Published online Dec 26, 2023. doi: 10.4252/wjsc.v15.i12.1077
Peer-review started: September 6, 2023
First decision: October 23, 2023
Revised: November 17, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 26, 2023
Processing time: 111 Days and 0.1 Hours
Mesenchymal stem cells (MSCs) have protective effects on the cornea, lacrimal gland, retina, and photoreceptor cell damage, which may be mediated by exosomes (exos) released by MSCs.
To investigate the ameliorating effect of exos derived from different MSCs on retinal ganglion cell (RGC) injury induced by hydrostatic pressure.
The RGC injury model was constructed by RGC damage under different hydrostatic pressures (40, 80, 120 mmHg). Then RGCs were cultured with adipose-derived stem cell (ADSC)-Exos and bone marrow-derived stem cell (BMSC)-Exos. Cell Counting Kit-8, transmission electron microscopy, flow cytometry, immunofluorescence, real-time quantitative polymerase chain reaction, and western blotting were performed to detect the ameliorating effect of exos on pressure-induced RGC injury.
ADSC-Exos and BMSC-Exos were successfully isolated and obtained. The gibbosity of RGCs was lower, the cells were irregularly ellipsoidal under pressure, and the addition of ADSC-Exos and BMSC-Exos significantly restored RGC morphology. Furthermore, the proliferative activity of RGCs was increased and the apoptosis of RGCs was inhibited. Moreover, the levels of lactate dehydrogenase and apoptosis-related proteins were increased, and the concentrations of antiapoptotic proteins and neurotrophic factors were decreased in damaged RGCs. However, the above indicators were significantly improved after ADSC-Exos and BMSC-Exos treatment.
These findings indicated that ADSC-Exos and BMSC-Exos could ameliorate RGC injury caused by hydrostatic pressure by inhibiting apoptosis and increasing the secretion of neurotrophic factors.
Core Tip: We discovered for the first time that adipose-derived stem cell-exosomes (ADSC-Exos) significantly ameliorate retinal ganglion cell (RGC) injury caused by hydrostatic pressure by inhibiting apoptosis and increasing the secretion of neurotrophic factors. ADSC-Exos manifested better ameliorating effects than bone marrow-derived stem cell (BMSC)-Exos in ameliorating the RGC injury induced by hydrostatic pressure. BMSC-Exos ameliorate optic nerve injury caused by hydrostatic pressure by inhibiting apoptosis and increasing the secretion of neurotrophic factors.
- Citation: Zheng ZK, Kong L, Dai M, Chen YD, Chen YH. ADSC-Exos outperform BMSC-Exos in alleviating hydrostatic pressure-induced injury to retinal ganglion cells by upregulating nerve growth factors. World J Stem Cells 2023; 15(12): 1077-1092
- URL: https://www.wjgnet.com/1948-0210/full/v15/i12/1077.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i12.1077
Glaucoma is the main cause of irreversible blindness around the world and is a chronic degenerative optic nerve disease[1]. The early pathological changes of glaucoma include reduced axonal transport, axon loss, and dendrite reconstruction, leading to retinal ganglion cell (RGC) apoptosis and thinning of the retinal nerve fiber layer, eventually resulting in visual field defects and loss of vision[2,3]. Apoptosis of RGCs in glaucoma has been demonstrated in vivo in several animal models and in humans[4,5]. The mechanism of RGC apoptosis in glaucoma, including oxidative injury[6], inflammatory response[4], and glutamate toxicity[7], particularly how elevated intraocular pressure (IOP) leads to RGC apoptosis, is yet to be fully elucidated. There are three treatment methods for glaucoma caused by high IOP, including using drugs to reduce the production of aqueous humor, surgically aqueous humor drainage, and laser stimulation for cell proliferation and differentiation to repair damaged eye tissue[8]. However, existing IOP-lowering treatments cannot prevent the reduction of RGCs[9], and there is the limitation of repeated treatment, even worse.
The only hope for glaucoma patients with RGC loss to regain vision is preventing RGC apoptosis[10]. Current research is focused on developing neuroprotective measures, particularly the use of stem cell therapy to protect RGCs[11]. Stem cells produce many molecules responsible for cell signaling, such as cytokines, growth factors, morphogens, chemokines, and extracellular vesicles (EVs), improving various cellular mechanisms[12-15]. However, the direct transplantation of stem cells has some risks such as safety, tumorigenesis, and immune rejection[16]. Recent studies have shown that stem cells play a therapeutic role in a paracrine manner, with stem cell-derived exosomes (exos) being the major contributors to stem cell function, carrying and transporting cytokines, proteins, and nucleic acids from stem cells[17]. It also has stronger target specificity and lower carcinogenic and immune risks[18], which can avoid the problems existing in the direct use of stem cell therapy, and increasing the use of stem cell-derived exos is the focus of research to enhance the efficacy of stem cell therapy.
Mesenchymal stem cells (MSCs) are fibroblast-like, self-renewing cells that are present in almost all postpartum tissues and organs, including bone marrow, adipose tissue, blood, umbilical cord, amniotic fluid, and dental pulp and are responsible for regulating the normal development and maintenance of mesenchymal tissue[19]. Bone marrow, adipose tissue, and human MSCs of the umbilical cord have protective effects on cornea, lacrimal gland, retina, and photoreceptor cell damage[20]. This ameliorating effect may be mediated at least in part by exos. MSC-Exos can maintain activity for at least 4 wk after vitreous injection and can integrate into tissues more quickly to provide nutritional factors for RGCs due to their smaller diameter[15]. Bone marrow stem cell (BMSC)-derived exos are also more effective than directly transplanted BMSCs in the treatment of glaucoma because BMSCs have a poor ability to integrate into the retina, remain in the vitreous after injection, and cannot deliver neurotrophic factors to RGCs[21]. Mead et al[22] found that the neural function of RGCs was significantly improved after intravitreal injection of BMSC-Exos in a glaucoma rat model. A similar result was reported in the hereditary DBA/2J mouse model of glaucoma[23]. Moreover, studies have shown that BMSC-Exos injected in an optic nerve compression rat model by intravitreal injection can integrate into the cell bodies and axons of retinal neurons and promote axon regeneration by activating the Akt pathway dependent on microRNA (miRNA)[24]. These findings indicate that BMSC-Exos have an ameliorative effect on glaucoma. Moreover, Sheykhhasan et al[25] demonstrated that adipose-derived stem cell (ADSC)-Exo and CoQ10 administration could ameliorate memory deficits by modulating SOX2 and brain-derived neurotrophic factor (BDNF) expression in a rat model of Alzheimer’s disease. Numerous preclinical studies have confirmed the therapeutic potential of ADSC-Exos in neurodegenerative diseases[26,27]. Glaucoma is a neurodegenerative disease in which the role of ADSC-Exos has not been studied yet. Therefore, this study aimed to investigate the protective effect of BMSC-Exos and ADSC-Exos on RGC injury, which is the main feature of glaucoma, induced by hydrostatic pressures.
ADSCs and BMSCs were a kind gift from Kunming Cell Bank of Chinese Academy of Sciences (Kunming, China). Cells in the third generation with good logarithmic phase growth were selected. The cell culture medium was discarded, and the cells were washed 3 times with phosphate-buffered solution (PBS) and replaced with serum-free medium. After 48 h in culture, the cells were adjusted to a concentration of 1 × 105 cells/mL. The cells were then centrifuged at 3000 r/min for 10 min at room temperature to collect the supernatant. The supernatant was centrifuged at 20000 r/min for 20 min at 4 °C to remove dead cells and debris, and the supernatant was then filtered through a sterile, 0.22 μm pore size syringe filter to obtain the crude extract of exos. Then the crude extract was centrifuged at 50000 r/min at 4 °C for 1 h. The white precipitate could be seen at the bottom of the tube after centrifugation, and the supernatant was removed. The white precipitate was cleaned with 0.9% sodium chloride injection several times, and the resulting suspension was collected and centrifuged at 4 °C for 10 min at 3000 r/min. Exo protein quantification was performed using a BCA protein assay kit (Beyotime, Shanghai, China). Dynamic light scattering was used to detect the size distribution of the exos. The structure of exos was observed by transmission electron microscopy (TEM) (HITACHI, Japan).
Mice 1-3 d old were sacrificed by intraperitoneal injection of 4.3% chloral hydrate, and the eyeballs were removed under aseptic conditions and rinsed three times. Under the microscope, the retinal neuroepithelial layer tissue was isolated. The tissue was rinsed 3 times and digested in 0.5 g/L trypsin for 30 min at 37 °C. The digestion was terminated by the addition of DMEM containing 10% foetal bovine serum (FBS) and filtered through a 40-μm filter. Centrifuge at room temperature for 5 min, discard the supernatant, and add complete DMEM. After adjusting the cell density, the cells were inoculated into cell culture flasks. The cells were placed in a 5% CO2 incubator at 37 °C for 7-10 d. The obtained cells were taken and identified by immunofluorescence staining using a Thy1.1 antibody. The project was approved by the Yunnan University Ethics Committee (approval number YUN20230463).
Then, the cells were grouped into different experiments as follows: (1) Normal control (NC) group, 40 mmHg group, 40 mmHg + ADSC-exo group, and 40 mmHg + BMSC-exo group; (2) NC group, 80 mmHg group, 80 mmHg + ADSC-exo group, and 80 mmHg + BMSC-exo group; and (3) NC group, 120 mmHg group, 120 mmHg + ADSC-exo group, and 120 mmHg + BMSC-exo group.
RGC culture dishes were placed within the pressure chamber[30,31], and the incubating gas mix was pressurized. The cells were exposed to conditions of elevated ambient hydrostatic pressure (40 mmHg, 80 mmHg, 120 mmHg) over and above atmospheric pressure for a period of 2 h. The pressure conditions were then restored to atmospheric, and the culture dishes were removed from the chamber. Under normal cell culture conditions (37 °C, 5% CO2), the culture was continued for 24 h.
The concentrations of ADSC-Exos and BMSC-Exos were measured by a BCA kit (P0010, Beyotime). P3-generation SCs with good digestive state were taken from each group to make a uniform cell suspension and put 100 μL per well in 96-well cell culture plates. ADSC-Exos (20 μg/mL)[32] were added after the stem cells were completely attached to the wall. BMSC-Exos (20 μg/mL)[33] were cultured for 24 h, and the cells of each group were collected for subsequent experiments. Then, cell morphology was observed under an inverted phase contrast microscope (Olympus).
After the cells were treated for 24 h according to the above groups, the cells were digested and collected with Trypsin-EDTA Solution (Beyotime, Shanghai, China), centrifuged in a 1.5 mL EP tube, and the supernatant was discarded. Then, 2.5% glutaraldehyde fixing solution was quickly added to completely cover the cell specimens, which were refrigerated at 4 °C overnight. The fixing solution was poured away, and the samples were rinsed with PBS first and then fixed with 1% osmic acid solution. The samples were subjected to gradient dehydration with an ethanol solution and finally treated with pure acetone for 20 min. The samples were treated with a mixture of equal volume embedding medium and acetone for 1 h. Then, the samples were treated with a mixture of embedding medium and acetone (V/V = 3/1) for 3 h. Next, the embedding medium was used to treat the sample overnight. The samples with osmotic treatment were embedded and heated at 70 °C overnight to prepare the needed samples. The samples were sliced in a Leica EM UC7 ultramicrotome, and slices of 70-90 nm were obtained, double-dyed with lead citrate solution and 50% ethanol saturated solution of uranyl acetate, dried and observed under a transmission electron microscope (Tecnai G2 spirit).
RGCs were collected in each group, washed twice with PBS, fixed at room temperature for 30 min with 4% paraformaldehyde, permeated with 0.1% Triton X-100 for 10 min, washed with PBS 3 times, and sealed with 10% goat serum at room temperature for 1 h. The blocking buffer was removed, and β-III tubulin primary antibody (ab78078, Abcam) was added and incubated overnight at 4 °C. After washing with PBS 3 times, sheep anti-rabbit IgG fluorescent secondary antibody (P0186, Beyotime) was added to the dark, and incubated at room temperature for 2 h. After 3 washes with PBS, 5-10 nonrepetitive visual fields were randomly taken under a fluorescence microscope (Nikon fluorescence microscope), and the number of retinal ganglion cells was quantified by ImageJ software (ImageJ, RRID:SCR_003070).
The cells of each group were digested with Trypsin-EDTA Solution, and the cells were suspended in 10% FBS medium. Each well was inoculated with 1 × 105 cells in 96-well plates. The 96-well plates were cultured in a constant temperature incubator containing 5% CO2 at 37 °C for 0, 24, 48, 72, and 96 h. At the corresponding time, 10 μL Cell Counting Kit-8 (CCK-8) reagent (Beyotime) was added, and the absorption value at 450 nm was detected by a microplate reader after continuous culture for 2 h.
Cells in each group were digested with trypsin-EDTA solution, collected, washed with PBS, resuspended in 300 μL of binding buffer, and incubated with 10 μL of Annexin V-FITC at room temperature for 15 min in the dark. Before the operation, 5 μL PI was added and then 200 μL binding buffer was added. The apoptosis rate of cells was detected by a flow cytometer.
The cells were inoculated into 24-well plates with 5 × 104 cells per well, and each well was cultured with 500 μL medium for 12 h. Then, the cells were fixed with 4% paraformaldehyde at room temperature. PBS containing 0.1% Triton X-100 was added, and permeabilization was performed at room temperature for 10 min. The cells were sealed with 5% BSA at room temperature for 30 min. The liquid was removed and rabbit CREB antibody (Beyotime) and rabbit p-CREB antibody (Beyotime) diluted with 1% BSA were added. Next, the cells were incubated at 4 °C overnight and washed with PBS. The goat anti-rabbit secondary antibody was incubated at room temperature for 1 h and washed with PBS. Finally, the samples were dyed with DAPI solution at room temperature for 5 min and washed with PBS. A fluorescence microscope was used to observe and photograph the cells.
One hundred microliters of the cell supernatant in each group was collected for the lactate dehydrogenase (LDH) concentration test according to the LDH kit (Keygen, Nanjing, China) instructions. Then, the reaction mixture was added and incubated in the dark for 30 min at room temperature. The LDH concentration was quantified by measuring the absorbance at 490 nm.
The cells of each group were collected and lysed with RIPA lysis buffer containing a protease inhibitor, and the protein concentration was determined with a BCA protein concentration detection kit. The sample was loaded at 30-50 μg/well, separated by 10% sodium-dodecyl sulfate gel electrophoresis for 1 h, transferred to a polyvinylidene fluoride membrane, sealed with PBS buffer containing 5% skim milk powder for 2 h, added to the corresponding primary antibody, and incubated overnight at 4 °C. Then, the cells were rinsed with PBST buffer 3 times for 5 min each, the corresponding secondary antibody was added, and the cells were incubated at 37 °C for 4 h. Next, PBST buffer was used to rinse the cells 3 times. Color development was achieved by using the ECL Western Blot Detection Kit (Thermo Scientific). β-Actin was used as the internal reference. All antibodies were purchased from Abcam Company in Shanghai, China. Image J was used to analyze the gray value of the target protein.
TRIzol reagent (TRIzol Invitrogen) was employed to extract total RNA from cells, which were then treated with DNA enzymes for reverse transcription using a reverse transcription kit (Takara Biotechnology). Two microliters of cDNA product was placed into an EP tube and amplified by a SYBR Green Master Mix reaction system. The Ct value of the template was determined using GAPDH as the internal parameter, and the relative expression level of the target mRNA was quantified by the cycle counting method (2-ΔΔCt). The primer sequences are shown in Table 1.
| Genes | Primer sequences |
| GAPDH-F | TGTGACAGTGACTTGGGACA |
| GAPDH-R | AGGTGGAAGAGTGGGAGTTG |
| Bcl2-F | TCGCAGAGATGTCCAGTCAG |
| Bcl2-R | ATCTCCCTGTTGACGCTCTC |
| Caspase-3-F | AAGGAGCAGCTTTGTGTGTG |
| Caspase-3-R | TGTCTCAATGCCACAGTCCA |
| Bax-F | TGCAGAGGATGATTGCTGAC |
| Bax-F | GATCAGCTCGGGCACTTTAG |
| CNTF-F | GGTGACTTCCATCAGGCAAT |
| CNTF-R | TGACACGGAGGTCATGGATA |
| BDNF-F | TAATGCAGCATGATGGGAAA |
| BDNF-R | TCACAGTGAAAGCACCTTGC |
| NGF-F | CATGGGGGAGTTCTCAGTGT |
| NGF-R | GCACCCACTCTCAACAGGAT |
| TrkA-F | GTCTGGTGGGTCAGGGACTA |
| TrkA-R | AAAGCTCCACACATCGCTCT |
| TrkB-F | ACTCGCTTCTGGCATTGTCT |
| TrkB-R | TGTTAGTTGTGGTGGGCAAA |
| CREB-F | TTTTACCCAGGTGCCACTTC |
| CREB-R | TGGGGCATTATAACCGATGT |
| Caspase 9-F | TGCCCTTGCCTCTGAGTAGT |
| Caspase 9-R | AACAAAGAAACGCCCACAAC |
All data in this study were analyzed using SPSS 22.0 statistical software. Data are shown as the means ± SE (the number of samples was 3 per group, and each sample was repeated three times). Comparisons between the two groups were made using independent t tests, and comparisons between multiple groups were made using one-way ANOVA. P < 0.05 was considered to be a statistically significant difference.
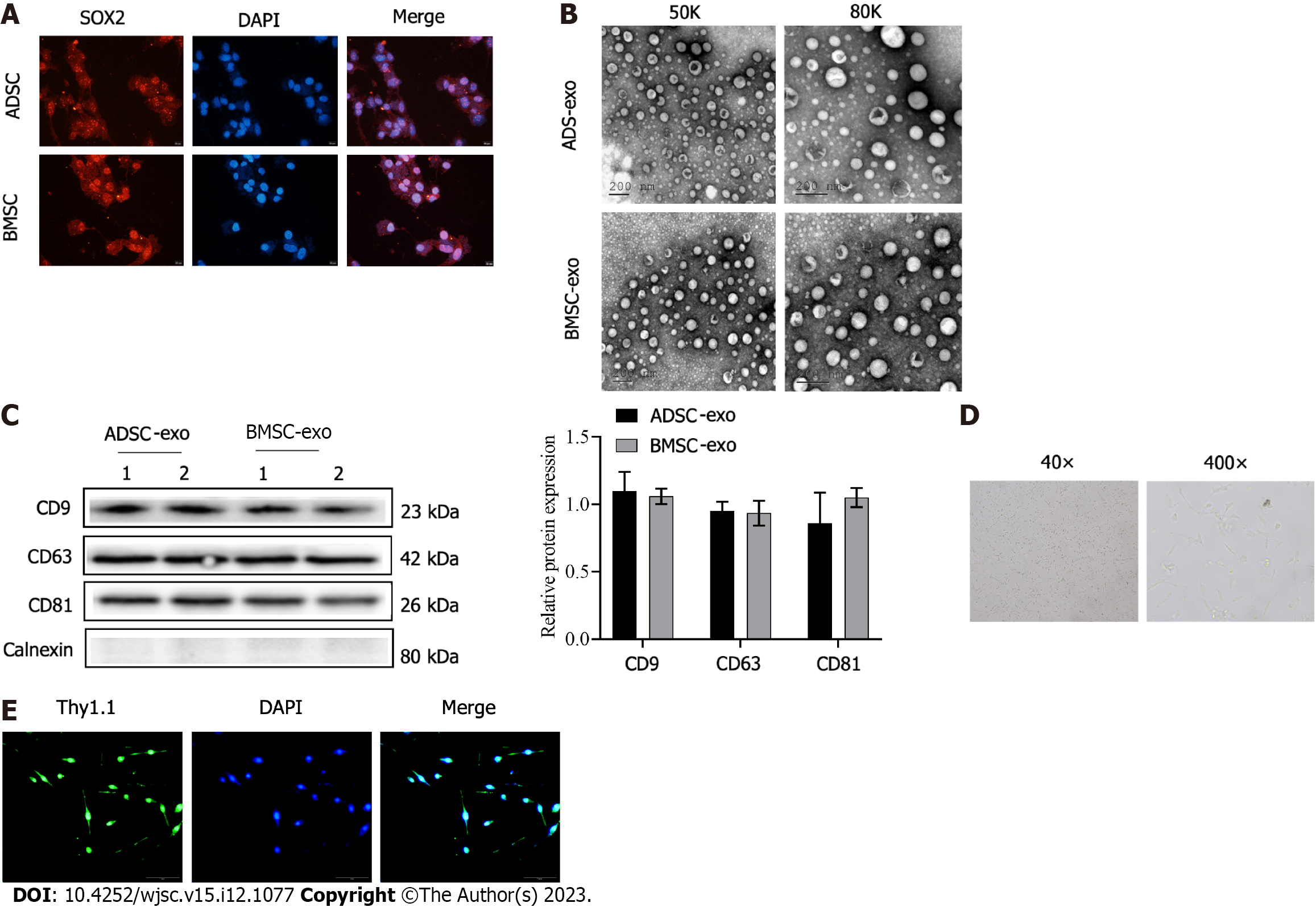
The immunofluorescence labeled SOX2, which is the marker protein of stem cells was highly positive in the two kinds of stem cells (Figure 1A). Then, exos were further isolated from the stem cell solution, and suborbicular vesicles in the form of complex exo were observed by electron microscopy (Figure 1B). The exo marker molecules CD9, CD63, and CD81 detected by western blot were positive (Figure 1C), which proved that ADSC- and BMSC-Exos were successfully isolated and obtained. Finally, primary RGCs were extracted. Figure 1D shows light microscope images of RGCs. The isolated RGCs were examined by Thy1.1 immunofluorescence (Figure 1E).
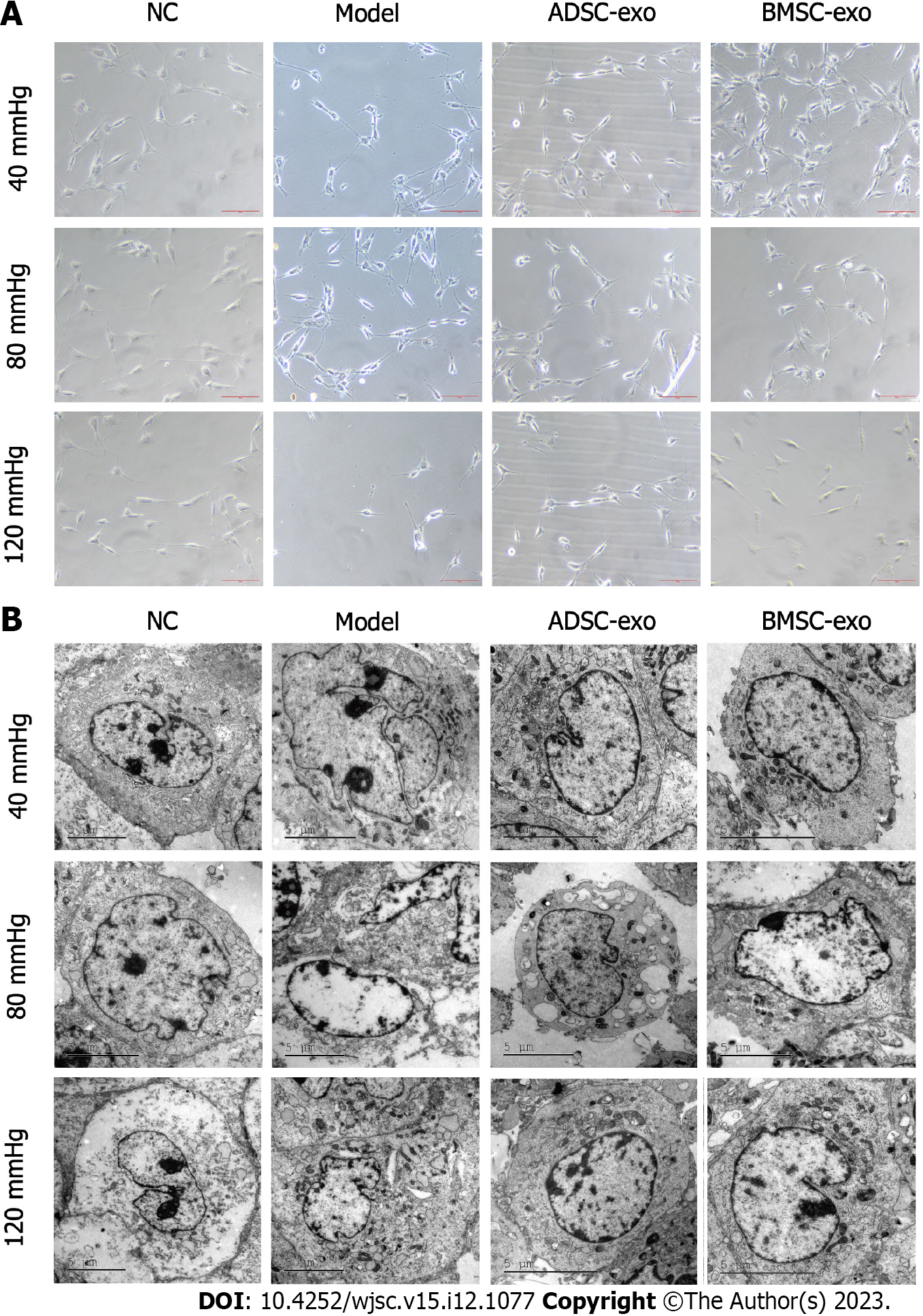
RGC growth observation: The RGC damage models under 40, 80, and 120 mmHg pressure were constructed, and cultured with ADSC-Exos (20 μg/mL) and BMSC-Exos (20 μg/mL) for 24 h, and then the morphology of RGCs was observed. The results of phase contrast microscopy showed that the gibbosity of RGCs was lower and that the cells were irregularly ellipsoidal under pressure. The addition of ADSC-Exos significantly restored the morphology of RGCs, but BMSC-Exos failed to restore the morphology of RGCs in the 120 mmHg pressure group (Figure 2A). After pressure was applied, transmission electron microscopy observations showed that RGCs had nuclear swelling, structural disorder, atrophy, malformation, and nucleolar shrinkage or disappearance. The addition of ADSC-Exos and BMSC-Exos significantly restored RGC morphology (Figure 2B).
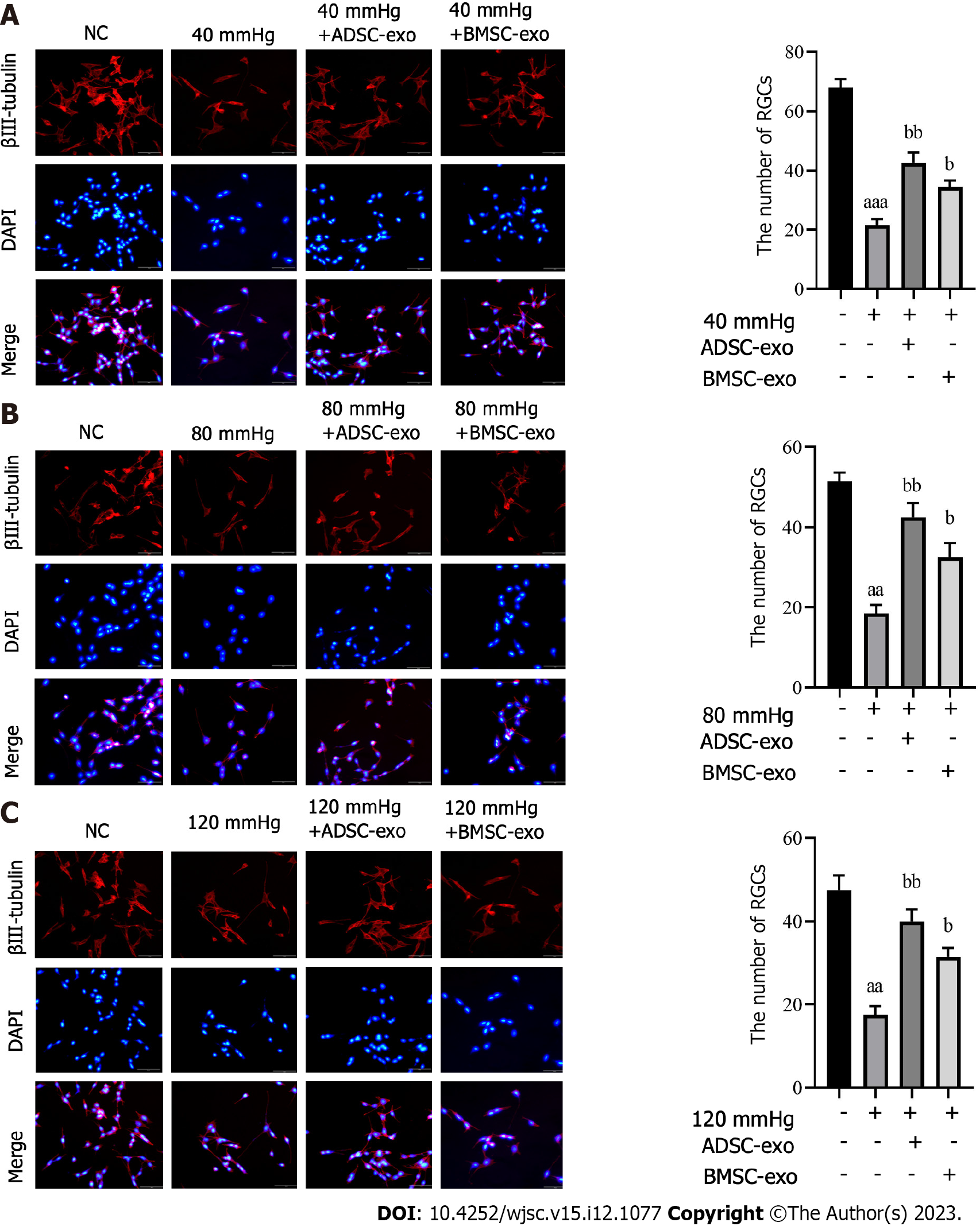
The number of RGCs: To further explore the effect of ADSC-Exos and BMSC-Exos on RGCs, we performed neuron-specific β III-tubulin expression by immunofluorescence. The results showed that the number of RGCs was significantly reduced after pressure was applied, while the number of RGCs was markedly increased after ADSC-Exos and BMSC-Exos were administered (Figures 3A-C).
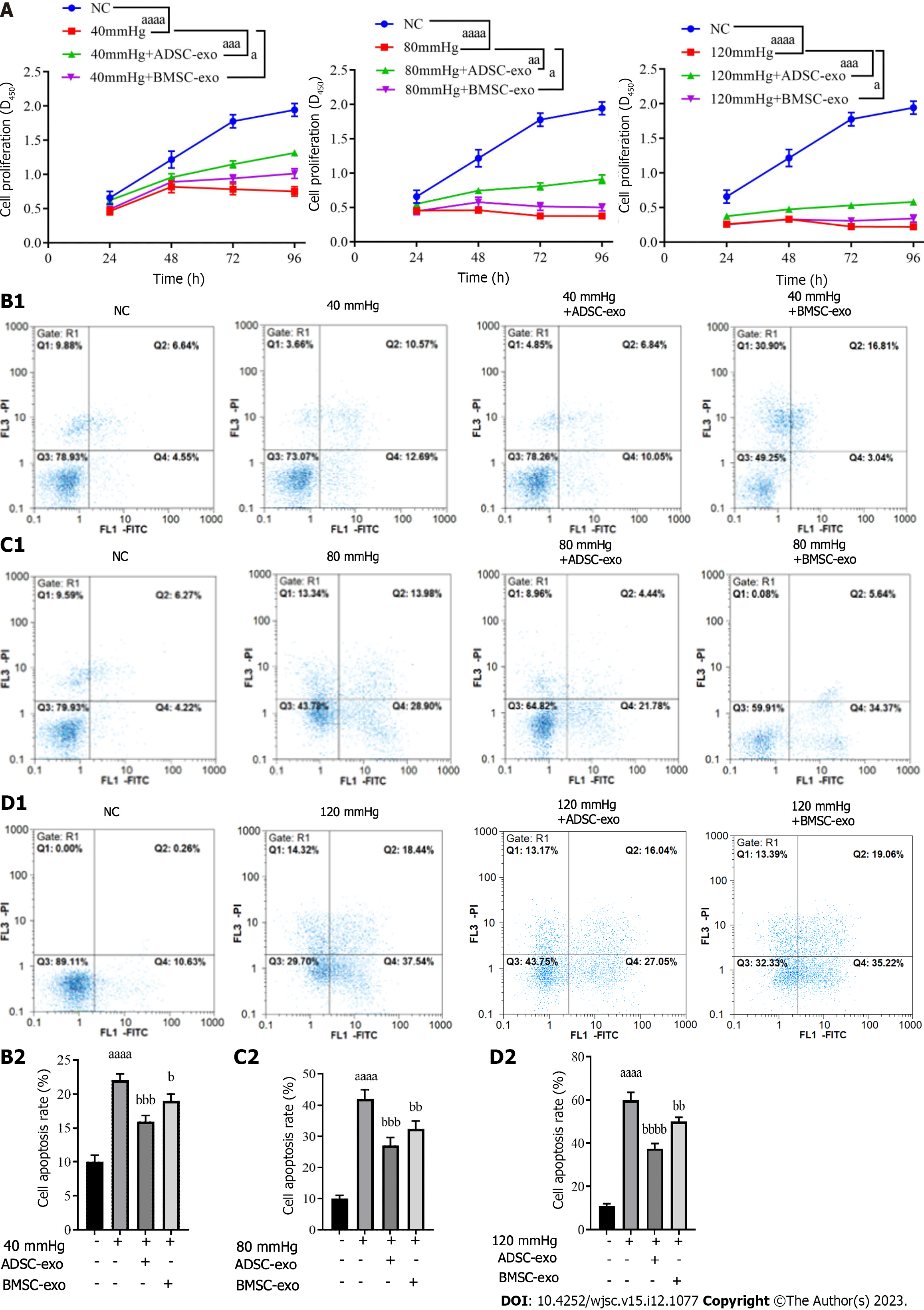
RGC proliferation and apoptosis: The CCK-8 results showed that RGC proliferation activity in the pressure injury group was significantly lower than that in the NC group, while proliferation activity was elevated after ADSC- and BMSC-Exos treatment (Figure 4A). The flow cytometry results indicated that compared with that in the NC group, the apoptosis rate in the pressure injury group was significantly increased, while the apoptosis rate was significantly decreased after ADSC- and BMSC-Exos treatment (Figure 4B).
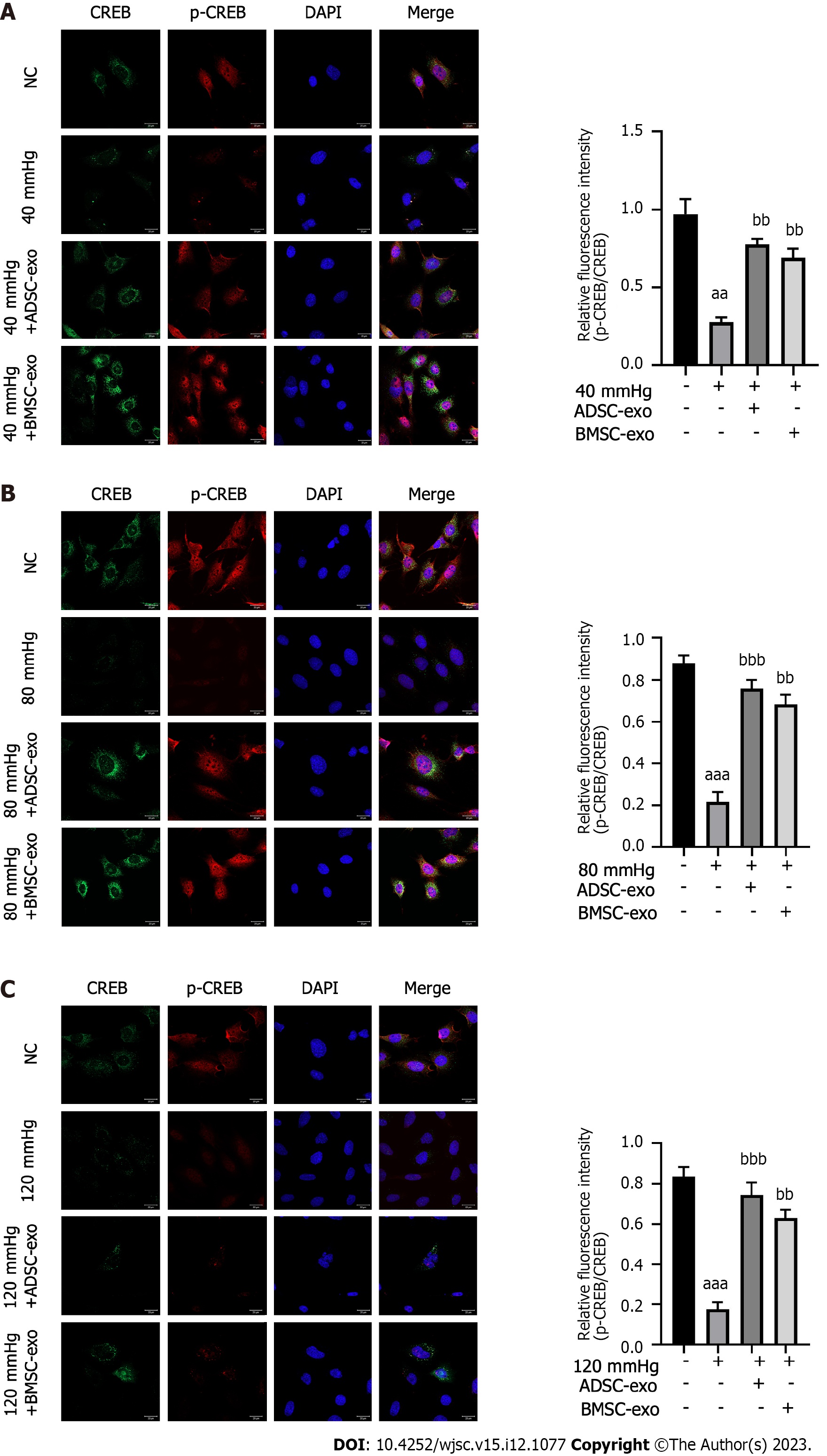
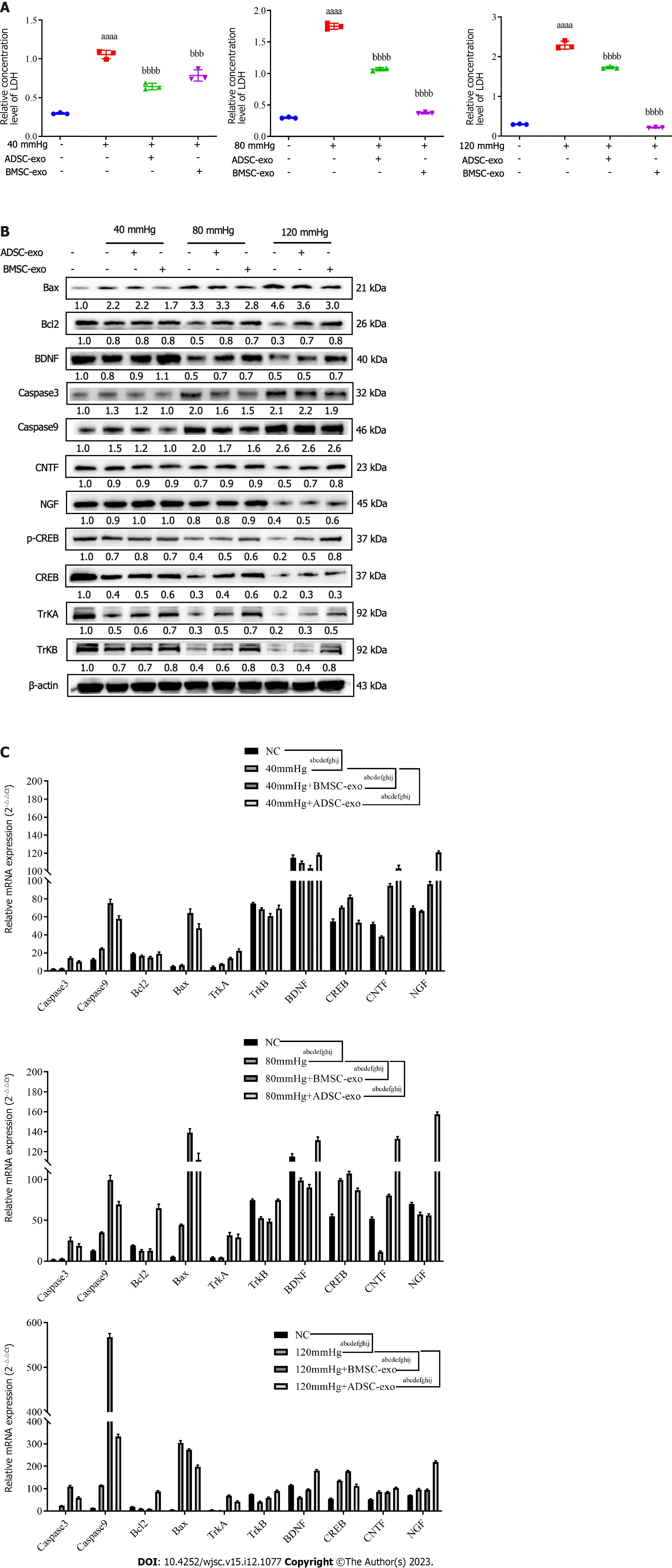
CREB inhibits apoptosis by upregulating the expression of the antiapoptotic genes Bcl-2 and Bcl-xL through a transcription-dependent mechanism. Therefore, this study further explored the effects of exos from different sources on the apoptosis-related protein CREB in RGCs. As shown in Figures 5A-C, the ratio of p-CREB/CREB in the pressure injury group was significantly reduced compared with that in the NC group, while the ratio of p-CREB/CREB was significantly increased after ADSC- and BMSC-Exos treatment.
LDH is present in all cells. When RGCs are damaged, they are released rapidly. When LDH is elevated, RGCs are damaged or dysfunctional, which leads to apoptosis. Therefore, this study further explored the effects of exos from different sources on LDH in RGCs. The LDH test kit results showed that compared with that in the NC group, the LDH level was significantly increased in the pressure injury group, while the LDH level was significantly decreased after ADSC-Exos and BMSC-Exos treatment (Figure 6A). western blot and real-time quantitative polymerase chain reaction results indicated that compared with the NC group, the anti-apoptotic protein (Bcl-2) and neurotrophic factor (BDNF, NGF, CNTF, TRKA, TRKB) were significantly decreased and the pro-apoptotic protein (Bax, caspase3, caspase9, CREB) was significantly increased in the pressure injury group, while the above indices could be reversed after ADSC-Exos and BMSC-Exos treatment (Figures 6B and C).
Hydrostatic pressure is known to impact various aspects of cellular anatomy and physiology. Morphological changes in cell shape, alignment and processes and cytoskeletal actin redistribution have been demonstrated in rat RGCs[34,35]. Agar et al[36] showed that neurons may undergo apoptosis in direct response to increased pressures at clinically relevant levels. Cell cultures were subjected to elevated hydrostatic pressures in an in vitro system based on established pressure chamber models[37,38]. Experimental pressure conditions were selected to be relevant to the IOPs seen in clinical settings, with levels of 100 mmHg analogous to acute glaucoma, 30 mmHg for chronic glaucoma and 15 mmHg for the so-called ‘normal’ IOP. Therefore, hydrostatic pressures of 40, 80, and 120 mmHg were chosen for this study and the intervention of ADSC- and BMSC-Exos could improve RGC damage by inhibiting RGC apoptosis and promoting neural restoration of RGCs.
Stem cells are a kind of cell population with the potential for self-renewal, high proliferation and differentiation, and multidirectional differentiation. MSCs are derived from connective tissue and can be differentiated into osteoblasts, chondrocytes, fat cells, and so on. In neurodegenerative diseases, MSC secretes a variety of neurotrophic factors that directly promote the survival and growth of nerve cells and act on the microenvironment of nerve tissue[39,40]. MSCs isolated from rat femoral bone marrow injected into a vitreous body can integrate into the retina, survive for at least 5 wk, significantly reduce IOP and have a protective effect on RGCs[41]. Liu et al[42] found that BMSC transplantation can significantly reduce photoreceptor cell death and preserve retinal structure in retinal detachment models. Hu et al[43] reported that BMSCs promoted the survival of RGCs in the transplanted eye compared with the control eye. MSCs play a role in neuroprotection mainly by regulating the intraocular microenvironment. This suggests that BMSCs have an ameliorating effect. However, the structural integration of MSCs into retinal tissue is complex and difficult due to the barrier action of the inner boundary membrane of the retina and the intercellular junctions[44]. In addition, transplanting MSCs in tissue reconstruction carries risks of tumorigenesis and immunological rejection. Transplanting MSC-Exos may overcome these obstacles in future applications. For example, MSC-Exos have an ameliorating effect on RGCs in some optic neuropathy rat models[45,46]. Therefore, exos derived from stem cells are emerging as a promising method for glaucoma treatment.
This study elucidated that BMSC-Exos significantly reversed RGC damage induced by hydrostatic pressure in a RGC injury model in vitro. The protective effect was mainly reflected in the fact that ADSC-Exos and BMSC-Exos promoted the proliferation of RGCs, the regeneration of RGC axons, and the secretion of RGC neurotrophic factors while inhibiting RGC apoptosis. We emphasized its potential in the prevention or treatment of glaucomatous optic neuropathy. Our study confirmed previous findings that MSC-Exos have an ameliorating effect against RGC damage in some optic neuropathies. BMSC-Exos have been found to increase RGC survival and promote axon regeneration. This ameliorating effect of BMSC-Exos was also observed in a genetic modification DBA/2J glaucoma mouse model[23]. Furthermore, in rats with optic nerve compression, Pan et al[47] found that umbilical cord-MSC-Exos enhanced RGC survival. Yu et al[48] confirmed that MSC-derived EVs can help alleviate optic nerve injury caused by chronic ocular hypertension, and this effect is achieved by inhibiting cell apoptosis. In addition, we discovered for the first time that ADSC-Exos significantly reversed high-pressure-induced RGC damage. It was reported that after ADSCs and ADSC-the trabecular meshwork (ADSC-TM) were transplanted into the eyes of infantile mice, the cells integrated into TM tissue expressed TM cell markers, and maintained normal IOP, outflow facility, and extracellular matrix[49]. The results demonstrated the possibility of applying autologous or allogeneic ADSC and ADSC-TM cells as a potential therapeutic approach to restore the structure and function of the TM in glaucoma. Therefore, combined with literature reports and our study, ADSC-Exos are expected to be an adjunct therapeutic agent for glaucoma in the future.
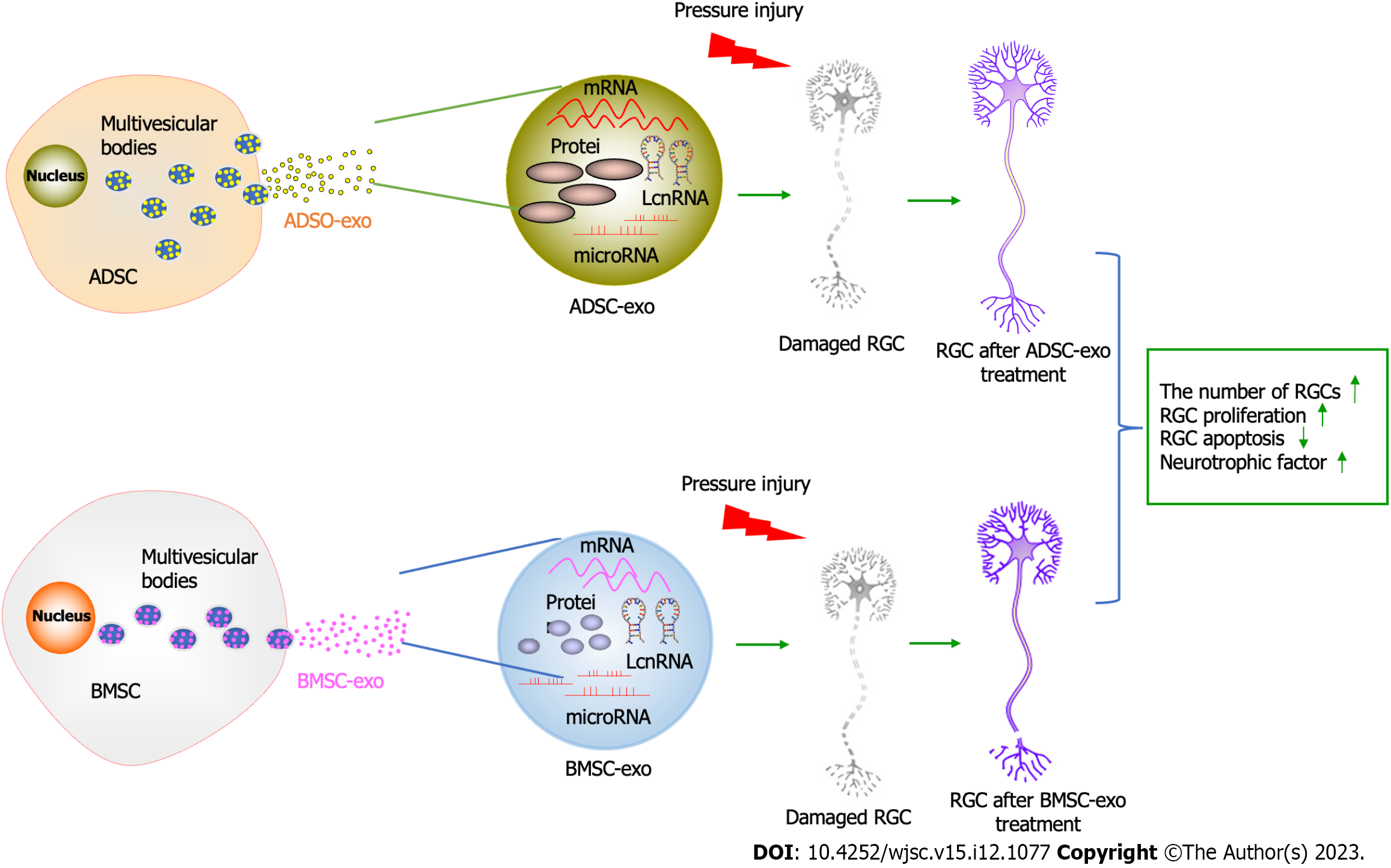
Surprisingly, ADSC-Exos manifested better protective effects than BMSC-Exos in the present study. There is evidence that ADSCs are most conducive to clinical utilization. In addition, adipose tissue is relatively abundant in the human body compared with other tissues. ADSCs can be isolated from adipose tissue. In addition, Kern et al[50], through comparative analysis of MSCs from bone marrow, umbilical cord blood, or adipose tissue, concluded that 500 times more stem cells were obtained from adipose tissue than from the same amount of bone marrow. Moreover, ADSCs are easier to obtain from adipose tissue due to their subcutaneous location than BMSCs. Patients tend to choose less traumatic sites for collecting tissue. Significant discrepancies are observed in cellular exos from different sources, including the type and content of mRNAs, miRNAs, and proteins[51]. Importantly, ADSCs have a higher proliferation capacity than BMSCs[52]. Moreover, ADSC-Exos possess the advantages of ADSCs. Hence, ADSC-Exos seem to be more favorable than BMSC-Exos because they are easily obtained and have a high proliferation capacity. ADSC-Exos could also be considered for the treatment of glaucoma. The graphic abstract is shown in Figure 7.
Some limitations should be stated. First, we lacked the comparison of neurotrophic factors and miRNAs between ADSC-Exos and BMSC-Exos by bioinformatics and other basic biological methods. Second, the effects of ADSC-Exos and BMSC-Exos on RGCs should be further validated by an in vivo experiment. Last but not least, more in vivo and in vitro experiments are needed to provide theoretical evidence for that MSC-Exos protect RGCs.
These findings indicated that ADSC-Exos and BMSC-Exos could ameliorate RGC injury caused by hydrostatic pressure by inhibiting apoptosis and increasing the secretion of neurotrophic factors.
Mesenchymal stem cells (MSCs) have a protective effect against damage to the cornea, lacrimal gland, retina and photoreceptor cells, and this protective effect can be mediated through exosomes (exos) released by MSCs.
Experimental pressure conditions were selected to be relevant to intraocular pressures seen in clinical settings, with levels of 100 mmHg analogous to acute glaucoma, 30 mmHg for chronic glaucoma and 15 mmHg for the so-called ‘normal’ intraocular pressure. Bone marrow-derived stem cell (BMSC)-Exos have an ameliorative effect on glaucoma. However, the effect of adipose-derived stem cell (ADSC)-Exos which is the ideal source of exos on optic neural degeneration has not been studied yet.
This study investigated the ameliorating effect of exos derived from different MSCs on retinal ganglion cell (RGC) injury induced by hydrostatic pressure.
The RGC injury model was constructed by RGC damage under different hydrostatic pressures (40, 80, 120 mmHg). Then RGCs were cultured with ADSC-Exos and BMSC-Exos. Cell Counting Kit-8, transmission electron microscopy, flow cytometry, immunofluorescence, real-time quantitative polymerase chain reaction, and western blotting were performed to detect the ameliorating effect of exos on pressure-induced RGC injury.
ADSC-Exos and BMSC-Exos were successfully separated. RGCs were in the shape of irregular ellipsoids, and the addition of ADSC-Exos and BMSC-Exos significantly restored the morphology of RGCs. In addition, the proliferative activity of RGCs was increased and cell apoptosis was inhibited. Increased levels of lactate dehydrogenase and apoptosis-related proteins, and increased concentrations of anti-apoptotic proteins and neurotrophic substances.
The results indicate that ADSC-Exos and BMSC-Exos can improve hydrostatic pressure-induced RGC damage by inhibiting apoptosis and increasing neurotrophic factor secretion.
The results of this study will be verified by the experiment in vivo in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Egypt; Li SC, United States; Sheykhhasan M, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | He S, Stankowska DL, Ellis DZ, Krishnamoorthy RR, Yorio T. Targets of Neuroprotection in Glaucoma. J Ocul Pharmacol Ther. 2018;34:85-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Chen W, Liu P, Liu D, Huang H, Feng X, Fang F, Li L, Wu J, Liu L, Solow-Cordero DE, Hu Y. Maprotiline restores ER homeostasis and rescues neurodegeneration via Histamine Receptor H1 inhibition in retinal ganglion cells. Nat Commun. 2022;13:6796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 3. | Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2709] [Article Influence: 246.3] [Reference Citation Analysis (0)] |
| 4. | Li Q, Cheng Y, Zhang S, Sun X, Wu J. TRPV4-induced Müller cell gliosis and TNF-α elevation-mediated retinal ganglion cell apoptosis in glaucomatous rats via JAK2/STAT3/NF-κB pathway. J Neuroinflammation. 2021;18:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Galvao J, Davis BM, Cordeiro MF. In vivo imaging of retinal ganglion cell apoptosis. Curr Opin Pharmacol. 2013;13:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Liu L, Sha XY, Wu YN, Chen MT, Zhong JX. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen Res. 2020;15:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Kanamoto T, Okumichi H, Rimayanti U, Kiuchi Y. Cullin5 reduces retinal cell death induced by glutamate toxicity. Curr Eye Res. 2011;36:66-70. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Karl MO. The potential of stem cell research for the treatment of neuronal damage in glaucoma. Cell Tissue Res. 2013;353:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Bull ND, Irvine KA, Franklin RJ, Martin KR. Transplanted oligodendrocyte precursor cells reduce neurodegeneration in a model of glaucoma. Invest Ophthalmol Vis Sci. 2009;50:4244-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Daniel S, Clark AF, McDowell CM. Subtype-specific response of retinal ganglion cells to optic nerve crush. Cell Death Discov. 2018;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Yadav KS, Sharma S, Londhe VY. Bio-tactics for neuroprotection of retinal ganglion cells in the treatment of glaucoma. Life Sci. 2020;243:117303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Mizuta Y, Akahoshi T, Guo J, Zhang S, Narahara S, Kawano T, Murata M, Tokuda K, Eto M, Hashizume M, Yamaura K. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res Ther. 2020;11:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Yu Z, Wen Y, Jiang N, Li Z, Guan J, Zhang Y, Deng C, Zhao L, Zheng SG, Zhu Y, Su W, Zhuo Y. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Christ A, Christa A, Klippert J, Eule JC, Bachmann S, Wallace VA, Hammes A, Willnow TE. LRP2 Acts as SHH Clearance Receptor to Protect the Retinal Margin from Mitogenic Stimuli. Dev Cell. 2015;35:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M, Roth S. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 16. | Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018;2018:6901983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Mead B, Tomarev S. Extracellular vesicle therapy for retinal diseases. Prog Retin Eye Res. 2020;79:100849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 19. | Sharma A, Jaganathan BG. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. Biologics. 2021;15:299-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692-11712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 21. | Mesentier-Louro LA, Zaverucha-do-Valle C, Rosado-de-Castro PH, Silva-Junior AJ, Pimentel-Coelho PM, Mendez-Otero R, Santiago MF. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016;2016:5078619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Mead B, Amaral J, Tomarev S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Invest Ophthalmol Vis Sci. 2018;59:702-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Mead B, Ahmed Z, Tomarev S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Invest Ophthalmol Vis Sci. 2018;59:5473-5480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Mead B, Tomarev S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl Med. 2017;6:1273-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 25. | Sheykhhasan M, Amini R, Soleimani Asl S, Saidijam M, Hashemi SM, Najafi R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer's disease. Biomed Pharmacother. 2022;152:113224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 26. | Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson's disease. Mol Ther Nucleic Acids. 2021;23:1334-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 27. | Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47:864-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 28. | Chan-Juan H, Sen L, Li-Qianyu A, Jian Y, Rong-Di Y. MicroRNA-30b regulates the polarity of retinal ganglion cells by inhibiting semaphorin-3A. Mol Vis. 2019;25:722-730. [PubMed] |
| 29. | Xu C, Lu H, Li F, Su G. Protein Expression Profile on Differentiation of Bone Marrow Mesenchymal Stem Cells into Retinal Ganglion-Like Cells. J Comput Biol. 2020;27:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Coroneo MT, Li S, Agar A, Hill MA. Pressure related apoptosis in human & neuronal cell lines. Investig Ophthalmol Vis Sci. 2001;42:S23-S23. |
| 31. | Agar A, Li S, Agarwal N, Coroneo MT, Hill MA. Retinal ganglion cell line apoptosis induced by hydrostatic pressure. Brain Res. 2006;1086:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Ren S, Chen J, Guo J, Liu Y, Xiong H, Jing B, Yang X, Li G, Kang Y, Wang C, Xu X, Liu Z, Zhang M, Xiang K, Li C, Li Q, Machens HG, Chen Z. Exosomes from Adipose Stem Cells Promote Diabetic Wound Healing through the eHSP90/LRP1/AKT Axis. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 33. | Yang W, Huang C, Wang W, Zhang B, Chen Y, Xie X. Bone mesenchymal stem cell-derived exosomes prevent hyperoxia-induced apoptosis of primary type II alveolar epithelial cells in vitro. PeerJ. 2022;10:e13692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | Liu B, Ma X, Guo D, Guo Y, Chen N, Bi H. Neuroprotective effect of alpha-lipoic acid on hydrostatic pressure-induced damage of retinal ganglion cells in vitro. Neurosci Lett. 2012;526:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Ju WK, Kim KY, Lindsey JD, Angert M, Patel A, Scott RT, Liu Q, Crowston JG, Ellisman MH, Perkins GA, Weinreb RN. Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, and induces apoptotic cell death in differentiated RGC-5 cells. Mol Vis. 2009;15:120-134. [PubMed] |
| 36. | Agar A, Yip SS, Hill MA, Coroneo MT. Pressure related apoptosis in neuronal cell lines. J Neurosci Res. 2000;60:495-503. [PubMed] [DOI] [Full Text] |
| 37. | Chalam K, Vinjamaram S, Shan V, Tripathi B, Tipathi R. An in vitro system for studying the effect of ambient hydrostatic pressure on growth, morphology, and biochemical aspects of ganglion cells. Investig Ophthalmol Vis Sci. 2003;44. |
| 38. | Mattana J, Singhal PC. Applied pressure modulates mesangial cell proliferation and matrix synthesis. Am J Hypertens. 1995;8:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Sadatpoor SO, Salehi Z, Rahban D, Salimi A. Manipulated Mesenchymal Stem Cells Applications in Neurodegenerative Diseases. Int J Stem Cells. 2020;13:24-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Staff NP, Jones DT, Singer W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin Proc. 2019;94:892-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 41. | Roubeix C, Godefroy D, Mias C, Sapienza A, Riancho L, Degardin J, Fradot V, Ivkovic I, Picaud S, Sennlaub F, Denoyer A, Rostene W, Sahel JA, Parsadaniantz SM, Brignole-Baudouin F, Baudouin C. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res Ther. 2015;6:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Liu X, Xie J, Yang L, Li Y, He Y, Liu Z, Zhang Y, Su G. Bone marrow mesenchymal stem cells enhance autophagy and help protect cells under hypoxic and retinal detachment conditions. J Cell Mol Med. 2020;24:3346-3358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Hu Y, Tan HB, Wang XM, Rong H, Cui HP, Cui H. Bone marrow mesenchymal stem cells protect against retinal ganglion cell loss in aged rats with glaucoma. Clin Interv Aging. 2013;8:1467-1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Wang Y, Lv J, Huang C, Li X, Chen Y, Wu W, Wu R. Human Umbilical Cord-Mesenchymal Stem Cells Survive and Migrate within the Vitreous Cavity and Ameliorate Retinal Damage in a Novel Rat Model of Chronic Glaucoma. Stem Cells Int. 2021;2021:8852517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Cui Y, Liu C, Huang L, Chen J, Xu N. Protective effects of intravitreal administration of mesenchymal stem cell-derived exosomes in an experimental model of optic nerve injury. Exp Cell Res. 2021;407:112792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Seyedrazizadeh SZ, Poosti S, Nazari A, Alikhani M, Shekari F, Pakdel F, Shahpasand K, Satarian L, Baharvand H. Extracellular vesicles derived from human ES-MSCs protect retinal ganglion cells and preserve retinal function in a rodent model of optic nerve injury. Stem Cell Res Ther. 2020;11:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, Luo X, Xu J, Yang X, Sun X. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat. 2019;96:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Yu F, Wang Y, Huang CQ, Lin SJ, Gao RX, Wu RY. Neuroprotective effect of mesenchymal stem cell-derived extracellular vesicles on optic nerve injury in chronic ocular hypertension. Neural Regen Res. 2023;18:2301-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 49. | Zhou Y, Xia X, Yang E, Wang Y, Marra KG, Ethier CR, Schuman JS, Du Y. Adipose-derived stem cells integrate into trabecular meshwork with glaucoma treatment potential. FASEB J. 2020;34:7160-7177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 51. | Wang Y, Yu T, Hu F. Hypocapnia Stimuli-Responsive Engineered Exosomes Delivering miR-218 Facilitate Sciatic Nerve Regeneration. Front Bioeng Biotechnol. 2022;10:825146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Lv X, He J, Zhang X, Luo X, He N, Sun Z, Xia H, Liu V, Zhang L, Lin X, Lin L, Yin H, Jiang D, Cao W, Wang R, Zhou G, Wang W. Comparative Efficacy of Autologous Stromal Vascular Fraction and Autologous Adipose-Derived Mesenchymal Stem Cells Combined With Hyaluronic Acid for the Treatment of Sheep Osteoarthritis. Cell Transplant. 2018;27:1111-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |