Published online Aug 26, 2022. doi: 10.4252/wjsc.v14.i8.577
Peer-review started: March 18, 2022
First decision: May 11, 2022
Revised: May 24, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: August 26, 2022
Processing time: 160 Days and 20.3 Hours
The latest achievements in the field of pancreas transplantation and stem cell therapy require an effort by the scientific community to clarify the ethical implications of pioneering treatments, often characterized by high complexity from a surgical point of view, due to transplantation of multiple organs at the same time or at different times, and from an immunological point of view for stem cell therapy. The fundamental value in the field of organ transplants is, of course, a solidarity principle, namely that of protecting the health and life of people for whom transplantation is a condition of functional recovery, or even of survival. The nature of this value is that of a concept to which the legal discipline of transplants entrusts its own ethical dignity and for which it has ensured a constitutional recognition in different systems. The general principle of respect for human life, both of the donor and of the recipient, evokes the need not to put oneself and one’s neighbor in dangerous conditions. The present ethical reflection aims to find a balance between the latest therapeutic advances and several concepts including the idea of the person, the respect due to the dead, the voluntary nature of the donation and the consent to the same, the gratuitousness of the donation, the scientific progress and the development of surgical techniques, and the policies of health promotion.
Core Tip: Recent research in pancreatic transplantation is involving many branches of medicine. The objective is represented by the achievement of euglycemia and the anatomo-functional restoration of the β-cells, avoiding exogenous insulin administration. Transplantation of the pancreas or pancreatic islets (eventually associated with renal transplantation) and stem cell therapy constitute the new frontiers in this field. However, such rapid scientific growth must be followed by an extensive discussion on the ethical implications to make experimental and clinical practices adequate and sustainable.
- Citation: Padovano M, Scopetti M, Manetti F, Morena D, Radaelli D, D’Errico S, Di Fazio N, Frati P, Fineschi V. Pancreatic transplant surgery and stem cell therapy: Finding the balance between therapeutic advances and ethical principles. World J Stem Cells 2022; 14(8): 577-586
- URL: https://www.wjgnet.com/1948-0210/full/v14/i8/577.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i8.577
Currently, the management of chronic diseases represents an issue of growing interest in relation to the incidence, the increase in life expectancy, and the loads deriving from prolonged care needs. An important problem related to chronic conditions is represented by the complications that inevitably and, sometimes even in the presence of adequate therapy, worsen the patient’s health. The complications of chronic diseases also affect health care by increasing the number of hospitalizations, the length of hospital stays, and health management costs. Therefore, the possibility of avoiding these implications constitutes a continuous challenge for healthcare professionals, spurring scientists to continuously identify new therapies aimed at overcoming the critical condition and consequent prevention of complications.
Chronic pancreatic diseases and specifically diabetes mellitus denote a burden for the health professions, as the worldwide incidence is continuously increasing; insulin therapy achieves good glycemic control but does not allow the restoration of damaged β-cells or prevent any complications related to glycemic changes. Over the years, countless approaches have been tried to definitively restore pancreatic function; first pancreas transplantation and later stem cell transplantation represent cutting-edge treatments and are the subject of great interest in this field of medicine.
Advances in pancreatic transplantology are currently at the center of heated debates in the medical, ethical, and legal fields. Therefore, similar treatments require an effort to clarify the ethical implications of pioneering procedures, often characterized by high complexity both from a surgical point of view, due to transplantation of multiple organs at the same time or at different times, and from an immunological point of view for stem cell therapy.
The present paper aims to outline an overview of the current perspectives of pancreatic replacement therapy with the aim of highlighting the ethical issues relating to the different procedures and providing useful information to professionals.
The formulation of the ethical considerations was based on a preliminary literature search on PubMed and Reference Citation Analysis (https://www.referencecitationanalysis.com/) databases. Only contributions in English have been included; no time limits were applied. The review was conducted using MeSH terms, Boolean operators, and free text terms to broaden the search. The study design included case series, animal studies, retrospective and prospective studies, as well as reviews, clinical trials, and meta-analysis. No unpublished or gray literature was searched.
Diabetes mellitus is an endemic pathology whose treatment poses several challenges to health professionals and determines a conspicuous healthcare expenditure globally. Worldwide, as documented by the International Diabetes Federation, the spreading of the disease is constantly increasing[1-3], with a prevalence for the years 2021 of about 537 million people. Type 1 diabetes mellitus (T1DM) affects about 10% of the general population, with greater prevalence among children and young adults; even in this age range, the incidence is continuously growing[4], with an overall annual increase of approximately 3%. In 2021, it was found that more than 3 out of 4 adults with diabetes live in low- and middle-income countries and that around 240 million adults have affected undiagnosed diabetes; concerning mortality data, it appears that in 2021 deaths related to diabetes and its complications were 6.7 million (1 every 5 s)[5].
T1DM is among the most common autoimmune disorders and is characterized by multifactoriality, with imposing importance of genetic predisposition; in detail, particular human leukocyte antigen polymorphisms are frequently predisposed[6]. From a pathophysiological point of view, the etiology must be related to the specific immune-based destruction of insulin-producing β-cells, sparing glucagon-producing α-cells, and somatostatin-producing δ-cells[7]. Specifically, this destruction is caused by an incorrect recognition as autoantigens of some cellular components (i.e. insulin, islet antigen 2, zinc transporter 8, and glutamic acid decarboxylase 65[8,9]). According to some authors, recognition as “foreign” proteins can occur because of posttranslational modifications, represented for example by citrullination, deamination, and disulfide bridge formation[10].
The peculiar clinical heterogeneity makes diagnosis difficult and often delayed, being frequently placed at the onset of symptoms[11]. Given the diagnostic delay and systemic involvement, subjects with T1DM have a tripled rate of hospitalization in relation to long-term complications[12], a deterioration in the quality of life, as well as a decrease in life span from 1 year to 13 years.
Although it is not a curative treatment, not regenerating the islets of Langerhans, the administration of exogenous insulin through daily injections or computerized pumps, represents the most common treatment for obtaining a condition of euglycemia. Actually, the administration of exogenous insulin and a healthy lifestyle reduce blood glucose, but despite this, vascular complications resulting in irreversible organic damage are inevitable in most patients[13,14]. Sometimes, even in the case of correct administration, episodes of hyperglycemia can occur and if persistent can lead to irreversible complications systemically. Occasionally, even in patients with good glycemic control, injections carried out repeatedly and erroneously in the same site can determine a lipodystrophic condition, at the basis of an abnormal release of the inoculated insulin with consequent episodes of hypoglycemia. The latter complication is life-threatening and is associated with a mortality rate of 3%-6%[15].
Therefore, the development of innovative treatments is not only a research challenge but a necessity for the entire population[16-18]. This indispensability also derives from the widespread involvement of young patients who in the absence of a curative therapeutic strategy must face T1DM and all the associated complications throughout life.
Whole pancreas and islet allotransplantation as well as stem cell therapy are currently the only treatments for T1DM capable of re-establishing a normoglycemic state without an exogenous insulin supply. At the same time, these treatments are effective in reducing or eliminating dietary restrictions and hyperglycemia-related organ damage.
Whole pancreas and islet allotransplantation have undergone in recent years a remarkable development related to the refinement of the selection criteria of the donor and the recipient, to the evolution of surgical techniques, and to the use of new immunosuppressive drugs that have led to a remarkable improvement of the organ and patient survival[19,20].
Synthetically, these treatments can be performed in patients with T1DM with good renal function, chronic kidney disease, and previous kidney transplant. As concern of the different therapeutic possibilities available, the choice of the ideal treatment must be weighted according to the state of the underlying diabetic disease, the presence of diabetes complications, age, general conditions, and surgical risk[21,22].
The most relevant clinical element to indicate pancreatic transplant alone is the presence of unstable diabetes (with frequent hyperglycemia, recurrent and unpredictable episodes of ketoacidosis, asymptomatic hypoglycemia with life-threatening despite correct insulin therapy) that is associated with at least two rapidly progressing complications (proliferative retinopathy, peripheral and/or autonomic neuropathy, and vasculopathy)[23]. The presence of adequate renal function must be ascertained in pancreatic transplant alone candidate patients since the subsequent immunosuppressive therapy, including potentially nephrotoxic drugs, may lead to a reduction in the functional reserve.
Simultaneous pancreas and kidney transplantation is indicated in patients with T1DM on dialysis or pre-dialysis[24]. An increasing number of scientific evidence reveal substantial advantages in the execution of the transplant before the start of dialysis. In fact, such a choice avoids complications related to uremia, reduces the costs of dialysis, and intervenes earlier on other chronic complications of diabetes[25,26]. Surgery is also affected by lower postoperative morbidity and mortality.
Furthermore, pancreas after kidney transplantation is proposed to patients with T1DM who have previously been transplanted only with kidney from cadaver or living donor, especially if a difficult metabolic control and rapid evolution of the degenerative complications of diabetes coexist. Among the main advantages, since an immunosuppressive therapeutic regimen is already in place, there is a reduction of risks with the persistence of the operative risk only[27]. Moreover, the latter is significantly lower than the risks related to the further development of secondary complications of diabetes, including the recurrence of diabetic nephropathy in the transplanted kidney[28].
Finally, allogenic pancreatic islet transplantation has the same indications as whole pancreas transplantation (sometimes associated with renal transplant) but is performed through infusion into the portal vein[29,30].
Currently, whole pancreas and islet allotransplantations still have important limitations related to donor scarcity, post-transplant graft loss, and immunosuppressive therapy[31,32]. For similar reasons, further research is underway to expand accessibility to pancreatic replacement therapy.
Stem cell transplantation represents a cutting-edge treatment and falls within the broad context of regenerative medicine that allowing for the definitive recovery of damaged organs and tissues is experiencing a period of maximum expansion; this branch is involved in many areas of medicine finding application also in pancreatic diseases. As previously stated, regenerative medicine is heavily investigated in the treatment of T1DM, but some studies highlight its applicability also in type 2 diabetes mellitus[33]. To date, two main approaches have been tested to ensure the restoration of insulin-producing β-cells. The most common approach consists in the implantation in the recipient of pancreatic progenitor cells, which in vivo differentiate into β-cells and over months reach a functional mass composed of progenitor and other pancreatic cells[34]. The other approach involves the in vitro generation of β-cells and their subsequent implantation to reduce the volume of the transplant and restore pancreatic function in a short time[35-37].
The selection of the cell type for the implementation of the protocols provides for different possibilities. The stem cells can be classified in embryonic and non-embryonic “somatic” stem cells. Both groups share peculiar properties as the ability for unlimited or prolonged self-renewal and the capacity to differentiate into more specialized cell types[38,39].
The embryonic stem cells, obtained from the internal cell mass of the blastocyst[40,41], represent the gold standard concerning pluripotency; in fact, through different procedures, these cells can self-renew or differentiate into multiple cell lines, such as hematopoietic progenitors and endothelial cells, as well as cardiac, neuronal, and pancreatic tissues[42-46].
Non-embryonic stem cells include mesenchymal stem cells (MSCs), which are endowed with multipotency, and are therefore able to self-renew and differentiate into different cell lines[47]. The sources from which MSCs are obtained are represented by the bone marrow and adipose tissue[48]. Such cells find wide application in the therapy of many degenerative and auto-immune diseases[49,50]. Therefore, although the exact mechanism is currently not delineated, MSCs are used in the treatment of T1DM due to an immunomodulatory and reparative action on damaged tissues[51]; in detail, MSCs allow euglycemia to be reached restoring the β-cells and reducing the autoimmune response through an immunomodulatory function[52-54]. Specifically, the ability to regulate the immune response constitutes the main mechanism with an antidiabetic effect since, by ensuring the survival of the β-cells, it contributes to the achievement of euglycemia.
From a molecular point of view, they carry out an inhibitory action against T, B, and natural killer cells, while they perform an activating action against regulatory T cells and modulate the function of dendritic cells[55,56]; furthermore, these cells create a local immunosuppressive environment. In addition to the above property, MSCs can differentiate into lineage-specific progeny; an in vitro study has demonstrated the greater ability of adipose-derived MSCs to differentiate into β-cells[57]. As part of this regenerative capacity, some authors have described the secretion of cytokines and growth factors capable of stimulating β-cells[58]. MSCs exhibit low immunogenicity as they do not express costimulating molecules such as human leukocyte antigen-DR as well as CD80, CD86, CD40, and CD40L[59-62]. Therefore, in consideration of the immunomodulatory capacities, MSCs avoids allogenic rejection in humans and animal models[63], and they can be transplanted without the need for the recipient to take immunosuppressive therapy[64].
To re-establish pluripotency in mature stromal cells, some scientists developed cutting-edge protocols that resulted in the spread of inducible pluripotential stem cells[65,66]. Such a method introduces a new treatment for T1DM to carry out the autologous transplant[67-69].
The ethics of transplant surgery is at the center of a bundle of anthropological, ethical, juridical, medical, and political questions. The application of transplant surgery requires reflections on the lawfulness and on the limits to the practice even more so in complex transplants such as the pancreatic one. For these reasons, the ethical assessment of pancreatic transplantation cannot disregard the evaluation of the general principles underlying the organ replacement therapy.
The fundamental value in the field of organ transplants is, of course, a solidarity principle, namely that of protecting the health and life of people for whom transplantation is a condition of functional recovery or even of survival[70]. The nature of this value is that of a concept to which the legal discipline of transplants entrusts its own ethical dignity and for which it has ensured a constitutional recognition in different systems. The general principle of respect for human life, both the donor and the recipient, evokes the need not to put oneself and one’s neighbor in dangerous conditions. In particular, if the transplant from a living donor involves problems of incompatibility in the recipient or a high risk for the life of the donor, the availability of one’s body for the good of another must be subordinated to the preservation of the integrity of the person involved. Nevertheless, the pursuit of the solidarity purpose requires, in addition to the attention for the implications concerning the living donor, the acceptance of some basic principles related to the corpse donation such as respect for the will manifested before death, the dignity of the person, and the values of relatives of the deceased.
The affirmation of the principle of solidarity and the positive overcoming of the principles of protection of life and integrity of the body also implies the gratuitousness of donation. In fact, especially in the case of the living donor, organ donation should be inspired by the firm will to save a life and not by the mere possibility of receiving a reward. With regard to the donation from corpses, gratuitousness is instead guaranteed, in addition to the principle of charity, by the concept of respect for the deceased person. The gratuity is not resolved in the pure and simple lack or renunciation of a pecuniary compensation, but it is characterized by the intent of solidarity and the achievement of a predetermined result not left to the free determination of the donor. Moreover, gratuitousness constitutes a guarantee of the freedom of consent, removed from the constraints of economic necessity[71].
Finally, the principle of justice constitutes an important ethical assumption in relation to the context of deficiency in which transplant surgery is expressed. Given that the supply of organs is inferior to the demand, it is crucial that the identification of the recipients is based, in addition to the medical considerations, on strict criteria that prevent the greater economic capacity from becoming the most important, if not the unique, condition of accessibility to the organs available[72]. In the allocation of organs, impartiality cannot be absolute since each choice is based on values and is therefore subject to reference criteria. Any decision on the matter involves the “cutting” of something and the “waste” of something else, precisely because each allocative choice is based on the preference of one solution over another. However, the inevitable partiality must not result in preconceived discrimination and the selection criteria must be conceived considering the analysis of the situations, the possibilities of realization, and the management complexity[73].
The development of stem cell therapies requires continuous discussions and reflections in the medical, bioethical, religious, and judicial fields. Precisely, a broad debate that includes all aspects of the new therapeutic frontier from bench to bedside is needed.
As far as the experimental aspect is concerned, the possible use of stem cells in the treatment of T1DM is investigated in about 70 studies (www.clinicaltrials.gov); of these, 54 are in phases I and II of experimentation. In consideration of the pioneering nature of the research, the pursuit of methodological rigor aimed at obtaining reliable and reproducible results is fundamental[74]. Nonetheless, transparency in the sharing of results is desirable on the part of researchers[75]. In fact, scientific disclosure in such sensitive areas for health and public opinion must presuppose a clear discussion of the results, not generating false expectations and detailed in the aspects relating to the complications of the treatment. With reference to the last aspect, scientific and commercial competition can generate pressure regarding the declaration of results concerning problems such as tumorigenicity, neoangiogenic potential, and unwanted differentiation of stem cells[76].
The validation of stem cell-based therapies in the treatment of T1DM undoubtedly limits the problem of donor scarcity without eliminating the issues on access to care. In fact, the limited availability of this therapeutic possibility and the high costs currently pose serious sustainability problems. The problem is strongly accentuated by the epidemiological peculiarities of diabetes mellitus that presents a greater diffusion of decompensated and refractory to therapy forms among the socioeconomically disadvantaged groups of the population. Therefore, both in experimental and clinical practice, the inspiration to the principles of equity and justice is of great importance to guarantee equal therapeutic possibilities to all patients. Despite some still undefined regulatory and technological aspects, a prospect of certain usefulness in terms of improving access to care is represented by biobanking; the deve
Given the greater incidence of T1DM in pediatric and juvenile age, the prognostic aspects of the disease as well as the complexity of the therapies, further ethical aspects worthy of further study concern self-determination, information, and shared planning of care pathways. To jointly pursue the lengthening of life expectancy and the improvement of the quality of the same, it is of fundamental importance in such patients to carry out a global assessment of frailty, foreseeable risks, expected benefits, and life prospects[81]. In the context of stem cell-based therapies, it is all the more important to provide adequate responses to the patient’s requests since the quality of care necessarily depends on the conscious choice of a treatment capable of improving life and recovery expectancies[82,83]. Then, in a specialized therapeutic field such as regenerative therapy, it is possible to respect the principles of self-determination, dignity, and identity of the patient by promoting the global acceptance of the disease, clearly stating the therapeutic objectives, and sharing the concept of proportionality of the treatments.
Pancreatic replacement therapy requires a significant scientific, regulatory, and ethical commitment. The enhancement of the efforts implemented by the different stakeholders presupposes an attitude aimed in general at avoiding injury to the person and at providing support to the rapid evolution of knowledge.
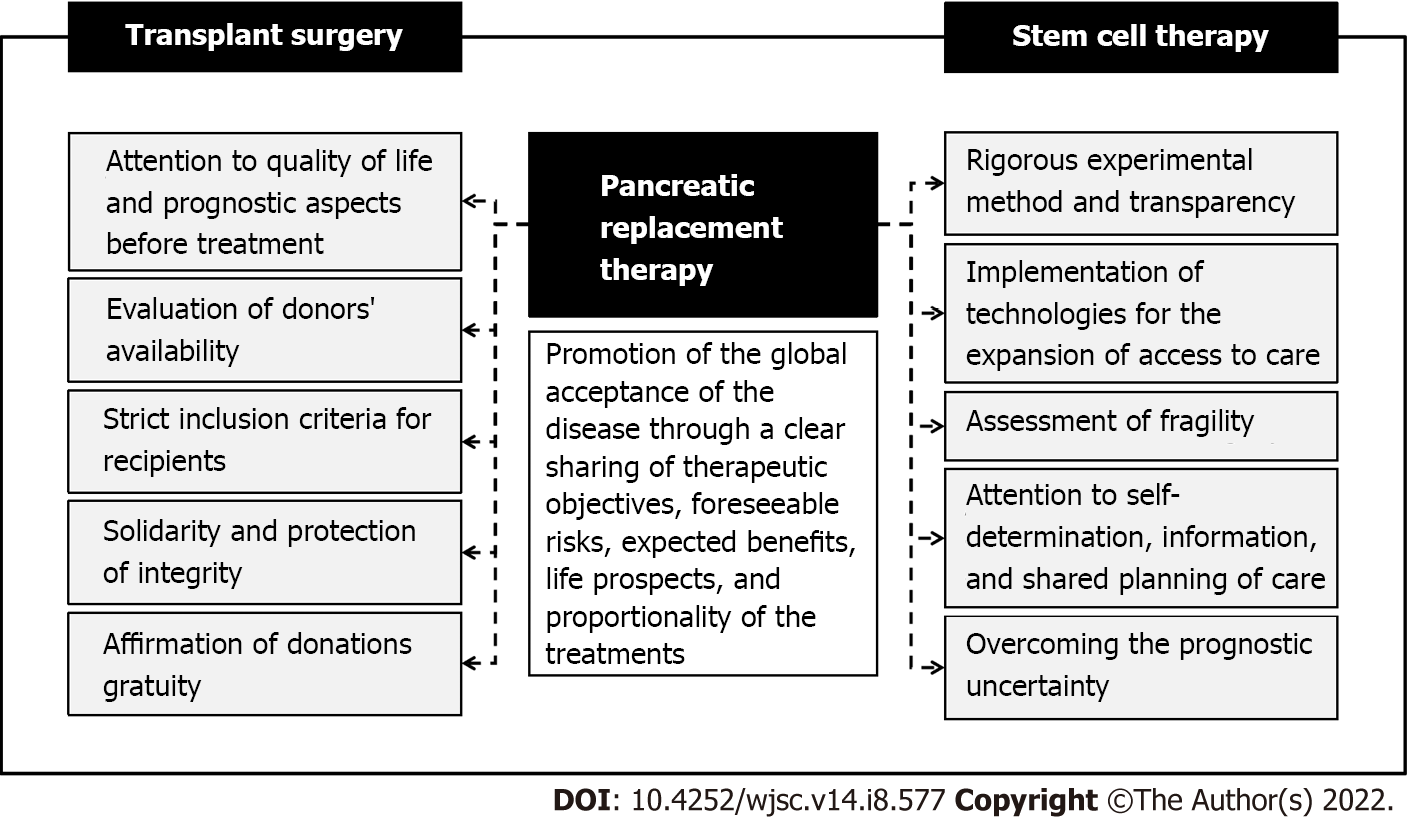
In a nutshell, having to express a position that can be useful to those involved in the experimentation and clinical use of pancreatic replacement therapies, a good practice should presuppose (Figure 1): (1) Adoption of a rigorous experimental method aimed at transparency to clearly state results and avoid the onset of false expectations; (2) Objective sharing of results with avoidance of pressures deriving from scientific and instrumental competition; (3) Comprehensive assessment including quality of life before treatment as well as prognostic aspects in terms of post-treatment morbidity and mortality; (4) Calibration of the therapeutic choice based on the availability of donors, the possibility of post-transplant graft loss, and the feasibility of immunosuppressive therapy; (5) Foundation of therapeutic choices on the protection of health and life, both of the donor and the recipient, in compliance with the principles of solidarity as well as respect for the dignity and integrity of the person; (6) Guarantee of gratuity to avoid donations for profit and ensure the freedom of donation; (7) Codification of strict criteria for inclusion in pancreatic replacement therapy paths through the evaluation of concrete cases, feasibility, and management complexity; (8) Avoidance of socioeconomic discrimination in the evaluation of therapeutic choices; (9) Guarantee of equal therapeutic possibilities to all patients inspiring experimental and clinical practice on the principles of equity and justice as well as having regard for the disadvantaged groups of the population; (10) Implementation of technologies for the spreading of innovative therapies and the expansion of access to care (e.g., biobanking); (11) Attention to aspects related to fragility, self-determination, information, and shared planning of care pathways, especially in the pediatric field; and (12) Promotion of the global acceptance of the disease through a clear sharing of therapeutic objectives, foreseeable risks, expected benefits, life prospects, and proportionality of the treatments.
The rapid advancement of knowledge and the implementation of therapeutic possibilities in the field of pancreatic replacement therapy imply different concerns relating to experimental and clinical practice. The heterogeneity of therapeutic strategies determines a wide range of ethical aspects differently inherent to transplant surgery and stem cell-based therapies. At present, it is essential to proceed with rigor to support the encouraging results obtained from scientific research from an ethical and legislative point of view. Therefore, a joint commitment of the different stakeholders is desirable so that in the near future it will be possible to proceed on a large scale to a definitive treatment against an extremely insidious disease, such as diabetes, in terms of prevalence and prognosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ilkafah I, Surabaya; Sipos F, Hungary S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Scully T. Diabetes in numbers. Nature. 2012;485:S2-S3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | El-Badawy A, El-Badri N. Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus: A Meta-Analysis. PLoS One. 2016;11:e0151938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Cañibano-Hernández A, Sáenz Del Burgo L, Espona-Noguera A, Ciriza J, Pedraz JL. Current advanced therapy cell-based medicinal products for type-1-diabetes treatment. Int J Pharm. 2018;543:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1186] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 5. | International Diabetes Federation. IDF diabetes atlas 2021. Tenth edition 2021. [cited 16 March 2022]. Available from: https://diabetesatlas.org/atlas/tenth-edition/. |
| 6. | Noble JA. Immunogenetics of type 1 diabetes: A comprehensive review. J Autoimmun. 2015;64:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25:80-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Arvan P, Pietropaolo M, Ostrov D, Rhodes CJ. Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harb Perspect Med. 2012;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Han S, Donelan W, Wang H, Reeves W, Yang LJ. Novel autoantigens in type 1 diabetes. Am J Transl Res. 2013;5:379-392. [PubMed] |
| 10. | van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A, Gomez-Touriño I, Arif S, Peakman M, Drijfhout JW, Roep BO. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Aghazadeh Y, Nostro MC. Cell Therapy for Type 1 Diabetes: Current and Future Strategies. Curr Diab Rep. 2017;17:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 297] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Wu D, Wan J, Huang Y, Guo Y, Xu T, Zhu M, Fan X, Zhu S, Ling C, Li X, Lu J, Zhu H, Zhou P, Lu Y, Wang Z. 3D Culture of MIN-6 Cells on Decellularized Pancreatic Scaffold: In Vitro and In Vivo Study. Biomed Res Int. 2015;2015:432645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Gaetani R, Aude S, DeMaddalena LL, Strassle H, Dzieciatkowska M, Wortham M, Bender RHF, Nguyen-Ngoc KV, Schmid-Schöenbein GW, George SC, Hughes CCW, Sander M, Hansen KC, Christman KL. Evaluation of Different Decellularization Protocols on the Generation of Pancreas-Derived Hydrogels. Tissue Eng Part C Methods. 2018;24:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Aye MM, Atkin SL. Patient safety and minimizing risk with insulin administration - role of insulin degludec. Drug Healthc Patient Saf. 2014;6:55-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia. 2016;59:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, Leese G, Leslie P, McCrimmon RJ, Metcalfe W, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, Sattar NA, Traynor JP, Colhoun HM; Scottish Diabetes Research Network epidemiology group; Scottish Renal Registry. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 18. | Petrie D, Lung TW, Rawshani A, Palmer AJ, Svensson AM, Eliasson B, Clarke P. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia. 2016;59:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Dholakia S, Oskrochi Y, Easton G, Papalois V. Advances in pancreas transplantation. J R Soc Med. 2016;109:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Boggi U, Amorese G, Marchetti P. Surgical techniques for pancreas transplantation. Curr Opin Organ Transplant. 2010;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Sánchez-Velázquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N, Javed AA, Inoue Y, Beghdadi N, Kalisvaart M, Vigia E, Walsh CD, Lovasik B, Busquets J, Scandavini C, Robin F, Yoshitomi H, Mackay TM, Busch OR, Hartog H, Heinrich S, Gleisner A, Perinel J, Passeri M, Lluis N, Raptis DA, Tschuor C, Oberkofler CE, DeOliveira ML, Petrowsky H, Martinie J, Asbun H, Adham M, Schulick R, Lang H, Koerkamp BG, Besselink MG, Han HS, Miyazaki M, Ferrone CR, Fernández-Del Castillo C, Lillemoe KD, Sulpice L, Boudjema K, Del Chiaro M, Fabregat J, Kooby DA, Allen P, Lavu H, Yeo CJ, Barroso E, Roberts K, Muiesan P, Sauvanet A, Saiura A, Wolfgang CL, Cameron JL, Boggi U, Yoon DS, Bassi C, Puhan MA, Clavien PA. Benchmarks in Pancreatic Surgery: A Novel Tool for Unbiased Outcome Comparisons. Ann Surg. 2019;270:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 22. | Samoylova ML, Borle D, Ravindra KV. Pancreas Transplantation: Indications, Techniques, and Outcomes. Surg Clin North Am. 2019;99:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Shin S, Jung CH, Choi JY, Kwon HW, Jung JH, Kim YH, Han DJ. Long-term effects of pancreas transplant alone on nephropathy in type 1 diabetic patients with optimal renal function. PLoS One. 2018;13:e0191421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ziaja J, Chudek J, Kolonko A, Kamińska D, Kujawa-Szewieczek A, Kuriata-Kordek M, Król R, Klinger M, Wiecek A, Patrzałek D, Cierpka L. Does simultaneously transplanted pancreas improve long-term outcome of kidney transplantation in type 1 diabetic recipients? Transplant Proc. 2011;43:3097-3101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Rajkumar T, Mazid S, Vucak-Dzumhur M, Sykes TM, Elder GJ. Health-related quality of life following kidney and simultaneous pancreas kidney transplantation. Nephrology (Carlton). 2019;24:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Marcacuzco A, Jiménez-Romero C, Manrique A, Calvo J, Cambra F, Caso Ó, García-Sesma Á, Nutu A, Justo I. Outcome of patients with hemodialysis or peritoneal dialysis undergoing simultaneous pancreas-kidney transplantation. Comparative study. Clin Transplant. 2018;32:e13268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Fridell JA, Niederhaus S, Curry M, Urban R, Fox A, Odorico J. The survival advantage of pancreas after kidney transplant. Am J Transplant. 2019;19:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Yin H, Arpali E, Leverson GE, Sollinger HW, Kaufman DB, Odorico JS. Ipsilateral vs contralateral placement of the pancreas allograft in pancreas after kidney transplant recipients. Clin Transplant. 2018;32:e13337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Rickels MR, Robertson RP. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr Rev. 2019;40:631-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 30. | Johnson PR, Jones KE. Pancreatic islet transplantation. Semin Pediatr Surg. 2012;21:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Mensink JW, de Boer JD, Kopp WH, Braat AE. How Far Can We Expand Donor Age Criteria for Pancreas Transplantation? Transplantation. 2018;102:e494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Chan KM, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Lee WC. Difficult Situation of Pancreas Transplantation in the Setting of Scarce Organ Donation. Transplant Proc. 2017;49:2324-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Chen W, Feng B, Cao H. The Clinical Efficacy and Safety of Stem Cell Therapy for Diabetes Mellitus: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:141-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D'Amour KA. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl Med. 2015;4:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 35. | Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V, Blelloch R, Szot GL, Arvan P, Hebrok M. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 462] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 36. | Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1162] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 37. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1526] [Article Influence: 152.6] [Reference Citation Analysis (1)] |
| 38. | Mosca JD, Hendricks JK, Buyaner D, Davis-Sproul J, Chuang LC, Majumdar MK, Chopra R, Barry F, Murphy M, Thiede MA, Junker U, Rigg RJ, Forestell SP, Böhnlein E, Storb R, Sandmaier BM. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res. 2000;S71-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1173] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 40. | Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 460] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 42. | Huang Q, Huang Y, Liu J. Mesenchymal Stem Cells: An Excellent Candidate for the Treatment of Diabetes Mellitus. Int J Endocrinol. 2021;2021:9938658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 756] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 44. | Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 577] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 45. | Kaufman DS, Thomson JA. Human ES cells--haematopoiesis and transplantation strategies. J Anat. 2002;200:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391-4396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 605] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 47. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] |
| 48. | Ghoneim MA, Refaie AF, Elbassiouny BL, Gabr MM, Zakaria MM. From Mesenchymal Stromal/Stem Cells to Insulin-Producing Cells: Progress and Challenges. Stem Cell Rev Rep. 2020;16:1156-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn's Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018;12:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 50. | Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 51. | Cui Y, Liu S, Zhang X, Ding X, Duan X, Zhu Z, Zhang J, Liang H, Wang D, Zhang G, Yu Z, Yang J, Sun T. Metabolomic Analysis of the Effects of Adipose-Derived Mesenchymal Stem Cell Treatment on Rats With Sepsis-Induced Acute Lung Injury. Front Pharmacol. 2020;11:902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Murai N, Ohtaki H, Watanabe J, Xu Z, Sasaki S, Yagura K, Shioda S, Nagasaka S, Honda K, Izumizaki M. Intrapancreatic injection of human bone marrow-derived mesenchymal stem/stromal cells alleviates hyperglycemia and modulates the macrophage state in streptozotocin-induced type 1 diabetic mice. PLoS One. 2017;12:e0186637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Xv J, Ming Q, Wang X, Zhang W, Li Z, Wang S, Li Y, Li L. Mesenchymal stem cells moderate immune response of type 1 diabetes. Cell Tissue Res. 2017;368:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Vanikar AV, Trivedi HL, Thakkar UG. Stem cell therapy emerging as the key player in treating type 1 diabetes mellitus. Cytotherapy. 2016;18:1077-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 56. | Fiorina P, Voltarelli J, Zavazava N. Immunological applications of stem cells in type 1 diabetes. Endocr Rev. 2011;32:725-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Karimi S, Ai J, Khorsandi L, Bijan Nejad D, Saki G. Vildagliptin Enhances Differentiation of Insulin Producing Cells from Adipose-Derived Mesenchymal Stem Cells. Cell J. 2019;20:477-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 58. | Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N, Rostami P, Mohsenipour R, Amirkashani D, Bandarian F, Arjmand B, Larijani B. Placenta derived Mesenchymal Stem Cells transplantation in Type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metab Disord. 2021;20:1179-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 60. | Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 61. | Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev (Orlando). 2013;27:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Wang H, Qiu X, Ni P, Lin X, Wu W, Xie L, Lin L, Min J, Lai X, Chen Y, Ho G, Ma L. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int J Mol Med. 2014;33:263-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 598] [Cited by in RCA: 645] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 64. | Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ. Human chorionic-plate-derived mesenchymal stem cells and Wharton's jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18200] [Article Influence: 957.9] [Reference Citation Analysis (0)] |
| 66. | Dadheech N, James Shapiro AM. Human Induced Pluripotent Stem Cells in the Curative Treatment of Diabetes and Potential Impediments Ahead. Adv Exp Med Biol. 2019;1144:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768-15773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 407] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 68. | Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1775] [Cited by in RCA: 1608] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 69. | Beikmohammadi L, Bandehpour M, Hashemi SM, Kazemi B. Generation of insulin-producing hepatocyte-like cells from human Wharton's jelly mesenchymal stem cells as an alternative source of islet cells. J Cell Physiol. 2019;234:17326-17336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Saunders B. Altruism or solidarity? Bioethics. 2012;26:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Frati P, La Russa R, Santurro A, Fineschi B, Di Paolo M, Scopetti M, Turillazzi E, Fineschi V. Bioethical issues and legal frameworks of surrogacy: A global perspective about the right to health and dignity. Eur J Obstet Gynecol Reprod Biol. 2021;258:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Andrews WS, Kane BJ, Hendrickson RJ. Organ allocation and utilization in pediatric transplantation. Semin Pediatr Surg. 2017;26:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | van Dijk G, Hilhorst M, Rings E. Liver, pancreas and small bowel transplantation: current ethical issues. Best Pract Res Clin Gastroenterol. 2014;28:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Lovell-Badge R, Anthony E, Barker RA, Bubela T, Brivanlou AH, Carpenter M, Charo RA, Clark A, Clayton E, Cong Y, Daley GQ, Fu J, Fujita M, Greenfield A, Goldman SA, Hill L, Hyun I, Isasi R, Kahn J, Kato K, Kim JS, Kimmelman J, Knoblich JA, Mathews D, Montserrat N, Mosher J, Munsie M, Nakauchi H, Naldini L, Naughton G, Niakan K, Ogbogu U, Pedersen R, Rivron N, Rooke H, Rossant J, Round J, Saitou M, Sipp D, Steffann J, Sugarman J, Surani A, Takahashi J, Tang F, Turner L, Zettler PJ, Zhai X. ISSCR Guidelines for Stem Cell Research and Clinical Translation: The 2021 update. Stem Cell Reports. 2021;16:1398-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 75. | Frati P, Scopetti M, Santurro A, Gatto V, Fineschi V. Stem Cell Research and Clinical Translation: A Roadmap about Good Clinical Practice and Patient Care. Stem Cells Int. 2017;2017:5080259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Scopetti M, Santurro A, Gatto V, La Russa R, Manetti F, D'Errico S, Frati P, Fineschi V. Mesenchymal stem cells in neurodegenerative diseases: Opinion review on ethical dilemmas. World J Stem Cells. 2020;12:168-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Pavón A, Beloqui I, Salcedo JM, Martin AG. Cryobanking Mesenchymal Stem Cells. Methods Mol Biol. 2017;1590:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Harris DT. Biobanking and Regenerative Medicine: An Overview. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Yong KW, Choi JR, Wan Safwani WK. Biobanking of Human Mesenchymal Stem Cells: Future Strategy to Facilitate Clinical Applications. Adv Exp Med Biol. 2016;951:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Karch SB, Fineschi V, Francia P, Scopetti M, Padovano M, Manetti F, Santurro A, Frati P, Volpe M. Role of induced pluripotent stem cells in diagnostic cardiology. World J Stem Cells. 2021;13:331-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 81. | Scopetti M, Santurro A, Gatto V, Padovano M, Manetti F, D'Errico S, Fineschi V. Information, Sharing, and Self-Determination: Understanding the Current Challenges for the Improvement of Pediatric Care Pathways. Front Pediatr. 2020;8:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Rhondali W, Perez-Cruz P, Hui D, Chisholm GB, Dalal S, Baile W, Chittenden E, Bruera E. Patient-physician communication about code status preferences: a randomized controlled trial. Cancer. 2013;119:2067-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1080] [Article Influence: 47.0] [Reference Citation Analysis (0)] |