P6. Cells cultured in high glucose at P10 had lower proliferation and higher senescence than their counterparts in the previous passage, while no change in apoptosis was observed. On the other hand, MSCs cultured under hypoxia showed decreased senescence, increased apoptosis and no significant change in proliferation when compared to the same conditions at P8.
These results indicate that stress factors had distinct effects on the biological processes of MSCs at different passages, and suggest that senescence may be a protective mechanism for MSCs to survive under stress conditions at higher passage numbers.
Core Tip: Mesenchymal stem cells (MSCs) are increasingly being used for the treatment of various diseases due to their immunomodulatory and regenerative properties, but serial passages needed for MSCs expansion and stress factors found in the diseased tissues may compromise their therapeutic potential. Investigating the effects of stress factors (high glucose and severe hypoxia) on the biological characteristics of MSCs at different passages will help optimize the clinical uses of MSCs and their expected outcomes.
- Citation: Almahasneh F, Abu-El-Rub E, Khasawneh RR, Almazari R. Effects of high glucose and severe hypoxia on the biological behavior of mesenchymal stem cells at various passages. World J Stem Cells 2024; 16(4): 434-443
- URL: https://www.wjgnet.com/1948-0210/full/v16/i4/434.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i4.434
Mesenchymal stem cells (MSCs) are stromal cells that have the ability to self-renew and differentiate into multiple lineages. Due to their potent regenerative and immunomodulatory properties, MSCs have been extensively studied as potential therapies for a large number of acute and chronic diseases[1]. To preserve their biological and therapeutic properties, MSCs must be cultured in optimal environmental conditions, which are rarely found in the diseased tissues into which MSCs are transplanted[2,3]. Indeed, the successful therapeutic effects of MSCs are often of short duration due to these hostile microenvironments[4]. Although MSCs are able to adapt to many conditions, their biological response to stressful microenvironments can be unexpected[5].
High glucose and severe hypoxia are two main stress conditions that affect the biological functions of MSCs and the efficacy of their clinical applications. High glucose cell media were found to decrease proliferation rates, induce senescence and increase apoptosis in rat MSCs[6,7], while low glucose was associated with increased survival[8]. When used to treat pathologic conditions characterized by hyperglycemia, such as diabetes mellitus (DM), MSCs have low survival rate and poor outcome[3,9].
Moderate hypoxic conditions (oxygen gradient 2%-9%) are considered the physiologic environment for MSC growth[10] and are known to positively affect their behavior[11]. On the other hand, exposure of MSCs to severe hypoxia (O2 less than 1%) was found to promote their senescence and apoptosis while reducing proliferation[12,13]. MSCs are obtained in limited numbers from donors, so they have to undergo expansion in vitro by serial passage[14]. It is important to recognize the effects of serial passage on the quality of cultured MSCs in order to ensure the accuracy of stem cell research, as well as consistent and effective MSC based therapies[15]. Serial passage of MSCs from different origins (umbilical cord, placenta, adipose tissue) was found to affect their biological characteristics, including growth rate, senescence, gene expression, migration and differentiation[15-17]. For example, long-term serial passaging of MSCs from different sources induced cellular senescence and decreased their proliferation potential[18]. The results of previous studies, however, are discrepant and the details of these effects need further clarifications. In addition, little is known about the response of MSCs to stress conditions at different passages, which dictates the need for further investigations in this field.
Human adipose-tissue derived MSCs (hAD-MSCs) were commercially purchased from Lonza (Cat# PT5006, Lot# 21TL138912) and expanded using Dulbecco’s Modified Eagle’s Medium low glucose (DMEM-low glucose, Euroclone), which contained 5.6 mmol/L glucose and were supplemented with 10% fetal bovine serum (FBS, Gibco)[19], 0.1 mg/mL streptomycin and 100 units/mL penicillin G in standard cell culture incubators (5% CO2/95% air; 37 °C). Medium was changed every 72 h and cells were sub-cultured when confluence exceeded 60%. For high glucose conditions, cells were cultured in DMEM high glucose (DMEM-High Glucose, Euroclone) which contained 25 mmol/L glucose and were supplemented with 10% FBS, 0.1 mg/mL streptomycin and 100 units/mL penicillin G for 5 d in standard cell culture incubators (5% CO2/95% air; 37 °C). High glucose complete medium was changed every 72 h. For the preparation of the high glucose media, we followed a previously reported protocol[20,21]. MSCs that were cultured in low glucose were considered as our study control. MSCs of passage 6 (P6), P8, and P10 were used to perform the experiments.
A stock solution of cobalt chloride (CoCl2), a hypoxia-mimetic chemical agent[22], was prepared by dissolving the chemical in distilled water (stock solution concentration was 100 mmol/L). For hypoxia induction, the stock solution of CoCl2 was further diluted in the culture media to yield a working concentration of 250 μM. The hypoxia treatment for hAD-MSCs was performed for 48 h in standard cell culture incubators (5% CO2/95% air; 37 °C)[22].
The proliferation of hAD-MSCs at P6, P8, and P10 after being exposed to normal (low glucose) and treatment conditions (high glucose and hypoxia) was measured using the commercial kit (WST-1 Assay Kit, Abcam, Cat# ab65473). The WST-1 assay is based on the cleavage of tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. The greater the percentage of viable cells, the higher the amounts of formazan generated. Briefly, MSCs were seeded in 96 well plate (5 × 104 cells/well) after being cultured in low glucose and high glucose for 5 d. For hypoxia induction, MSCs were seeded in 96 well plate (5 × 104 cells/well) and CoCl2 was added to the cell culture media at 250 μM and cells were incubated in CoCl2 for 48 h. Next, 10 μL of WST-1 solution was added to each well of the different treatment groups. After an incubation time of 2 h in the standard cell culture incubator (5% CO2/95% air; 37 °C), the absorbance values were measured at 450 nm using Cytation 5 Multimode Reader (BioTek, United States).
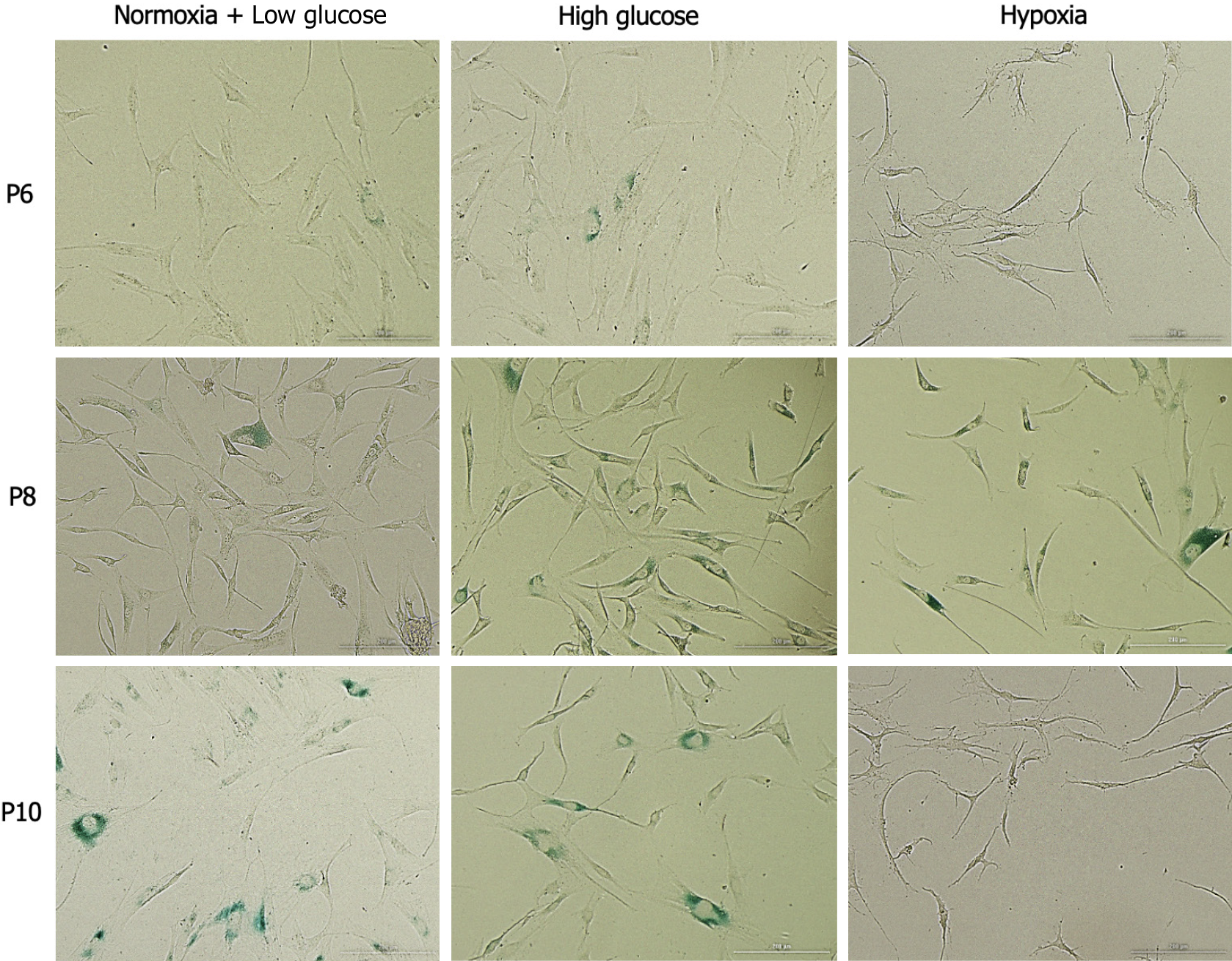
Senescence-associated beta-galactosidase (SA-β-gal) activity was detected with a senescent cell staining kit (Abcam, Cat# ab65351). Briefly, MSCs at P6, P8, and P10 were seeded in 24-well plate (1 × 105 cells/well) after being cultured in low and high glucose conditions. For hypoxia treatment, MSCs at P6, P8, and P10 were seeded in 24-well plate (1 × 105 cells /well) followed by the addition of CoCl2 in the culture media at 250 μM and incubation for 48 h. Media were aspirated and MSCs in different treatment groups were washed with 1X phosphate buffered saline (PBS) and then fixed with the fixative solution supplied in the kit for 10 min. 20X X-gal stock solution was prepared by weighing 20 mg X-gal and dissolving it in dimethyl sulfoxide. Next, cells were incubated in 250 μL staining solution containing X-gal, Staining Solution I/Staining Solution, and Staining Supplement for 24 h. Colored bright field images were obtained using Cytation 5 Multimode Reader (BioTek, United States) and the activity of X-gal was calculated.
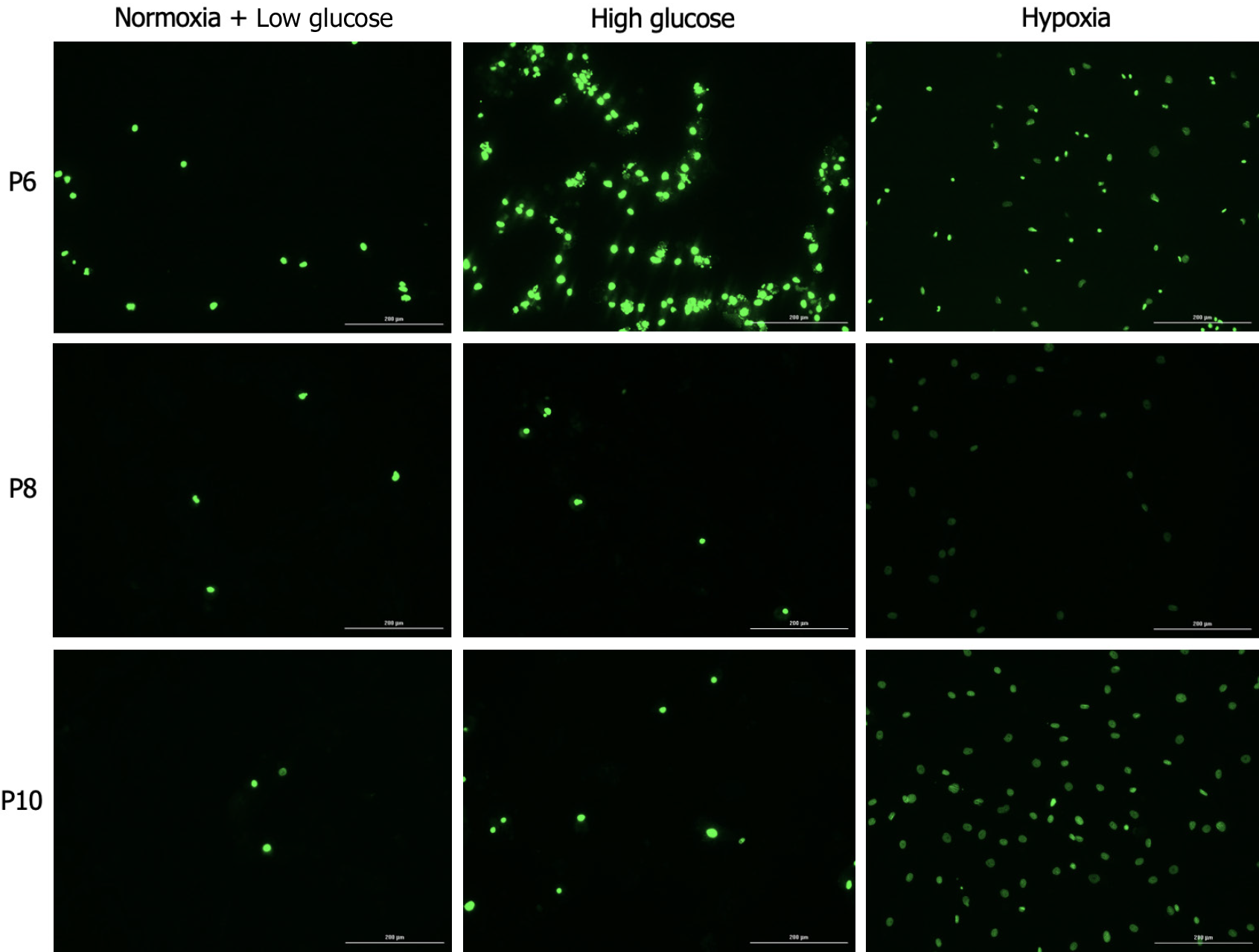
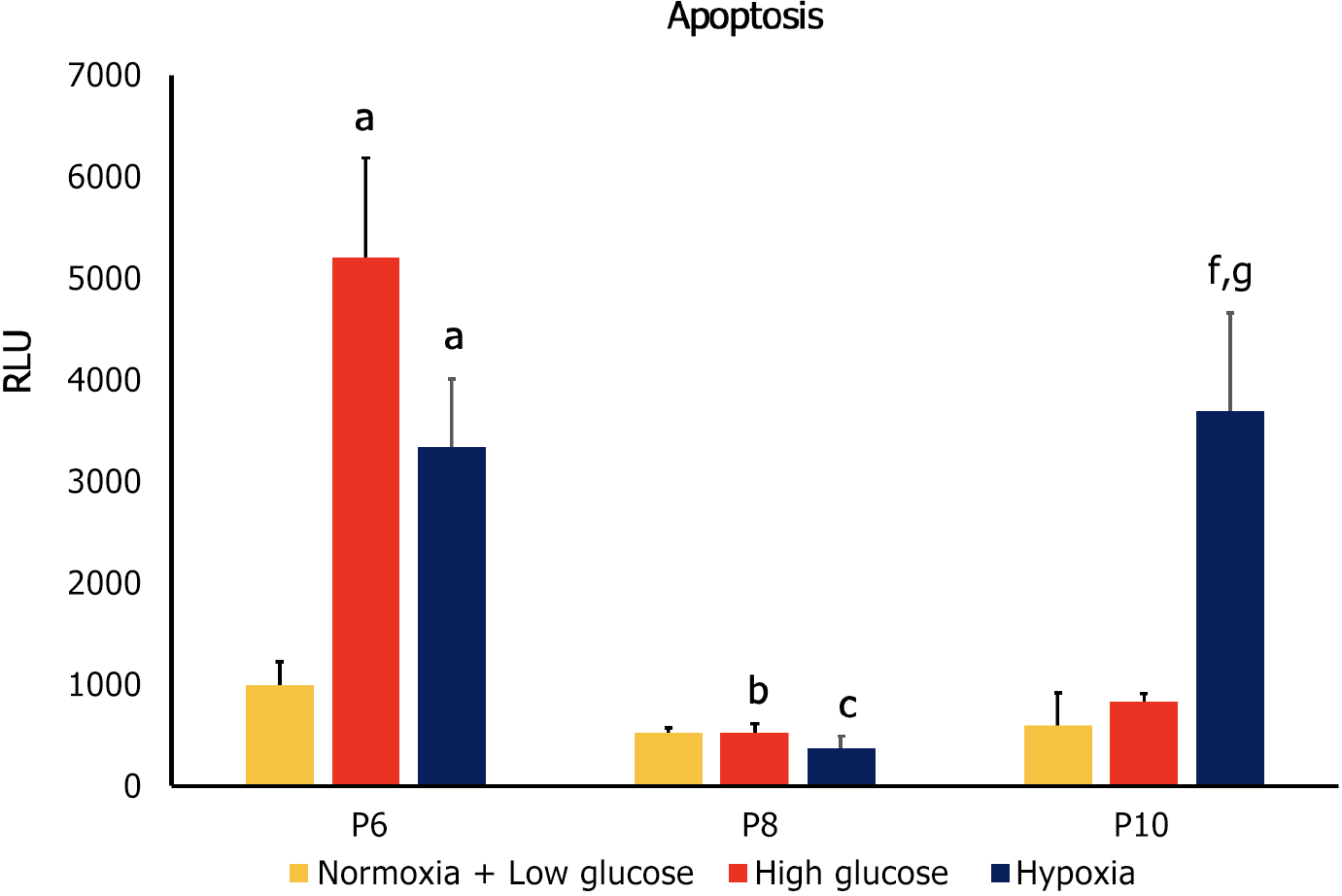
To detect apoptosis in hAD-MSCs at P6, P8, and P10 after being cultured in low glucose, high glucose, and hypoxia, we used RealTime-Glo™ Annexin V Apoptosis live assay (Promega, Cat# JA1011, Lot# 0000400486) following the manufacturer’s guidelines. Briefly, cells were seeded (1 × 104 cells/well) in 24 well plate after being cultured in low and high glucose for 5 d. For hypoxia treatment, cells were seeded (1 × 104 cells/well) in 24 well plate and incubated with 250 μM CoCl2 for 48 h. For apoptosis detection in different treatment conditions, the media were aspirated and each well was washed with PBS followed by the addition of 100 μL of fresh medium having Annexin V-LgBiT, Annexin V-SmBiT, CaCl2, and Annexin V NanoBiT Substrate to each well. After 1 h incubation, the fluorescent images of green color, which represented cells undergoing apoptosis, were detected at GFP filter using Cytation 5 Multimode Reader (BioTek, United States). Relative luminescence intensity, which is proportional to the level of apoptosis, was also detected using the luminescence filter in Cytation 5. The higher the luminescence intensity, the greater the apoptosis level.
Data were reported as mean ± SD. Comparison of data between multiple groups was performed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc multiple comparison test, and analysis between two groups was made using Student’s t-test (two-tailed). Statistical significance was determined as P < 0.05. Each figure represents one of at least three independent quantifiable experiments.
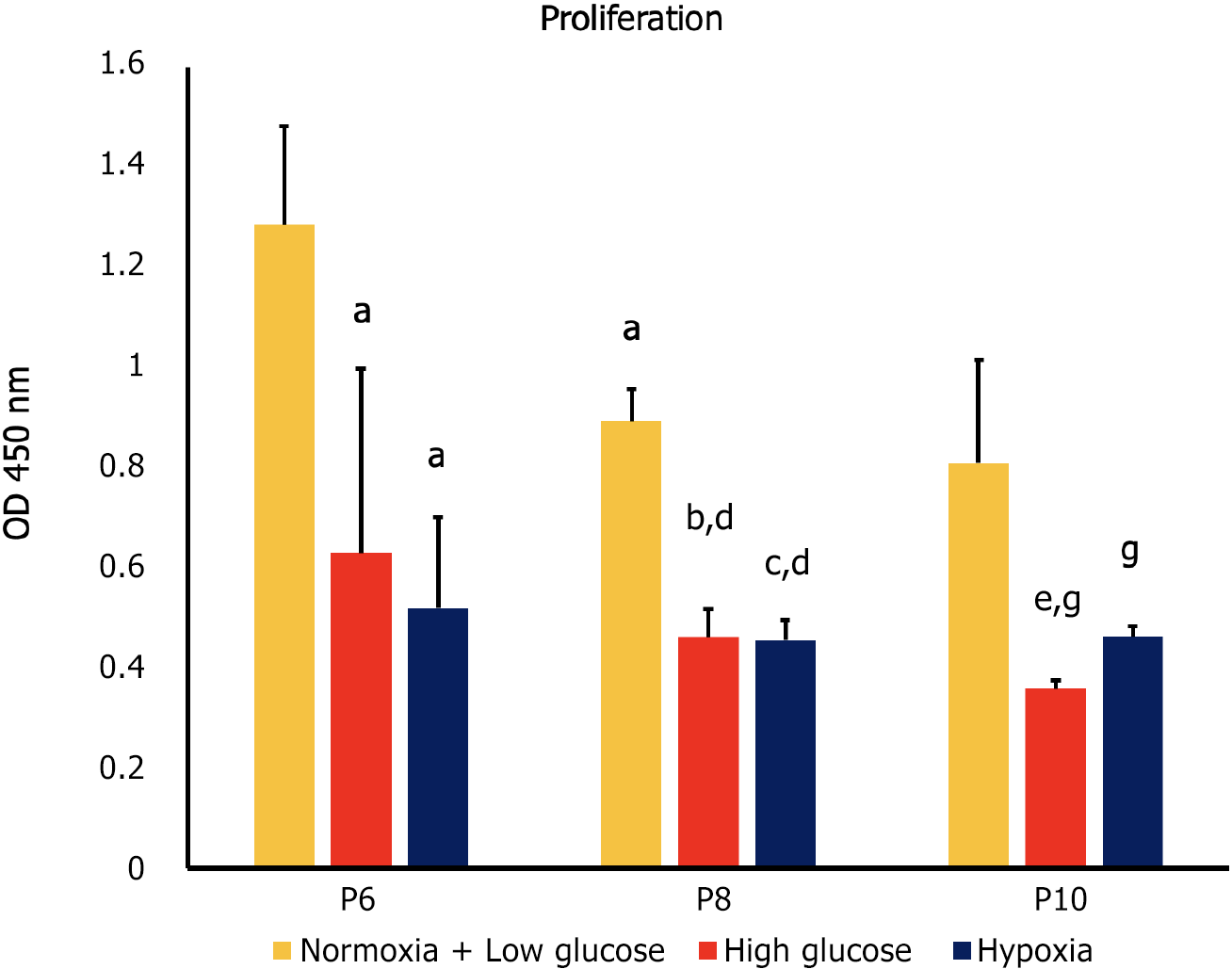
Cell proliferation is the controlled, well-defined increase in cell number resulting from cell division[23]. In our study, proliferation was quantified using a WST-1 assay protocol, which is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. Our results showed that high glucose and hypoxia were associated with a significant decrease in proliferation of MSCs compared to control cells at each of the studied passages (P6, P8 and P10; P < 0.05). At P8, proliferation decreased in all MSC groups compared to their counterparts at P6 (P < 0.05). On the other hand, at P10 only MSCs cultured in high glucose showed decreased proliferation compared to the same condition at P8 (P < 0.05; Figure 1).
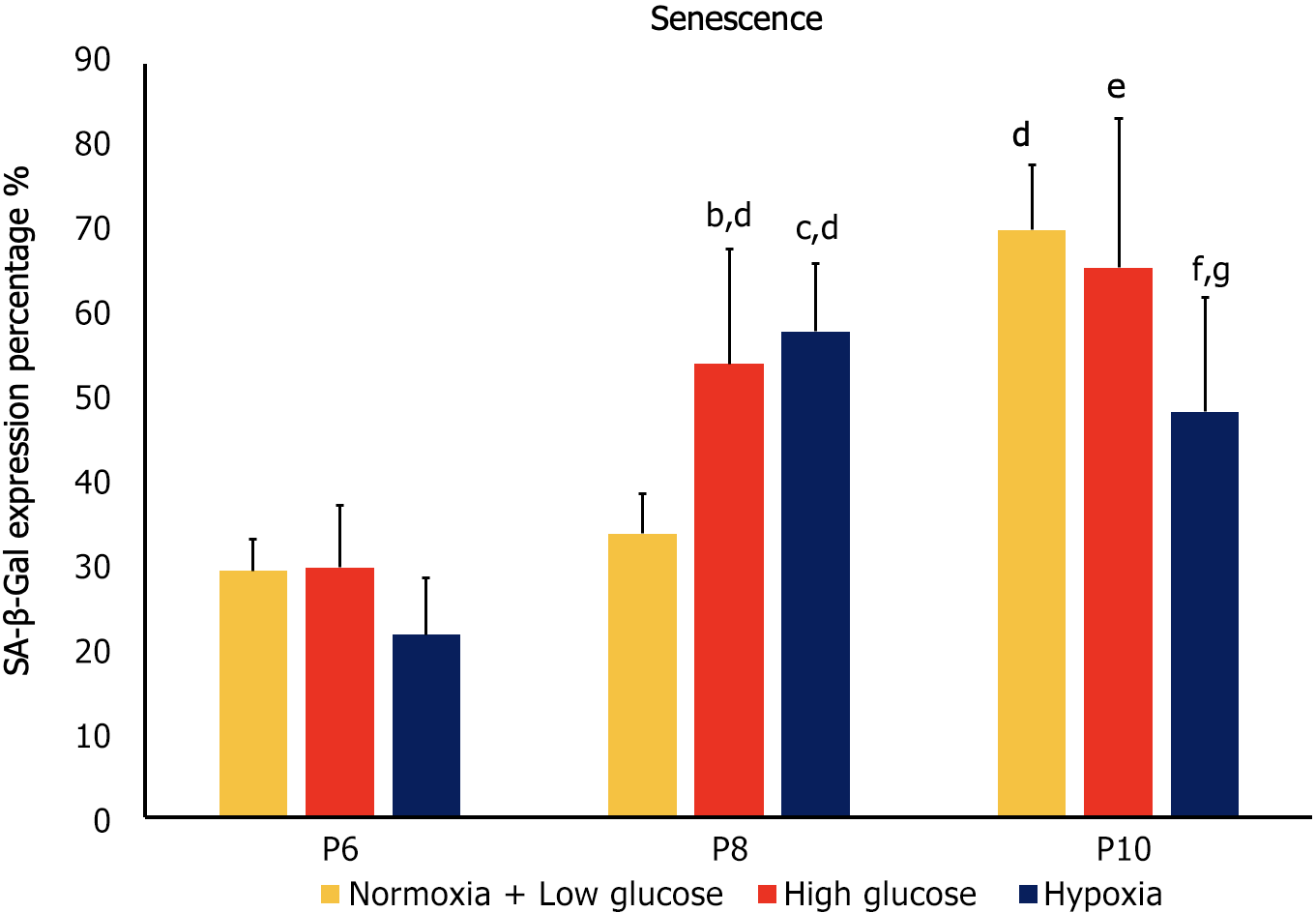
Cellular senescence is the process leading to a state of cell growth arrest[24]. Expression of SA-β-gal was used as a marker of senescence in hAD-MSCs. At P6, no significant differences in senescence were detected between the cells cultured in stress conditions and control cells. At P8, MSCs exposed to both hypoxia and high glucose exhibited significantly higher senescence compared to control at the same passage (P < 0.05), as well as to their counterparts at P6 (P < 0.05). On the other hand, at P10 control MSCs and MSCs cultured in high glucose showed significantly higher senescence compared to their counterparts at the previous passage (P < 0.05), while MSCs cultured under hypoxia exhibited significantly less senescence than control MSCs at P10 and compared to the cells cultured at the same condition at P8 (P < 0.05 both; Figures 2 and 3).
Apoptosis is a controlled form of programmed cell death that occurs in multicellular organisms to maintain homeostasis[25]. Apoptosis is also involved in the pathophysiology of various diseases[26] and it was found to compromise the therapeutic efficacy of MSCs[27]. Apoptosis of MSCs was detected using annexin V, a calcium-binding protein that binds to phosphatidylserine, a plasma membrane lipid that becomes exposed in apoptotic cells[28]. MSCs at P6 exhibited increased apoptosis under conditions of high glucose and hypoxia compared to control cells (P < 0.05). Apoptosis of the MSCs at P8 significantly decreased in both stress conditions compared to their counterparts at P6 (P < 0.05), while no significant difference was detected compared to the control group at the same passage. At P10 only cells cultured in hypoxia had a significantly higher apoptosis compared to control group at P10 and to counterpart cells at both P6 and P8 (P < 0.05 both; Figures 4 and 5).
At P6 high glucose and hypoxia were associated with significant decrease in proliferation and increase in apoptosis (P < 0.05 both), while they had no significant effect on senescence. At P8, MSCs cultured in both stress conditions showed significantly lower proliferation and higher senescence compared to control groups (P < 0.05 both), while no significant difference was observed in apoptosis. Compared to counterpart cells at P6, MSCs in stress conditions had a significant decrease in proliferation and apoptosis (P < 0.05 both) and a significant increase in senescence (P < 0.05).
At P10 both stress factors were associated with lower proliferation compared to control conditions at the same passage (P < 0.05), while only hypoxia significantly affected senescence (decrease; P < 0.05) and apoptosis (increase; P < 0.05). When compared to the previous passage, cells cultured in high glucose had lower proliferation, higher senescence (P < 0.05 both) and no change in apoptosis, while MSCs cultured under hypoxia showed decreased senescence, increased apoptosis (P < 0.05 both) and no significant change in proliferation.
The results of our study showed a reverse correlation between senescence and apoptosis in stress conditions at higher passages. At P8 increased senescence was associated with decreased apoptosis of the MSCs cultured both in high glucose and under hypoxia, and decreased senescence was associated with increased apoptosis in case of hypoxia at P10.
Our findings revealed that at P10 the effects of the two studied stress conditions on hAD-MSCs were different. When compared to the same condition at the previous passage, high glucose was associated with decreased proliferation (P < 0.05), increased senescence (P < 0.05), and no change in apoptosis. On the other hand, hypoxia was associated with decreased senescence (P < 0.05), increased apoptosis (P < 0.05), and no change in proliferation.
MSCs have shown promising results in the treatment of a number of chronic diseases, providing a new hope to patients with debilitating conditions. Due to their specific molecular composition, MSCs are able to regenerate damaged tissues and restore their functionality[29]. These effective outcomes, however, are often short-lived due to the hostile microenvironments in which MSCs are infused[5]. Severe hypoxia and high glucose are two of the stress factors that characterize these environments and negatively affect the outcomes of MSC infusions. Severe hypoxia, a condition associated with myocardial ischemia, chronic kidney disease and cancer, among others[30], was found to impair MSC proliferation and enhance senescence and apoptosis[12,13]. Similarly, MSCs cultured in high glucose concentrations were reported to have lower proliferation rates and greater senescence and apoptosis[6,7]. High glucose is of particular importance in the pathophysiology of DM and the development of its complications, such as retinopathy, nephropathy, and heart disease[31].
To obtain successful clinical applications, MSCs have to be generated in large numbers without compromising their properties; therefore, it is paramount to characterize the biological functions and the potency of MSCs in serial passages[32]. This will ensure the quality of cells during culture in vitro, their stability when used for specific purposes, the maximization of their potential and the reproducibility of techniques and technologies based on MSCs[15].
In this study, we investigated the effects of two stress factors - high glucose and severe hypoxia - on the proliferation, senescence and apoptosis of hAD-MSCs at different serial passages (P6, P8, and P10). Our findings revealed that hAD-MSCs cultured under conditions of high glucose and hypoxia exhibited decreased proliferation at all studied passages compared to the cells cultured in low glucose/normoxia conditions. These findings corroborate previous results indicating the association of stressful microenvironment with impaired proliferation[7,12]. A number of studies reported that hyperglycemia impaired proliferation of MSCs from various sources[33], and high glucose was found to induce aging of MSCs via the Akt/mTOR pathway[7]. Similarly, human MSCs, including hAD-MSCs, showed reduced proliferation rates when cultured under conditions of severe hypoxia[34,35].
The effect of stress factors on senescence compared to control conditions was observed starting at P8, where senescence was significantly higher, while at P10 only hypoxia induced a decrease in senescence. On the other hand, apoptosis increased at P6 under conditions of stress, did not differ at P8 and was significantly increased only under hypoxia at P10. These results are interesting when compared to previous reports. Despite a few exceptions[36,37], previous studies found that high glucose and severe hypoxia induced both senescence and apoptosis in different types of MSCs[10,20,38,39]. This supports the suggestion that senescence and apoptosis pathways are simultaneously involved in certain stress responses[40]. Our findings, on the other hand, indicate that hAD-MSCs apoptotic and senescent response to high glucose and hypoxia varied according to the stress factor and the passage number.
An interesting finding in our results is the association of senescence with decreased apoptosis, which was observed in both stress conditions at P8. Similarly, increased senescence at P10 prevented an increase in apoptosis in cells cultured in high glucose media, while decreased senescence was associated with increased apoptosis in case of hypoxia. Our findings indicate a protective role of senescence against apoptosis, an observation that had been reported in previous studies[41,42]. Senescent cells were found to activate a number of pro-survival factors, including members of the BCL-2 family, PI3K, p21, FOXO4, JNK, caspase, HSP90 and plasminogen activated inhibitor-2, leading to apoptosis resistance[40,42]. Resistance to apoptosis occurring through p53 signaling was found in senescent human fibroblasts[43]. Because cellular senescence is commonly observed in MSCs in response to stressful stimuli[41], it can be suggested that MSCs utilize senescence as a survival mechanism against stress factors. This finding is of particular importance, because it highlights the enhancement of senescence as a potential strategy to inhibit apoptosis in MSCs, thus improving their survival and therapeutic efficacy.
Most MSC-based therapeutic interventions utilize cells at P3 to P7[14], while higher passage numbers are avoided as passaging was found to modify some physiologic characteristics of MSCs[44], as well as their immunological behavior and cellular bioenergetics[45,46]. The results of our study, however, challenge this practice, as they suggest that even at higher passages (P8 and P10) hAD-MSCs are able to resist apoptosis when exposed to stress conditions by shifting into the senescent phenotype. This indicates the potential usefulness of high passage MSCs in specific clinical situations.
Interestingly, we observed a difference in MSC response to the different stress factors at the highest passage. At P10 high glucose was associated with increased senescence, while hypoxia induced a decrease in senescence, compared to the previous passage. Regarding apoptosis, high glucose had no significant effect on this parameter, while hypoxia was associated with higher apoptosis. Although a number of studies addressed the biological and functional characteristics of MSCs at different serial passages[47,48], to the best of our knowledge, no previous studies investigated the effects of stressful microenvironments on MSCs at various serial passages. Our findings thus provide a new insight for the optimization of MSCs use in the clinical setting, with different passages of MSCs to be considered for the treatment of conditions characterized by specific stressful microenvironments. This suggests that optimal MSCs therapy may benefit from a “passage - stress matching”.
Our study has some limitations. First, it involved only one type of MSCs (hAD-MSCs), which may make generalization of the results to MSCs from other sources inappropriate. In addition, only three passages where investigated (P6, P8, and P10), while it would be useful to study the biological behavior of MSCs under stress conditions at even higher passages. In the future, it would be recommended to expand investigation to other types of MSCs and to different stressful conditions. The biological behavior of MSCs could also be addressed in a wider context by measuring other parameters, such as cytotoxicity and differentiation.
The successful utilization of MSCs in the treatment of many diseases is hindered by a number of stress conditions that undermine the biological characteristics and survival rates of these cells. These properties are also affected by the serial passages MSCs undergo for culture. The results of our study revealed that severe hypoxia and high glucose induced distinct biological responses in hAD-MSCs at different passages. Therefore, the passage number of MSCs could represent a significant factor to be taken into consideration when choosing the optimal MSCs for specific therapeutic applications. Our study also found that hAD-MSCs at high passages still possess the ability to resist apoptosis, as showed by the reverse relationship between senescence and apoptosis. These findings reinforce the theory that senescence in MSCs may represent a protective mechanism against stress, and prompts a review of the practice of limiting the MSCs used in regenerative therapies into the earlier passages.
Mesenchymal stem cells (MSCs) have significant therapeutic potential. The biological properties of MSCs seem to be affected by serial passage and stress factors.
Despite their regenerative and immunomodulatory properties, the therapeutic applications of MSCs are sub-optimal and short-lived. The effects of stress factors on MSCs at various serial passages on their biological characteristics have not been recognized yet.
This study aimed to investigate the effects of stress factors (high glucose and severe hypoxia) on the biological characteristics of MSCs at different passages, in order to optimize the therapeutic applications of MSCs.
Proliferation, senescence and apoptosis of MSCs exposed to severe hypoxia and high glucose were evaluated measuring WST-1, senescence-associated beta-galactosidase, and annexin V, respectively.
Severe hypoxia and high glucose affected the biological responses of human adipose tissue-derived MSCs and these responses varied according to the serial passages. At high passages, a reverse relationship between senescence and apoptosis was observed, suggesting that senescence in MSCs may represent a protective mechanism against stress.
This study showed that the passage number of MSCs could represent a significant factor to be taken into consideration when choosing the optimal MSCs for specific therapeutic applications.
The practice of limiting MSCs used in regenerative therapies into the earlier passages should be reviewed to expand and optimize the therapeutic potential of MSCs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Jordan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo D, China; Liu Y, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Li Q, Wang Y, Deng Z. Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem Cell Res Ther. 2013;4:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Mahmoud M, Abu-Shahba N, Azmy O, El-Badri N. Impact of Diabetes Mellitus on Human Mesenchymal Stromal Cell Biology and Functionality: Implications for Autologous Transplantation. Stem Cell Rev Rep. 2019;15:194-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : In vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones. 2015;20:237-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 340] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 6. | Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Zhang D, Lu H, Chen Z, Wang Y, Lin J, Xu S, Zhang C, Wang B, Yuan Z, Feng X, Jiang X, Pan J. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol Med Rep. 2017;16:1685-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Liang C, Li H, Tao Y, Zhou X, Li F, Chen G, Chen Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Zheng Y, Huang L, He J. Research Progress on Mesenchymal Stem Cells for the Treatment of Diabetes and Its Complications. Int J Endocrinol. 2023;2023:9324270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv Pharm Bull. 2015;5:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 746] [Cited by in RCA: 893] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 12. | Cicione C, Muiños-López E, Hermida-Gómez T, Fuentes-Boquete I, Díaz-Prado S, Blanco FJ. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013;2013:232896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Xing J, Ying Y, Mao C, Liu Y, Wang T, Zhao Q, Zhang X, Yan F, Zhang H. Hypoxia induces senescence of bone marrow mesenchymal stem cells via altered gut microbiota. Nat Commun. 2018;9:2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Sareen N, Sequiera GL, Chaudhary R, Abu-El-Rub E, Chowdhury SR, Sharma V, Surendran A, Moudgil M, Fernyhough P, Ravandi A, Dhingra S. Early passaging of mesenchymal stem cells does not instigate significant modifications in their immunological behavior. Stem Cell Res Ther. 2018;9:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Lian J, Lv S, Liu C, Liu Y, Wang S, Guo X, Nan F, Yu H, He X, Sun G, Ma X. Effects of Serial Passage on the Characteristics and Cardiac and Neural Differentiation of Human Umbilical Cord Wharton's Jelly-Derived Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:9291013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Wall ME, Bernacki SH, Loboa EG. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007;13:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Jiang CF, Hsu SH, Sun YM, Tsai MH. Quantitative Bioimage Analysis of Passaging Effect on the Migratory Behavior of Human Mesenchymal Stem Cells During Spheroid Formation. Cytometry A. 2020;97:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Hong SH, Lee MH, Koo MA, Seon GM, Park YJ, Kim D, Park JC. Stem cell passage affects directional migration of stem cells in electrotaxis. Stem Cell Res. 2019;38:101475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Khasawneh RR, Al Sharie AH, Abu-El Rub E, Serhan AO, Obeidat HN. Addressing the impact of different fetal bovine serum percentages on mesenchymal stem cells biological performance. Mol Biol Rep. 2019;46:4437-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Abu-El-Rub E, Almahasneh F, Khasawneh RR, Alzu'bi A, Ghorab D, Almazari R, Magableh H, Sanajleh A, Shlool H, Mazari M, Bader NS, Al-Momani J. Human mesenchymal stem cells exhibit altered mitochondrial dynamics and poor survival in high glucose microenvironment. World J Stem Cells. 2023;15:1093-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (1)] |
| 21. | Khasawneh RR, Abu-El-Rub E, Almahasneh FA, Alzu'bi A, Zegallai HM, Almazari RA, Magableh H, Mazari MH, Shlool HF, Sanajleh AK. Addressing the impact of high glucose microenvironment on the immunosuppressive characteristics of human mesenchymal stem cells. IUBMB Life. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 22. | Teti G, Focaroli S, Salvatore V, Mazzotti E, Ingra' L, Mazzotti A, Falconi M. The Hypoxia-Mimetic Agent Cobalt Chloride Differently Affects Human Mesenchymal Stem Cells in Their Chondrogenic Potential. Stem Cells Int. 2018;2018:3237253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Yang Y, Hu M, Zhang Y, Li H, Miao Z. CD29 of human umbilical cord mesenchymal stem cells is required for expansion of CD34(+) cells. Cell Prolif. 2014;47:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol. 2017;27:2652-2660.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 614] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 25. | Renehan AG, Booth C, Potten CS. What is apoptosis, and why is it important? BMJ. 2001;322:1536-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY). 2012;4:330-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 27. | Giacomini C, Granéli C, Hicks R, Dazzi F. The critical role of apoptosis in mesenchymal stromal cell therapeutics and implications in homeostasis and normal tissue repair. Cell Mol Immunol. 2023;20:570-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 28. | Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb Protoc. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 29. | Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, Marofi F, Shamlou S, Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 30. | Chen PS, Chiu WT, Hsu PL, Lin SC, Peng IC, Wang CY, Tsai SJ. Pathophysiological implications of hypoxia in human diseases. J Biomed Sci. 2020;27:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 31. | Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 788] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 32. | Fariha MM, Chua KH, Tan GC, Tan AE, Hayati AR. Human chorion-derived stem cells: changes in stem cell properties during serial passage. Cytotherapy. 2011;13:582-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Luo M, Zhao Z, Yi J. Osteogenesis of bone marrow mesenchymal stem cell in hyperglycemia. Front Endocrinol (Lausanne). 2023;14:1150068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 34. | Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Müller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 35. | Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | He X, Yang Y, Yao MW, Ren TT, Guo W, Li L, Xu X. Full title: High glucose protects mesenchymal stem cells from metformin-induced apoptosis through the AMPK-mediated mTOR pathway. Sci Rep. 2019;9:17764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 38. | Yin M, Zhang Y, Yu H, Li X. Role of Hyperglycemia in the Senescence of Mesenchymal Stem Cells. Front Cell Dev Biol. 2021;9:665412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Gao H, Nepovimova E, Heger Z, Valko M, Wu Q, Kuca K, Adam V. Role of hypoxia in cellular senescence. Pharmacol Res. 2023;194:106841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 40. | Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 648] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 41. | Deryabin PI, Shatrova AN, Borodkina AV. Apoptosis resistance of senescent cells is an intrinsic barrier for senolysis induced by cardiac glycosides. Cell Mol Life Sci. 2021;78:7757-7776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Hu L, Li H, Zi M, Li W, Liu J, Yang Y, Zhou D, Kong QP, Zhang Y, He Y. Why Senescent Cells Are Resistant to Apoptosis: An Insight for Senolytic Development. Front Cell Dev Biol. 2022;10:822816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 43. | Seluanov A, Gorbunova V, Falcovitz A, Sigal A, Milyavsky M, Zurer I, Shohat G, Goldfinger N, Rotter V. Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. Mol Cell Biol. 2001;21:1552-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H, Ljungman P, Gustafsson B, Karlsson H, Le Blanc K, Ringdén O. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Stab BR 2nd, Martinez L, Grismaldo A, Lerma A, Gutiérrez ML, Barrera LA, Sutachan JJ, Albarracín SL. Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front Aging Neurosci. 2016;8:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Ziegler DV, Wiley CD, Velarde MC. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell. 2015;14:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 47. | Zhang Y, Li Y, Li W, Cai J, Yue M, Jiang L, Xu R, Zhang L, Li J, Zhu C. Therapeutic Effect of Human Umbilical Cord Mesenchymal Stem Cells at Various Passages on Acute Liver Failure in Rats. Stem Cells Int. 2018;2018:7159465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Zhao YT, Qin Y, Yang JS, Huang DG, Hu HM, Wang XD, Wu SF, Hao DJ. Wharton's Jelly-derived mesenchymal stem cells suppress apoptosis of nucleus pulposus cells in intervertebral disc degeneration via Wnt pathway. Eur Rev Med Pharmacol Sci. 2020;24:9807-9814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |