Published online Sep 26, 2022. doi: 10.4252/wjsc.v14.i9.700
Peer-review started: March 28, 2022
First decision: June 11, 2022
Revised: June 20, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: September 26, 2022
Processing time: 179 Days and 1.2 Hours
Heart diseases are the primary cause of death all over the world. Following myocardial infarction, billions of cells die, resulting in a huge loss of cardiac function. Stem cell-based therapies have appeared as a new area to support heart regeneration. The transcription factors GATA binding protein 4 (GATA-4) and myocyte enhancer factor 2C (MEF2C) are considered prominent factors in the development of the cardiovascular system.
To explore the potential of GATA-4 and MEF2C for the cardiac differentiation of human umbilical cord mesenchymal stem cells (hUC-MSCs).
hUC-MSCs were characterized morphologically and immunologically by the presence of specific markers of MSCs via immunocytochemistry and flow cytometry, and by their potential to differentiate into osteocytes and adipocytes. hUC-MSCs were transfected with GATA-4, MEF2C, and their combination to direct the differentiation. Cardiac differentiation was confirmed by semiquantitative real-time polymerase chain reaction and immunocytochemistry.
hUC-MSCs expressed specific cell surface markers CD105, CD90, CD44, and vimentin but lack the expression of CD45. The transcription factors GATA-4 and MEF2C, and their combination induced differentiation in hUC-MSCs with significant expression of cardiac genes i.e., GATA-4, MEF2C, NK2 homeobox 5 (NKX2.5), MHC, and connexin-43, and cardiac proteins GATA-4, NKX2.5, cardiac troponin T, and connexin-43.
Transfection with GATA-4, MEF2C, and their combination effectively induces cardiac differentiation in hUC-MSCs. These genetically modified MSCs could be a promising treatment option for heart diseases in the future.
Core Tip: Transcription factors have great potential to direct cell fate decisions during embryonic development. In this study, we investigated the overexpression of cardiac transcription factors in human umbilical cord mesenchymal stem cells to enhance their differentiation into cardiac-like cells. The synergistic effect of GATA binding protein 4 and myocyte enhancer factor 2C transcription factors increased the expression of cardiac genes and proteins. The results of this study will aid in the development of new therapeutic strategies aimed at curing heart diseases.
- Citation: Razzaq SS, Khan I, Naeem N, Salim A, Begum S, Haneef K. Overexpression of GATA binding protein 4 and myocyte enhancer factor 2C induces differentiation of mesenchymal stem cells into cardiac-like cells. World J Stem Cells 2022; 14(9): 700-713
- URL: https://www.wjgnet.com/1948-0210/full/v14/i9/700.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i9.700
Heart failure is the most challenging issue after myocardial infarction[1,2]. The environmental and genetic risk factors cause the deregulation of cardiomyocytes as well as endothelial, smooth muscle, and inflammatory cells of heart tissue[3]. Cardiomyocytes largely fail in adult life to divide or enter the cell cycle[4,5]. Therefore, the adult heart has a limited endogenous repair and regeneration mechanism[6-8]. Current interventions rely on heart transplantation, mechanical assistance devices, and medicinal therapies for the management of damaged organ. However, these options cannot revert the normal functioning of the heart. The future therapeutic strategy for cardiac diseases is to regenerate damaged tissue for restoring complete heart function[9,10].
Cell based therapies are promising for damaged heart tissue. Stem cells possess the remarkable potential to stimulate endogenous myocardial repair and regeneration processes[11-15]. However, the low viability of transplanted stem cells due to inadequate supply of blood and inflamed myocardium has been a major challenge[12-14]. Adult mesenchymal stem cells (MSCs) have the potential to make bone, muscle, nerve, cardiac, and fat cells[16,17]. Furthermore, MSCs help in the formation of new blood vessels, induce apoptotic resistance, and provide anti-fibrotic effects[18,19]. One of the recently emp-loyed innovative approaches is the use of forward programming with tissue type-specific transcription factors for the differentiation of stem cells[20].
The successful cell fate reprogramming requires a temporospatial expression pattern of transcription factors[21]. Heart development is a complex process that requires the coordination of a series of events such as specification, proliferation, and differentiation[22]. Cardiac transcription factors, including GATA binding protein 4 (GATA-4), myocyte enhancer factor 2A (MEF2A), NK2 homeobox 5 (NKX2.5), and serum response factor (Srf), have a paradoxical role in the differentiation and homeostasis of myocardial cells[23]. It has been documented that three cardiac transcription factors, GATA-4, NKX2.5, and T-Box transcription factor 5 (TBX5), programmed extra-cardiac mesoderm of mouse embryo into cardiac tissue[24]. Also, a combination of GATA-4, NKX2.5, TBX5, and BAF60C can induce the differentiation of embryonic stem cells into cardiac lineage[25]. Altogether, these research studies display that transcription factor mediated stem cell reprogramming is a valuable strategy that directs cardio-myogenic differentiation of various stem cell types.
The current study aimed to examine the effects of overexpressing two cardiac transcription factors, GATA-4 and MEF2C, in cardiac differentiation of human umbilical cord MSCs (hUC-MSCs). After introducing the transcription factors either individually or in combination, hUC-MSCs were analyzed for the expression of cardiac genes and proteins. These genetically modified MSCs could be a promising treatment option for cardiovascular diseases.
The current research project was approved by the institutional bioethical committee of University of Karachi (protocol #: ICB KU-92/2020).
Human umbilical cords (n = 12) were collected from healthy pregnant females at the Dow University of Health Science, OJHA campus, Karachi, Pakistan after obtaining the consent from the donors.
Human umbilical cord tissue was longitudinally cut and thoroughly washed with sterile phosphate-buffered saline (PBS). The human cord tissue was cut into 2-5 mm in size and placed in 1 × (0.25%) trypsin (GIBCO, United States) for 20 min at 37 °C. Partially digested cord tissues were kept in a T-25 tissue culture flask having 3-5 mL of DMEM (GIBCO, United States) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin/streptomycin, and 1 mmol sodium pyruvate. Explants were placed at 37 °C with 5% CO2 (Heracell, United States). The medium was changed every third day. MSCs attached to the tissue culture flask during 15-20 d of the first culture. After adhesion of the MSCs, tissues were discarded and fresh DMEM was added for the proliferation of cells. Once the MSCs reached 70% to 80% confluence, they were detached using 1 × (0.25%) trypsin. hUC-MSCs at passage (P) 1-2 were used for experiments.
Isolated hUC-MSCs were characterized by immunocytochemistry to detect the specific markers of MSCs. Briefly, 4% paraformaldehyde (PFA) was added to the cells and then incubated with 0.1% Triton X-100. The permeabilized cells were then kept in a blocking solution for 1 h. After incubation, the solution was discarded and cells were kept at 4 °C with anti-mouse primary antibodies against CD90, CD105, vimentin, CD44, and CD45. After overnight incubation, cells were thoroughly washed 4-5 times with PBS. Alexa fluor 488 conjugated goat anti-mouse secondary antibody was added to each well. The negative control cells were incubated only with the secondary antibody. DAPI (4’,6-diamidino-2-phenylindole) was used to stain the cell nuclei. Lastly, cells were mounted and observed under a fluorescence microscope (NIE, Nikon, Japan).
MSCs were washed 2-3 times with PBS and incubated with dissociation buffer at 37 °C for 40 min. The cells were pelleted down through centrifugation and then the cell pellet was mixed in FACS solution containing 1% BSA, 1 mmol EDTA, and 0.1% Na-azide. The tubes were centrifuged for 5 min and then the blocking solution was added to all the tubes. Primary antibodies against CD44, CD90, and CD73 were added and the tubes were incubated at 4 °C. After washing with FACS solution, Alexa fluor 488 conjugated goat anti-mouse secondary antibody was added. Unlabeled and isotype labeled cells were used as controls. Data were analyzed using BD FACS Diva software.
Approximately 4 × 105 hUC-MSCs were seeded in a 6-well plate for 24 h. After confirming cell proliferation, cells were washed with sterile PBS. For osteogenesis, low glucose DMEM supplemented with 10 mmol glycerol-2-phosphate, 0.2 mmol ascorbic acid, 0.1 μmol dexamethasone, 10% FBS, 100 μg/mL streptomycin, 100 units/mL penicillin, and 2 mmol L-glutamine were added into the cell culture plate. The medium was replaced every 4th day till 21 d. After the completion of 21 d incubation period, ice cold 75% ethanol was used for cell fixation, and then the cells were stained with 2% Alizarin stain.
For adipogenesis, hUC-MSCs were cultured in adipogenic induction and maintenance medium for 21 d. Adipogenic induction medium contains 10 μg/mL insulin, 100 μmol indomethacin, 1 μmol dexamethasone, 10% FBS, 100 μg/mL streptomycin, and 100 units/mL penicillin in low glucose DMEM. After 21 d, 4% PFA was used for cell fixation and then cells were stained with 0.5% Oil Red O. Finally, images were taken under a phase contrast microscope (CKX41, Olympus, Japan).
GATA-4 and MEF2C plasmids were purchased from Addgene (plasmid No. 46030 and No. 46031, respectively). Plasmid DNA was isolated by using a maxiprep plasmid DNA isolation kit (Thermo Scientific, United States). Briefly, Escherichia coli were harvested by centrifugation at 5000 × g. The pellet was mixed in resuspension solution and then lysis solution was added. The suspension was incubated at room temperature for 3 min and a neutralization solution followed by endotoxin binding reagent was added to the tube. The tube was incubated at room temperature for a further 5 min and 96% ethanol was added. The supernatant was collected through centrifugation, mixed with 96% ethanol, and then shifted to the purification column. The tube was centrifuged at 2000 × g for 3 min. Wash solution 1 was added to the column and centrifuged at 3000 × g. This step was repeated with wash solution 2. The plasmid DNA was eluted in elution buffer and quantified using a nano-drop spectrophotometer. hUC-MSCs were transfected separately with GATA-4 and MEF2C, and co-transfected with 1 μg each of GATA-4 and MEF2C plasmids using lipofectamine 3000 kit (Invitrogen, United States). Briefly, the plasmid vector (1 μg for GATA-4 or MEF2C) was diluted in serum free DMEM, and 2 μL of P3000 reagent was added per 1 μg of plasmid DNA. Lipofectamine TM 3000 reagent and DNA were mixed and kept at room temperature for 15 min. Cells at 70%-80% confluence were incubated with DNA-lipid complex at 37 °C for 24 h. After 24 h, lipofectamine was replaced with FBS containing DMEM. The cells were kept for 2 wk at 37 °C using an air jacketed CO2 incubator. The medium was changed every 3 to 4 d. The following experimental groups were used in this study: Untreated control, GATA-4 transfected, MEF2C transfected, and combination group of GATA-4 + MEF2C transfected hUC-MSCs.
The overexpression of the GATA-4 and MEF2C genes in transfected hUC-MSCs was confirmed by semiquantitative real-time polymerase chain reaction (RT-PCR). RNA was extracted from transfected and control hUC-MSCs using TRIzol reagent. For RNA isolation, cells were harvested and the pellet was gently mixed with TRIzol reagent. In the next step, chloroform was added to the tube and incubated at room temperature for 15 min. The cell suspension was centrifuged at 12000 × g for 15 min. Isopropyl alcohol was added to the separated aqueous phase followed by centrifugation at 12000 × g. The RNA pellet was air dried and then resuspended in RNAase-free water. The RNA absorbance was calculated at 260 nm. cDNA was synthesized using a cDNA synthesis kit (Invitrogen, United States) and then amplified using primers corresponding to GATA-4 and MEF2C genes. Human beta-actin was used as a housekeeping gene. Reverse transcription reaction products were initially denatured for 30 s at 94 °C, followed by 40 cycles of amplification: Denaturation at 94 °C for 3 s and annealing at 60 °C for 30s. Primer sequences and melting temperatures of each gene are enlisted in Table 1.
| Gene | Primer sequence (5’-3’) | Annealing temperature (°C) |
| Beta-actin | Forward: 5’-TGGGCATGGGTCAGAAGGATTC-3’ | 60 |
| Reverse: 5’-AGGTGTGGTGCCAGATTTTCTC-3’ | ||
| Myocyte enhancer factor 2C | Forward: 5’-CGAGATGCCAGTCTCCATCC-3’ | 60 |
| Reverse: 5’-CAGAGAAGGGTGAGCCAGTG-3’ | ||
| NKX2.5 | Forward: 5’-AGTGTGCGTCTGCCTTTCC-3’ | 60 |
| Reverse: 5’-CACAGCTCTTTCTTTTCGGCTC-3’ | ||
| MHC | Forward: 5’-GACAGGTGCAGCAAAA CAGG-3’ | 60 |
| Connexin-43 | Forward: 5’-CTTCATGCTGGTGGTGTCC-3’ | 60 |
| Reverse: 5’-ACCACTGGTCGCATGGTAAG-3’ | ||
| GATA-4 | Forward: 5’-CTGCCCTCCGTCTTCTGC-3’ | 60 |
| Reverse: 5’-CTCGCAGGTCAAGGAGCC-3’ |
For gene expression, RT-PCR of untreated and transfected hUC-MSCs was performed at day 14 of transfection. For cardiac protein expression, immunocytochemistry staining of untreated and transfected hUC-MSCs was performed also on day 14 of transfection. Primary antibodies for cardiac specific proteins, i.e., GATA-4, connexin-43, NKX2.5, and cTnT, were used. The negative control cells were incubated only with the secondary antibody. Finally, cells were mounted and images were taken under a fluorescence microscope (NIE, Nikon, Japan). The fluorescence intensities were calculated through Image J software (NIH, United States).
Data were analyzed by using IBM SPSS Statistics 20 software. One way ANOVA and Tukey’s post hoc test were used for comparisons among multiple groups. All data were collected from three independent experiments. A P value less than 0.05 (aP < 0.05) was considered statistically significant.
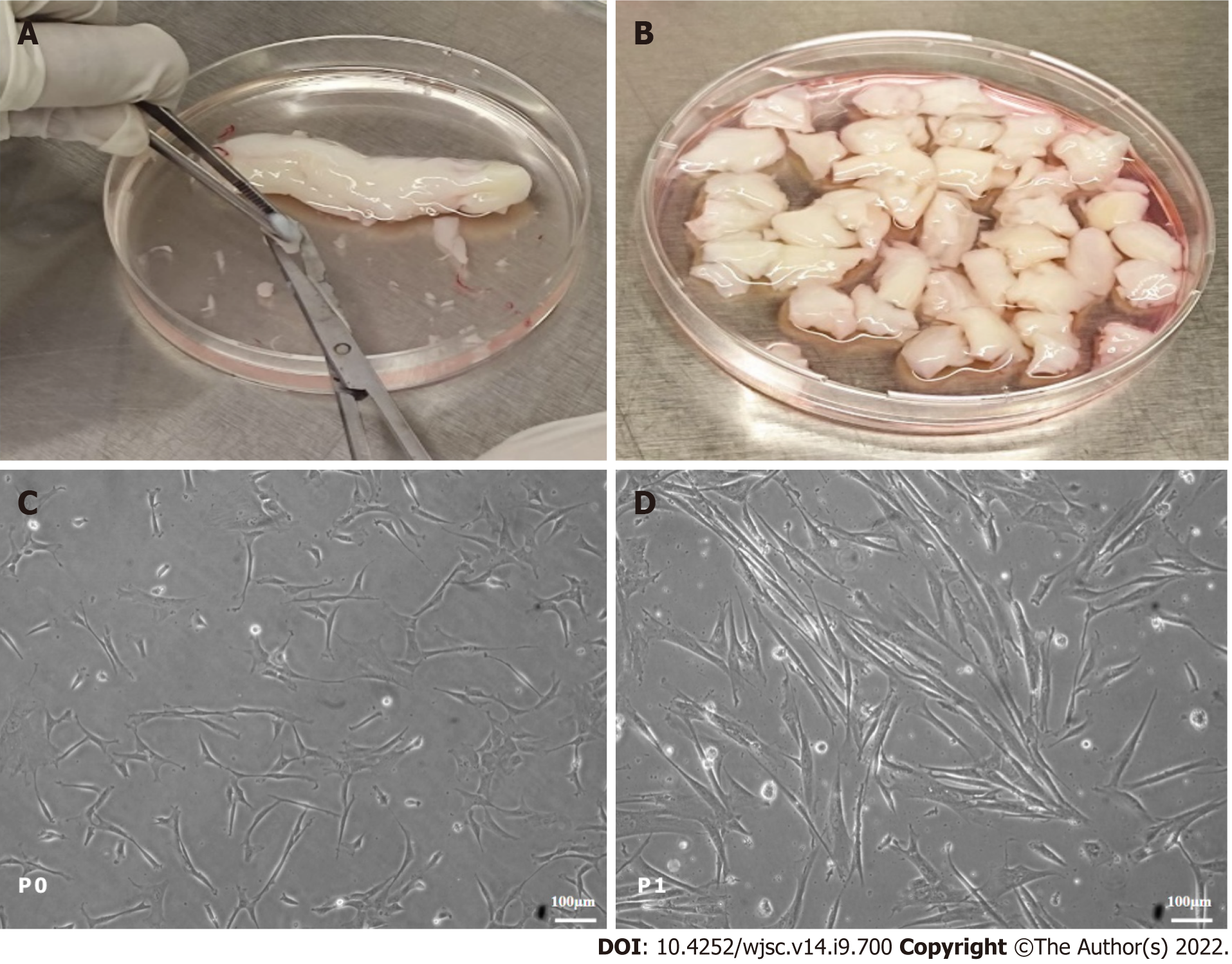
Adherent cells started to grow during 15 d to 20 d of isolation and are termed P0 cells, as shown in Figure 1. The P0 cells were sub-cultured once they reached 80% confluence and termed P1 cells. The hUC-MSCs appeared in colonies and showed a fibroblast-like morphology (Figure 1). P1 to P2 cells were used in this study.
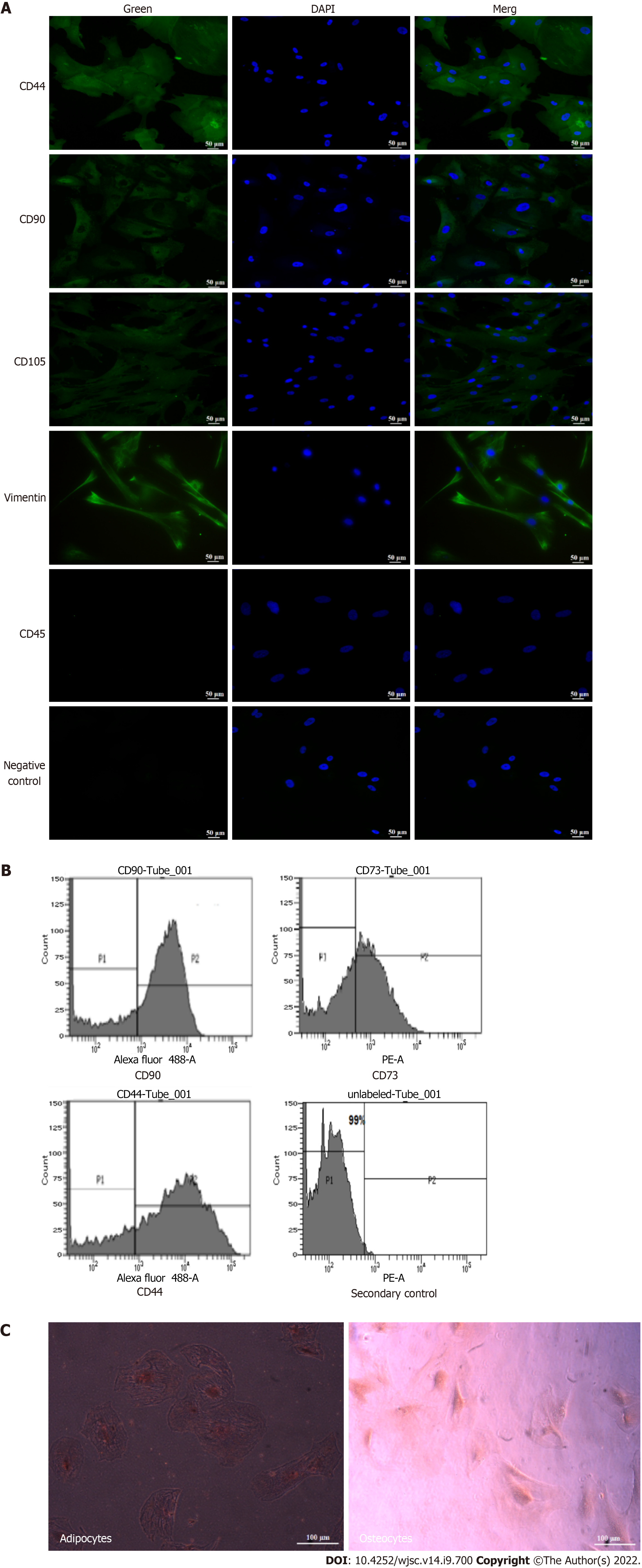
Immunocytochemistry analysis showed positive expression of the MSC markers CD105, CD90, CD44, and vimentin, while CD45, a hematopoietic marker, was not expressed in these cells (Figure 2A). The immunophenotypic analysis showed positive expression of CD90, CD73, and CD44 in hUC-MSCs (Figure 2B). The osteogenic and adipogenic differentiation was confirmed, respectively, by Alizarin Red staining which revealed mineral deposits, and Oil Red O staining which revealed lipid droplets (Figure 2C).
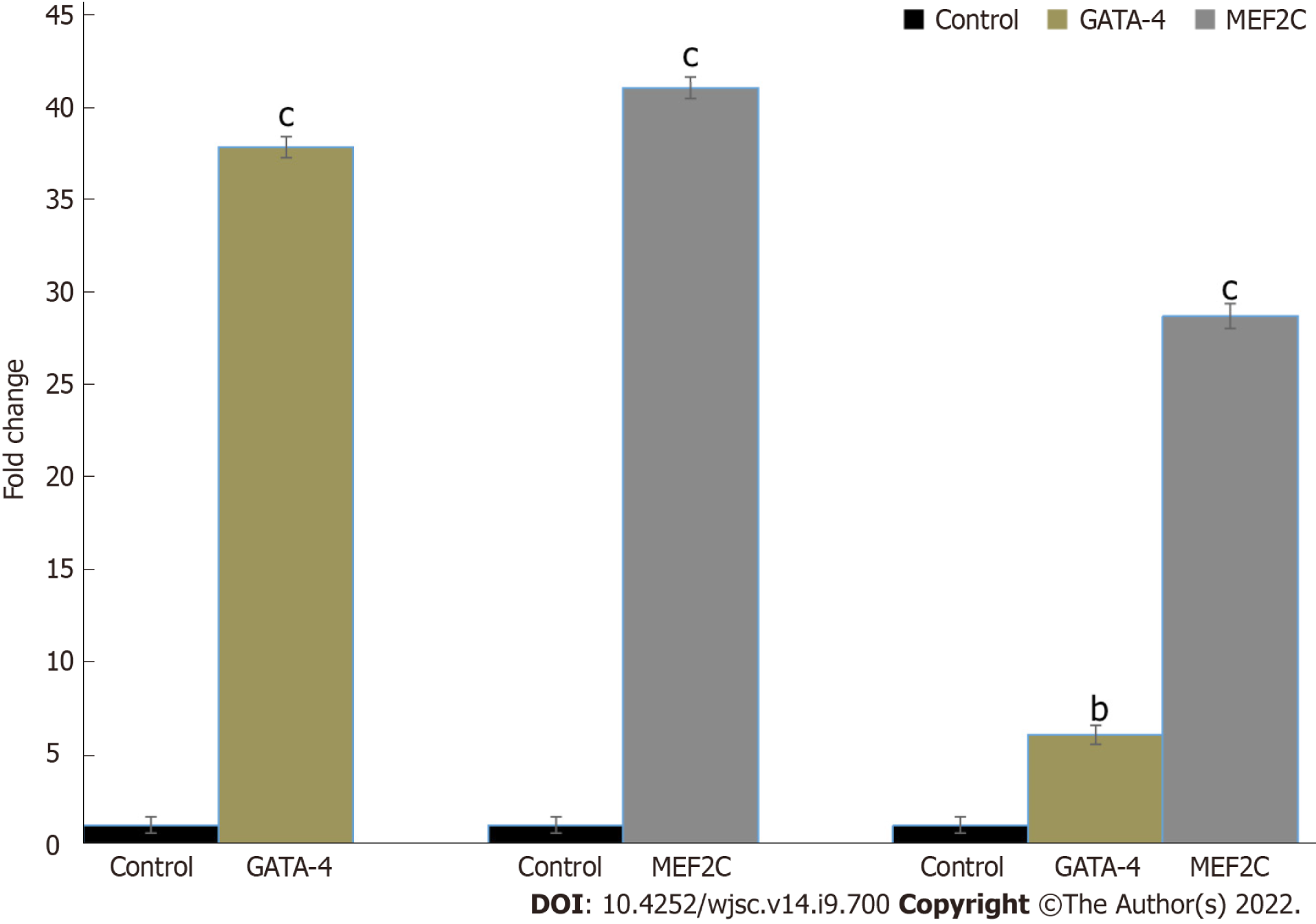
hUC-MSCs were successfully transfected with GATA-4 and MEF2C genes. RT-PCR analysis showed a significant increase in GATA-4 and MEF2C expression after 24 h of transfection compared with the control (Figure 3).
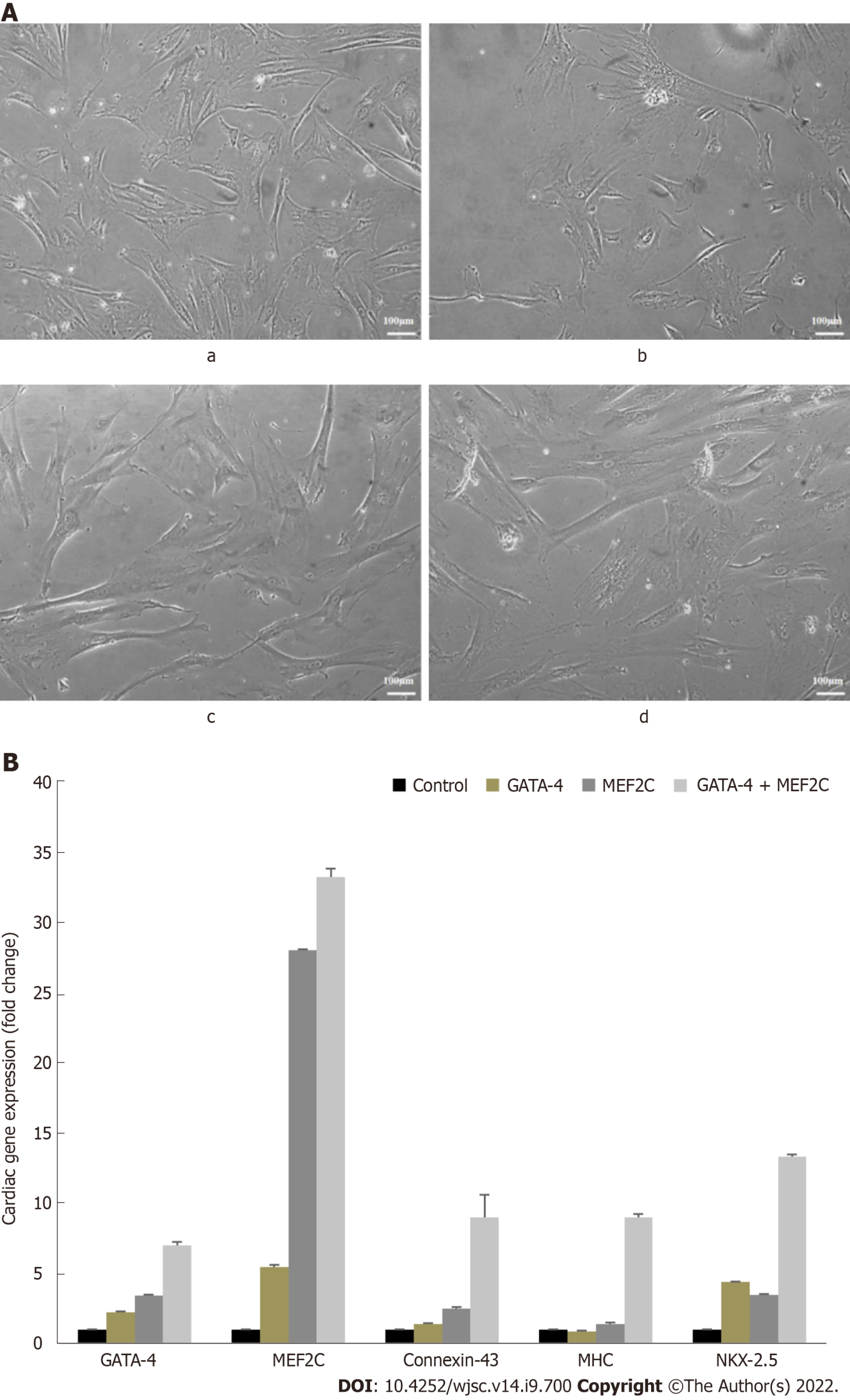
After 14 d of culture, the transfected cells displayed extended cytoplasmic processes and myotube like structures which are the typical features of cardiomyocytes (Figure 4A). hUC-MSCs transfected with GATA-4, MEF2C, and their combination showed significant expression of cardiac genes including MEF2C, NKX2.5, GATA-4, connexin-43, and myosin heavy chain (MHC) (Figure 4B). Moreover, the combination group for the evaluation of the synergistic effect of both transcription factors showed significant expression of cardiac genes as compared to the individual groups (Figure 4B).
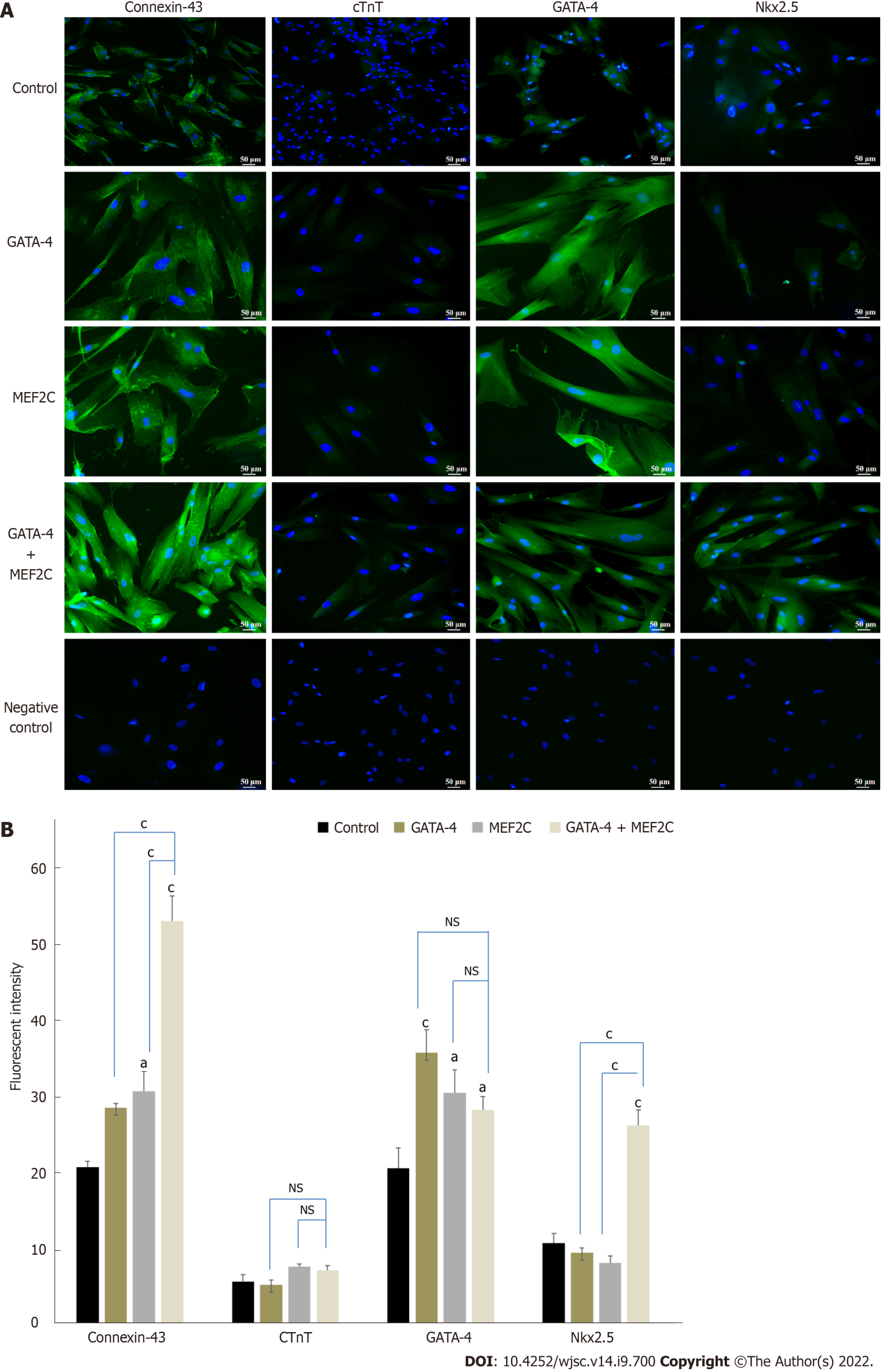
Cardiac differentiation of transfected hUC-MSCs was further confirmed using immunocytochemistry. hUC-MSCs transfected with GATA-4, MEF2C, or their combination exhibited positive expression of cardiac specific proteins, including connexin-43, cTnT, GATA-4, and NKX2.5 as compared to the untreated control at day 14 (Figure 5A). Moreover, the fluorescence intensity of hUC-MSCs transfected with GATA-4, MEF2C, and their combination was also calculated using Image J software. Statistical analysis showed significant up-regulation of GATA-4, connexin-43, and NKX2.5 in all three treatment groups as compared to the untreated control. However, the late cardiac marker cTnT was not up-regulated at day 14 (Figure 5B).
This study determined the effects of two cardiac transcription factors, GATA-4 and MEF2C, on the differentiation of hUC-MSCs towards cardiac lineage in vitro. GATA-4 is an important transcription factor that regulates the proliferation, survival, and fate commitment of many cell types[26]. Moreover, GATA-4 plays a vital role in the process of heart development[27]. The myocyte enhancer factor 2C (MEF2C) acts as a transcriptional regulator in cardiovascular growth[28]. It is demonstrated by various studies that MEF2C acts together with GATA factors to induce gene transcription in cardiomyocytes[27]. Based on their widely documented role in the structure and function of the heart, we hypothesized that GATA-4 and MEF2C overexpression may have the potential to induce the differentiation of hUC-MSCs into cardiac-like cells.
In this study, hUC-MSCs were isolated by the explant method[29]. The characterization studies of isolated cells were performed according to the standard criteria of the International Society for Stem Cell Research (ISSCR)[30]. The isolated cells showed a fibroblast-like morphology and positive expression of CD105, CD90, CD44, and vimentin, whereas they lack the expression of the hematopoietic marker CD45. MSCs specific markers CD73, CD90, and CD44 were also verified by flow cytometry analysis. Moreover, cord derived MSCs showed the differentiation potential of adipocytes and osteocytes. The results of our study confirmed that the cord derived cells possess the main characteristics of MSCs. Next, we analyzed the overexpression of GATA-4 and MEF2C mRNA in control and transfected hUC-MSCs. The expression of GATA-4 and MEF2C was maximum 24 h after transfection. Based on these gene expression data, we selected 24 h transfected hUC-MSCs for further experiments. hUC-MSCs were transfected with GATA-4 and MEF2C separately and in combination for 24 h, and then their cardiac differentiation potential at day 14 was analyzed. We observed elongated cells with extended cyto-plasmic processes in the transfected groups in comparison with the control group. The transfected cells had a morphology similar to cardiomyocytes and these results are also in line with earlier studies[31,32].
The cardiac differentiation of transfected cells at day 14 was analyzed via analysis of mRNA expression of early and late cardiac specific markers, such as GATA-4, MEF2C, NKX2.5, connexin-43, and MHC. Cardiac markers were initiated to express in the GATA-4 and MEF2C transfected cells, while their significant up-regulation was prominent in the combination group. The cardiac transcription factor GATA-4 facilitates the binding of various transcriptional factors and co-activators including GATA-6, NKX2.5, Srf, MEF2, dHAND, YY1, and NFAT[33]. MEF2C participates in the growth and maturation of myocardial cells with GATA-4[34]. It has been found that the overexpression of transcription factors induces cardiomyocyte differentiation in stem cells[35,36]. The combination of precardiac mesodermal transcription factors (Csx/NKX2.5 and GATA-4) has been reported to induce cardiac differentiation of 9-15c stem cells[37]. It has been found that GATA-4, MEF2C, and TBX5 generated cardiomyocyte like cells from mouse heart fibroblast[38]. The gene expression data revealed that GATA-4, MEF2C, and their combination were capable of directing stem cell fate into cardiomyocytes in vitro. Additionally, in the combination group, the significantly higher expression of cardiac specific genes indicates their synergistic effect on cardiac differentiation.
To complement gene expression data, we analyzed cardiac specific proteins in the GATA-4, MEF2C, their combination, and control groups. The combination group showed significant up-regulation of connexin-43, NKX2.5, and GATA-4 proteins at day 14 of transfection. The transcription factor NKX2.5 is expressed at the early and late stages of heart development[39]. NKX2.5 transcription is regulated by binding with GATA-4 and MEF2C[40,41]. The late stage marker troponin T regulates cardiac rhythm and maintains thin filaments in cardiac and skeletal muscles[42]. The heart rhythm regulation and coordinated contraction are controlled by a complex network of interconnected cardiomyocytes. Gap junction proteins help cardiomyocytes to communicate with their surrounding cells[43]. Connexin-43 is the major connexin protein involved in the propagation of electrical signals essential for the structural and functional maintenance of cardiac cells[44].
Collectively, the results of the current study demonstrate that hUC-MSCs overexpressing GATA-4 and/or MEF2C have the potential to generate cardiac-like cells. These genetically modified MSCs may be used as a new therapeutic approach for the regeneration of heart tissue.
It is concluded from this study that overexpression of the cardiac transcription factors in hUC-MSCs enhanced their differentiation potential into cardiac-like cells. The expression of early and late cardiac genes was significantly higher in all treatment groups. However, the combination group showed enhanced synergistic effect on cardiac differentiation. GATA-4 and MEF2C delivery seems to have the potential for the development of a cell-based treatment approach for cardiovascular diseases. However, further research is needed to explore the therapeutic effects of transfected hUC-MSCs in in vivo models.
Myocardial infarction is the leading cause of death worldwide. Following myocardial infarction, billions of cardiomyocytes die, resulting in a significant loss in cardiac function. Cell-based therapies have emerged as a new area to support heart regeneration. GATA binding protein 4 (GATA-4) and myocyte enhancer factor 2C (MEF2C) are considered important transcription factors in the formation of cardiac cells during the embryonic development.
Stem cell based therapies are considered a promising approach for repairing the damaged heart. However, the underlying mechanisms that control stem cell mediated cardiac cell fate decisions are still poorly understood. Since GATA-4 and MEF2C are the critical regulators of cardiac differentiation, use of these factors for transfection of mesenchymal stem cells (MSCs) may enhance the potential of these stem cells for cardiac differentiation.
Considering the critical role of cardiac transcription factors in maintaining the structure and function of the heart during the development process, their role in cardiac differentiation is highly anticipated. These genetically modified MSCs could be a promising future therapeutic option for heart diseases.
Human umbilical cord-MSCs (hUC-MSCs) were isolated and characterized morphologically and immunologically. The cord derived MSCs were identified by the presence of specific markers via immunocytochemistry and flow cytometry, and by their potential for osteogenic and adipogenic differentiation. hUC-MSCs were transfected with GATA-4, MEF2C, and their combination to direct cardiac differentiation. Cardiac differentiation was confirmed by semiquantitative real-time polymerase chain reaction and immunocytochemistry.
GATA-4, MEF2C, and their combination induced the differentiation of hUC-MSCs with significant expression of cardiac genes and proteins. Moreover, myotube like structure, which is the main characteristic of cardiomyocytes, was also observed in the transfected cells.
Overexpression of GATA-4 and MEF2C in hUC-MSCs induces the differentiation of stem cells into cardiac-like cells. This study is an attempt to provide deeper insights into the mechanism of trans-cription factors in the cardiac differentiation of stem cells.
The knowledge of the current study offers a promising therapeutic approach to improve treatment strategies for heart diseases. The genetically modified MSCs may serve as an ideal source for cardiac tissue repair and regeneration.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed AA, Egypt; Exbrayat JM, France S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Castellan RFP, Meloni M. Mechanisms and Therapeutic Targets of Cardiac Regeneration: Closing the Age Gap. Front Cardiovasc Med. 2018;5:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Hodgkinson CP, Kang MH, Dal-Pra S, Mirotsou M, Dzau VJ. MicroRNAs and Cardiac Regeneration. Circ Res. 2015;116:1700-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Bauer AJ, Martin KA. Coordinating Regulation of Gene Expression in Cardiovascular Disease: Interactions between Chromatin Modifiers and Transcription Factors. Front Cardiovasc Med. 2017;4:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Zacchigna S, Giacca M. Extra- and intracellular factors regulating cardiomyocyte proliferation in postnatal life. Cardiovasc Res. 2014;102:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Zhang D, Wang Y, Lu P, Wang P, Yuan X, Yan J, Cai C, Chang CP, Zheng D, Wu B, Zhou B. Author Correction: REST regulates the cell cycle for cardiac development and regeneration. Nat Commun. 2018;9:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Karakikes I, Senyei GD, Hansen J, Kong CW, Azeloglu EU, Stillitano F, Lieu DK, Wang J, Ren L, Hulot JS, Iyengar R, Li RA, Hajjar RJ. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med. 2014;3:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Broughton KM, Wang BJ, Firouzi F, Khalafalla F, Dimmeler S, Fernandez-Aviles F, Sussman MA. Mechanisms of Cardiac Repair and Regeneration. Circ Res. 2018;122:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 8. | Haneef K, Ali A, Khan I, Naeem N, Jamall S, Salim A. Role of interleukin-7 in fusion of rat bone marrow mesenchymal stem cells with cardiomyocytes in vitro and improvement of cardiac function in vivo. Cardiovasc Ther. 2018;36:e12479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 10. | Lazaros G, Oikonomou E, Tousoulis D. Established and novel treatment options in acute myocarditis, with or without heart failure. Expert Rev Cardiovasc Ther. 2017;15:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Saheli M, Pirhajati Mahabadi V, Mesbah-Namin SA, Seifalian A, Bagheri-Hosseinabadi Z. DNA methyltransferase inhibitor 5-azacytidine in high dose promotes ultrastructural maturation of cardiomyocyte. Stem Cell Investig. 2020;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Gnecchi M. Cell Therapy for Heart Regeneration: Learning from the Past to Build a Brighter Future. Stem Cells Transl Med. 2018;7:702-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KA, Guarita-Souza LC, Foldes G. Stem cell death and survival in heart regeneration and repair. Apoptosis. 2016;21:252-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Hu M, Guo G, Huang Q, Cheng C, Xu R, Li A, Liu N, Liu S. The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: the role of injured cardiomyocytes-derived exosomes. Cell Death Dis. 2018;9:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Dixit P, Katare R. Challenges in identifying the best source of stem cells for cardiac regeneration therapy. Stem Cell Res Ther. 2015;6:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Shen H, Wang Y, Zhang Z, Yang J, Hu S, Shen Z. Mesenchymal Stem Cells for Cardiac Regenerative Therapy: Optimization of Cell Differentiation Strategy. Stem Cells Int. 2015;2015:524756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:762695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Mao Q, Lin C, Gao J, Liang X, Gao W, Shen L, Kang L, Xu B. Mesenchymal stem cells overexpressing integrin-linked kinase attenuate left ventricular remodeling and improve cardiac function after myocardial infarction. Mol Cell Biochem. 2014;397:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | Al-Maqtari T, Hong KU, Vajravelu BN, Moktar A, Cao P, Moore JB 4th, Bolli R. Transcription factor-induced activation of cardiac gene expression in human c-kit+ cardiac progenitor cells. PLoS One. 2017;12:e0174242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Laemmle LL, Cohen JB, Glorioso JC. Constitutive Expression of GATA4 Dramatically Increases the Cardiogenic Potential of D3 Mouse Embryonic Stem Cells. Open Biotechnol J. 2016;10:248-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Grunert M, Dorn C and Rickert-Sperling S. Congenital Heart Diseases: The Broken Heart. In: Rickert-Sperling, S., Kelly, R., Driscoll, D. (eds), Cardiac transcription factors and regulatory networks. ViennaL Springer, 2016: 139-152. [DOI] [Full Text] |
| 23. | Schlesinger J, Schueler M, Grunert M, Fischer JJ, Zhang Q, Krueger T, Lange M, Tönjes M, Dunkel I, Sperling SR. The cardiac transcription network modulated by Gata4, Mef2a, Nkx2.5, Srf, histone modifications, and microRNAs. PLoS Genet. 2011;7:e1001313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 25. | Dixon JE, Dick E, Rajamohan D, Shakesheff KM, Denning C. Directed differentiation of human embryonic stem cells to interrogate the cardiac gene regulatory network. Mol Ther. 2011;19:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Hao C, Lu Z, Zhao Y, Chen Z, Shen C, Ma G, Chen L. Overexpression of GATA4 enhances the antiapoptotic effect of exosomes secreted from cardiac colony-forming unit fibroblasts via miRNA221-mediated targeting of the PTEN/PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020;11:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Vanpoucke G, Goossens S, De Craene B, Gilbert B, van Roy F, Berx G. GATA-4 and MEF2C transcription factors control the tissue-specific expression of the alphaT-catenin gene CTNNA3. Nucleic Acids Res. 2004;32:4155-4165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Materna SC, Sinha T, Barnes RM, Lammerts van Bueren K, Black BL. Cardiovascular development and survival require Mef2c function in the myocardial but not the endothelial lineage. Dev Biol. 2019;445:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Beeravolu N, Brougham J, Khan I, McKee C, Perez-Cruet M, Chaudhry GR. Human umbilical cord derivatives regenerate intervertebral disc. J Tissue Eng Regen Med. 2018;12:e579-e591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 31. | Ali SR, Ahmad W, Naeem N, Salim A, Khan I. Small molecule 2'-deoxycytidine differentiates human umbilical cord-derived MSCs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. Mol Cell Biochem. 2020;470:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Naeem N, Haneef K, Kabir N, Iqbal H, Jamall S, Salim A. DNA methylation inhibitors, 5-azacytidine and zebularine potentiate the transdifferentiation of rat bone marrow mesenchymal stem cells into cardiomyocytes. Cardiovasc Ther. 2013;31:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Peterkin T, Gibson A, Loose M, Patient R. The roles of GATA-4,-5 and-6 in vertebrate heart development. Semin Cell Dev Biol. 2005;16:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Kolodziejczyk SM, Wang L, Balazsi K, DeRepentigny Y, Kothary R, Megeney LA. MEF2 is upregulated during cardiac hypertrophy and is required for normal post-natal growth of the myocardium. Curr Biol. 1999;9:1203-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol. 2010;299:H1772-H1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Armiñán A, Gandía C, García-Verdugo JM, Lledó E, Mullor JL, Montero JA, Sepúlveda P. Cardiac transcription factors driven lineage-specification of adult stem cells. J Cardiovasc Transl Res. 2010;3:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Yamada Y, Sakurada K, Takeda Y, Gojo S, Umezawa A. Single-cell-derived mesenchymal stem cells overexpressing Csx/Nkx2.5 and GATA4 undergo the stochastic cardiomyogenic fate and behave like transient amplifying cells. Exp Cell Res. 2007;313:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 39. | Serpooshan V, Liu YH, Buikema JW, Galdos FX, Chirikian O, Paige S, Venkatraman S, Kumar A, Rawnsley DR, Huang X, Pijnappels DA, Wu SM. Nkx2.5+ Cardiomyoblasts Contribute to Cardiomyogenesis in the Neonatal Heart. Sci Rep. 2017;7:12590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Kinnunen S, Välimäki M, Tölli M, Wohlfahrt G, Darwich R, Komati H, Nemer M, Ruskoaho H. Nuclear Receptor-Like Structure and Interaction of Congenital Heart Disease-Associated Factors GATA4 and NKX2-5. PLoS One. 2015;10:e0144145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Palmer S, Groves N, Schindeler A, Yeoh T, Biben C, Wang CC, Sparrow DB, Barnett L, Jenkins NA, Copeland NG, Koentgen F, Mohun T, Harvey RP. The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an insulin-like growth factor 1-dependent manner. J Cell Biol. 2001;153:985-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Clause KC, Tchao J, Powell MC, Liu LJ, Huard J, Keller BB, Tobita K. Developing cardiac and skeletal muscle share fast-skeletal myosin heavy chain and cardiac troponin-I expression. PLoS One. 2012;7:e40725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Stoppel WL, Kaplan DL, Black LD 3rd. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliv Rev. 2016;96:135-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 44. | Scuderi GJ, Butcher J. Naturally Engineered Maturation of Cardiomyocytes. Front Cell Dev Biol. 2017;5:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |