Published online Jun 26, 2022. doi: 10.4252/wjsc.v14.i6.420
Peer-review started: February 16, 2022
First decision: April 19, 2022
Revised: April 21, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: June 26, 2022
Processing time: 127 Days and 22.6 Hours
Treatments involving stem cell (SC) usage represent novel and potentially interesting alternatives in facial nerve reanimation. Current literature includes the use of SC in animal model studies to promote graft survival by enhancing nerve fiber growth, spreading, myelinization, in addition to limiting fibrotic dege
To investigate the histological, neurophysiological, and functional outcomes in facial reanimation using SC, compared to autograft.
Our study is a systematic review of the literature, consistently conducted according to the preferred reporting items for systematic reviews and meta-analyses statement guidelines. The review question was: In facial nerve reanimation on rats, has the use of stem cells revealed as effective when compared to autograft, in terms of histological, neurophysiological, and functional outcomes? Random-effect meta-analysis was conducted on histological and neurophysiological data from the included comparative studies.
After screening 148 manuscript, five papers were included in our study. 43 subjects were included in the SC group, while 40 in the autograft group. The meta-analysis showed no significative differences between the two groups in terms of myelin thickness [CI: -0.10 (-0.20, 0.00); I2 = 29%; P = 0.06], nerve fibers diameter [CI: 0.72 (-0.93, 3.36); I2 = 72%; P = 0.6], compound muscle action potential amplitude [CI: 1.59 (0.59, 3.77); I2 = 89%; P = 0.15] and latency [CI: 0.66 (-1.01, 2.32); I2 = 67%; P = 0.44]. The mean axonal diameter was higher in the autograft group [CI: 0.94 (0.60, 1.27); I2 = 0%; P ≤ 0.001].
The role of stem cells in facial reanimation is still relatively poorly studied, in animal models, and available results should not discourage their use in future studies on human subjects.
Core Tip: Our meta-analysis of studies comparing the use of autograft and stem cells for facial nerve reanimation in rats suggest that there appears to be no advantages in favor of stem cells, according to the evaluated histological and neurophysiological outcomes. Stem cell treatments have proven to be an interesting and viable option in numerous fields of surgery that have vast supporting scientific and clinically applicable literature. The role of stem cells in facial reanimation is still relatively new and poorly studied due to the liming nature and number of studies carried out exclusively in animal models.
- Citation: Ricciardi L, Pucci R, Piazza A, Lofrese G, Scerrati A, Montemurro N, Raco A, Miscusi M, Ius T, Zeppieri M. Role of stem cells-based in facial nerve reanimation: A meta-analysis of histological and neurophysiological outcomes. World J Stem Cells 2022; 14(6): 420-428
- URL: https://www.wjgnet.com/1948-0210/full/v14/i6/420.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i6.420
Facial nerve (FN) palsy (FNP) represents a relevant issue, which poses a great burden on socio-economical health-related costs[1]. This condition constitutes a limitation in social relations, eventually affecting psycho-mental health[2]. Other than facial movements limitations, a severe FN disfunction results in functional disorders such as ipsilateral corneal ulcerations and unvoluntary drooling[3]. There are several medical specialists that need to be involved in the management of these patients, which include neurologists, neurosurgeons, ophthalmologists, maxillo-facial surgeons, ENTs, psychiatrics, and physiotherapists. A multidisciplinary management from numerous specialists tends to make this topic of wide interest with a large audience of readers, including different medical and paramedical fields[4,5].
Traumatic injuries, infectious diseases, metabolic disorders, and iatrogenic causes may determine different grades of FNP, requiring specific treatments according to the single case specifics[4]. In the short-to-midterm facial palsy, conservative management is usually preferred in cases of facial nerve anatomical preservation, while reconstructive techniques, such as nerve grafting, or flap harvesting are considered in patients showing facial nerve interruption or non-spontaneous restoration for longer than 6 mo[4]. The functional-aesthetic outcome, however, is often lower than expected after reconstructive surgeries. In addition, cranial nerves need to be partially sacrificed for the proximal coaptation of the nerve graft.
A current frontier in facial nerve reanimation are potentially represented by stem cells (SC). The role of SC in facilitating and accelerating nerve fibers spreading throughout grafts, ameliorating the myelinization, and reducing fibrotic degeneration have been recently reported in animal models[6-9]. The aim of our systematic review of the literature and meta-analysis of the comparative studies available in current literature was to investigate the histological, neurophysiological, and functional outcomes in facial reanimation using SC, compared to autograft.
The present study is a systematic review of the literature, consistently conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.
The review questions, according to the PRISMA statement, was formulated following the PICO (P: patients; I: intervention; C: comparison; O: outcomes) scheme, as it follows: In facial nerve reanimation on rats (P), has the use of stem cells (I) revealed as effective when compared to autograft (C), in terms of histological, neurophysiological, and functional outcomes (O)?
Screened papers were considered for eligibility if they: focused on the use of SC for FN reanimation in rats; included a comparative group with autograft; reported the type of SC, histological analysis of myelinization of nerve fibers, neurophysiological analysis of the compound muscle action potential (CMAP) amplitude and latency, data on the residual mobility, and the length of the follow-up (FU). Exclusion criteria included language other than English, non-comparative studies, and non-reported quantitative data for analysis. Papers reporting incomplete or not pool- able data, such as means missing of standard deviations or medians missing of interquartile ranges, were excluded or included only for the follow-up periods during which the data were complete.
Four different medical databases (PubMed, Scopus, Cochrane Library, Mendeley) were screened for identifying pertinent papers, using Reference Citation Analysis (https://www.referencecitationanalysis.com/). The search terms “stem cell”, “facial nerve”, “regeneration”, “repair”, “functional restoration”, “reanimation” were combined using the Boolean operators “AND” and “OR”. In the first review round, Title and Abstract of the papers were independently screened by two authors (R.P. and A.P.). Duplicated papers were excluded from the screening. In the second review round, papers included for the Full text analysis were screened, and considered for inclusion according to the inclusion criteria. The references of the included papers were then screened for papers erroneously missed in the first review round (forward search). Papers not considered as eligible were excluded with reason. Any discordance in the screening process were solved by consensus with a third senior author (L.R.). Included papers were considered for data analysis and evidence synthesis.
Title, list of authors, year and journal of publication were collected for every included paper. Animal type, number per each treatment group, surgical strategy, and the type of cells used were databased.
The following outcomes were extracted from the included papers: (1) Histological outcomes: myelin thickness, density of myelinated fibers, number of axons, axonal density, axonal diameter; (2) Neurophysiological outcomes: CMAP amplitude (mV), CMAP latency (ms), CMAP duration (ms); (3) Functional outcomes: residual mobility of the vibrissae; and (4) Complications.
Data of the study populations were summarized using proportion and weighed means. The mean and standard deviations in individual studies were estimated from the median and interquartile ranges, when needed, according to the method described by Wan et al[10]. Pooled mean differences for continuous variables were computed between outcome groups with a random effects model[11]. Comprehensive meta-analysis software (Review Manager - RevMan 5.4.1 The Cochrane Collaboration, 2020) was used for pooling data. P-value was considered significant at α < 0.05.
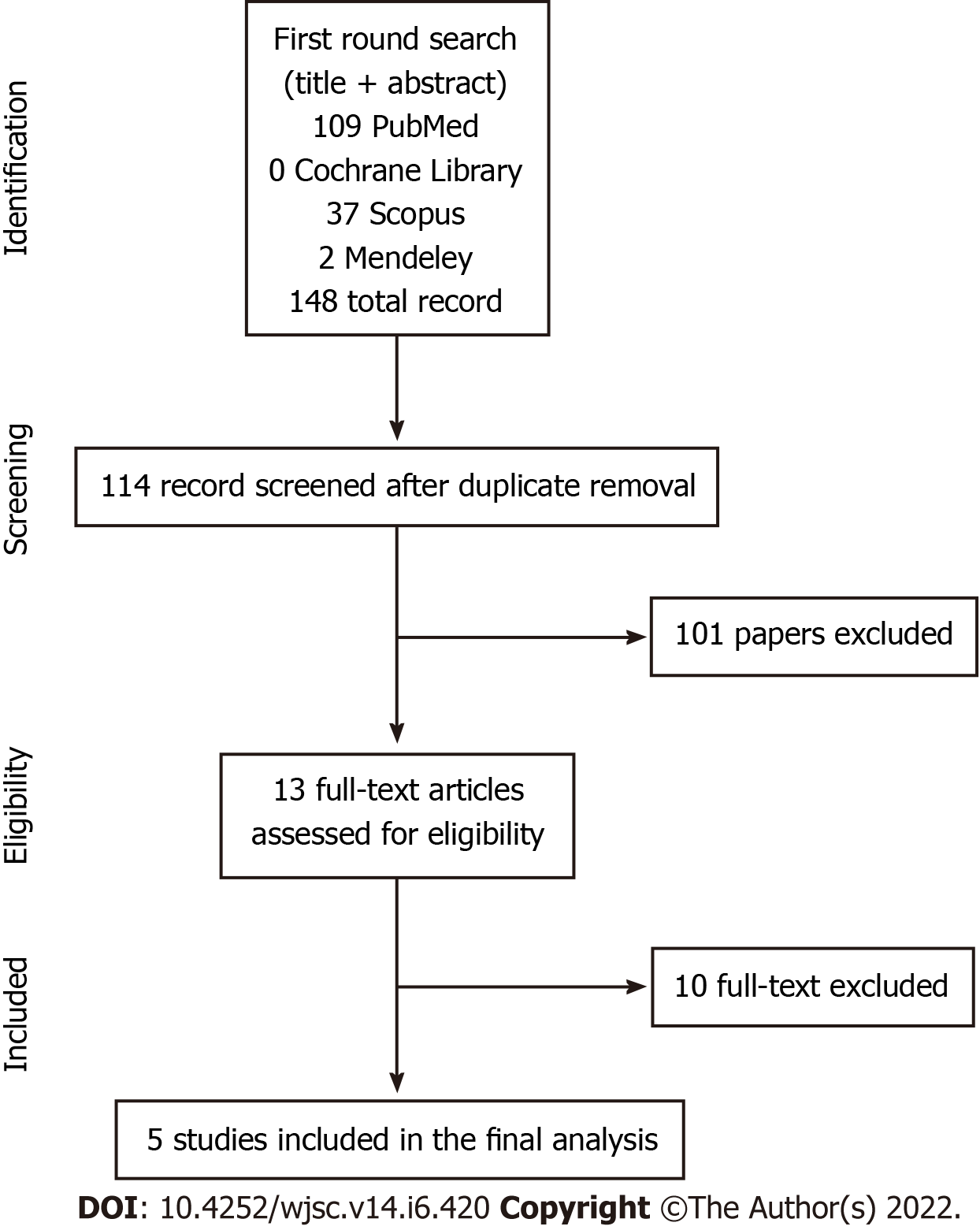
The first round of search on the selected database identified 148 abstracts to be screened. According to our inclusion criteria, five papers met these criteria and were included in the final meta-analysis of comparative studies[12-16]. See Figure 1 - Search strategy.
From the five included papers, 43 subject were included in the study group (SC), while 40 were included in the control group (Autograft). In the study group, adipose-derived stem cells (ASC) were used in 26 subject, stem cells from human exfoliated deciduous teeth (SHED) in 10, and bone marrow stem cells (BMSC) in 7.
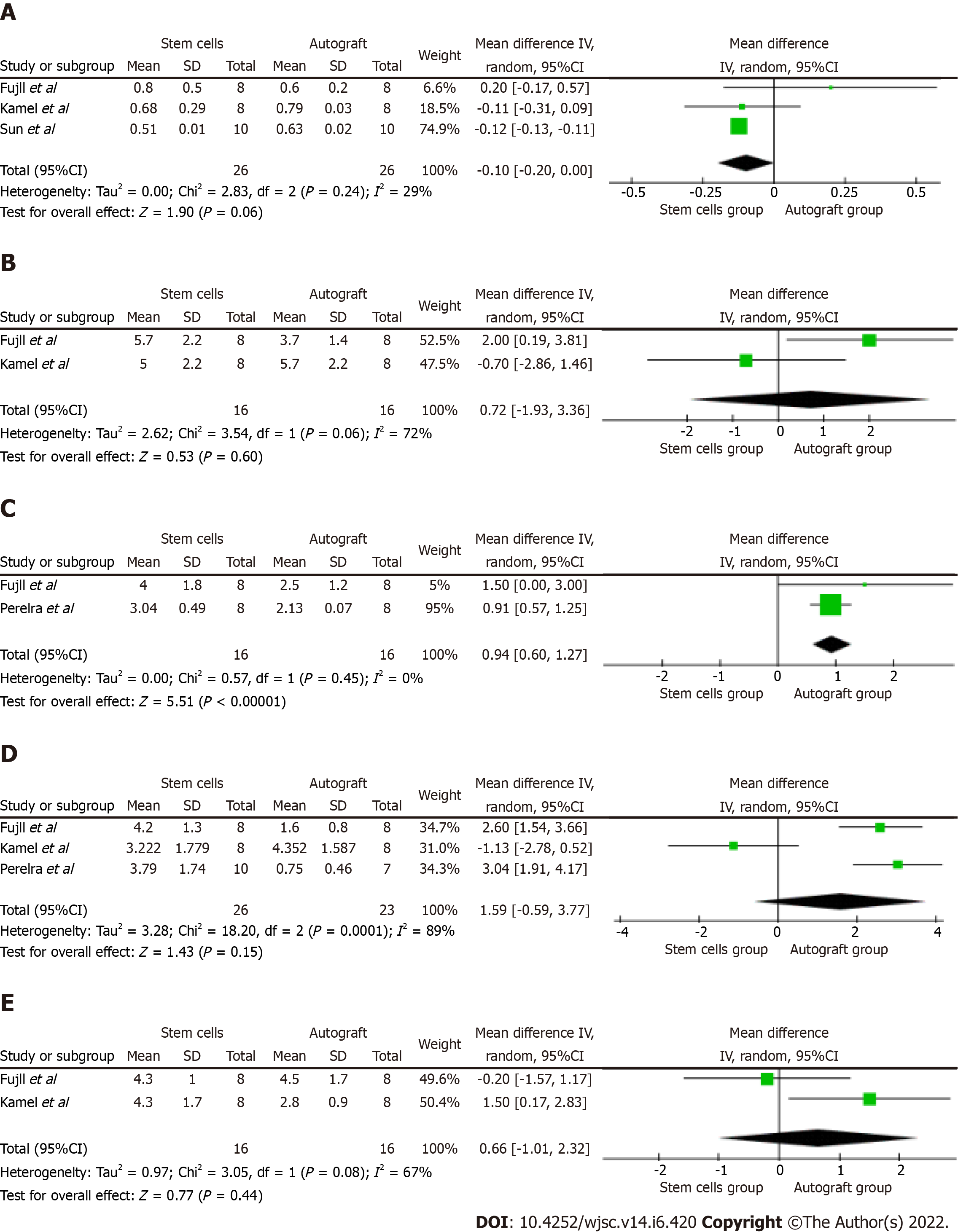
The evaluation of myelin thickness was reported in three[12,13,15] of the five included studies, on a total of 28 subjects from the study group and 28 from the control group. The meta-analysis showed no significative differences in terms of myelin thickness between the two groups, and a low heterogeneity between the contributing studies [CI: 0.10 (-0.20, 0.00); I2 = 29%; P = 0.06] (Figure 2A).
The nerve fiber diameters were evaluated in two[12,15] of the five included studies, which included data of 16 subjects from the study group and 16 from the control group. Our data analysis showed no significative differences in terms of nerve fibers diameter between the two groups, and a high heterogeneity between the contributing studies [CI: 0.72 (-0.93, 3.36); I2 = 72%; P = 0.6] (Figure 2B).
Two studies[12,16] reported data on the axonal diameter, including a total of 16 subjects in the study group and 16 in the control group. The pooled analysis showed a significatively higher axonal diameter in the control group, and a very low heterogeneity between the contributing studies [CI: 0.94 (0.60, 1.27); I2 = 0%; P < 0.001] (Figure 2C).
The CMAP amplitude was reported in three[12,15,16] of the five included studies, which was evaluated in 26 subjects from the study group and 23 from the control group. Our data analysis showed that no significative differences existed between the treatment groups, although a high heterogeneity was measured between the contributing studies [CI: 1.59 (0.59, 3.77); I2 = 89%; P = 0.15] (Figure 2D).
Quantitative data on CMAP latency were reported in two[12,15] of the five included studies that included 16 subjects from the study group and 16 from the control group. The data analysis showed that no significative differences existed in terms of CMAP latency between the study and the control group, although a medium-to-high heterogeneity was calculated between the contributing studies [CI: 0.66 (-1.01, 2.32); I2 = 67%; P = 0.44] (Figure 2E).
Data on density of myelinated fibers, CMAP duration, and residual mobility was only reported in 1 of the 5 studies, thus preventing any data pooling for analysis.
Previous studies have reported data on the use of mesenchymal stem cells[6], dental pulp stem cells[17], gingiva-derived mesenchymal stem cells[18], bone-marrow derived stem cells, and adipose-tissue derived stem cells[7] in nerve regeneration after peripheral nerve injury. These treatments have demonstrated several advantages with regards to nerve fiber spreading, myelinization, and regeneration of the optimal perineural environment, thus tending to reduce fibrosis and inflammatory-mediated disorders[7-9,18]. Adipose tissue has been used in peripheral nerve reconstruction after sciatic nerve transection in animal models, which seemed to provide relevant advantages in terms of nerve fiber density, axon area, and myelin area[19]. It is important, however, to demonstrate that histology translates to functional benefits, which was investigated by Schweizer et al[19] and Tuncel et al[20], using a swim test and a walking track analysis, respectively, thus confirming clinical effectiveness.
Standard reconstructive techniques use autograft for filling the gap between the proximal stump and the distal nerve in facial nerve reanimation[4]. Studies have shown that autograft use tend to be superior to acellular grafts in these cases, in which sensory nerves are considered as the gold standard graft for motor nerves reconstruction[8]. In designing our meta-analysis, we thus included only studies comparing autografts and stem cells for facial nerve reanimation in rats.
Our meta-analysis analysis of comparative studies highlighted that the use of BMSC, ASC, or SHED do not improve the histological and neurophysiological outcomes in facial nerve reanimation in rats in a short-term follow-up, compared to the use of autograft. Our analysis showed that the use of SC was able to slightly increase the mean myelin thickness, although the difference with the autograft group was not significative (P = 0.06). The mean axonal diameter, however, was significantly higher when using autografts (P < 0.001). It is important to note that the axons in the autograft groups were not distinguished in newer spreading axons and native fibers, thus need careful interpretation. Similarly, the mean nerve fibers diameter was not different between the study and control group (P = 0.06), although it was slightly wider in the autograft group.
With regards to the neurophysiological outcomes, our pooled analysis showed no differences between the study and control group in terms of CMAP amplitude (P = 0.15) and latency (P = 0.44). The innervated muscles did not seem to benefit from the use of stem cells for facial nerve reanimation, in terms of earlier and effective reinnervation. This should be carefully and critically interpretated, since the timing for reinnervation plays a relevant role in peripheral nerves surgery. The reinnervation itself may result as useless once the interested muscle has already experience a non-reversable degeneration once the long-term denervation occurred. It is important to note that the study protocols of the papers assessed in our study, set a maximum follow-up 13 wk, which may be too short and not sufficient in evaluating reinnervation after treatment. Nerve fibers regenerate 1mm per day in humans, which is assumed to be similar with regrowth rates in animals. Reinnervation may occur within up to 12 mo in patients undergoing surgery for facial reanimation[4]; therefore, data on reinnervation should be carefully interpreted, especially if based on short follow-up observation times. Limiting results with stem cells may be due to short healing time assessments as opposed to lack of efficacy. Only future studies based on extensive follow-up periods after treatment can provide true answers.
A critical analysis and discussion of our results are fundamental to provide a correct interpretation of our study. The histological findings and the non-reported significative differences between the use of autograft or stem cells could be of limited clinical use. The short follow-up time (of a maximum of 13 wk) may prove not to be sufficient in assessing medium-to-long term differences in new generated fibers spreading throughout the conduct. Furthermore, fibrotic degeneration may be influenced when using stem cells, and fibrosis, which normally occurs much later, may not have been an important factor in short time outcomes, yet of utmost importance in long term functional and histological results that could not be considered in these short follow-up studies.
The neurophysiological results showed no differences in terms of CMAP amplitude and latency. Once again, the short-term follow-up must be considered when evaluating the nerve conduction and the muscle activation. Accordingly, the similar neurophysiological outcomes between the two treatment groups cannot be considered as reliable for clinical application.
Functional outcomes in terms of residual movements and spontaneous movements restoration were not quantitatively reported, thus preventing any meta-data analysis. As reported in numerous papers, the functional outcomes should be considered as the primary outcome in facial reanimation techniques since it has a primary impact on the needs and satisfaction of the patient. The reinnervation and the histological pattern might play a marginal role in cases of non-functional restoration of muscles function.
Based on the results of our meta-analysis, the use of autograft should still be preferred in facial reinnervation, due to the non-significative differences compared to the use of stem cells. Current studies in literature based on animal subjects are limiting in terms of type of assessments, number of cases and short follow-up time evaluations, thus not sufficient in discouraging the clinical use of SC for facial reanimation.
Our study has several limitations that need to be disclosed for a proper data interpretation. Only five studies only were included in the analysis; the type of rats was not the same throughout the studies; the surgical technique was not the same in the study protocols; different type of stem cells were used in each study; the follow-up time for outcomes evaluation was not homogeneous between the studies; there were no quantitative data on residual movements after treatment, thus preventing any analysis of functional outcomes.
Our meta-analysis of studies comparing the use of autograft and stem cells for facial nerve reanimation in rats suggest that there appears to be no advantages in favor of stem cells, according to the evaluated histological and neurophysiological outcomes. A higher heterogeneity amongst the included studies, short follow-up time periods and the limitations of our investigation should be carefully considered for a proper data interpretation. Stem cell treatments have proven to be an interesting and viable option in numerous fields of surgery that have vast supporting scientific and clinically applicable literature. The role of stem cells in facial reanimation is still relatively new and poorly studied due to the liming nature and number of studies carried out exclusively in animal models. Future studies based on longer follow-up with homogenous criteria, preferably on human subjects, can pave the way to stem cell therapy in patients with nerve palsy.
Treatments involving stem cell (SC) usage represent novel and potentially interesting alternatives in facial nerve reanimation. Current literature includes the use of SC in animal model studies to promote graft survival by enhancing nerve fiber growth, spreading, myelinization, in addition to limiting fibrotic degeneration after surgery. However, the effectiveness of the clinical use of SC in facial nerve reanimation has not been clarified yet.
To investigate the histological, neurophysiological, and functional outcomes in facial reanimation using SC, compared to autograft.
The objectives of our systematic review of the literature and meta-analysis of the comparative studies available in current literature was to investigate the histological, neurophysiological, and functional outcomes in facial reanimation using SC, compared to autograft.
Our study is a systematic review of the literature, consistently conducted according to the preferred reporting items for systematic reviews and meta-analyses statement guidelines. The review question was: In facial nerve reanimation on rats, has the use of stem cells revealed as effective when compared to autograft, in terms of histological, neurophysiological, and functional outcomes? Random-effect meta-analysis was conducted on histological and neurophysiological data from the included comparative studies.
After screening 148 manuscript, five papers were included in our study. 43 subjects were included in the SC group, while 40 in the autograft group. The meta-analysis showed no significative differences between the two groups in terms of myelin thickness [CI: -0.10 (-0.20, 0.00); I2 = 29%; P = 0.06], nerve fibers diameter [CI: 0.72 (-0.93, 3.36); I2 = 72%; P = 0.6], Compound Muscle Action Potential amplitude [CI: 1.59 (0.59, 3.77); I2 = 89%; P = 0.15] and latency [CI: 0.66 (-1.01, 2.32); I2 = 67%; P = 0.44]. The mean axonal diameter was higher in the autograft group [CI: 0.94 (0.60, 1.27); I2 = 0%; P ≤ 0.001].
The role of stem cells in facial reanimation is still relatively poorly studied, in animal models, and available results should not discourage their use in future studies on human subjects.
The role of stem cells in facial reanimation is still relatively new and poorly studied due to the liming nature and number of studies carried out exclusively in animal models. Future studies based on longer follow-up with homogenous criteria, preferably on human subjects, can pave the way to stem cell therapy in patients with nerve palsy.
The present study is a systematic review of the literature, consistently conducted according to the Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delgado-Gallegos JL, Mexico; Grawish ME, Egypt; Long X, China; Shalaby MN, Egypt S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Li MK, Niles N, Gore S, Ebrahimi A, McGuinness J, Clark JR. Social perception of morbidity in facial nerve paralysis. Head Neck. 2016;38:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Dey JK, Ishii LE, Byrne PJ, Boahene KD, Ishii M. Seeing is believing: objectively evaluating the impact of facial reanimation surgery on social perception. Laryngoscope. 2014;124:2489-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Lassaletta L, Alfonso C, Del Rio L, Roda JM, Gavilan J. Impact of facial dysfunction on quality of life after vestibular schwannoma surgery. Ann Otol Rhinol Laryngol. 2006;115:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Ricciardi L, Stifano V, Pucci R, Stumpo V, Montano N, Della Monaca M, Lauretti L, Olivi A, Valentini V, Sturiale CL. Comparison between VII-to-VII and XII-to-VII coaptation techniques for early facial nerve reanimation after surgical intra-cranial injuries: a systematic review and pooled analysis of the functional outcomes. Neurosurg Rev. 2021;44:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Hoffman WY. Reanimation of the paralyzed face. Otolaryngol Clin North Am. 1992;25:649-667. [PubMed] |
| 6. | Zhang RC, Du WQ, Zhang JY, Yu SX, Lu FZ, Ding HM, Cheng YB, Ren C, Geng DQ. Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review. Neural Regen Res. 2021;16:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Li Y, Kamei Y, Kambe M, Ebisawa K, Oishi M, Takanari K. Peripheral Nerve Regeneration Using Different Germ Layer-Derived Adult Stem Cells in the Past Decade. Behav Neurol. 2021;2021:5586523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Podsednik A, Cabrejo R, Rosen J. Adipose Tissue Uses in Peripheral Nerve Surgery. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Lopes B, Sousa P, Alvites R, Branquinho M, Sousa AC, Mendonça C, Atayde LM, Luís AL, Varejão ASP, Maurício AC. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 10. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 7045] [Article Influence: 640.5] [Reference Citation Analysis (0)] |
| 11. | DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1795] [Article Influence: 94.5] [Reference Citation Analysis (1)] |
| 12. | Fujii K, Matsumine H, Osaki H, Ueta Y, Kamei W, Niimi Y, Hashimoto K, Miyata M, Sakurai H. Accelerated outgrowth in cross-facial nerve grafts wrapped with adipose-derived stem cell sheets. J Tissue Eng Regen Med. 2020;14:1087-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Sun F, Zhou K, Mi WJ, Qiu JH. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci Lett. 2011;499:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Costa HJ, Bento RF, Salomone R, Azzi-Nogueira D, Zanatta DB, Paulino Costa M, da Silva CF, Strauss BE, Haddad LA. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013;1510:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Kamei W, Matsumine H, Osaki H, Ueta Y, Tsunoda S, Shimizu M, Hashimoto K, Niimi Y, Miyata M, Sakurai H. Axonal supercharged interpositional jump-graft with a hybrid artificial nerve conduit containing adipose-derived stem cells in facial nerve paresis rat model. Microsurgery. 2018;38:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Pereira LV, Bento RF, Cruz DB, Marchi C, Salomone R, Oiticicca J, Costa MP, Haddad LA, Mingroni-Netto RC, Costa HJZR. Stem Cells from Human Exfoliated Deciduous Teeth (SHED) Differentiate in vivo and Promote Facial Nerve Regeneration. Cell Transplant. 2019;28:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Luzuriaga J, Polo Y, Pastor-Alonso O, Pardo-Rodríguez B, Larrañaga A, Unda F, Sarasua JR, Pineda JR, Ibarretxe G. Advances and Perspectives in Dental Pulp Stem Cell Based Neuroregeneration Therapies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review. Front Immunol. 2021;12:667221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 19. | Schweizer R, Schnider JT, Fanzio PM, Tsuji W, Kostereva N, Solari MG, Plock JA, Gorantla VS. Effect of Systemic Adipose-derived Stem Cell Therapy on Functional Nerve Regeneration in a Rodent Model. Plast Reconstr Surg Glob Open. 2020;8:e2953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Tuncel U, Kostakoglu N, Turan A, Çevik B, Çayli S, Demir O, Elmas C. The Effect of Autologous Fat Graft with Different Surgical Repair Methods on Nerve Regeneration in a Rat Sciatic Nerve Defect Model. Plast Reconstr Surg. 2015;136:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |