Published online Jul 26, 2022. doi: 10.4252/wjsc.v14.i7.539
Peer-review started: March 6, 2022
First decision: April 19, 2022
Revised: April 24, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 26, 2022
Processing time: 141 Days and 20.7 Hours
Cancer stem cells (CSCs) have been implicated in tumorigenesis and tumor recurrence and metastasis are key therapeutic targets in cancer treatment. MicroRNAs display therapeutic potential by controlling the properties of CSCs; however, whether an association exists between miR-3682-3p and CSCs is unknown.
To investigate the mechanism by which miR-3682-3p promotes stemness maintenance in hepatocellular carcinoma (HCC).
MiR-3682-3p expression in HCC cell lines and 34 pairs of normal and HCC specimens was assayed by quantitative polymerase chain reaction. The functional role of miR-3682-3p was investigated in vitro and in vivo. Dual-luciferase reporter and chromatin immunoprecipitation assays were performed for target asse
We found that miR-3682-3p plays a key role in HCC pathogenesis by promoting HCC cell stemness. The upregulation of miR-3682-3p enhanced CSC spheroid-forming ability, side population cell fractions, and the expression of CSC factors in HCC cells in vitro and the tumorigenicity of transplanted HCC cells in vivo. Furthermore, silencing miR-3682-3p prolonged the survival of HCC-bearing mice. Mechanistically, we found that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression through PI3K/AKT; c-Myc, in turn, activates miR-3682-3p, forming a positive feedback loop. Intriguingly, miR-3682-3p expression was induced by hepatitis B virus X protein (HBx) and was involved in HBx-induced tumor stemness-related pathogenesis.
Our findings reveal a novel mechanism by which miR-3682-3p promotes stemness in HCC stem cells. Silencing miR-3682-3p may represent a novel therapeutic strategy for HCC.
Core Tip: In this work, we identified miR-3682-3p as a key inducer of cancer stem cell properties, thereby promoting the pathogenesis of hepatocellular carcinoma (HCC). In brief, we found that the upregulation of miR-3682-3p enhanced the spheroid forming ability, the fraction of side population cells, and the expression of cancer stem cell factors in HCC cells in vitro as well as the tumorigenicity of transplanted HCC cells in vivo; furthermore, silencing miR-3682-3p significantly prolonged the survival time of HCC-bearing mice. Mechanistically, we found that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression through PI3K/AKT; c-Myc, in turn, activates miR-3682-3p, resulting in the formation of a positive feedback loop. Taken together, we identified a novel positive feedback regulatory loop involving HBx, miR-3682-3p, FOXO3, β-catenin, and c-Myc that plays a pivotal role in the stemness of HCC. Our findings revealed a novel mechanism by which miR-3682-3p promotes stem cell maintenance in HCC, and silencing miR-3682-3p may represent a novel therapeutic strategy for the treatment of this cancer.
- Citation: Chen Q, Yang SB, Zhang YW, Han SY, Jia L, Li B, Zhang Y, Zuo S. miR-3682-3p directly targets FOXO3 and stimulates tumor stemness in hepatocellular carcinoma via a positive feedback loop involving FOXO3/PI3K/AKT/c-Myc. World J Stem Cells 2022; 14(7): 539-555
- URL: https://www.wjgnet.com/1948-0210/full/v14/i7/539.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i7.539
Hepatocellular carcinoma (HCC) is a commonly diagnosed malignant tumor in China and is associated with very high morbidity and mortality[1,2]. Despite notable advances in multimodal treatment strategies over recent decades, such as radiotherapy, immunotherapy, and noninvasive surgical resection, the prognosis of patients with HCC, with a 5-year relative overall survival rate of approximately only 12%, remains unsatisfactory[3,4]. This highlights the urgent need to elucidate the mechanisms underlying HCC tumorigenicity and metastasis and identify reliable biomarkers to guide early HCC diagnosis and therapeutic intervention[5,6]. Increasing evidence has indicated that cancer stem cells (CSCs) underlie the insensitivity of HCC to radiotherapy and chemotherapy and play a key role in HCC metastasis and recurrence[7]. Like most stem cells, CSCs have the potential for self-renewal and the ability to differentiate into multiple cell types, resulting in tumor heterogeneity, and ultimately leading to tumor recurrence and metastasis[8]. Accordingly, targeting CSCs may represent a promising therapeutic target for cancer treatment[9,10].
The hepatitis B virus X protein (HBx) can reportedly disrupt several pathways and associated functions by regulating the expression and activity of numerous genes, epigenetics-related molecules [such as microRNAs (miRNAs)], and long non-coding RNAs (lncRNAs)[11]. MiRNAs are endogenous non-coding RNA molecules that can bind to the 3′-untranslated regions (3′-UTRs) of target mRNAs and regulate their expression at the post-transcriptional level[12]. More than 2500 miRNAs have been identified in humans to date, and many likely remain to be identified[13-15]. It is well documented that miRNAs play a significant role in regulating CSC function and cancer progression at multiple levels[16,17]. MiR-3682-3p exerts both oncogenic and tumor-suppressive functions, depending on the cancer type. For instance, high miR-3682-3p expression is correlated with esophageal cancer[18]. Exosomal miR-3682-3p targets angiopoietin 1 by altering Ras-MEK1/2-ERK signaling, suggesting that exosomal-derived miR-3682-3p may act as a tumor suppressor in HCC[19]. However, a different study reported that miR-3682-3p induces the proliferation of HCC cells and inhibits their apoptosis through the FAS pathway, indicating that miR-3682-3p may instead exert oncogenic effects in HCC[20].
Given these conflicting reports on the role of miR-3682-3p in HCC, we performed early validation of miR-3682-3p expression using in situ hybridization, and found that the expression level of miR-3682-3p was significantly elevated in HCC tissue compared with that in controls, and was positively correlated with both malignancy and worse prognosis in HCC. Our data further indicated that high miR-3682-3p expression levels were positively correlated with hepatitis B virus (HBV) infection. However, whether miR-3682-3p also affects stemness in HCC remains unknown. In this study, we demonstrated that miR-3682-3p directly targets and inhibits the expression of FOXO3. The downregulation of FOXO3 Levels subsequently leads to the activation of a PI3K/AKT/β-catenin/c-Myc/miR-3682-3p positive feedback loop that promotes the stemness of HCC cells. In summary, our findings revealed a role for miR-3682-3p in promoting the stemness of HCC cells and suggested that this miRNA may represent a potential therapeutic target for the treatment of HCC.
A total of 34 patients with HCC who underwent surgical resection in the Department of Hepatobiliary Surgery of the Affiliated Hospital of Guizhou Medical University from June 2017 to June 2019 were selected for this study. All patients provided informed consent. The study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. HCC and paired paracancerous tissue specimens (at least 3 cm from the edge of the tumor) were collected after resection and were preserved in liquid nitrogen. HCC was pathologically confirmed in all cases. All the selected patients were undergoing first-time surgical resection of the primary lesion, and none had received radiotherapy, chemotherapy, or hormone therapy before surgery.
The HCC tissue microarray was purchased from the Shanghai Molecular Medicine Engineering Center (Shanghai, China) with approval from its Ethics Committee.
The cell lines used in this study (LO2, LM3, Huh7, Hep3B, HepG2, and MHCC97H) were provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone Laboratories, Inc., Logan, UT, United States) supplemented with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2.
Total RNA was extracted from cultured HCC cells using Trizol reagent and reverse transcribed into cDNA, which served as a template for quantitative polymerase chain reaction (qPCR). Gene expression levels were detected separately using a standard SYBR Green RT-PCR kit (Thermo Scientific, Waltham, MA, United States) according to the manufacturer’s instructions. The sequences of the primers used are detailed in Supplementary Table 1. Relative gene expression levels were calculated using the 2-ΔΔCt method and are reported as fold changes.
Purchased miR-3682-3p overexpressing lentivirus with green fluorescence constructed and synthesized by Ji kai (Shanghai, China) and the corresponding control lentivirus in vacuo. The virus dosage was calculated at a cell fusion rate of 60%. Lentivirus was transfected into HCC cells at an MOI of 80 using a transfection reagent. The medium was changed after 8 h of transfection. Green fluorescence could be detected after 72 h of transfection under a fluorescence microscope, at which point the cells were collected for PCR-based detection of the miR-3682-3p level to determine the transfection efficiency (Supplementary Table 2).
A total of 5 × 103 HCC cells were inoculated into six-well ultra-low adsorption plates (Corning, Painted Post, NY, United States) containing 2% B27 (BD PharMingen, Carlsbad, CA, United States), 20 ng/mL epidermal growth factor, 20 ng/mL fibroblast growth factor in DMEM/F12 serum-free medium (Invitrogen). The cells were cultured for 14 d and then imaged and counted under a light microscope.
Cells in the logarithmic growth phase were digested into single-cell suspensions and washed twice with PBS. A total of 1 × 106 cells/mL were resuspended in each of two tubes containing medium supplemented with 2% FBS. Verapamil (20 μL) was added to one tube (to inhibit dye efflux) and incubated for 30 min at 37 °C protected from light. Vybrant DyeCycle Violet stain (Sigma-Aldrich) at a final concentration of 5 µg/mL was then added to both tubes followed by incubation with shaking for 90 min at 37 °C in the dark, and with mixing every 10 min. The samples were subsequently centrifuged at 1500 × g, the supernatant was discarded, and the cells were resuspended in 1 mL of PBS. Finally, 5 μL of 7-ADD (BD PharMingen) was added to each tube followed by flow cytometric detection. Each set of experiments was repeated three times.
HCC cells were inoculated into 24-well plates, and the cells were fused to 70% with Lipofectamine 2000 (Invitrogen, Guangzhou, China). Plasmids containing the wild-type or mutated (mut) FOXO3 3′-UTR were co-transfected with miR-3682-3p mimic/inhibitor and the cells were assayed for luciferase activity after 48 h. To investigate the effect of c-Myc on miR-3682-3p transcription, vectors containing mutated c-Myc binding sites were constructed and co-transfected with c-Myc expression plasmids into HCC cells, following which the cells were assayed for luciferase activity.
Total protein was extracted from each group of HCC cells. Protein concentrations were determined by BCA. Equal amounts of protein were separated by 10% SDS-PAGE, transferred to PVDF membranes (Millipore, Bedford, MA, United States), incubated overnight at 4 °C with primary antibodies, and then labeled with secondary antibodies at room temperature for 1 h. Bands were developed using luminol-enhanced chemiluminescence (Thermo Scientific). The primary antibodies used for western blotting are described in Supplementary Table 3.
The chromatin immunoprecipitation (ChIP) assay was performed using a ChIP kit (Thermo Scientific) according to the manufacturer's instructions. Briefly, genomic DNA was extracted from HCC cells, the DNA was fragmented, and a c-Myc antibody was added to the reaction system for immunoprecipitation. IgG served as a negative control. The purified DNA was PCR-amplified and the products were separated on a 2% agarose gel. The primers used for PCR amplification are shown in Supplemen
Total cellular protein was extracted using the Pierce Co-immunoprecipitation (Co-IP) Kit (Thermo Scientific). A total of 2 mg of protein was incubated with 5 μg of specific antibody or IgG (used as a negative control) overnight at 4 °C. After washing, the protein samples were subjected to western blotting.
All animal experiments were performed according to the requirements of the Guizhou Medical University Animal Experiment Ethics Committee (2100555). Cyclophosphamide (200 μL) was injected daily for the first three days of the experiment to disrupt the immune system of BALB/c nude mice (SPF, Beijing, China). Then, the mice were divided into two groups of 20 mice each and administered HCC cells (1 × 106, 2 × 106, 4 × 106, and 8 × 106 cells, n = 5 mice per concentration) overexpressing or not miR-3682-3p by subcutaneous injection. The mice were euthanized after 21 d and the tumors were excised. Equal amounts of HCC cells (8 × 106) were subcutaneously injected into four-week-old BALB/c nude mice (n = 10 per group) to establish a HCC xenograft model. After one week, the mice received either saline or 10 nmol of a miR-3682-3p antagomir (RiboBio, Guangzhou, China) intraperitoneally twice a week for three weeks. The Kaplan-Meier method was used to evaluate the survival of the mice.
FOXO3, OCT4, and SOX2 protein expression levels were assessed in tissue microarrays (Proteintech, Wuhan, China) according to the manufacturer’s protocol as well as in animal tissues. The indirect streptavidin peroxidase method was used. Immunohistochemistry was scored by two registered pathologists from the Affiliated Hospital of Guizhou Medical University based on the intensity of coloration and area of the sections. The stained area was scored from 0 to 4 (0: < 5% staining; 1: 5%-25% staining; 2: 26%-50% staining; 3: 50%-75% staining; and 4: > 75% staining). The product of the two sets of scores was counted, and final staining scores of < 6 and ≥ 6 were considered to reflect low and high expression, respectively.
SPSS v.25.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Data were expressed as means ± standard deviation. Differences between two groups were compared using t-tests. Patient clinicopathological parameters were analyzed using the χ2 test, patient survival was analyzed using Kaplan-Meier survival curves, and comparisons of survival and recurrence rates between groups were performed using the log-rank test. P values < 0.05 were considered significant. One-way Cox regression was used to analyze all clinicopathological indicators and factors with P values < 0.05 were used for multivariate regression analysis.
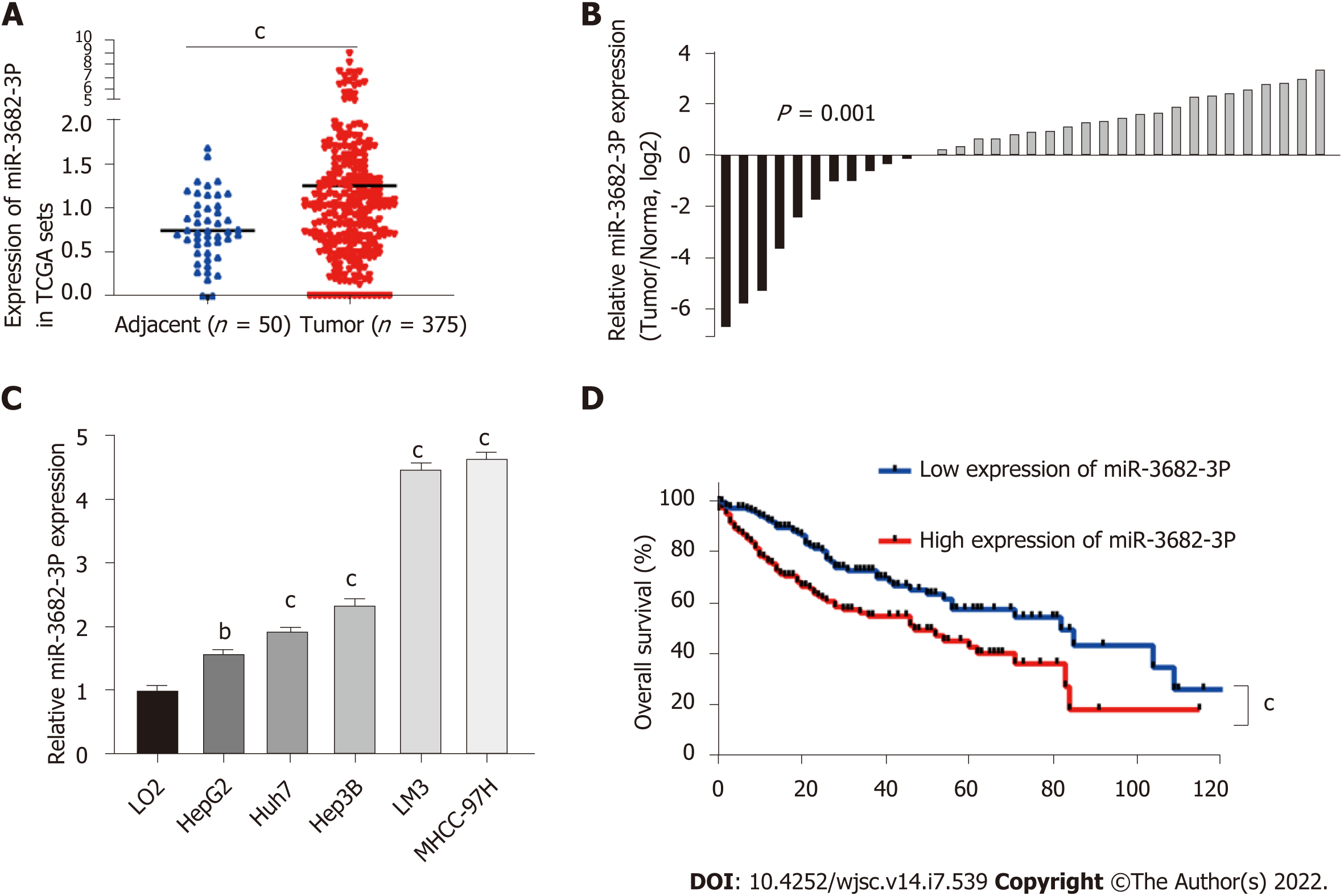
Data from The Cancer Genome Atlas (TCGA) were accessed to identify miRNAs related to the survival of HCC patients. We identified miR-3682-3p as being distinctly highly expressed in HCC specimens from TCGA datasets (P = 0.001) (Figure 1A). The results of our previous in situ hybridization analysis in primary HCC tissues from a cohort of 90 HCC patients had suggested that a significant correlation exists between miR-3682-3p expression and tumor size and stage, intrahepatic GGT levels, and HBV surface antigen status. Additionally, high miR-3682-3p expression levels were associated with a shorter survival time in HCC patients[21]. To verify the above results, we obtained HCC and adjacent paracancerous tissue from a different cohort of 34 patients. RT-qPCR analysis showed that miR-3682-3p expression was significantly higher in HCC tissue than in adjacent liver tissue (P = 0.001) (Figure 1B) and was also significantly higher in HCC cell lines than in an immortalized hepatocyte line (LO2). Intriguingly, the expression of miR-3682-3p was upregulated in HBV-positive HCC cells (Hep3B, MHCC-97H, and HCCLM3) compared with that in HBV-negative (Huh7 and HepG2) HCC cells (Figure 1C). Furthermore, survival analysis of TCGA dataset suggested that HCC patients with low miR-3682-3p expression survived longer than those with high miR-3682-3p expression (P < 0.001) (Figure 1D). In summary, our results suggested that miR-3682-3p expression is upregulated in HCC tissues and is positively correlated with poor prognosis in HCC patients.
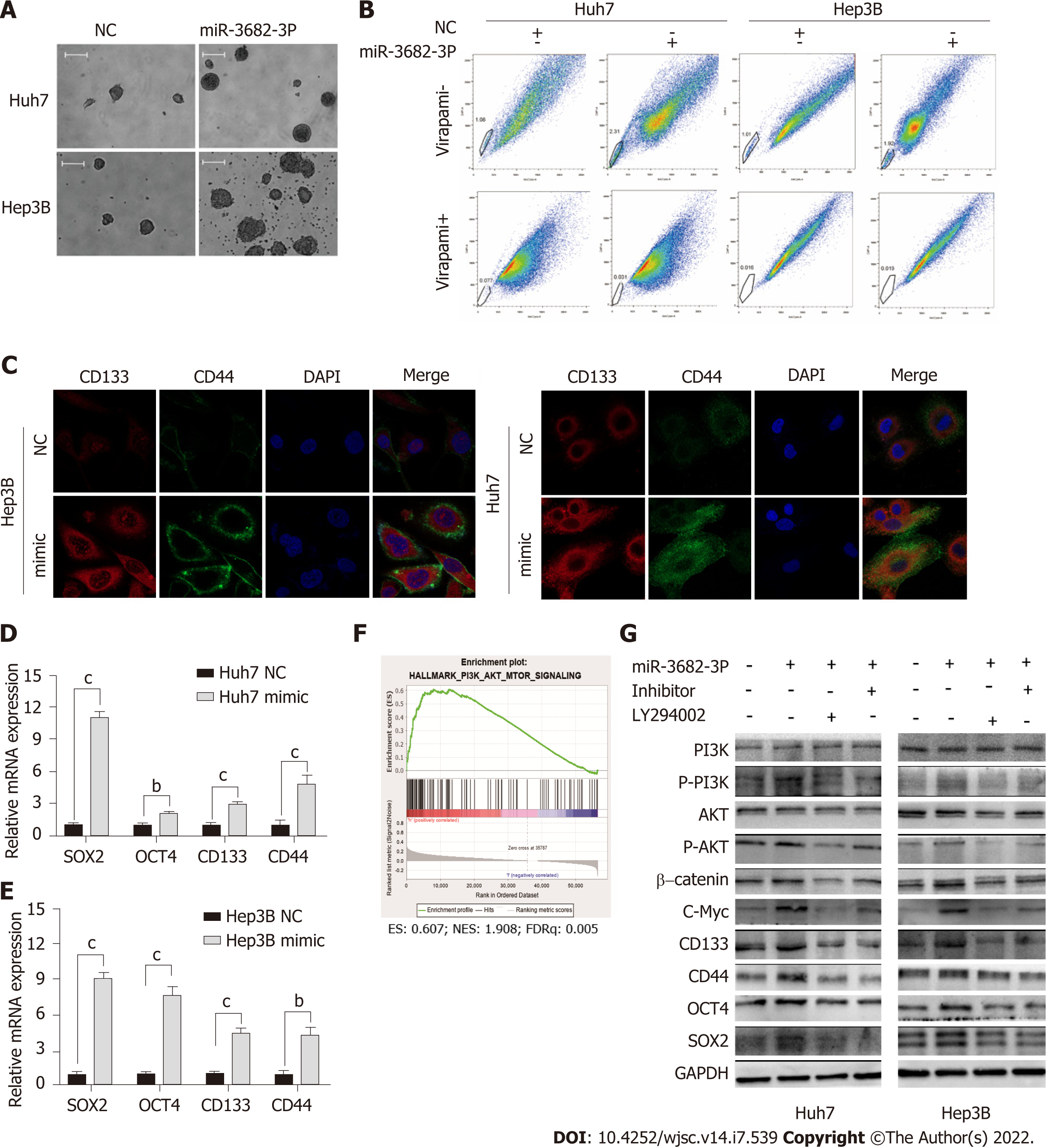
To investigate the effect of miR-3682-3p on CSCs, miR-3682-3p mimics, miR-3682-3p inhibitors, or miR-3682-3p-expressing lentiviral vectors were transfected into HCC cells. We first examined the transfection efficiency of miR-3682-3p by RT-qPCR (Supplementary Figure 1A and B). Overexpression of miR-3682-3p increased the sphere-forming ability of HCC cells (Figure 2A and Supplementary Figure 1C) as well as the percentage of side population cells (Figure 2B and Supplementary Figure 1D). Immunofluorescence staining and RT-qPCR further confirmed that miR-3682-3p increased the stemness of HCC cells (Figure 2C-E). In contrast, miR-3682-3p downregulation in LM3 and MHCC-97H cells following treatment with the miR-3682-3p inhibitor elicited the opposite result (Supplementary Figure 1E-H).
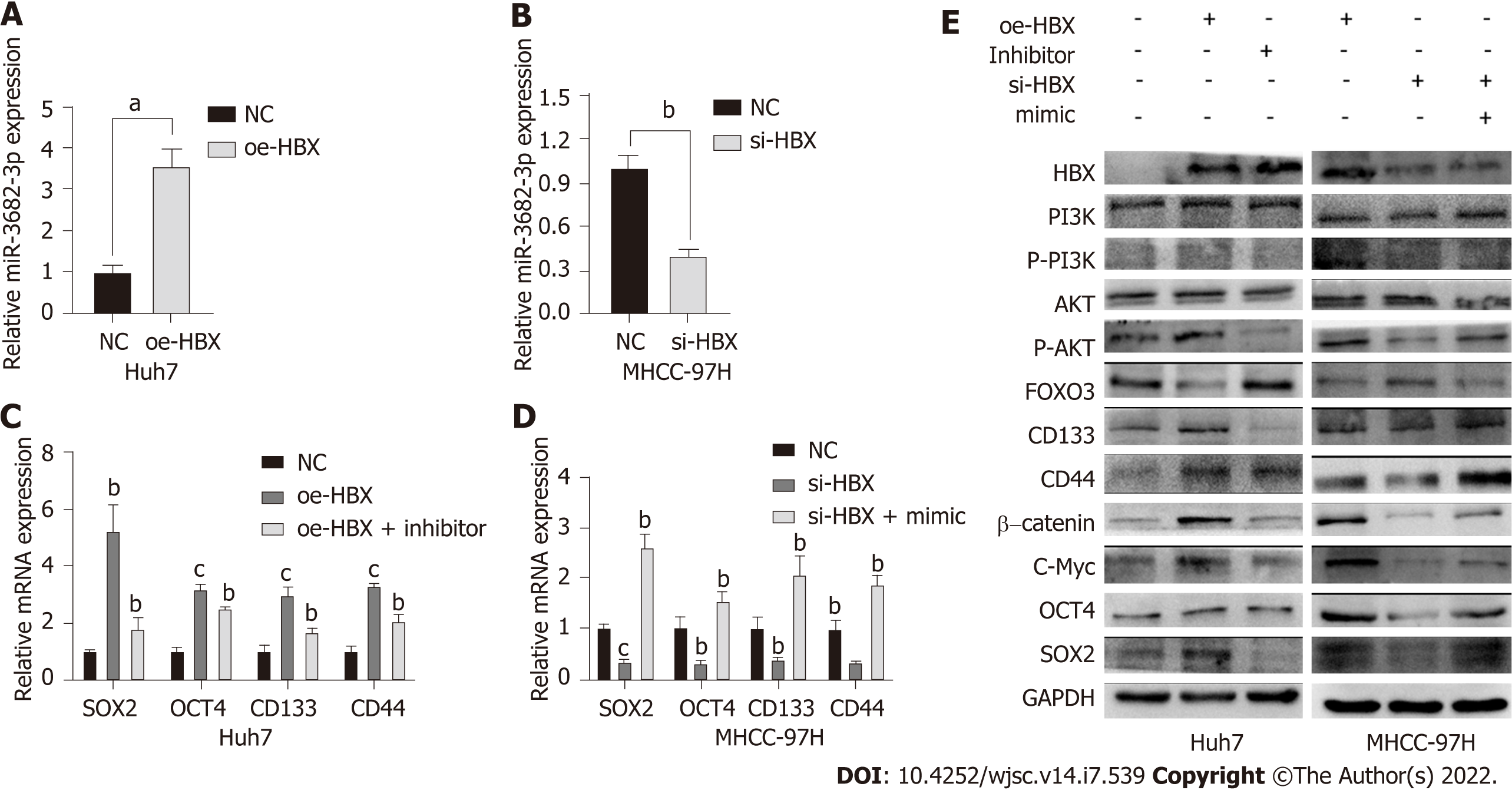
To investigate whether miR-3682-3p modulated the signaling pathway involved in the promotion of tumor stemness, we performed gene set enrichment analysis (GSEA), and found that miR-3682-3p expression was closely associated with PI3K/AKT activation in HCC (Figure 2F). Western blot was further performed to confirm this possibility, with the results showing that miR-3682-3p overexpression led to the upregulation of the expression of PI3K/AKT pathway-related proteins, including c-Myc, as well as that of stemness-related molecules such as CD44, CD133, SOX2, and OCT4. The simultaneous application of a PI3K inhibitor (LY294002) and the miR-3682-3p inhibitor in HCC cells overexpressing miR-3682-3p reversed the above-mentioned changes in protein levels (Figure 2G). Together, these data demonstrated that miR-3682-3p promotes a CSC-like phenotype in HCC cells through the PI3K/AKT/c-Myc signaling axis, thereby further contributing to the progression of HCC.
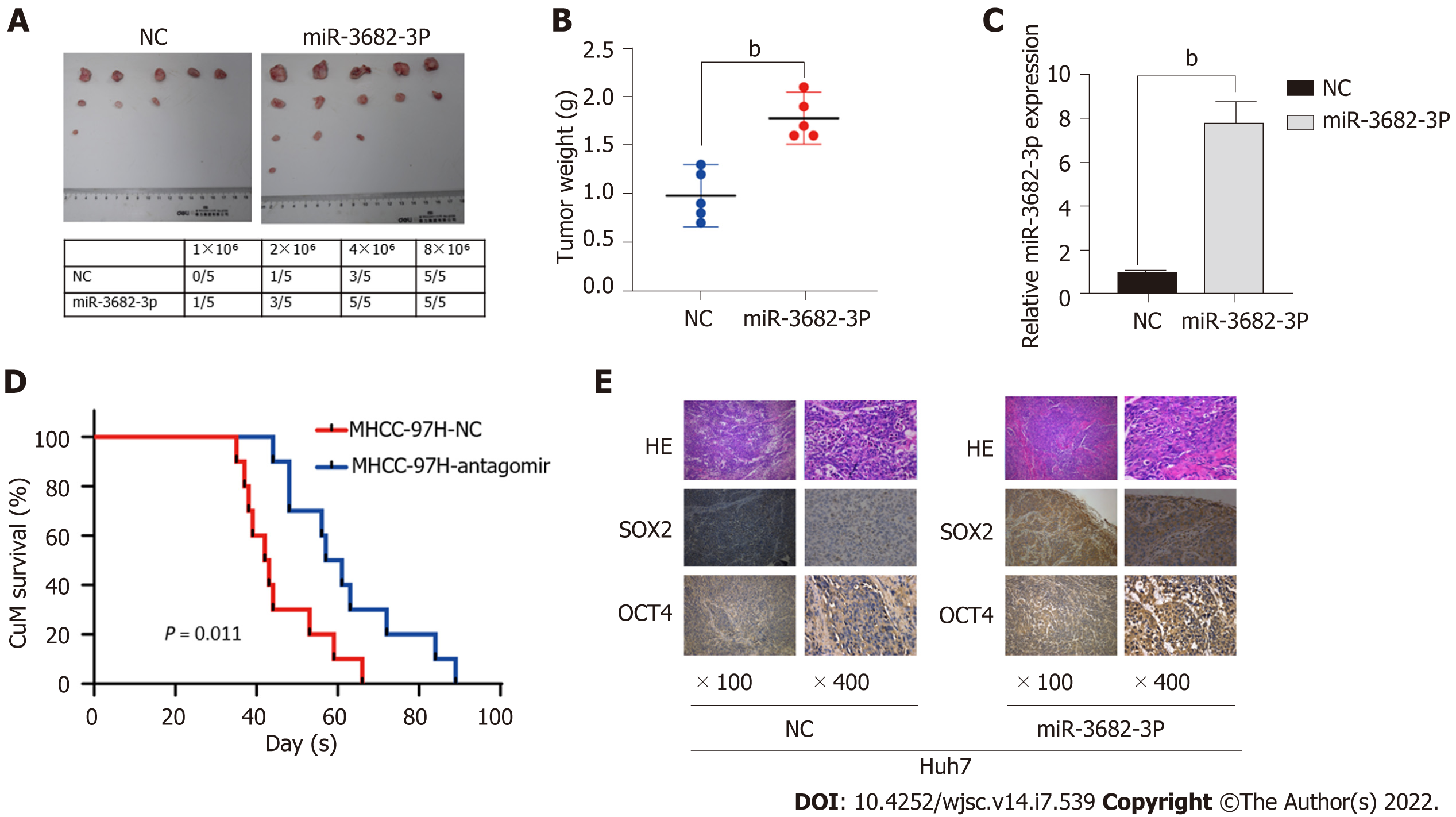
Because we found that MiR-3682-3p could promote a CSC-like phenotype in vitro, we next assessed whether miR-3682-3p exerted a similar effect in vivo. For this, different concentrations (1 × 106, 2 × 106, 4 × 106, and 8 × 106) of HCC cells stably transfected with miR-3682-3p were subcutaneously injected into BALB/C nude mice. As shown in Figure 3A, the associated tumorigenicity rates were 20% (1/5), 60% (3/5), 100% (5/5), and 100% (5/5), respectively. For equal concentrations of control cells, the tumorigenicity rates were 0% (0/5), 20% (1/5), 60% (3/5), and 100% (5/5), respectively. Similarly, the tumor weight was greater in the miR-3682-3p overexpression group than in the control group (Figure 3B). RT-qPCR results confirmed that the miR-3682-3p expression levels were higher in xenografts derived from cells stably overexpressing miR-3682-3p than in negative control cell-derived xenografts (Figure 3C). Additionally, Kaplan-Meier curve analysis indicated that miR-3682-3p antagomir administration prolonged the survival time of mice compared with that of control animals (Figure 3D). The expression of SOX2 and OCT4 in nude mouse xenograft tumors was then detected by immunohistochemistry, and the same results were obtained (Figure 3E). Overall, these results provided further evidence that miR-3682-3p promotes the tumorigenicity of HCC cells in vivo.
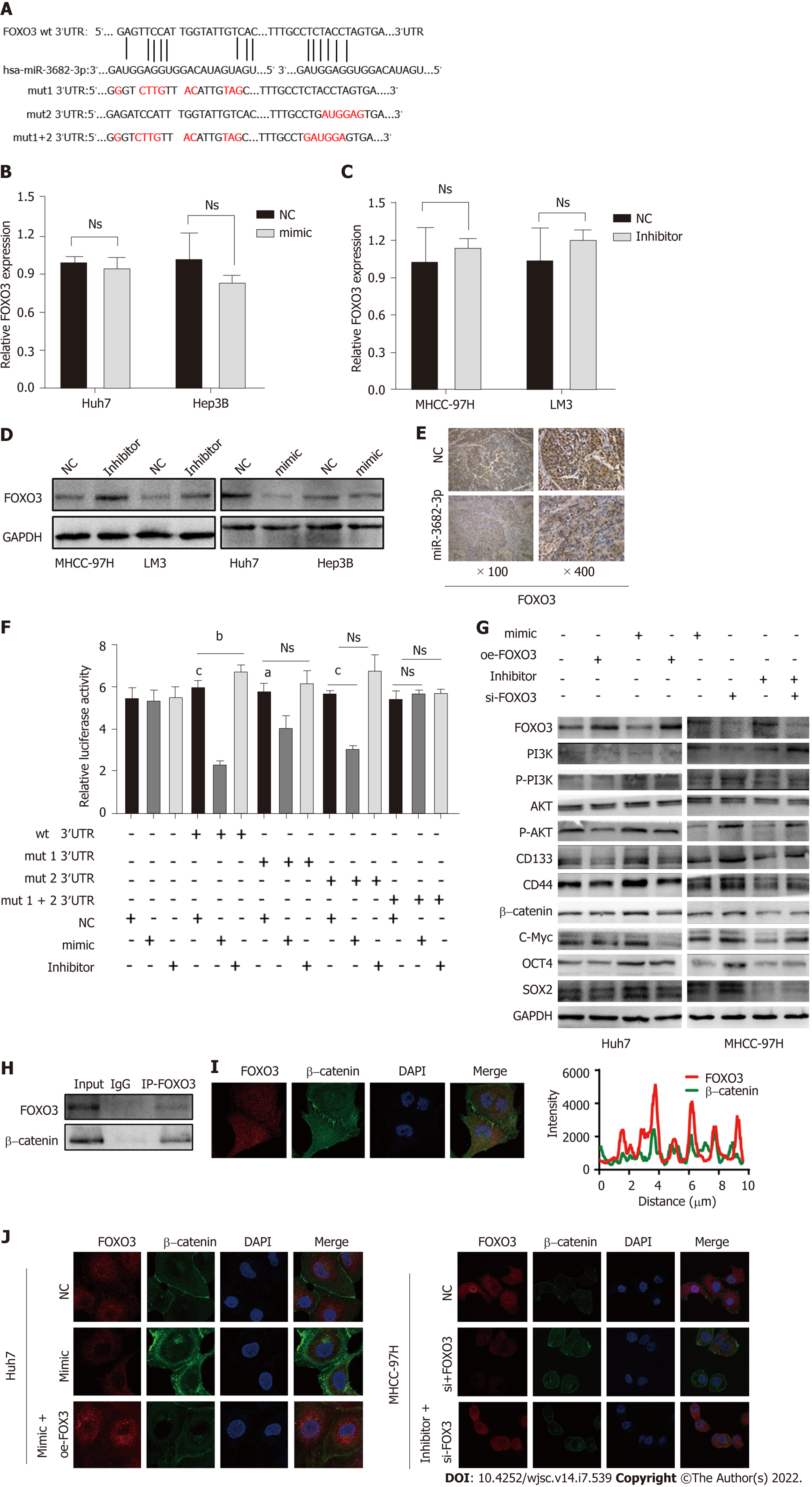
To understand how miR-3682-3p affects the stemness of HCC cells, miRNA walk 2.0 was used for the prediction of miR-382-3p targets, resulting in the identification of FOXO3 as a potential target gene of this miRNA (Figure 4A). MiR-3682-3p overexpression led to the suppression of FOXO3 protein levels, but did not affect FOXO3 transcript levels; meanwhile, the knockdown of miR-3682-3p elicited the opposite effect (Figure 4B-D). The interaction between miR-3682-3p and FOXO3 was further validated using a dual-luciferase reporter assay (Figure 4E). Additionally, immunohistochemical analysis of xenografts derived from miR-3682-3p-overexpressing HCC cells demonstrated that FOXO3 expression was downregulated in miR-3682-3p-overexpressing tumors (Figure 4F). To further demonstrate that FOXO3 mediates the oncogenic activity of miR-3682-3p, we evaluated the effect of FOXO3 on miR-3682-3p-modulated signals in HCC cells. We found that the overexpression of FOXO3 eliminated the promotive effects of miR-3682-3p on the expression of phosphorylated (p)-PI3K, p-AKT, β-catenin, SOX2, OCT4, CD133, CD44, and c-Myc (Figure 4G). Moreover, FOXO3 inhibited miR-3682-3p-induced stimulation of CSC-like phenotypes in HCC cells (Supplementary Figure 2A-D). Taken together, these results indicated that FOXO3 inhibits the miR-3682-3p-induced stemness of HCC cells.
It has been documented that FOXO3 interacts with β-catenin and plays a pivotal role in a variety of tumors[22,23]. Here, we identified a possible interaction between FOXO3 and β-catenin by CoIP (Figure 4H). Interestingly, we further found that FOXO3 co-localized with β-catenin in the cytoplasm (Figure 4I), while further immunofluorescence staining results showed that FOXO3 inhibited β-catenin nuclear translocation (Figure 4J).
In brief, the above findings indicated that miR-3682-3p directly binds to the 3′-UTR of FOXO3, thereby activating the PI3K/AKT/β-catenin/c-MYC signaling axis and promoting the stemness of HCC cells.
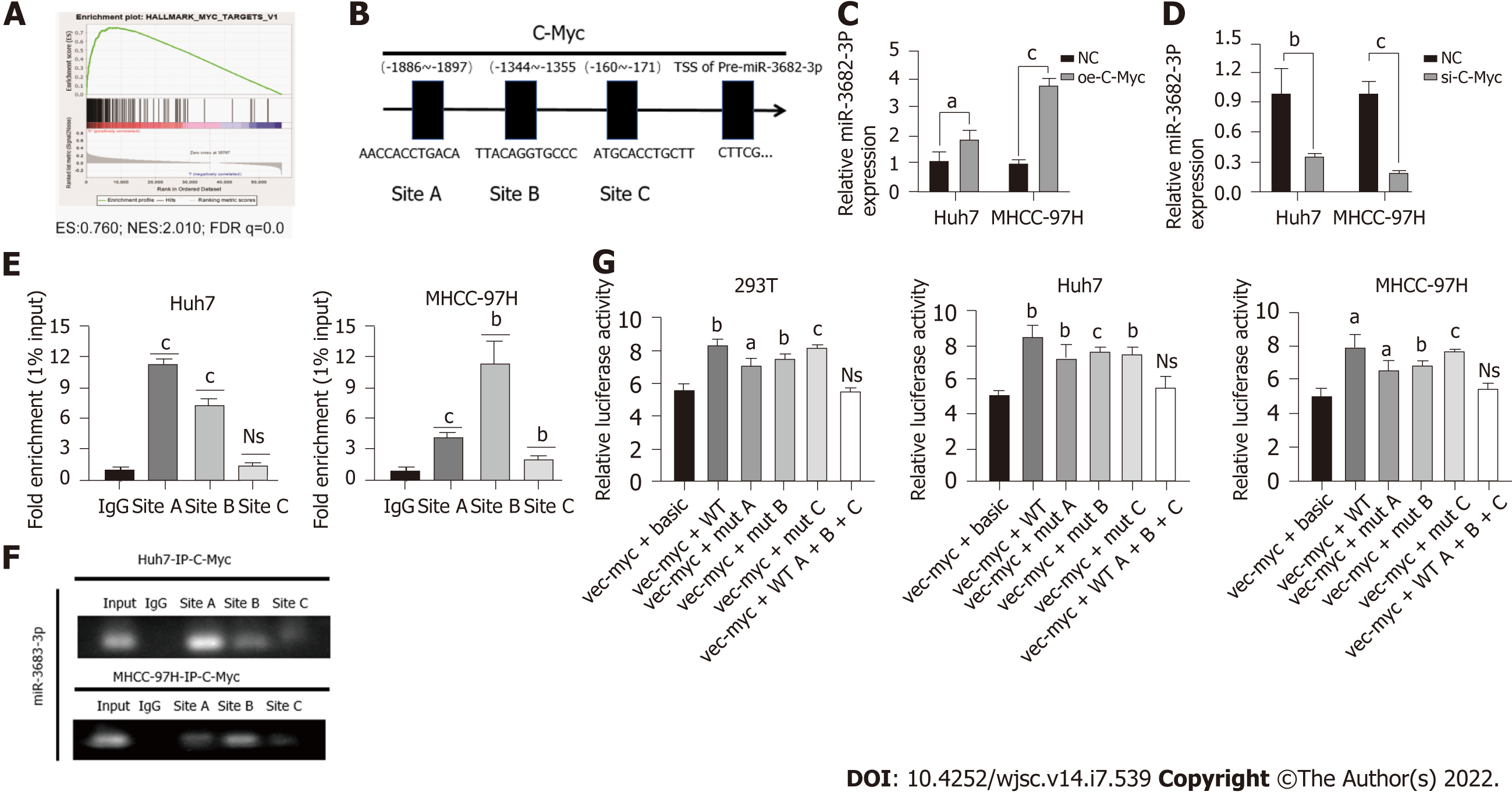
Transcription factors can promote the transcription of downstream miRNAs, which then bind to and regulate the expression of their target genes. To determine the mechanism underlying the upregulation of miR-3682-3p expression in HCC, we performed GSEA, and found that miR-3682-3p was positively correlated with c-Myc (Figure 5A). Transcription factor binding site prediction using the UCSC and JASPAR databases indicated that c-Myc can bind three regions in the miR-3682-3p promoter (Figure 5B). RT-qPCR analysis showed that upregulation of c-Myc increased the expression levels of miR-3682-3p, whereas c-Myc downregulation elicited the opposite result (Figure 5C and D). ChIP and luciferase reporter assays further confirmed that c-Myc can bind to the three sites on the miR-3682-3p promoter (Figure 5E-G). In summary, these data suggested that in HCC, c-Myc regulates miR-3682-3p tran
HBx is an HBV-encoded protein that displays oncogenic properties and is known to play a critical role in the development of many tumors. We have previously shown that miR-3682-3p expression is positively correlated with HBV infection. Here, we sought to determine whether there is a correlation between HBx and the miR-3682-3p/FOXO3/PI3K/AKT/β-catenin/c-Myc feedback loop. Our results demonstrated that the overexpression or knockdown of the HBx-encoding gene respectively increased and decreased the expression level of miR-3682-3p (Figure 6A and B). We further showed that miR-3682-3p mediated the oncogenic effects resulting from the HBx-mediated induction of PI3K/AKT/c-Myc signaling (Figure 6C and D), i.e., HBx promoted stemness by upregulating miR-3682-3p expression.
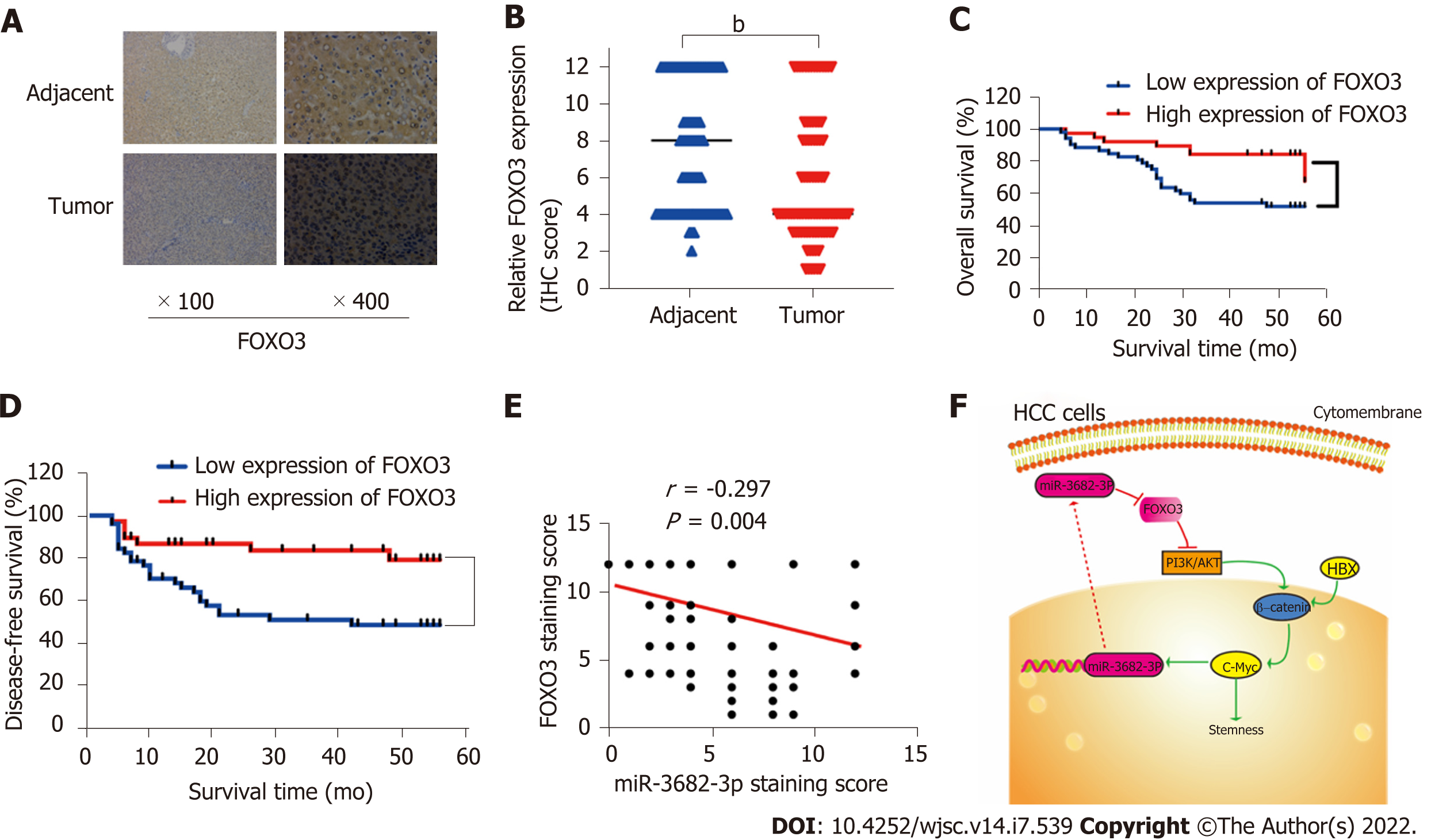
Finally, to obtain additional evidence to support our conclusions, tissue microarrays containing 90 HCC and paired adjacent non-tumor tissue samples were subjected to FOXO3 immunohistochemical analysis and scored for cell staining (Figure 7A and B). Survival analysis indicated that low FOXO3 expression correlated with reduced overall survival time in HCC patients (Figure 7C and D), while high FOXO3 expression elicited the opposite effect (Figure 7C and D). Subsequently, FOXO3 expression was evaluated in relation to the clinicopathological characteristics of HCC (Table 1). A one-step univariate Cox regression analysis indicated that AJCC staging (P = 0.022), relapse (P = 0.002), GGT (P = 0.035), Edmondson-Steiner grading (P < 0.001), and FOXO3 expression (P = 0.006). However, no differences were identified using multivariate Cox regression analysis (Table 2). Combined with our early fluorescence in situ hybridization analysis scores, our immunohistochemical analysis revealed that FOXO3 expression was negatively correlated with miR-3682-3p expression (Figure 7E).
| Characteristics | n | FOXO3 expression | P value | |
| Low | High | |||
| Age (yr) | ||||
| > 50 | 50 | 30 (60.00) | 20 (40.00) | 0.633 |
| ≤ 50 | 40 | 22 (55.00) | 18 (45.00) | |
| Gender | ||||
| Male | 80 | 46 (57.50) | 34 (42.50) | 1.000 |
| Female | 10 | 6 (60.00) | 4 (40.00) | |
| AJCC stage | ||||
| I | 52 | 25 (48.08) | 27 (51.92) | 0.029 |
| II-III | 38 | 27 (71.05) | 11 (28.95) | |
| HBsAg | ||||
| Negative | 19 | 8 (42.11) | 11 (57.89) | 0.119 |
| Positive | 71 | 44 (61.97) | 27 (38.03) | |
| Recurrence | ||||
| No | 41 | 19 (46.34) | 22 (53.76) | 0.032 |
| Yes | 49 | 33 (67.35) | 16 (32.65) | |
| AFP (μg/L) | ||||
| > 400 | 33 | 19 (57.6) | 14 (42.4) | 0.976 |
| ≤ 400 | 57 | 33 (57.9) | 24 (42.1) | |
| Total bilirubin (μmol/L) | ||||
| >20 | 15 | 5 (33.33) | 10 (66.67) | 0.036 |
| ≤ 20 | 75 | 47 (62.67) | 28 (37.33) | |
| ALT (U/L) | ||||
| > 45 | 31 | 20 (64.52) | 11 (35.48) | 0.348 |
| ≤ 45 | 59 | 32 (54.24) | 27 (45.76) | |
| GGT | ||||
| > 40 | 59 | 37 (62.7) | 22 (37.3) | 0.191 |
| ≤ 40 | 31 | 15 (48.4) | 16 (51.6) | |
| Edmondson-Steiner grade | ||||
| I-II | 61 | 28 (45.90) | 33 (54.10) | 0.001 |
| III-IV | 29 | 24 (82.76) | 5 (17.24) | |
| Tumor number | ||||
| Single | 79 | 45 (56.97) | 34 (45.03) | 0.675 |
| Multiple | 11 | 7 (63.64) | 4 (36.36) | |
| Tumor size (cm) | ||||
| > 5 | 28 | 24 (85.71) | 4 (14.29) | 0.001 |
| ≤ 5 | 62 | 28 (45.16) | 34 (54.84) | |
| Characteristics | Overall survival | |||||
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| FOXO3 expression | 0.311 | (0.134-0.721) | 0.006 | 0.504 | (0.164-1.553) | 0.233 |
| Low vs high | ||||||
| Age (yr) | 0.721 | (0.352-1.476) | 0.371 | |||
| ≤ 50 vs > 50 | ||||||
| Gender | 0.520 | (0.124-2.176) | 0.371 | |||
| Male vs female | ||||||
| AJCC stage | 2.277 | (1.123-4.616) | 0.022 | 1.203 | (0.555-2.604) | 0.640 |
| I vs II-III | ||||||
| HBsAg | 0.667 | (0.257-1.736) | 0.407 | |||
| Negative vs positive | ||||||
| Recurrence | 87.223 | (4.860-1565.443) | 0.002 | |||
| No vs yes | ||||||
| AFP (ng/mL) | 0.888 | (0.434-1.818) | 0.746 | |||
| ≤ 400 vs > 400 | ||||||
| Total bilirubin (μmol/L) | 1.113 | (0.428-2.893) | 0.826 | |||
| ≤ 20 vs > 20 | ||||||
| ALT (U/L) | 1.046 | (0.504-2.171) | 0.903 | |||
| ≤ 45 vs > 45 | ||||||
| GGT (U/L) | 0.386 | (0.159-0.937) | 0.035 | 0.594 | (0.233-1.516) | 0.276 |
| ≤ 40 vs > 40 | ||||||
| Edmondson-Steiner grade | 3.664 | (1.791-7.497) | < 0.001 | 1.839 | (0.560-3.445) | 0.478 |
| I-II vs III-IV | ||||||
| Tumor number | 1.810 | (0.742-4.417) | 0.192 | |||
| Singlevs vs multiple | ||||||
| Tumor size (cm) | 0.504 | (0.250-1.018) | 0.056 | |||
| ≤ 5 vs > 5 | ||||||
HCC is one of the most commonly diagnosed cancers worldwide[24]. Despite improvements in the treatment of HCC, this disease continues to be associated with high morbidity and mortality[5,25]. CSCs play key roles in tumorigenesis and tumor metastasis, and targeting CSCs has the potential to treat a variety of cancers[26]. Multiple hepatic CSC biomarkers have already been identified. In this study, we have added a genetic signature of these liver CSCs and revealed the signaling pathways that regulate HCC[27].
Epigenetic modification is a key regulatory mechanism in tumor pathogenesis and involves the participationof numerous miRNAs[28]. Recent studies have shown that specific miRNAs exhibit good therapeutic potential associated with the control of cancer cell stemness[29]. In addition, strategies to upregulate or inhibit miRNA levels in tumors, such as the design of miRNA mimics or antagomiRs, have shown promising preliminary clinical results in tumor treatment, especially those targeting CSCs. Studies have revealed that miR-3682-3p plays a major role in a variety of cancers, including HCC. In addition, miR-3682-3p has been implicated as a key player in bladder cancer drug resistance by inhibiting its activation by BML1 and thereby affecting[30]. However, no study to date has investigated stemness in HCC or whether miR-3682-3p has a role in this process. To address this, we investigated the role of miR-3682-3p in regulating the properties of HCC stem cells as well as the underlying mechanism. We found that the inhibition of miR-3682-3p suppressed the development of HCC, demonstrating that miR-3682-3p may serve as a prognostic marker for this cancer.
FOXO3 is a key member of the large FOX family of transcription factors[31]. In mammals, FOXO3 is known to have a wide range of biological functions[32]. Studies have confirmed that FOXO3 exerts an oncogenic role in a variety of cancers, including breast cancer[33], through the promotion of tumor cell proliferation, metastasis, stemness, and drug resistance, among other biological behaviors. Several studies have illustrated the interactive relationship between FOXO3 and β-catenin and the functional role of FOXO3 in the PI3K/AKT signaling axis[22]. In the present study, we found that miR-3682-3p directly targets FOXO3 and suppresses its expression, thereby enhancing P3K/AKT/c-Myc and downstream signaling, including upregulating the expression of known stemness markers, and, consequently, promoting the stemness of HCC cells.
c-Myc is an integral member of the MYC family of transcription factors. It is known to be involved in the progression of various cancers[34,35], including through the regulation of the expression of numerous miRNAs, thereby promoting tumor stemness, metastasis, proliferation, and chemoresistance [36,38]. We have previously shown the existence of three binding sites for c-Myc in the miR-3682-3p promoter using online bioinformatics tools. The results of the current study suggested that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression; c-Myc, in turn, activates miR-3682-3p, thereby forming a positive feedback loop.
Despite the consensus that HBx is closely related to HBV-associated HCC[9,39], the role of miRNAs in HBx-associated HCC is poorly understood[40]. HBx is known to influence the development of HCC by regulating β-catenin entry into the nucleus, which, in turn, affects downstream signaling[39-41]. In the present study, we found that miR-3682-3p expression was positively correlated with HBV infection. We further found that HBx can promote miR-3682-3p expression, thereby downregulating that of FOXO3 and promoting stemness in HCC.
We identified a novel positive feedback regulatory loop involving miR-3682-3p, FOXO3, β-catenin, and c-Myc that plays a pivotal role in the stemness of HCC (Figure 7F). Our findings unveiled a novel mechanism by which miR-3682-3p promotes stem cell maintenance in HCC progression and elucidated for the first time the regulatory relationship between HBV and miR-3682-3p.
Cancer stem cells (CSCs) have been implicated in tumorigenesis and tumor recurrence and metastasis are key therapeutic targets in cancer treatment. MicroRNAs display therapeutic potential by controlling the properties of CSCs; however, whether an association exists between miR-3682-3p and CSCs is unknown.
However, whether an association exists between miR-3682-3p and CSCs is unknown. Here, we investigated whether miR-3682-3p has a role in hepatocellular carcinoma (HCC).
To investigate the mechanism by which miR-3682-3p promotes stemness maintenance in HCC.
MiR-3682-3p expression in HCC cell lines and 34 pairs of normal and HCC specimens was assayed by qPCR. The functional role of miR-3682-3p was investigated in vitro and in vivo. Dual-luciferase reporter and chromatin immunoprecipitation assays were performed for target assessment, and western blotting was utilized to confirm miR-3682-3p/target relationships.
We found that miR-3682-3p plays a key role in HCC pathogenesis by promoting HCC cell stemness. The upregulation of miR-3682-3p enhanced CSC spheroid-forming ability, side population cell fractions, and the expression of CSC factors in HCC cells in vitro and the tumorigenicity of transplanted HCC cells in vivo. Furthermore, silencing miR-3682-3p prolonged the survival of HCC-bearing mice. Mechanistically, we found that miR-3682-3p targets FOXO3 and enables FOXO3/β-catenin interaction, which promotes c-Myc expression through PI3K/AKT; c-Myc, in turn, activates miR-3682-3p, forming a positive feedback loop. Intriguingly, miR-3682-3p expression was induced by hepatitis B virus X protein (HBx) and was involved in HBx-induced tumor stemness-related pathogenesis.
Our findings reveal a novel mechanism by which miR-3682-3p promotes stemness in HCC stem cells. Silencing miR-3682-3p may represent a novel therapeutic strategy for HCC.
This study has shed some light on the mechanism of action of miR-3682-3p in promoting stemness maintenance in HCC and provides a potential target for the treatment of HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delgado-Gallegos JL, Mexico; Elshimi E, Egypt A-Editor: Liu X, China S-Editor: Gong ZM L-Editor: A P-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11942] [Article Influence: 2985.5] [Reference Citation Analysis (4)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2969] [Article Influence: 742.3] [Reference Citation Analysis (7)] |
| 3. | Lim H, Ramjeesingh R, Liu D, Tam VC, Knox JJ, Card PB, Meyers BM. Optimizing Survival and the Changing Landscape of Targeted Therapy for Intermediate and Advanced Hepatocellular Carcinoma: A Systematic Review. J Natl Cancer Inst. 2021;113:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Finn RS, Zhu AX. Evolution of Systemic Therapy for Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Harding-Theobald E, Louissaint J, Maraj B, Cuaresma E, Townsend W, Mendiratta-Lala M, Singal AG, Su GL, Lok AS, Parikh ND. Systematic review: radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:890-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Lin YL, Li Y. Study on the hepatocellular carcinoma model with metastasis. Genes Dis. 2020;7:336-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Zeng Z, Lu Q, Liu Y, Zhao J, Zhang Q, Hu L, Shi Z, Tu Y, Xiao Z, Xu Q, Huang D. Effect of the Hypoxia Inducible Factor on Sorafenib Resistance of Hepatocellular Carcinoma. Front Oncol. 2021;11:641522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Ma XL, Hu B, Tang WG, Xie SH, Ren N, Guo L, Lu RQ. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Lin X, Li AM, Li YH, Luo RC, Zou YJ, Liu YY, Liu C, Xie YY, Zuo S, Liu Z, Fang WY. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 10. | Xiao Y, Sun Y, Liu G, Zhao J, Gao Y, Yeh S, Gong L, Chang C. Androgen receptor (AR)/miR-520f-3p/SOX9 signaling is involved in altering hepatocellular carcinoma (HCC) cell sensitivity to the Sorafenib therapy under hypoxia via increasing cancer stem cells phenotype. Cancer Lett. 2019;444:175-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Gao Y, Gu J, Wang Y, Fu D, Zhang W, Zheng G, Wang X. Hepatitis B virus X protein boosts hepatocellular carcinoma progression by downregulating microRNA-137. Pathol Res Pract. 2020;216:152981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut. 2021;70:784-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 301] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 13. | Li J, Guan X, Fan Z, Ching LM, Li Y, Wang X, Cao WM, Liu DX. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Cao L, Huang C, Cui Zhou D, Hu Y, Lih TM, Savage SR, Krug K, Clark DJ, Schnaubelt M, Chen L, da Veiga Leprevost F, Eguez RV, Yang W, Pan J, Wen B, Dou Y, Jiang W, Liao Y, Shi Z, Terekhanova NV, Cao S, Lu RJ, Li Y, Liu R, Zhu H, Ronning P, Wu Y, Wyczalkowski MA, Easwaran H, Danilova L, Mer AS, Yoo S, Wang JM, Liu W, Haibe-Kains B, Thiagarajan M, Jewell SD, Hostetter G, Newton CJ, Li QK, Roehrl MH, Fenyö D, Wang P, Nesvizhskii AI, Mani DR, Omenn GS, Boja ES, Mesri M, Robles AI, Rodriguez H, Bathe OF, Chan DW, Hruban RH, Ding L, Zhang B, Zhang H; Clinical Proteomic Tumor Analysis Consortium. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell. 2021;184:5031-5052.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 345] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 15. | Zhao Q, Liu Y, Wang T, Yang Y, Ni H, Liu H, Guo Q, Xi T, Zheng L. MiR-375 inhibits the stemness of breast cancer cells by blocking the JAK2/STAT3 signaling. Eur J Pharmacol. 2020;884:173359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Wang L, Lv Y, Li C, Yang G, Fu B, Peng Q, Jian L, Hou D, Wang J, Zhao C, Yang P, Zhang K, Wang L, Wang Z, Wang H, Xu W. Transformable Dual-Inhibition System Effectively Suppresses Renal Cancer Metastasis through Blocking Endothelial Cells and Cancer Stem Cells. Small. 2020;16:e2004548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Lu LL, Wen D, Liu DL, Dong LL, Gao DM, Bian XY, Zhou J, Fan J, Wu WZ. MiR-612 regulates invadopodia of hepatocellular carcinoma by HADHA-mediated lipid reprogramming. J Hematol Oncol. 2020;13:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Zhao Y, Xu L, Wang X, Niu S, Chen H, Li C. A novel prognostic mRNA/miRNA signature for esophageal cancer and its immune landscape in cancer progression. Mol Oncol. 2021;15:1088-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Dong SS, Dong DD, Yang ZF, Zhu GQ, Gao DM, Chen J, Zhao Y, Liu BB. Exosomal miR-3682-3p Suppresses Angiogenesis by Targeting ANGPT1 via the RAS-MEK1/2-ERK1/2 Pathway in Hepatocellular Carcinoma. Front Cell Dev Biol. 2021;9:633358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Yao B, Niu Y, Li Y, Chen T, Wei X, Liu Q. High-matrix-stiffness induces promotion of hepatocellular carcinoma proliferation and suppression of apoptosis via miR-3682-3p-PHLDA1-FAS pathway. J Cancer. 2020;11:6188-6203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Liu S, Wen Y, Quan B, Lin J, Zhu Z, Tang J, Han S. High expression of mir-3682-3p is an unfavorable prognostic factor of hepatocellular carcinoma. Nanfang Yike Daxue Xuebao. 2021;41:1885-1891. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Cao MQ, You AB, Zhu XD, Zhang W, Zhang YY, Zhang SZ, Zhang KW, Cai H, Shi WK, Li XL, Li KS, Gao DM, Ma DN, Ye BG, Wang CH, Qin CD, Sun HC, Zhang T, Tang ZY. miR-182-5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J Hematol Oncol. 2018;11:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 23. | Salem M, Shan Y, Bernaudo S, Peng C. miR-590-3p Targets Cyclin G2 and FOXO3 to Promote Ovarian Cancer Cell Proliferation, Invasion, and Spheroid Formation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Semmler G, Meyer EL, Kozbial K, Schwabl P, Hametner-Schreil S, Zanetto A, Bauer D, Chromy D, Simbrunner B, Scheiner B, Stättermayer AF, Pinter M, Schöfl R, Russo FP, Greenfield H, Schwarz M, Schwarz C, Gschwantler M, Alonso López S, Manzano ML, Ahumada A, Bañares R, Pons M, Rodríguez-Tajes S, Genescà J, Lens S, Trauner M, Ferenci P, Reiberger T, Mandorfer M. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol. 2022;76:812-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 25. | Sanduzzi-Zamparelli M, Mariño Z, Lens S, Sapena V, Iserte G, Pla A, Granel N, Bartres C, Llarch N, Vilana R, Nuñez I, Darnell A, Belmonte E, García-Criado A, Díaz A, Muñoz-Martinez S, Ayuso C, Bianchi L, Fuster-Anglada C, Rimola J, Forner A, Torres F, Bruix J, Forns X, Reig M. Liver cancer risk after HCV cure in patients with advanced liver disease without non-characterized nodules. J Hepatol. 2022;76:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Zou Y, Lin X, Bu J, Lin Z, Chen Y, Qiu Y, Mo H, Tang Y, Fang W, Wu Z. Timeless-Stimulated miR-5188-FOXO1/β-Catenin-c-Jun Feedback Loop Promotes Stemness via Ubiquitination of β-Catenin in Breast Cancer. Mol Ther. 2020;28:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Gu Y, Ji F, Liu N, Zhao Y, Wei X, Hu S, Jia W, Wang XW, Budhu A, Ji J, Zhao B, Roessler S, Zheng X. Loss of miR-192-5p initiates a hyperglycolysis and stemness positive feedback in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Peng C, Chen J, Chen D, Yang B, He B, Hu W, Zhang Y, Liu H, Dai L, Xie H, Zhou L, Wu J, Zheng S. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 456] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 29. | Jiang N, Zou C, Zhu Y, Luo Y, Chen L, Lei Y, Tang K, Sun Y, Zhang W, Li S, He Q, Zhou J, Chen Y, Luo J, Jiang W, Ke Z. HIF-1ɑ-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and Notch signaling. Theranostics. 2020;10:2553-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 30. | Chen MK, Zhou JH, Wang P, Ye YL, Liu Y, Zhou JW, Chen ZJ, Yang JK, Liao DY, Liang ZJ, Xie X, Zhou QZ, Xue KY, Guo WB, Xia M, Bao JM, Yang C, Duan HF, Wang HY, Huang ZP, Qin ZK, Liu CD. BMI1 activates P-glycoprotein via transcription repression of miR-3682-3p and enhances chemoresistance of bladder cancer cell. Aging (Albany NY). 2021;13:18310-18330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Lin Z, Niu Y, Wan A, Chen D, Liang H, Chen X, Sun L, Zhan S, Chen L, Cheng C, Zhang X, Bu X, He W, Wan G. RNA m6 A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020;39:e103181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 32. | Yao S, Fan LY, Lam EW. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin Cancer Biol. 2018;50:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 33. | Liu H, Song Y, Qiu H, Liu Y, Luo K, Yi Y, Jiang G, Lu M, Zhang Z, Yin J, Zeng S, Chen X, Deng M, Jia X, Gu Y, Chen D, Zheng G, He Z. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27:966-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 34. | Fu Q, Song X, Liu Z, Deng X, Luo R, Ge C, Li R, Li Z, Zhao M, Chen Y, Lin X, Zhang Q, Fang W. miRomics and Proteomics Reveal a miR-296-3p/PRKCA/FAK/Ras/c-Myc Feedback Loop Modulated by HDGF/DDX5/β-catenin Complex in Lung Adenocarcinoma. Clin Cancer Res. 2017;23:6336-6350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Sun W, Li J, Zhou L, Han J, Liu R, Zhang H, Ning T, Gao Z, Liu B, Chen X, Ba Y. The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer. Theranostics. 2020;10:1981-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 36. | Deng X, Liu Z, Liu X, Fu Q, Deng T, Lu J, Liu Y, Liang Z, Jiang Q, Cheng C, Fang W. miR-296-3p Negatively Regulated by Nicotine Stimulates Cytoplasmic Translocation of c-Myc via MK2 to Suppress Chemotherapy Resistance. Mol Ther. 2018;26:1066-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Komoll RM, Hu Q, Olarewaju O, von Döhlen L, Yuan Q, Xie Y, Tsay HC, Daon J, Qin R, Manns MP, Sharma AD, Goga A, Ott M, Balakrishnan A. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. 2021;74:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 38. | Li J, Zhang S, Zou Y, Wu L, Pei M, Jiang Y. miR-145 promotes miR-133b expression through c-myc and DNMT3A-mediated methylation in ovarian cancer cells. J Cell Physiol. 2020;235:4291-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Huang P, Xu Q, Yan Y, Lu Y, Hu Z, Ou B, Zhang H, Mao K, Zhang J, Wang J, Xiao Z. HBx/ERα complex-mediated LINC01352 downregulation promotes HBV-related hepatocellular carcinoma via the miR-135b-APC axis. Oncogene. 2020;39:3774-3789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Lin X, Zuo S, Luo R, Li Y, Yu G, Zou Y, Zhou Y, Liu Z, Liu Y, Hu Y, Xie Y, Fang W. HBX-induced miR-5188 impairs FOXO1 to stimulate β-catenin nuclear translocation and promotes tumor stemness in hepatocellular carcinoma. Theranostics. 2019;9:7583-7598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 41. | Salerno D, Chiodo L, Alfano V, Floriot O, Cottone G, Paturel A, Pallocca M, Plissonnier ML, Jeddari S, Belloni L, Zeisel M, Levrero M, Guerrieri F. Hepatitis B protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription. Gut. 2020;69:2016-2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |