Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.165
Peer-review started: December 20, 2022
First decision: January 9, 2023
Revised: January 30, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 26, 2023
Processing time: 126 Days and 15.3 Hours
Cell transplantation therapy has certain limitations including immune rejection and limited cell viability, which seriously hinder the transformation of stem cell-based tissue regeneration into clinical practice. Extracellular vesicles (EVs) not only possess the advantages of its derived cells, but also can avoid the risks of cell transplantation. EVs are intelligent and controllable biomaterials that can participate in a variety of physiological and pathological activities, tissue repair and regeneration by transmitting a variety of biological signals, showing great potential in cell-free tissue regeneration. In this review, we summarized the origins and characteristics of EVs, introduced the pivotal role of EVs in diverse tissues regeneration, discussed the underlying mechanisms, prospects, and challenges of EVs. We also pointed out the problems that need to be solved, application directions, and prospects of EVs in the future and shed new light on the novel cell-free strategy for using EVs in the field of regenerative medicine.
Core Tip: Extracellular vesicles (EVs) play a critical role in tissue repair and regeneration medicine via cell-to-cell communication. In this review, we elaborate and discuss both the recent research progress and the advancements in the therapeutic effects and limitations of EVs in tissue regeneration and engineering medicine. Moreover, we summarize the underlying molecular mechanisms related to EV repair effects and point out the problems that need to be solved, application directions, and prospects of EVs in the future.
- Citation: Wang DR, Pan J. Extracellular vesicles: Emerged as a promising strategy for regenerative medicine. World J Stem Cells 2023; 15(4): 165-181
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/165.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.165
Tissue and organ loss are challenging complications that are usually caused by severe diseases such as cancer and serious accidents and impose great burdens on patients’ lives. Tissue regeneration and engineering medicine, which aims to repair lost cells and damaged organs caused by diseases or accidents, is achieving great progress sparked by numerous studies of stem cells, biomaterials and so on. Stem cells, especially mesenchymal stem cells (MSCs), play a critical role in regeneration medicine due to their strong self-renewal ability and diverse differentiation potential. However, there are several shortcomings of using MSCs in regeneration medicine, including cell source limitations, ethical controversies, low survival rates after cell transplantation, immune rejection, and risk of tumorigenesis after transplantation[1,2]. To solve the shortcomings of cell-based therapy, the paracrine action of cells has become a focus of research attention.
Recent research has focused on the secretions of cells and tissues. Extracellular vesicles (EVs) are heterogeneous lipid bilayer-surrounded vesicles secreted by various cell types, including immune cells, endothelial cells, epithelial cells, neuronal cells, cancerous cells, Schwann cells (SCs), and MSCs, that behave as crucial mediators of intercellular communication[3-6]. EVs participate in various types of physiological and pathological activities, including immune responses, homeostasis maintenance, inflammation, angiogenesis, and cancer progression, by transferring biological signals[7,8]. According to their size and origin, EVs can be classified in several ways. On the basis of their origin, EVs can be divided into three categories: Apoptotic bodies generated during cell apoptosis; microvesicles originating from budding cellular membranes; and exosomes derived from multivesicular bodies in fusion with the plasma membrane[4,9]. EVs also can be divided into small EVs, medium-sized EVs, and large EVs[3,10,11].
Increasing evidence indicates that EVs play a critical role in tissue repair and regeneration medicine via cell-to-cell communication. Moreover, several studies imply that the beneficial effects of MSCs on tissue regeneration may be attributed to their paracrine action by secreting EVs rather than MSC engraftment and proliferation[3,12,13]. Moreover, EVs themselves possess the ability to recruit endogenous cells and lead to their enrichment by releasing several chemokines, which may contribute to angiogenesis and tissue repair[14,15]. Therefore, EVs are an appropriate and hopeful new source for tissue repair and regeneration.
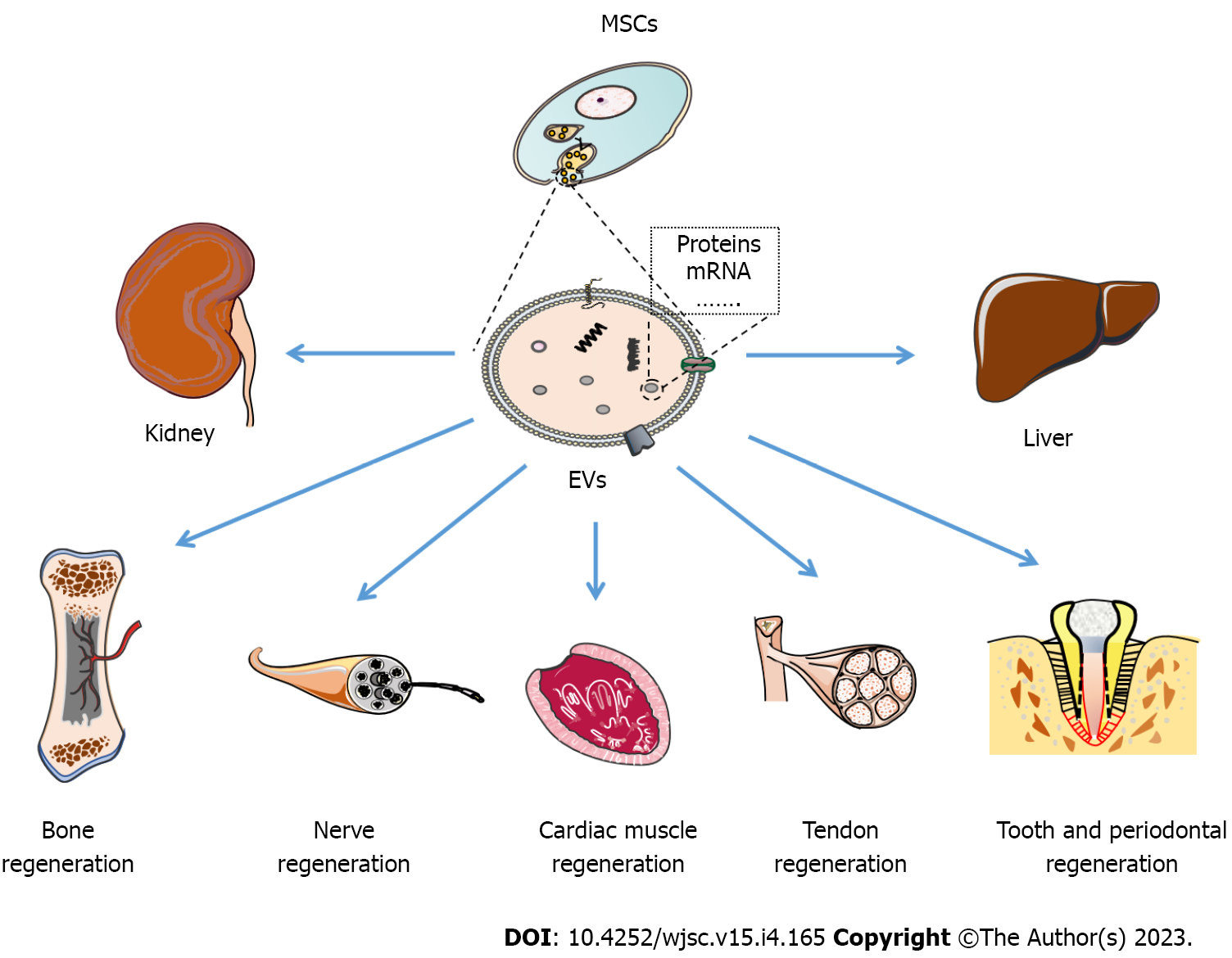
In this review, we elaborate and discuss both the recent research progress and the advancements in the therapeutic effects and limitations of EVs in tissue regeneration and engineering medicine (Figure 1). Moreover, we summarize the underlying molecular mechanisms related to EV repair effects (Table 1) and point out the problems that need to be solved, application directions (Table 2), and prospects of EVs in the future.
| Source of EVs | Mechanism | |
| Kidney | MSC-EVs | Inhibiting oxidation, apoptosis, and inflammation |
| HLSC-EVs | Regulating angiogenesis, the cell cycle, regeneration, autophagy, proliferation[36-38,46] | |
| BMSC-EVs | ||
| Liver disease | hiPSCs-EVs | Inhibiting hepatocyte apoptosis |
| Hepatocyte-EVs | Supporting hepatocyte function | |
| MSC-EVs | Promoting angiogenesis | |
| Reducing inflammatory responses[48-53] | ||
| Cardiac muscle | ESCs-EVs | Vascularization |
| iPSCs-EVs | Amelioration of apoptosis and hypertrophy | |
| MSCs-EVs | Promoting cell proliferation and migration[55-60] | |
| CDCs-EVs | ||
| BMSC-EVs | ||
| Tendon | BMSC-EVs | Modulating macrophage phenotypes |
| ADSC-EVs | Anti-inflammatory reaction | |
| Enhancing proliferation, migration, tenogenic differentiation of TSCs | ||
| Regulating angiogenesis | ||
| Modulating immune responses[62-68] | ||
| Wound healing | BMSC-EVs | Promoting re-epithelialization |
| ADSC-EVs | Promoting collagen maturity and angiogenesis | |
| hENSC-EV | Enhancing cell proliferation and migration[69-74] | |
| HUVECs-EVs | ||
| iPSC-EVs | ||
| Tooth and periodontal tissue | BMSC-EVs | Modulating the inflammatory immune response |
| ADSC-EVs | Enhancing cell proliferation and migration | |
| DPSC-EVs | Promoting odontogenic differentiation | |
| DFC-EVs | Stem cell recruitment[76-83] | |
| PDLSC-EVs | ||
| HERS-EVs | ||
| SC-EVs | ||
| Nerve | SKP-SC-EVs | Mediating axon regeneration |
| BMSC-EVs | Regulating the phenotype of Schwann cells | |
| ADSC-EVs | Promoting angiogenesis | |
| GMSC-EVs | Regulating inflammatory reactions[84-94] | |
| Bone | ADSC-EVs | Angiogenesis |
| BMSC-EVs | Osteoblast proliferation | |
| SMSC-EVs | Intercellular communication | |
| PRP-EVs | Immune regulation[96-104] |
| Research title | Interventions | Status |
| Treatment of Patients with Bone Tissue Defects Using Mesenchymal Stem Cells Enriched by Extracellular Vesicles | MSCs enriched by extracellular vesicles | Not yet recruiting |
| Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles Infusion Treatment for ARDS | Bone Marrow MSC Derived Extracellular | Not yet recruiting |
| Efficacy of Platelet- and Extracellular Vesicle-rich Plasma in Chronic Postsurgical Temporal Bone Inflammations | Plateletand extracellular vesicle-rich plasma | Completed |
| Use of Autologous Plasma Rich in Platelets and Extracellular Vesicles in the Surgical Treatment of Chronic Middle Ear Infections | Plateletand EVs-rich plasma | Recruiting |
| Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS | ExoFlo | Completed |
| Safety of Mesenchymal Stem Cell Extracellular Vesicles (BMMSC-EVs) for the Treatment of Burn Wounds | Drug: AGLE-102 (BMMSC-EVs) | Not yet recruiting |
| Treatment of Non-ischemic Cardiomyopathies by Intravenous ExtracellularVesicles of CardiovascularProgenitor Cells | Extracellular vesicle-enriched secretome of cardiovascular progenitor cells differentiated from induced pluripotent stem cells | Not yet recruiting |
| Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles Infusion Treatment for Mild-to-Moderate COVID-19: A Phase II Clinical Trial | Drug: ExoFlo. Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles | Not yet recruiting |
| A Safety Study of IV Stem Cellderived Extracellular Vesicles (UNEX-42) in Preterm Neonates at High Risk for BPD | UNEX-42 is a preparation of extracellular vesicles that are secreted from human bone marrow-derived mesenchymal stem cells suspended in phosphate-buffered saline | Terminated |
| ExoFlo™ Infusion for Post-Acute COVID-19 and Chronic Post COVID-19 Syndrome | Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles | Not yet recruiting |
| Study of ExoFlo for the Treatment of Medically Refractory Ulcerative Colitis | ExoFlo. Intravenous administration of bone marrow mesenchymal stem cell derived extracellular vesicles | Recruiting |
| Study of ExoFlo for the Treatment of Medically Refractory Crohn's Disease | ExoFlo. Intravenous administration of bone marrow mesenchymal stem cell derived extracellular vesicles | Recruiting |
| Bone Marrow Mesenchymal Stem Cell Derived EVs for COVID-19 Moderate-to-Severe ARDS: A Phase III Clinical Trial | EXOFLO. Bone Marrow Mesenchymal Stem Cell Derived EVs | Recruiting |
| Pilot Study of Human Adipose Tissue Derived Exosomes Promoting Wound Healing | Adipose tissue derived exosomes | Not yet recruiting |
| Exosome Effect on Prevention of Hairloss | Placental Mesenchymal Stem Cells-derived Exosome | Recruiting |
| Expanded Access for Use of ExoFlo in Abdominal Solid Organ Transplant Patients | Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles Infusion Treatment | Not yet recruiting |
| Safety of Injection of Placental Mesenchymal Stem Cell Derived Exosomes for Treatment of Resistant Perianal Fistula in Crohn's Patients | Placenal MSC derived exosomes | Active, not recruiting |
| Safety and Efficacy of Injection of Human Placenta Mesenchymal Stem Cells Derived Exosomes for Treatment of Complex Anal Fistula | Placenta MSCs derived exosomes | Recruiting |
| Safety and Tolerability Study of MSC Exosome Ointment | Exosome ointment | Completed |
| The Pilot Experimental Study of the Neuroprotective Effects of Exosomes in Extremely Low Birth Weight Infants | Exosomes derived from MSCs | Not yet recruiting |
The discovery of EVs dates back to 1940, when a brand-new subcellular factor was identified in cell-free plasma by high-speed centrifugation. The subcellular fraction was identified and shown to consist of small vesicles by electron microscopy in the 1960s. The term “exosomes” was introduced when vesicles were isolated from cell supernatant in 1987, and these exosomes were found to be related to the removal of obsolete transmembrane proteins[10]. Currently, EVs are secreted by both normal cells and cancerous cells and act as a means of cell-to-cell communication. Signals are communicated through vesicle membrane proteins or by vesicle contents such as proteins, miRNAs, or long noncoding RNAs (lncRNAs)[16].
EVs are classified by several traits, including their density, dimensions, and origin. Three subclasses of EVs have been reported recently. Although the sizes of these three main types of EVs may overlap, their biogenesis is different. Exosomes, whose dimensions range from 30-150 nm, are formed within the endosomal network, where endosomes target some protein/lipids for recycling or exocytosis. During early endosome transformation into late endosomes, proteins that are fated to be degraded or exported are packaged into vesicles. Late endosomes, which contain small vesicles, fuse with the plasma membrane and finally lead to the secretion of small vesicles, named exosomes, into the extracellular space[16]. Microvesicles/Ectosomes originate from vesicles budding outward and fission of the plasma membrane directly, which is a dynamic interplay between redistribution and cytoskeletal protein contraction. The size of microvesicles/ectosomes is larger than that of exosomes, ranging from 50-2000 nm. Apoptotic bodies, ranging from 500-40000 nm, are formed during programmed cell death and contain organelles.
Differential ultracentrifugation (UC), the gold standard EVs isolation approach, is the most reported and classical way to isolate EVs. Successive centrifugation was applied to eliminate large dead cells and cell debris. The supernatant acquired after successive centrifugation was used for another UC step at 100000 g to obtain the pellet related to EVs. The pellet was washed with PBS to obtain purified EVs[17]. UC has several advantages including low cost and low contamination risk with extra isolation reagents. UC is suitable for large volume preparation for its products is of high purity. However, UC is complicated, time-consuming and labor intensive. High speed centrifugation also may lead to potential mechanical damage. There are several isolation methods that can extract EVs in a more efficient way, including polymer-based precipitation[18], size exclusion chromatography (SEC)[19], ultrafiltration (UF)[20], flow field-flow fractionation[21], immunoaffinity capture[22], and microchip-based techniques[23]. Polymer-based precipitation is a commonly used strategy for EVs isolation. The principle of polymer-based precipitation is high hydrophilic water-excluding polymers can alternate the solubility of EVs. Highly hydrophilic polymers interact with water molecules surrounding the EVs to create a hydrophobic micro-environment, resulting in EVs precipitation[24]. Based on above principle, polyethylene glycol is well used in several popular commercial EVs isolation kits[25]. Polymer-based precipitation has high efficiency, but its products is easy to be contaminated by protein aggregates. SEC is according to the size of particle to realize the isolation. After adding to porous materials, substances eluted out in accordance with their particle size, with big particles eluted earlier[26]. Compared to UC, SEC is realized by the performance of passive gravity flow, which highly protect the structure and integrity of EVs[27]. UF uses filter membrane to isolate EVs from cell culture medium. Compared to traditional UC method, UF-based EVs isolation shortens time and presents relatively low requirements on experimental facilities. However, UF also has several shortages including the EVs production maybe limited due to clogging and membrane trapping[28,29]. Immunoaffinity capture is based on specific binding between EVs markers and immobilized antibodies such as Rab5, CD81, CD63, CD9, CD82, annexin, and Alix[30]. Immunoaffinity capture can harvest high-purity EVs with no chemical contamination. However, it is waste of antibodies and low yields[26]. The combination of the above methods is also applied in the isolation of EVs. Moreover, there are several kits that are available for EV isolation, including ExoQuick and Total Exosome Isolation kits. However, the purity and quality of isolated EVs are extremely important because some soluble proteins or lipoproteins may be coisolated with EVs, leading to inaccurate experimental results. Therefore, isolation methods are of critical importance. Among all the methods, UC and UF followed by SEC are reported to be the most appropriate isolation methods because they can isolate high-purity and high-quality EVs[31,32].
EVs carry several specific surface markers, including proteins related to the cell membrane, annexin, flotillin and auxiliary proteins. Annexins and tetraspanins such as CD9, CD63 and CD81, which are often used for the identification of EVs, are located in the membrane of EVs. Moreover, EVs express ALIX, tumor susceptibility gene 101, VPS4 and heat shock proteins (HSP70 and HSP 90), which are associated with the biogenesis of EVs. EVs are encapsulated in a bilayer membrane, which can help in the safe transfer of their contents to secondary cells. Once released into the extracellular environment, EVs interact with recipient cells in three ways: (1) Endocytic uptake; (2) direct fusion with cells; and (3) adhesion to the cell surface and transmission of contents[4]. Among their contents, EVs are reported to contain large-scale genetic materials, such as mRNA, that play critical roles in cell-to-cell communication. miRNAs that are transferred by EVs also have an impact on biological functions, including cell proliferation, migration, and differentiation of recipient cells. EVs also carry several types of lipids that are related to EV structure, function and biogenesis[3]. In conclusion, EVs are heterogeneous and are composed of a bilayer membrane surrounding cargos that are indispensable for cell interactions.
Acute kidney injury (AKI) and chronic kidney disease (CKD) are two major causes of renal failure and exert great pressure on public health. AKI, the most common features of which are the rapid loss of renal tubular cells and a decline in renal function, usually leads to hospitalization[33]. As research has progressed, evidence has shown that MSC-EVs play a major role in treating AKI. EVs were first proven to be effective against AKI in 2009[34]. The intravenous administration of EVs not only alleviated or even reversed the detrimental effect on renal function caused by glycerol injection in an AKI model but also improved renal function and morphology by stimulating the proliferation of tubular epithelial cells (TECs)[35]. In a renal ischemia-reperfusion (I/R) injury AKI model, MSC-EVs accumulated in the renal tubules and facilitated with the recovery of kidney function through the Keap1-Nrf2 signaling pathway as well as the mitochondrial function of TECs[36]. Studies have also shown that EVs can reduce the presence of luminal cell debris, tubular hyaline casts and necrosis of tubular cells in an AKI model induced by toxins[37]. Moreover, EVs released by cells that were cultured under hypoxia could stimulate angiogenesis and help the formation of the peritubular microvasculature[38]. Furthermore, MSC-EVs attenuated mtDNA damage and inflammation after AKI through the mitochondrial transcription factor A (TFAM) pathway. MSC-EVs could attenuate renal lesion formation, mitochondrial damage, and inflammation in mice with AKI. TFAM overexpression (TFAM-OE) improved the rescue effect of MSC-EVs on mitochondrial damage and inflammation to some extent[39]. In general, MSC-EVs can relieve AKI not only by inhibiting oxidation, apoptosis, and inflammation, but also through regulating angiogenesis, cell cycle, autophagy, and cell proliferation[38]. There are multiple underlying mechanisms. Currently, it is believed that the repair effects of EVs on AKI are largely related to their transfer of genetic material and proteins[40].
CKD is a complex and long-term disease. The main trigger that causes CKD is diabetes. Hyperglycemia leads to glomerular and tubulointerstitial fibrosis, and the progression of fibrosis is the main reason for renal dysfunction[41]. Renal glomerulosclerosis and tubulointerstitial fibrosis are hallmarks of all types of CKD, including diabetic nephropathy (DN). Recent studies have shown that EVs are effective for the prevention of DN. For example, EVs can protect podocytes and TECs from apoptosis by secreting protective proteins, including transforming growth factor (TGF)-β1 and angiogenin[42]. Another study found that EVs derived from human liver stem cells (HLSCs) and MSCs could reduce or even revert the progression of profibrotic processes and finally ameliorate renal dysfunction and attenuate renal histopathological changes[43]. Moreover, studies have shown that EVs can ameliorate DN by inducing autophagy induction through the mTOR pathway. Rat bone marrow-derived EVs present nephroprotective and antifibrotic effects by upregulating autophagy through suppressing the mTOR pathway in a DN model[44]. Furthermore, HLSC-derived EVs could also prevent the development of CKD. HLSC-EVs not only present a regenerative and anti-inflammatory role but also downregulate profibrotic genes, including alpha smooth muscle actin, Col1a1 and TGF-β1, and modulate miRNAs that are related to the fibrotic pathway in the kidney of an aristolochic acid-induced CKD model[45]. In conclusion, MSC-EVs can eliminate the pathogenic damage of CKD by targeting renal fibrosis, reducing tubular atrophy and inflammation, and promoting angiogenesis to facilitate with tissue regeneration[46]. EVs present a promising approach for renal repair and regeneration. Some evidence based on clinical trials has shown that EVs are safe and effective for CKD patients[47].
Liver dysfunction is classified into acute and chronic diseases, including hepatitis, alcoholic liver disease, fatty liver disease, cirrhosis, and hepatocellular carcinoma. Liver failure can manifest with several symptoms, such as jaundice, encephalopathy, cerebral edema, sepsis, and gastrointestinal bleeding, and its prognosis is relatively limited. Currently, liver transplantation is still the gold-standard therapy for liver diseases; however, it has many limitations, such as nonspecific treatment approaches, donor organ shortages and lifelong immunosuppressive therapy[48]. Although the liver possesses a great capacity for regeneration through the proliferation of mature liver cells, it can lose its function and lead to severe results when the injury progresses into a state of functional impairment, which may finally lead to liver failure or even death[49]. EVs can prevent further damage to injured liver cells. Studies have reported that EVs can reduce liver injury based on alanine aminotransferase and aspartate aminotransferase levels after CCl4-induced liver impairment in vivo. Moreover, EVs promoted hepatocyte regeneration gene expression and PCNA+ expression and induced quiescent hepatocytes to re-enter the cell cycle, ultimately assisting with hepatocyte proliferation. Another report showed that EVs derived from human-induced pluripotent stem cells (hiPSCs) could alleviate hepatic I/R injury by suppressing inflammatory responses, attenuating the oxidative stress response and inhibiting cell apoptosis[50]. Furthermore, Du et al[51] found that hiPSC-derived EVs could alleviate hepatic I/R injury by activating sphingosine kinase and the sphingosine-1-phosphate pathway in hepatocytes and ultimately promote cell proliferation[51]. EVs can not only be therapeutic but can also serve as diagnostic tools for liver disease and regeneration in the near future. A report outlined that hepatocyte-derived EVs played a key role in hepatocyte-to-hepatocyte communication and provided a new method for liver disease diagnosis, and progenitor cell-derived EVs offered a new opportunity for the treatment of liver diseases[52]. In conclusion, recent evidence supports that MSC-derived EVs inhibit hepatocyte apoptosis, support hepatocyte function, promote angiogenesis and hepatocyte proliferation, and reduce inflammatory responses by preventing immunocyte infiltration and inflammatory cytokine secretion[48]. In addition, animal model-based studies suggest that EVs may represent a novel and effective cell-free therapeutic agent as an alternative to cell-based therapies for patients with liver diseases[53].
Myocardial repair and regeneration are important in the context of the increasing occurrence of heart failure and cardiac-related diseases. Key mechanisms related to cardiac repair and regeneration include survival and protection, inflammation reduction, angiogenesis, cardiomyogenesis and cell–cell communication. All these mechanisms work collectively and contribute to cardiac regeneration[54]. However, myocardial repair is slow and limited. Stem cell–based therapies have acted as an effective method for cardiac repair. Moreover, EVs, a key component of stem cell secretion, bring new hope to cell-free therapies for cardiac repair and regeneration. EVs of embryonic stem cells, iPSCs, MSCs, and cardiosphere-derived cells (CDCs) have been proven to be effective in cardiac repair[55]. Recent research has shown that EVs derived from murine iPSCs impart cytoprotective properties to cardiac cells in vitro and induce cardiac repair in vivo through vascularization, amelioration of apoptosis and hypertrophy and improvement in left ventricular function[56]. Moreover, iPSC-EVs contained numerous miRNAs (miR-17-92 cluster) and proteins [BMP-4, teratocarcinoma-derived growth factor 1 and vascular endothelial growth factor (VEGF)-C] that are related to cellular proliferation and differentiation, enhanced angiogenesis, and the prevention of apoptosis. Therefore, iPSC-EVs could induce angiogenesis, migration and anti-apoptosis of cardiac endothelial cells and finally induce superior infarct repair[56]. MSC-derived EVs also play a critical role in MSC-based therapy in cardiac diseases. Bian et al[57] found that intramyocardial injection of MSC-EVs could markedly enhance blood flow recovery in an acute myocardial infarction (MI) rat model. MSC-EVs could protect cardiac tissue from ischemic injury by promoting blood vessel formation. Receptors for growth factors, cytokines and signaling molecules are contained in EVs, among which Sonic hedgehog and platelet-derived growth factors have been proven to be effective for proangiogenic activities. The results of direct comparisons between MSCs and MSC-EVs have highlighted the beneficial effects of EVs. EVs also present a safer profile than their cells of origin because EV injection does not produce tumors[56]. Moreover, combinatorial treatment with both MSCs and their derived EVs exhibits advantages in MI treatment. Rat bone marrow mesenchymal stem cells (BMSCs) and their derived EVs improved cardiac function and reduced infarct size when compared to groups treated with BMSCs or EVs alone[57]. CDC exosomes were proven to enhance angiogenesis and promote cardiomyocyte survival and proliferation. Moreover, CDC exosomes improved cardiac function and imparted structural benefits after MI. miR-146a was the most highly enriched in CDC exosomes and mediated some of the therapeutic benefits of CDC exosomes[58]. EVs combined with biomaterials present better effects for the treatment of cardiac disease. Studies have shown that MSC-derived EVs incorporated into alginate hydrogels are a sustained delivery system that allows for better retention of EVs in the heart in vivo than EV single injection. EVs-Gel has a good effect on promoting angiogenesis, reducing the apoptosis and fibrosis of cardiac tissue, enhancing scar thickness and improving cardiac function[59]. Another study showed that self-assembling peptide hydrogel-encapsulated exosomes could also promote cardiac repair due to the better retention and stability of EVs[60]. In general, EVs combined with biomaterials provide a novel approach for cell-free therapy and optimization of the therapeutic effects of EVs in MI.
Tendons are soft tissues that connect muscles to bones. Tendon diseases and injuries have high morbidity owing to various sports and exercises. The effects of treatments for tendon diseases are limited due to the limited regenerative capacity of tendons. Current treatments for tendon injuries, including surgery and conservative treatments, always have side effects, including secondary injury and scar formation. Therefore, the ideal treatment is to promote tendon regeneration[61]. There are three important stages of tendon healing: Inflammation, proliferation, and remodeling. Suppression of the inflammatory response is critical for tendon repair[62,63]. Research has found that BMSC-EVs may help improving tendon healing by regulating macrophage phenotypes, creating anti-inflammatory environment, promoting apoptotic cell accumulation, and increasing the ratio of tendon resident stem/progenitor cells[64]. Moreover, EV-educated macrophages (EEMs), a kind of M2-like macrophage treated by EVs, present a more functional and regenerative ability to heal tendons. EVs isolated from MSCs were able to reduce the M1/M2 macrophage ratio and increase the number of endothelial cells 14 days after tendon injury. Injured tendons treated with exogenous EEMs presented improved mechanical properties, reduced inflammation and earlier angiogenesis[62]. Furthermore, Yu et al[29] found that BMSC-EVs could promote the proliferation, migration and tenogenic differentiation ability of tendon stem/progenitor cells (TSPCs). BMSC-derived EVs could activate the regenerative potential of endogenous TSPCs in tendon injury sites. EVs embedded in fibrin glue may allow the sustained release of EVs in injured regions and promote the regeneration of patellar tendon tissue in rats[15]. In addition to BMSCs, ADSC-EVs can also promote the proliferation and migration of tendon cells, reduce fatty infiltration, promote tendon-bone healing, improve the biomechanical properties of the tendon-bone junction and improve the mechanical strength of the repaired tendon[65,66]. The overexpression of H19 can enhance tendon regeneration potential. As an ideal drug carrier, EVs are a reliable delivery method to transmit lncRNAs. Reports have shown that engineered EVs with overload of H19 can regulate tendon regeneration by activating YAP through the H9-pp1-YAP axis[67]. In conclusion, MSC-EVs facilitate tendon regeneration by inhibiting apoptosis as well as enhancing the proliferation, migration and tenogenic differentiation of tendon stem cells and tenocytes. In addition, they can also regulate angiogenesis and modulate immune responses and extracellular matrix (ECM) remodeling of tendon tissue[68]. These results indicate that EVs have broad prospects in tendon repair and regeneration.
EVs are currently widely used in wound healing and skin regeneration. EVs can participate in four stages of wound healing, namely, hemostasis, inflammatory response, cell proliferation and remodeling[69]. Both ADSC-EVs and BMSC-EVs exert benefits on cells related to skin wound healing, including fibroblasts, keratinocytes and endothelial cells, in different ways. BMSC-EVs mainly promote proliferation, whereas ADSC-EVs have a major effect on angiogenesis. BMSC-EVs and ADSC-EVs presented synergistic effects on wound healing[70]. Great progress has been made in the field of wound healing with the rapid development of the combination of EVs and biomaterials. EVs and glycerol hydrogels have synergistic effects on the proliferation of human skin fibroblasts. The full-thickness excisional wound model in mice showed that the fibrosis, vascularization, and epithelial thickness of wounds reached a maximum level after treatment with EV-loaded hydrogels. Moreover, research has found that EVs derived from human endometrial stem cells (hENSCs) contain several growth factors, including VEGF, basic fibroblast growth factor and TGF-1, which benefit angiogenesis. hENSC-EV-loaded chitosan hydrogel has positive impacts on wound healing by promoting angiogenesis and tissue granulation formation. hENSC-EV-loaded chitosan hydrogel could be an ideal scaffold for skin wound dressing and skin tissue regeneration[71]. EVs derived from HUVECs (HUVECs-EVs) could promote the proliferation and migration activities of keratinocytes and fibroblasts, which are two critical cells for skin regeneration. Gelatin methacryloyl (GelMA) hydrogel scaffolds combined with HUVEC-EVs could not only repair wound defects but could also achieve sustained release of EVs, which promoted re-epithelialization, collagen maturity and angiogenesis that ultimately contributed to wound healing[72]. In addition to wound healing and skin regeneration, EVs also play critical roles in skin aging and several skin diseases. For example, ADSC-EVs can relieve atopic dermatitis. ADSC-EVs can also promote cuticle hydration and ceramide synthesis and significantly reduce the secretion of inflammatory cytokines [interleukin (IL)-4, IL-5, IL-13, and IL-17] when applied in vivo[73]. Moreover, ADSCs could protect against oxidative stress by promoting the proliferation of human dermal fibroblasts (HDFs) and inhibiting reactive oxygen species and MMP production via the secretion of various cytokines. ADSC-EVs might be potential therapeutic tools for addressing the problem of photoaging[74]. Studies have also shown that EVs derived from human iPSCs could regulate the genotypic and phenotypic changes of HDFs induced by UV photoaging and natural aging. EVs derived from human iPSCs affected cellular responses related to skin aging, including the expression levels of MMP-1 and type I collagen and the proliferation and migratory ability of HDFs. iPSC-EVs can mediate intracellular transportation and reconstruct the matrix in aging skin by enhancing the expression of structural proteins and regulating the expression of age-related proteins[75]. In general, EVs serve as efficient carriers of molecular cargos for wound healing and skin regenerative medicine.
EVs have been widely used in the repair of dental tissue, including dental pulp, dentin and periodontal tissue. Dental pulp stem cells (DPSCs)-EVs can bind to matrix proteins such as type I collagen and fibronectin, enabling them to be tethered to biomaterials. EVs are endocytosed by both DPSCs and human MSCs in a dose-dependent and saturable manner via the caveolar endocytic mechanism and trigger the P38 mitogen-activated protein kinase (MAPK) pathway. In addition, EVs can trigger the increased expression of genes required for odontogenic differentiation. In the generated pulp tissue, DPSC-EVs can also promote the expression of several growth-promoting factors, including TBG-β and BMP-2, which ultimately promote the repair of dental pulp tissue[76]. Moreover, DPSC-EVs can promote the proliferation and angiogenesis of vascular endothelial cells by activating the P38/MAPK pathway, which provides the possibility for the regeneration of vascular pulp tissue[77]. SC-EVs could promote DPSC proliferation and enhance neurite outgrowth, neuron migration, and vessel formation in vitro. SC-EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via the SDF-1/CXCR4 axis without exogenous cell transplantation[78]. In addition to SC-EVs, EVs derived from Hertwig’s epithelial root sheath cells could trigger regeneration of dental pulp-dentin-like tissue composed of hard (regenerative dentin-like tissue) and soft (blood vessel and neuron) tissue in an in vivo tooth root slice model by activating the Wnt pathway[79]. Periodontitis is the primary cause of tooth loss, but there is no effective treatment to repair inflammatory bone loss in periodontitis. In terms of periodontal tissue regeneration, studies have shown that EVs secreted by periodontal ligament stem cells (PDLSCs) are therapeutics for bone defects in periodontitis. EVs derived from healthy PDLSCs could rescue the osteogenesis capacity of endogenous stem cells under an inflammatory environment and promote the regeneration of alveolar bone by recovering the osteogenic differentiation ability of inflammatory PDLSCs through the inhinbitation of canonical Wnt signaling[80]. BMSC-EVs are also an ideal cell-free strategy for periodontal regeneration. BMSC-EVs could promote the regeneration of periodontal tissues through the OPG-RANKL-RANK signaling pathway to regulate the function of osteoclasts and affect macrophage polarization and TGF-β1 expression to modulate the inflammatory immune response, thereby inhibiting the development of periodontitis and immune damage in periodontal tissue[81]. Furthermore, the therapeutic effect of ADSC-EVs was the same as that of periodontal surgery in a rat periodontitis model[82]. Lipopolysaccharide (LPS)-induced dental follicle cell-derived EVs (L-D-EVs) could promote the proliferation of periodontal ligament cells. L-D-EV-loaded hydrogel applied in the treatment of periodontitis was beneficial to repairing lost alveolar bone in the early stage of treatment and maintaining the level of alveolar bone in the late stage of treatment in experimental periodontitis rats by decreasing the expression of the RANKL/OPG ratio in vivo[83]. EVs are presented as a novel cell-free therapeutic strategy for both dental pulp and periodontal regeneration.
Neurological dysfunction usually causes great physical and psychological distress for patients. Autologous nerve transplantation is widely accepted as the gold standard for peripheral nerve repair, but its inherent defects greatly reduce its availability. Regeneration of peripheral nerves after injury remains a great challenge for researchers. EVs play a fundamental role in the physiological and pathological processes of the nervous system. There is growing evidence that EVs can play a neurotherapeutic role by mediating axon regeneration, activating SCs, promoting angiogenesis, and regulating inflammatory reactions. EVs from skin-derived precursor SCs (SKP-SC-EVs) could promote neurite outgrowth of sensory and motor neurons via the AKT/mTOR/p70S6K pathway in vitro[84,85]. SKP-SC-EV-incorporating silicone conduit nerve grafts could significantly accelerate the recovery of motor, sensory, and electrophysiological functions of rats; facilitate outgrowth and myelination of regenerated axons; and alleviate denervation-induced atrophy of target muscles, which raises the possibility of cell-free therapy in nerve regeneration[86]. Moreover, repair SCs (rSCs) could also release pro-regenerative EVs. Neuronal activity enhances the release of rSC-derived EVs and their transfer to neurons via the ATP-P2Y signaling pathway[87]. Mechanical stimuli could control the intercellular communication between neurons and SCs by changing the composition of miRNA in SC-EVs. MS-SC-EVs transferd miR-23b-3p from mechanically stimulated SCs to neurons, and lead the inhibition of neuronal Nrp1 expression, which was indicated the beneficial effect of MS-SC-EVs on axonal regeneration, and provided evidence for the role of miR-23b-3p-enriched EVs in peripheral nerve injury repair[88]. BMSC-EVs could promote the functional recovery of sciatic nerve injury and increase the expression of GAP-43, a marker of axon regeneration[89]. BMSC-EVs can also promote nerve regeneration by regulating the miRNA mediated genes which related to regeneration, such as vascular endothelial growth factor A and S100b[90]. In addition to BMSC-EVs, ADSC-EVs also benefited nerve regeneration. ADSC-EVs could increase neurite outgrowth in vitro and enhance regeneration after sciatic nerve injury in vivo[91]. Furthermore, ADSC-EVs contained mRNAs of neurotrophic factors, including NGR, brain-derived neurotrophic factor, ciliary neurotrophic factor and glial cell-derived neurotrophic factor. ADSC-EVs could deliver mRNAs as well as microRNAs that facilitate with neurotrophic factor secretion and proliferation, support the SC repair phenotype, and provide a solid therapeutic evidence for nerve regeneration[92]. Gingiva-derived mesenchymal stem cell (GMSC)-derived EVs could obviously promote axonal regeneration and functional recovery of injured mouse sciatic nerves. GMSC-derived EVs promoted the expression of SC dedifferentiation/repair phenotype-related genes in vitro, particularly c-JUN, a key transcription factor that drives the activation of the repair phenotype of SCs during PNI and regeneration[93]. Revascularization treatment is a critical measure for nerve repair. A report revealed that EVs derived from hypoxic preconditioned HUVECs could facilitate MSC angiogenesis activity and the anti-inflammatory impacts of MSCs, which contribute to in vivo effective nerve tissue repair after rat spinal cord transection and provide inspiration for therapies based on stem cells and EVs[94]. In general, EVs are an effective therapeutic tool in nerve regeneration by mediating axon regeneration, regulating the phenotype of SCs, promoting angiogenesis, and regulating inflammatory reactions.
EVs play four potential roles in bone regeneration, namely, angiogenesis, osteoblast proliferation, intercellular communication, and immune regulation. EV-mediated intercellular communication between osteoblasts and osteoclasts may represent a novel mechanism of bone modeling and remodeling. EVs transmit signals between osteoblasts and osteoclasts to regulate bone remodeling. Osteoblasts release RANKL-containing EVs, which are transferred to precursor osteoclasts and promote osteoclast formation by stimulating RANKL-RANK signaling[95]. MSC-derived EVs (MSC-exos), with their inherent capacity to modulate cellular behavior, are emerging as a novel cell-free therapy for bone regeneration. Reports have revealed that EVs derived from osteoinductive BMSCs (BMSC-OL-EVs) contribute to bone regeneration via multicomponent exosomal miRNAs (let-7a-5p, let-7c-5p, miR-328a-5p and miR-31a-5p), which target Acvr2b/Acvr1 and regulate the competitive balance of Bmpr2/Acvr2b toward Bmpr-elicited Smad1/5/9 phosphorylation[96]. Moreover, lyophilized delivery of BMSC-OI-EVs on hierarchical mesoporous bioactive glass scaffolds showed the possibility of bone regeneration in a rat cranial defect model[96]. Umbilical MSC-derived exosomes (uMSCEXOs) also showed great promise in bone regeneration. uMSCEXOs encapsulated in hyaluronic acid hydrogel and combined with customized nanohydroxyapatite/poly-ε-caprolactone (nHP) scaffolds could repair cranial defects in rats by promoting the proliferation, migration, and angiogenic differentiation of endothelial progenitor cells via the miR-21/NOTCH1/DLL4 signaling axis[97]. Compared to EVs derived from uMSCs exposed to normoxia, EVs derived from uMSCs treated with hypoxia promoted angiogenesis, proliferation, and migration to a greater extent. Hypo-Exos facilitated with the recovery of bone fracture by exosomal miR-126 and the SPRED1/Ras/Erk signaling pathway. In addtion, hypoxia preconditioning promote the transferring of exosomal miR-126 through the activation of hypoxia-inducible factor-1α. Hypoxia preconditioning is an effective and promising approach for optimizing the therapeutic actions of MSC-derived exosomes for bone fracture healing[98]. Moreover, the comparison between adipose, bone marrow, and synovium-derived EVs (ADSC-EVs, BMSC-EVs, and SMSC-EVs, respectively) showed that ADSC-EVs, BMSC-EVs, and SMSC-EVs could facilitate the viability and migration of MSCs and possessed favorable capacities for chondrogenesis and osteogenesis. Among these three types of EVs, ADSC-EVs presented the best performance. The different effects between ADSC-EVs, BMSC-EVs, and SMSC-EVs could be attributed to the different factors associated with the focal adhesion, ECM-receptor interaction, actin cytoskeleton regulation, cAMP, and PI3K-Akt signaling pathways[99]. EVs constitutively expressing BMP2 could facilitate the effects of bone regeneration. BMP-engineered EVs potentiate the BMP2 signaling cascade, possibly due to an altered miRNA composition. EV functionality may be engineered by genetic modification of the parental MSCs to induce osteoinduction and bone regeneration[100]. EVs combined with biomaterials present a better effect on bone repair. Three-dimensional engineered scaffolds (PLAs) complexed with human gingival MSCs have therapeutic effects that can improve bone tissue regeneration[101]. EVs combined with tricalcium phosphate-modified scaffolds can promote osteogenic differentiation of cells and promote the recovery of cranial defects in vivo by activating the PI3K/Akt signaling pathway[102]. In addition to bone regeneration, MSC-EVs or platelet-rich plasma-derived EVs also have high therapeutic value for treating osteoarthritis by suppressing the inflammatory immune microenvironment. BMSC-derived exosomes can effectively promote cartilage repair and extracellular matrix synthesis and alleviate knee pain in OA rats[103]. In addition, the modification of EVs and the combination of EVs with biomaterials can enhance targeting effects and extend retention which contribute to an effectively treatment of OA[104].
Previous studies based on EVs have shed new light on the application of EVs in tissue regeneration, but there are some major problems that still need to be solved in the clinical translation of EVs. First, the storage of EVs is unstable. EVs can be stored at 80 °C for several months; however, the pH value of the storage solution and freeze-thaw cycles can affect EV activity. The transport and storage conditions of EVs need to be further studied. Second, there is no effective method to isolate purified EVs in large quantities. At present, EVs are extracted mainly by UC, immunoadsorption, precipitation or microfluidic separation and are easily contaminated by proteins. Nowadays, there are several strategies to enhance the purity and quality of harvested EVs. From the perspective of stimulation of EVs secretion, many factors including protein regulations, thermal and oxidative stress, oxygen concentration, low pH (< 6.0), radiation, starvation can enhance the secretion of EVs from cells[105]. From the perspective of the methods of EVs isolation, how to simplify the isolation procedure and improve the EVs yield are the main two obstructions in the research field of EVs. Recent report reveals that the isolation procedure of EVs may be simplified with the improvement of newly EVs separation techniques including immunoaffinity, chromatography and polymer precipitation[106]. Moreover, with the development of commercial EVs isolation kits, EVs also can be extracted from limited sample in a short period of time. Artificial EVs is another way to realize large scale EVs generation. Artificial EVs generation technologies use the physical forces or chemicals (nitrogen cavitation[107], extrusion via porous membrane[108], sonication[109]) to break cells and release the cellular components. With reconstitution of the released lipids, proteins, and nucleic acids, artificial EVs can be generated in large quantity[105].
Moreover, improving the therapeutic efficiency of EVs is also a great challenge for applying EVs to clinical use. EV surface engineering can realize the EVs selective enrichment in specific cells and potentially tissues by introduction of targeting moieties[110]. To improve the therapeutic effects of EVs, a variety of methods (active drug loading, passive modification, electroporation, acoustic degradation, chemical transfection, etc.) can be used to modify EVs. Moreover, Cytochalasin B treatment and osmotic pressure can enhance the production of EVs with improved drug loading capacity[111]. Engineered EVs are a new strategy to enhance the expression of targeted proteins of EV-related RNA. These engineered EVs with specific targeted biomolecules can be used specifically for different therapeutic purposes, including as in vivo tracers or in targeted cell tracking, which could improve the efficacy of disease therapy and tissue regeneration.
In conclusion, EVs are intelligent and controllable biomaterials that can participate in a variety of physiological and pathological activities, tissue repair and regeneration by transmitting a variety of biological signals, showing great potential in cell-free tissue regeneration. Engineered EVs, which represent a clean, highly purified, and highly controllable means of achieving the sustained release of drugs, have broad prospects in future tissue regeneration engineering.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hassaan NA, Egypt; Kabdesh I, Russia; Ou Q, China; Li SC, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 526] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 2. | MacPherson A, Kimmelman J. Ethical development of stem-cell-based interventions. Nat Med. 2019;25:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Tsiapalis D, O'Driscoll L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 4. | Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 5. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6157] [Article Influence: 513.1] [Reference Citation Analysis (0)] |
| 6. | Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 7. | Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 8. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4124] [Article Influence: 412.4] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Wang Y, Lv Q, Li X. Exosomes: From garbage bins to translational medicine. Int J Pharm. 2020;583:119333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1345] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 11. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7682] [Article Influence: 1097.4] [Reference Citation Analysis (1)] |
| 12. | Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 420] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 13. | Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Silva AM, Almeida MI, Teixeira JH, Maia AF, Calin GA, Barbosa MA, Santos SG. Dendritic Cell-derived Extracellular Vesicles mediate Mesenchymal Stem/Stromal Cell recruitment. Sci Rep. 2017;7:1667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, Yang P, Duan X, Zhang J, Fu X, Hu X, Ao Y. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 1054] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 17. | Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2443] [Cited by in RCA: 3723] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 18. | Rider MA, Hurwitz SN, Meckes DG Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci Rep. 2016;6:23978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 509] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 19. | Böing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 854] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 20. | Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 855] [Cited by in RCA: 1265] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 21. | Wang T, Anderson KW, Turko IV. Assessment of Extracellular Vesicles Purity Using Proteomic Standards. Anal Chem. 2017;89:11070-11075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Yoo CE, Kim G, Kim M, Park D, Kang HJ, Lee M, Huh N. A direct extraction method for microRNAs from exosomes captured by immunoaffinity beads. Anal Biochem. 2012;431:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Correction for Wu et al, Isolation of exosomes from whole blood by integrating acoustics and microfluidicss. Proc Natl Acad Sci U S A. 2020;117:28525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Soares Martins T, Catita J, Martins Rosa I, A B da Cruz E Silva O, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13:e0198820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 25. | García-Romero N, Madurga R, Rackov G, Palacín-Aliana I, Núñez-Torres R, Asensi-Puig A, Carrión-Navarro J, Esteban-Rubio S, Peinado H, González-Neira A, González-Rumayor V, Belda-Iniesta C, Ayuso-Sacido A. Polyethylene glycol improves current methods for circulating extracellular vesicle-derived DNA isolation. J Transl Med. 2019;17:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-3707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 707] [Cited by in RCA: 666] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 27. | Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 474] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 28. | He L, Zhu D, Wang J, Wu X. A highly efficient method for isolating urinary exosomes. Int J Mol Med. 2019;43:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Yu LL, Zhu J, Liu JX, Jiang F, Ni WK, Qu LS, Ni RZ, Lu CH, Xiao MB. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed Res Int. 2018;2018:3634563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 31. | Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L, Yan X. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9:1697028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 401] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 32. | Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front Cell Dev Biol. 2020;8:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 33. | Pozzoli S, Simonini M, Manunta P. Predicting acute kidney injury: current status and future challenges. J Nephrol. 2018;31:209-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 1027] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 35. | Herrera Sanchez MB, Bruno S, Grange C, Tapparo M, Cantaluppi V, Tetta C, Camussi G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res Ther. 2014;5:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Cao H, Cheng Y, Gao H, Zhuang J, Zhang W, Bian Q, Wang F, Du Y, Li Z, Kong D, Ding D, Wang Y. In Vivo Tracking of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improving Mitochondrial Function in Renal Ischemia-Reperfusion Injury. ACS Nano. 2020;14:4014-4026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 37. | Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 38. | Grange C, Iampietro C, Bussolati B. Stem cell extracellular vesicles and kidney injury. Stem Cell Investig. 2017;4:90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Zhao M, Liu S, Wang C, Wang Y, Wan M, Liu F, Gong M, Yuan Y, Chen Y, Cheng J, Lu Y, Liu J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano. 2021;15:1519-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 40. | Grange C, Skovronova R, Marabese F, Bussolati B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007;71:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 43. | Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, Brizzi MF. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9:4468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 44. | Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 45. | Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC, Brizzi MF, Tetta C, Camussi G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front Immunol. 2018;9:1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Birtwistle L, Chen XM, Pollock C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Erratum to: Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2017;21:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Psaraki A, Ntari L, Karakostas C, Korrou-Karava D, Roubelakis MG. Extracellular vesicles derived from mesenchymal stem/stromal cells: The regenerative impact in liver diseases. Hepatology. 2022;75:1590-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 49. | Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 50. | Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, Wu B, Wang Y, Ai K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 51. | Du Y, Li D, Han C, Wu H, Xu L, Zhang M, Zhang J, Chen X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem. 2017;43:611-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 52. | Balaphas A, Meyer J, Sadoul R, Morel P, Gonelle-Gispert C, Bühler LH. Extracellular vesicles: Future diagnostic and therapeutic tools for liver disease and regeneration. Liver Int. 2019;39:1801-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2572] [Article Influence: 214.3] [Reference Citation Analysis (0)] |
| 54. | Broughton KM, Wang BJ, Firouzi F, Khalafalla F, Dimmeler S, Fernandez-Aviles F, Sussman MA. Mechanisms of Cardiac Repair and Regeneration. Circ Res. 2018;122:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 55. | Adamiak M, Sahoo S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol Ther. 2018;26:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 56. | Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, Jankowska U, Girgis M, Sekula M, Davani A, Lasota S, Vincent RJ, Sarna M, Newell KL, Wang OL, Dudley N, Madeja Z, Dawn B, Zuba-Surma EK. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ Res. 2018;122:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 57. | Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 540] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 58. | Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 662] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 59. | Lv K, Li Q, Zhang L, Wang Y, Zhong Z, Zhao J, Lin X, Wang J, Zhu K, Xiao C, Ke C, Zhong S, Wu X, Chen J, Yu H, Zhu W, Li X, Wang B, Tang R, Huang J, Hu X. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics. 2019;9:7403-7416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 60. | Han C, Zhou J, Liang C, Liu B, Pan X, Zhang Y, Wang Y, Yan B, Xie W, Liu F, Yu XY, Li Y. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci. 2019;7:2920-2933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 61. | Sun J, Mou C, Shi Q, Chen B, Hou X, Zhang W, Li X, Zhuang Y, Shi J, Chen Y, Dai J. Controlled release of collagen-binding SDF-1α from the collagen scaffold promoted tendon regeneration in a rat Achilles tendon defect model. Biomaterials. 2018;162:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 62. | Vinhas A, Rodrigues MT, Gomes ME. Exploring Stem Cells and Inflammation in Tendon Repair and Regeneration. Adv Exp Med Biol. 2018;1089:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Shen H, Yoneda S, Abu-Amer Y, Guilak F, Gelberman RH. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res. 2020;38:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 64. | Shi Z, Wang Q, Jiang D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J Transl Med. 2019;17:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 65. | Chen SH, Chen ZY, Lin YH, Chen SH, Chou PY, Kao HK, Lin FH. Extracellular Vesicles of Adipose-Derived Stem Cells Promote the Healing of Traumatized Achilles Tendons. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Wang C, Hu Q, Song W, Yu W, He Y. Adipose Stem Cell-Derived Exosomes Decrease Fatty Infiltration and Enhance Rotator Cuff Healing in a Rabbit Model of Chronic Tears. Am J Sports Med. 2020;48:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Tao SC, Huang JY, Li ZX, Zhan S, Guo SC. Small extracellular vesicles with LncRNA H19 "overload": YAP Regulation as a Tendon Repair Therapeutic Tactic. iScience. 2021;24:102200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Lui PPY. Mesenchymal Stem Cell-Derived Extracellular Vesicles for the Promotion of Tendon Repair - an Update of Literature. Stem Cell Rev Rep. 2021;17:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Zhou X, Brown BA, Siegel AP, El Masry MS, Zeng X, Song W, Das A, Khandelwal P, Clark A, Singh K, Guda PR, Gorain M, Timsina L, Xuan Y, Jacobson SC, Novotny MV, Roy S, Agarwal M, Lee RJ, Sen CK, Clemmer DE, Ghatak S. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano. 2020;14:12732-12748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 70. | Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini F, Lopatina T, Brizzi MF, Camussi G. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 71. | Nooshabadi VT, Khanmohamadi M, Valipour E, Mahdipour S, Salati A, Malekshahi ZV, Shafei S, Amini E, Farzamfar S, Ai J. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J Biomed Mater Res A. 2020;108:2138-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 72. | Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J Mol Histol. 2020;51:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 73. | Shin KO, Ha DH, Kim JO, Crumrine DA, Meyer JM, Wakefield JS, Lee Y, Kim B, Kim S, Kim HK, Lee J, Kwon HH, Park GH, Lee JH, Lim J, Park S, Elias PM, Park K, Yi YW, Cho BS. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 74. | Gentile P, Garcovich S. Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) against Ultraviolet (UV) Radiation Effects and the Skin Photoaging. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Cha H, Hong S, Park JH, Park HH. Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 77. | Xian X, Gong Q, Li C, Guo B, Jiang H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J Endod. 2018;44:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Wang D, Lyu Y, Yang Y, Zhang S, Chen G, Pan J, Tian W. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 2022;140:610-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 79. | Zhang S, Yang Y, Jia S, Chen H, Duan Y, Li X, Wang S, Wang T, Lyu Y, Chen G, Tian W. Exosome-like vesicles derived from Hertwig's epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics. 2020;10:5914-5931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Lei F, Li M, Lin T, Zhou H, Wang F, Su X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 2022;141:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 81. | Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, Tian W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng Part A. 2021;27:962-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 82. | Mohammed E, Khalil E, Sabry D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 83. | Shi W, Guo S, Liu L, Liu Q, Huo F, Ding Y, Tian W. Small Extracellular Vesicles from Lipopolysaccharide-Preconditioned Dental Follicle Cells Promote Periodontal Regeneration in an Inflammatory Microenvironment. ACS Biomater Sci Eng. 2020;6:5797-5810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 84. | Cong M, Shen M, Wu X, Li Y, Wang L, He Q, Shi H, Ding F. Improvement of sensory neuron growth and survival via negatively regulating PTEN by miR-21-5p-contained small extracellular vesicles from skin precursor-derived Schwann cells. Stem Cell Res Ther. 2021;12:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 85. | Wu X, Wang L, Cong M, Shen M, He Q, Ding F, Shi H. Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann Transl Med. 2020;8:1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 86. | Yu M, Gu G, Cong M, Du M, Wang W, Shen M, Zhang Q, Shi H, Gu X, Ding F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021;134:190-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 87. | Saquel C, Catalan RJ, Lopez-Leal R, Ramirez RA, Necuñir D, Wyneken U, Lamaze C, Court FA. Neuronal activity-dependent ATP enhances the pro-growth effect of repair Schwann cell extracellular vesicles by increasing their miRNA-21 Loading. Front Cell Neurosci. 2022;16:943506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 88. | Xia B, Gao J, Li S, Huang L, Zhu L, Ma T, Zhao L, Yang Y, Luo K, Shi X, Mei L, Zhang H, Zheng Y, Lu L, Luo Z, Huang J. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics. 2020;10:8974-8995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 89. | Ma Y, Ge S, Zhang J, Zhou D, Li L, Wang X, Su J. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. Int J Clin Exp Pathol. 2017;10:10032-10039. [PubMed] |
| 90. | Zhao J, Ding Y, He R, Huang K, Liu L, Jiang C, Liu Z, Wang Y, Yan X, Cao F, Huang X, Peng Y, Ren R, He Y, Cui T, Zhang Q, Zhang X, Liu Q, Li Y, Ma Z, Yi X. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res Ther. 2020;11:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 91. | Bucan V, Vaslaitis D, Peck CT, Strauß S, Vogt PM, Radtke C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol Neurobiol. 2019;56:1812-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 92. | Haertinger M, Weiss T, Mann A, Tabi A, Brandel V, Radtke C. Adipose Stem Cell-Derived Extracellular Vesicles Induce Proliferation of Schwann Cells via Internalization. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 93. | Mao Q, Nguyen PD, Shanti RM, Shi S, Shakoori P, Zhang Q, Le AD. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng Part A. 2019;25:887-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 94. | Li L, Mu J, Zhang Y, Zhang C, Ma T, Chen L, Huang T, Wu J, Cao J, Feng S, Cai Y, Han M, Gao J. Stimulation by Exosomes from Hypoxia Preconditioned Human Umbilical Vein Endothelial Cells Facilitates Mesenchymal Stem Cells Angiogenic Function for Spinal Cord Repair. ACS Nano. 2022;16:10811-10823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 95. | Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, Shen Z, Fu Q. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 96. | Liu A, Lin D, Zhao H, Chen L, Cai B, Lin K, Shen SG. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272:120718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 97. | Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472-18487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 98. | Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 99. | Li Q, Yu H, Sun M, Yang P, Hu X, Ao Y, Cheng J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021;125:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 100. | Huang CC, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, Gajendrareddy P, Ravindran S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020;109:182-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 101. | Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A, Nanci A, Caputi S, Fontana A, Mazzon E, Trubiani O. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther. 2018;9:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 102. | Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 103. | He L, He T, Xing J, Zhou Q, Fan L, Liu C, Chen Y, Wu D, Tian Z, Liu B, Rong L. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 104. | Yin B, Ni J, Witherel CE, Yang M, Burdick JA, Wen C, Wong SHD. Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics. 2022;12:207-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 105. | Hahm J, Kim J, Park J. Strategies to Enhance Extracellular Vesicle Production. Tissue Eng Regen Med. 2021;18:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 106. | Guix FX, Corbett GT, Cha DJ, Mustapic M, Liu W, Mengel D, Chen Z, Aikawa E, Young-Pearse T, Kapogiannis D, Selkoe DJ, Walsh DM. Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 107. | Gao J, Chu D, Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Control Release. 2016;224:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 108. | Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lötvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698-7710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 758] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 109. | Mendez R, Banerjee S. Sonication-Based Basic Protocol for Liposome Synthesis. Methods Mol Biol. 2017;1609:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | Richter M, Vader P, Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev. 2021;173:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 111. | Nair A, Bu J, Rawding PA, Do SC, Li H, Hong S. Cytochalasin B Treatment and Osmotic Pressure Enhance the Production of Extracellular Vesicles (EVs) with Improved Drug Loading Capacity. Nanomaterials (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |