Published online Feb 26, 2021. doi: 10.4252/wjSC.v13.i2.177
Peer-review started: July 31, 2020
First decision: October 21, 2020
Revised: October 31, 2020
Accepted: November 11, 2020
Article in press: November 11, 2020
Published online: February 26, 2021
Processing time: 208 Days and 0.6 Hours
Motion sickness (MS) is a disease that occurs during unbalanced movement, characterized by gastrointestinal symptoms and autonomic nervous system activation. Current clinical treatments for MS are limited. Recent evidence indicates that the levels of pro-inflammatory cytokines increase during MS and are associated with an inner ear immune imbalance. In the present study, mesenchymal stem cells (MSCs) have been shown to exert strong immuno-suppressive effects.
To explore whether umbilical cord-derived mesenchymal stem cells (UC-MSCs) can prevent the occurrence of MS, and the underlying mechanism regulated by MSCs in a mouse model of MS.
A total of 144 (equal numbers of males and females) 5wkold BALB/c mice were randomly divided into five groups: Normal group (n = 16), MS group (n = 32), MSCs group (n = 32), MS + MSCs group (n = 32), and MS + AS101/MSCs group (n = 32). The MSCs group (n = 32), MS + MSCs group (n = 32), and MS + AS101/MSCs group (n = 32) were preventively transplanted with UC-MSCs or AS101-treated UC-MSCs (1 × 106 cells/mouse). Mice in the MS (n = 32), MS + MSCs, and MS + AS101/MSCs groups were subjected to rotation on a centrifuge for 10 min at 8 × g/min for MS model establishment on days 3, 5, 8, and 10 after UC-MSCs injection. The Morris water maze (MWM) test was used to observe the symptom of dizziness. Enzyme-linked immunosorbent assay (ELISA) and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were used to detect the levels of inflammatory cytokines in mice peripheral blood and the petrous part of the temporal bone samples. Western blot analysis was performed to analyze the JAK2/STAT3 signaling pathway in the cochlear tissues. Histological examination was performed by hematoxylin and eosin (HE) staining for conventional morphological evaluation in the petrous part of temporal bone samples.
The MWM test demonstrated that UC-MSCs improved the symptoms of MS. The MS + MSCs group was faster than the MS group on days 3 and 5 (P = 0.036 and P = 0.002, respectively). ELISA and RT-qPCR showed that the serum and mRNA levels of interleukin-10 (IL-10) in the cochlear tissues were increased after transplantation with UC-MSCs (MS + MSCs group vs MS group at 3 and 5 d, P = 0.002 and cP < 0.001, respectively). RT-qPCR results confirmed a significant increase in IL-10 levels at four time points (MS + MSCs group vs MS group, P = 0.009, P = 0.009, P = 0.048, and P = 0.049, respectively). This suggested that UC-MSCs reduced the sensitivity of the vestibular microenvironment by secreting IL-10. Moreover, Western blot analysis showed that the MSCs activated the JAK2/STAT3 signaling pathway in the cochlear tissues. The levels of IL-10, IL-10RA, JAK2, STAT3, and phosphorylated JAK2 and STAT3 in the MS + MSCs group were increased compared to those of the MS group (P < 0.05). The morphological changes in the four groups showed no significant differences. The role of IL-10 secretion on the ability of UC-MSCs to successfully improve the symptoms of MS was confirmed by the diminished therapeutic effects associated with treatment with the IL-10 inhibitor ammonium trichloro (dioxoethylene-o,o′) tellurate (AS101).
Prophylactic transplantation of UC-MSCs can alleviate the clinical symptoms of MS in mice, particularly at 3-5 d after preventive transplantation. The mechanism for UC-MSCs to reduce the sensitivity of vestibular cortex imbalance may be the secretion of IL-10. The next step is to demonstrate the possibility of curing MS in the vestibular environment by intermittent transplantation of MSCs. Above all, MSCs are expected to become a new method for the clinical prevention and treatment of MS.
Core Tip: This study demonstrated that prophylactic transplantation of umbilical cord-derived mesenchymal stem cells (UC-MSCs) can alleviate motion sickness (MS) in mice, particularly at 3-5 d after transplantation. After the MS model was established, molecular biology experiments confirmed the upregulation of interleukin-10 (IL-10) in the cochlea tissues. The transplanted UC-MSCs have activated the JAK2/STAT3 signaling pathway. They reduced the sensitivity of the vestibular cortex to imbalanced movements by secreting IL-10. Accordingly, MSC transplantation is expected to be a new strategy for the prevention and treatment of MS.
- Citation: Zhu HS, Li D, Li C, Huang JX, Chen SS, Li LB, Shi Q, Ju XL. Prior transfusion of umbilical cord mesenchymal stem cells can effectively alleviate symptoms of motion sickness in mice through interleukin 10 secretion. World J Stem Cells 2021; 13(2): 177-192
- URL: https://www.wjgnet.com/1948-0210/full/v13/i2/177.htm
- DOI: https://dx.doi.org/10.4252/wjSC.v13.i2.177
Despite the increase in convenient transportation, two thirds of all travelers suffer from motion sickness (MS)[1]. MS mainly manifests as acute nausea, vomiting, and other gastrointestinal symptoms, along with autonomic nervous excitement such as a pale complexion and decreased blood pressure, when traveling in boats, cars, and airplanes[2,3]. The appearance of these symptoms exacerbates the psychological burden of travel for the individual. MS has already been recognized in Ancient China, and the Chinese medical classics have distinguished several forms of travel sickness[4]. Currently, the main pathogenic mechanism of MS is based on the sensory conflict hypothesis, in which the vestibular cortex potentially plays a role in the internal dynamics. In addition, single-nucleotide polymorphisms in some genes and epigenetic modulation have been suggested to be related to MS[5]. In recent years, the role of the immune system in mediating pathologies of the vestibular microenvironment has received increasing attention[6]. The inflammatory reaction caused by immune disorders often has devastating effects on hearing and balance[7]. However, the current treatment for MS is predominantly medication such as anticholinergics (atropine and scopolamine) and antihistamines (betahistine)[8]. In addition, monoamine antagonists, stimulants, and sedatives have also been used in small amounts[9]. However, the efficacy of these medications showed great differences between individuals and adverse effects include drowsiness, blurred vision, and dry mouth[10].
Recent studies have shown that the levels of pro-inflammatory cytokines increase during MS[11]. A clinical trial on human MS showed an increased sensitivity of inflammatory cytokine-producing cells[12], and glucocorticoid treatment could reduce the release of inflammatory cytokines by suppressing nuclear factor-κB binding after an inflammatory stimulus. However, the side effects caused by glucocorticoid treatment, including osteoporosis and insulin resistance, cannot be ignored[13]. Alternatively, mesenchymal stem cells (MSCs) show potent anti-inflammatory effects without such side effects, and have been applied in the treatment of a variety of clinical diseases. MSCs have low immunogenicity and are thus widely used in treating immune-based disorders[14]. For example, MSC transplantation can reduce disease progression in patients with systemic lupus erythematous[15]. In addition, activated MSCs exert immunosuppressive effects in the treatment of patients with graft-versus-host disease (GvHD)[16]. However, there are no reports of MSC treatment for MS.
With the goal of avoiding the adverse reactions in MS treatment, the aim of the present study was to investigate the influence of the prophylactic transplantation of umbilical cord-derived MSCs (UC-MSCs) on MS symptoms in a mouse model. Given the potential role of inner ear immune imbalance in the pathogenesis of MS, we hypothesized that UC-MSCs may exert immunosuppressive effects in the vestibular microenvironment of mice. This experiment was divided into four time periods to determine the optimal prophylactic transplantation time of UC-MSCs, and to provide a framework for establishing a new treatment plan for the clinical prevention of MS.
UC-MSCs were prepared and identified as described previously[17]. The cells were cultured in dishes with alpha-minimal essential medium containing 10% fetal bovine serum (FBS; Gibco, United States), 1% penicillin-streptomycin (Gibco) at 37 °C with 5% CO2. The cells were used after three to five passages.
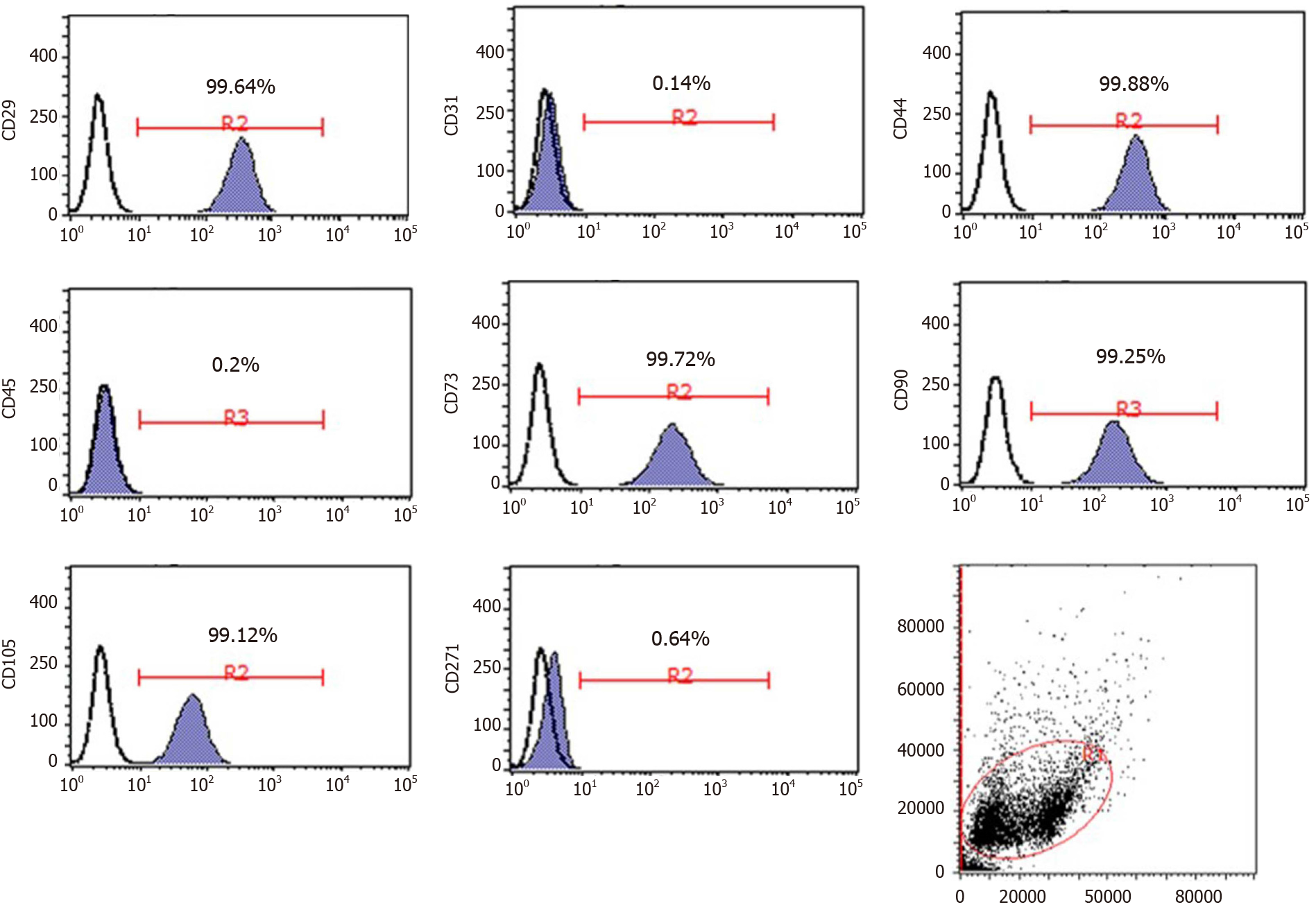
To confirm the identity of the cells, MSC cell surface markers were detected by flow cytometry. The cells were trypsinized and resuspended in phosphate-buffered saline (PBS) at the desired concentration of 1.0-2.0 × 106 cells/mL, followed by staining with corresponding antibodies against the following fluorescent-conjugated proteins: CD29-phycoerythrin (PE), CD31-PE, CD44-PE, CD45-fluorescein isothiocyanate (FITC), CD73-PE, CD90-FITC, CD105-PE, and CD271-PE. All antibodies were purchased from eBioscience, Inc. (1:20 dilutions; San Diego, CA, United States) and used according to the manufacturer’s protocol. Flow cytometry was performed using the Guava easyCyte 6HT system (EMD Millipore, Billerica, MA, United States), and the data were analyzed using Guava Incyte software (3.1 version, EMD Millipore). To investigate the differentiation potential, P3 cells were cultured under conditions appropriate for inducing osteogenic (HUXUC-90021, Cyagen, China), adipogenic (HUXUC-90031, Cyagen), and chondrogenic differentiation (HUXUC-90042, Cyagen). All differentiation-related experiments were performed according to the manufacturer’s protocol. At the end of 21 d, all cells were collected for subsequent analysis.
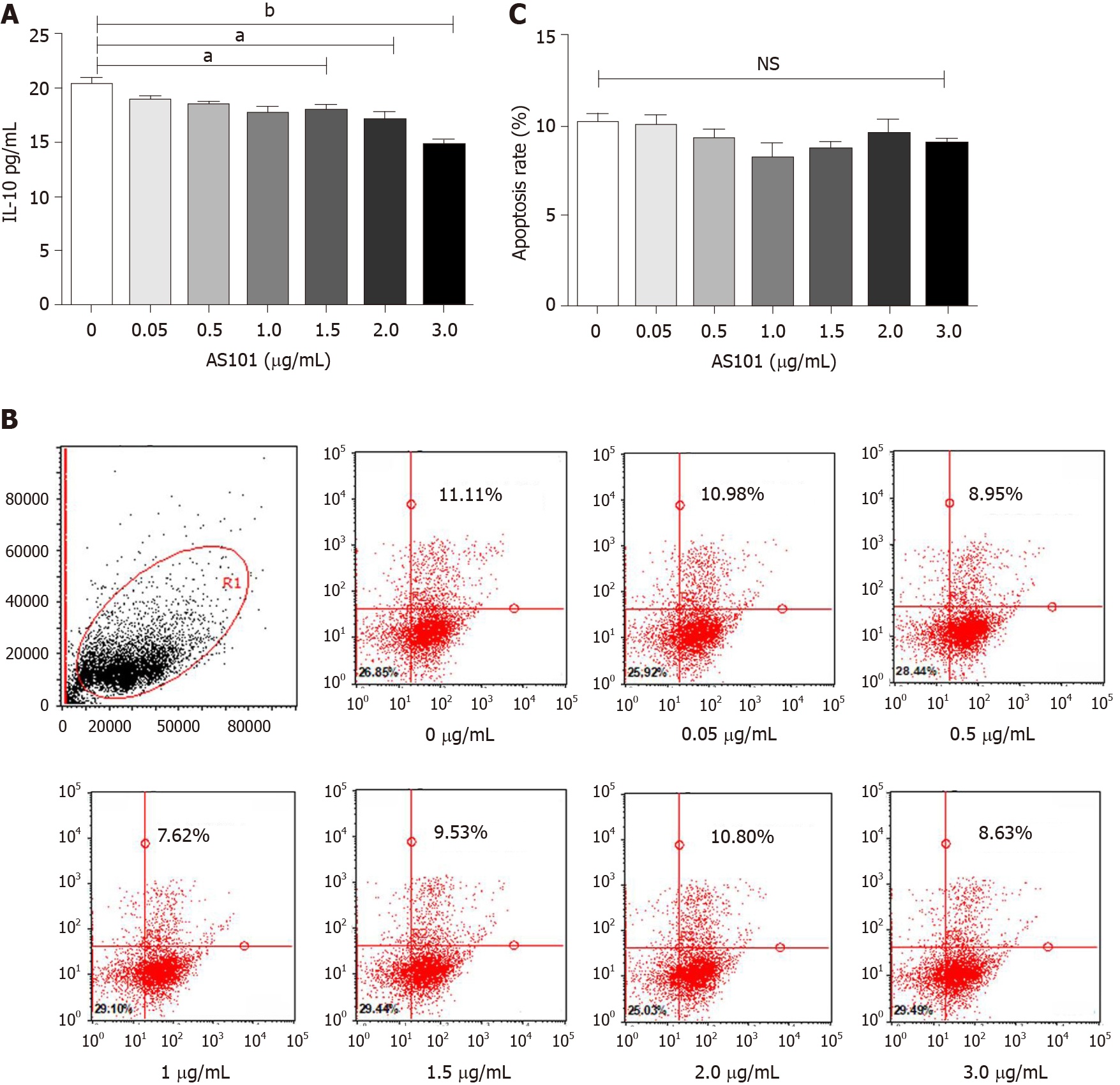
The cells were cultured at various doses with or without the interleukin (IL)-10 inhibitor ammonium trichloro(dioxoethylene-o,o′) tellurate (AS101; S8301, Selleck, China). After 1-h stimulation, the medium was replaced with fresh medium. Supernatants were collected after 24 h and the inhibition of IL-10 was evaluated with a human IL-10 enzyme-linked immunosorbent assay (ELISA) kit (Proteintech, KE00012, United States) according to the manufacturer’s instructions.
The UC-MSCs were washed twice with cold PBS after culturing with different doses of AS101 for 1 h, followed by resuspension in 1 × binding buffer at a density of 1 × 106 cells/mL. Apoptosis was detected by flow cytometry with the FITC Annexin V Apoptosis Detection Kit (BD, 556547, United States) according to the manufacturer’s instructions.
A total of 144 (equal numbers of males and females) 5wkold BALB/c mice weighing 19.4 ± 1.9 g were obtained from Beijing HFK Bioscience (Beijing, China). The animals were housed individually under controlled conditions (12/12-h light/dark cycle, 25 ± 2 °C). All animal protocols were approved by the Ethics Committee on Animal Experiment of Shandong University Qilu Hospital (DWLL-2019-023, Shandong, China).
The mice were randomly divided into the following five groups: Normal group (n = 16), MS group (n = 32, exposed to acceleration and given PBS via the tail vein), MSCs group (n = 32, given UC-MSCs via the tail vein, 1 × 106 cells/mouse), MS + MSCs group (n = 32, exposed to acceleration following transplantation with UC-MSCs via the tail vein, 1 × 106 cells/mouse), and MS + AS101/MSCs group (n = 32, exposed to acceleration following transplantation with AS101-treated UC-MSCs via the tail vein, 1 × 106 cells/mouse). All groups were further divided into four subgroups according to different time points. Mice in the MS, MS + MSCs, and MS + AS101/MSCs groups were subjected to rotation on a centrifuge for 10 min at 8 × g/min for MS model establishment, which was, respectively, performed on days 3 (n = 24), 5 (n = 24), 8 (n = 24), and 10 (n = 24) after UC-MSCs injection. After rotation, the symptoms of the mice were observed and recorded. The mice were immediately placed one by one into the Morris water maze (MWM), and the time taken to reach the platform was recorded. Finally, all mice were sacrificed by decapitation. Blood glucose levels under stress were determined with a glucose analyzer (Accu-Chek Performa, Roche, United States).
As mice have mild gastrointestinal symptoms, the MS index was used to determine the severity of MS according to the following evaluation criteria established by Yu et al[18]: Each fecal granule scored 1, none scored 0; urination scored 1.2, none scored 0; severe piloerection scored 1.2, slight piloerection scored 0.6, and none scored 0; tremor scored 1.2, none scored 0. The MS index was calculated as the sum of all scores.
The MWM consisted of a tank (diameter: 90 cm; height: 50 cm) filled with water to a depth of 30 cm, maintained at 22 °C, and the bottom of the tank was painted black. The maze was divided by two principal axes, with each line bisecting the maze perpendicular to one another. The end of each line demarcated four cardinal points that were used as the four start locations: North (N), south (S), east (E), and west (W). The pool area was conceptually divided into four quadrants (NE, NW, SW, and SE) of equal size. A fixed platform (diameter: 5 cm; height: 28 cm) with the top surface 0.5 cm below the water was placed inside the pool. Bright visual cues of different shapes were placed on the tank walls of each quadrant in plain sight of the mice. A trial limit of 120 s was used, which is standard for mice[19]; animals not finding the platform within this time limit were either placed on the platform or guided to it.
Before the start of the formal experiment, each mouse was trained for 5 d in the maze to ensure that each mouse was able to find the platform within 120 s. Uncoordinated mice were removed during training. The treated mice were subjected to the MWM test once a day to maintain their memory. After establishment of the MS model, the mice were placed in the MWM immediately and the time taken to reach the platform was recorded. Experimental data were recorded with a stopwatch and videos were synchronously recorded on electronic devices (Yishu VC-123, Shanghai, China). The Yishu animal behavioral experimental analysis system (Morris2.8.9.2, Shanghai, China) was used as MWM path analysis software.
After dissecting the skull base, the petrous part of temporal bone samples was fixed in 10% formaldehyde solution overnight and decalcified with 10% ethylen-ediaminetetraacetic acid for 3 d. Paraffinembedded slices of the petrous part of the temporal bone (4μm thick) were standardly processed. These slices were subjected to hematoxylin and eosin (HE) staining for conventional morphological evaluation. The slides were observed under a microscope (Olympus, BX53, Japan) and photographed (CellSens, Ver. 1.18, Japan).
Total RNA was extracted from the petrous part of the temporal bone samples using Trizol reagent (Sigma, T9424-100mL, USA). First-strand cDNA was synthesized using 1 μg of total RNA in 20 μL of reverse transcriptase reaction mixture using the ReverTra Ace qPCR RT Master Mix kit (TOYOBO, Osaka, Japan) with specific primers. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed using a Real Time Thermo cycler (Analytik Jena AG, qTOWER3G, Germany), and detection was performed with SYBR Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan) in a 20-μL reaction mixture to detect the mRNA levels of cytokines. The primer sequences used in RT-qPCR are shown in Supplementary Table 1. The thermal cycling program was as follows: 95 °C for 5 s, followed by 40 cycles of 95 °C for 5 s, 53 °C for 10 s, and 72 °C for 15 s. Data were analyzed using Sequence Detection Software 1.4 (Applied Biosystromals, CA, United States). The mRNA levels were normalized to the level of the Normal group as reference, which was set to 1.
The peripheral blood of the mice was centrifuged to obtain serum (600 × g, 30 min), which was then subjected to ELISA with the mouse IL-1β (KE10003, Proteintech, United States), IL-6 (KE10007, Proteintech), IL-10 (KE10008, Proteintech), and tumor necrosis factor-alpha (TNF-α) (KE10002, Proteintech) kits to determine the level of inflammatory cytokines according to the manufacturer’s instructions. The plates were read at a wavelength of 450 nm. All tests were performed in duplicate.
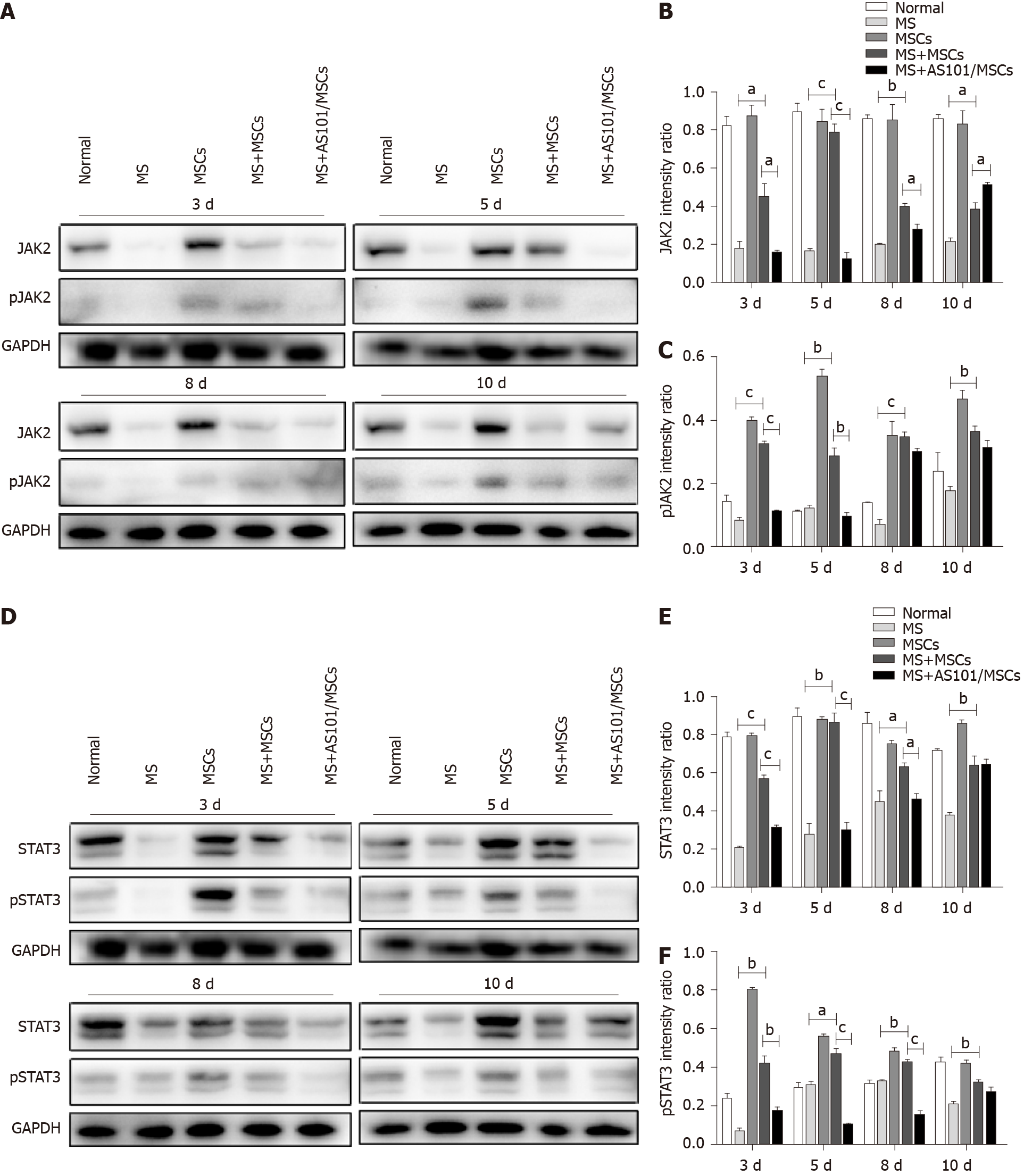
Western blot analysis was carried out according to a standard protocol described previously[17]. In brief, the total protein of the petrous part of the temporal bone samples was extracted using lysis buffer (P0013B, Beyotime, China), and the protein concentrations were measured using a BCA protein assay kit (P0012, Beyotime, China). The lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane. After blocking with 5% bovine serum albumin, the membrane was incubated with primary antibodies raised against GAPDH (1:1000; 5174S, Cell Signaling Technology, United States), Janus kinase 2 (JAK2; 1:1000; 3230S, Cell Signaling Technology), phospho-JAK2 (1:1000; 3776S, Cell Signaling Technology), signal transducer and activator of transcription 3 (STAT3; 1:2000; 4904S, Cell Signaling Technology), phospho-STAT3 (1:2000; 9145S, Cell Signaling Technology), IL-10 alpha receptor (IL-10RA; 1:1000; ab225820, Abcam, United States), and IL-10 (0.2 μg/mL; ab9969, Abcam). Goat anti-rabbit antibodies conjugated to horseradish peroxidase were used as secondary antibodies (1:500; SA00001-2, Proteintech, United States), and proteins were visualized using enhanced chemiluminescence (Tanon, Tanon-5200, Shanghai, China). The band intensity was quantified using ImageJ software. Experiments were performed in triplicate.
All data are presented as the mean ± SE of three separate experiments. The significance of differences among multiple groups was assessed by one-way analysis of variance (ANOVA). For significant ANOVA results, the Bonferroni test was performed between groups. Statistical analyses were performed using SPSS Statistics 21.0 (SPSS, Inc., Chicago, IL, United States). A two-sided probability level less than 0.05 (P < 0.05) was regarded as statistically significant.
After several passages, the adherent cells from the UC could form a monolayer of typical fibroblastic and plastic-adherent cells. Flow cytometry results demonstrated that the UC-MSCs showed the typical immunophenotypes of MSCs. The rates of expression of the positive stem markers CD29, CD44, CD73, CD90, and CD105 reached up to 99%, and the rates of expression of the negative endothelial cell marker (CD31), hematopoietic marker (CD45), and differentiated activated effector cell marker (CD271) were less than 1% (Figure 1).
These cultured cells could differentiate into osteoblasts, adipocytes, and chondrocytes under the corresponding induction conditions. The cells were stained with Alizarin red (Supplementary Figure 1A), Oil Red O (Supplementary Figure 1B), and Alcian blue (Supplementary Figure 1C).
Figure 2A shows that the UC-MSCs constitutively secreted high levels of IL-10. The addition of 3 μg/mL AS101 to the cell cultures for 1 h significantly decreased the level of IL-10 secretion (bP < 0.01; Figure 2A). Flow cytometry further showed that 0-3.0 μg/mL AS101 did not affect the apoptosis of UC-MSCs (Figure 2B and C). Therefore, we chose 3.0 μg/mL AS101 as the concentration for treatment of UC-MSCs in the MS + AS101/MSCs group.
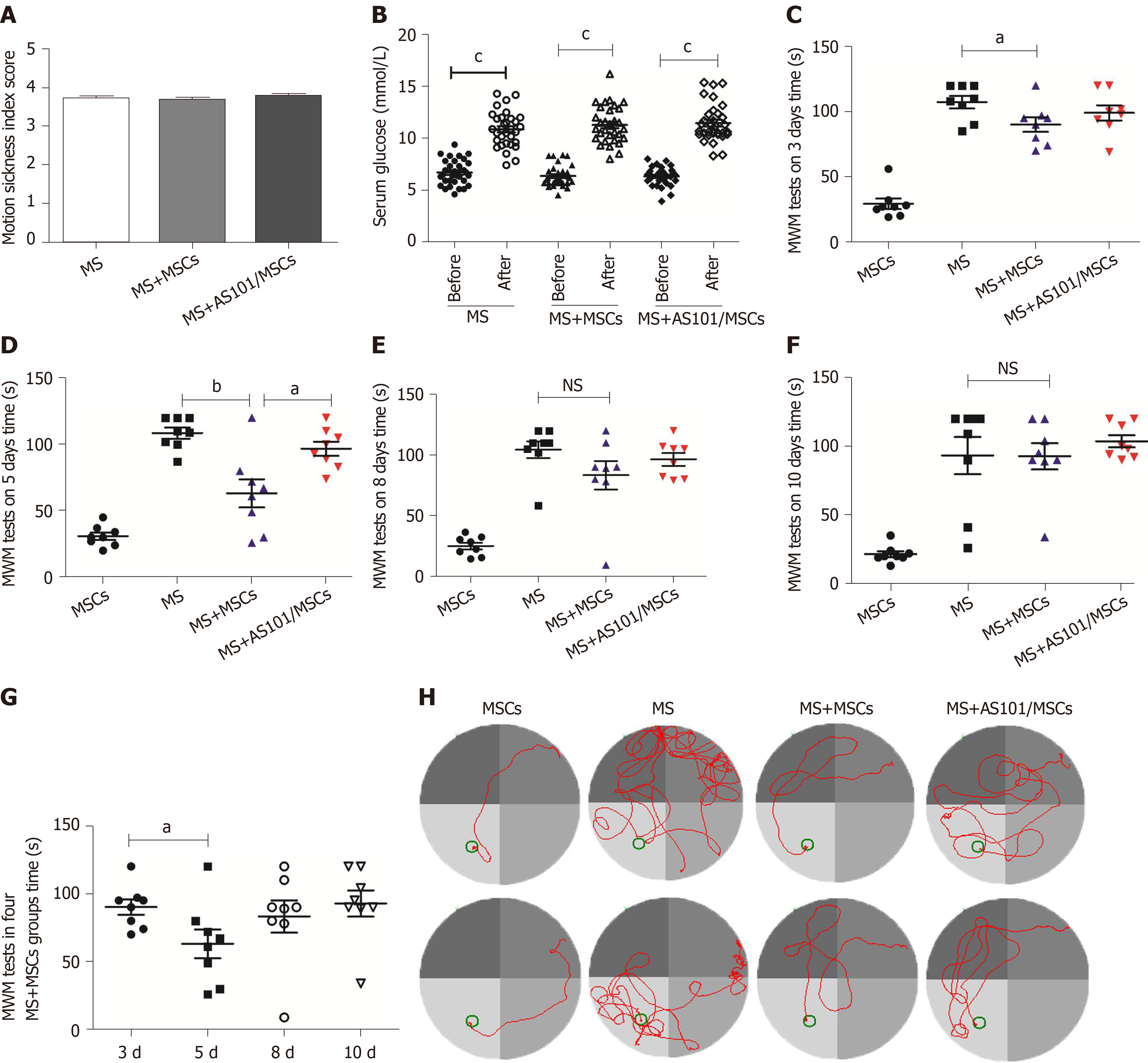
The MS indices for the MS, MS + MSCs, and MS + AS101/MSCs groups demonstrated clear MS symptoms. The mice showed significant fecal granules, urination, and severe piloerection and tremor symptoms; the mean MS index values were 3.70 ± 0.80, 3.68 ± 0.81, and 3.76 ± 0.10 in the three groups, respectively (Figure 3A). After exposure to rotation, serum glucose levels in the three groups increased significantly compared to the baseline levels (cP < 0.001) (Figure 3B).
The MWM tests showed that the mice found the platform faster within a specified time after 4 d of training. The mice in the MSCs group, which were not subjected to rotation, found the platform in the shortest time. The MS + MSCs group was faster than the MS group on days 3 and 5 (P = 0.036 and P = 0.002, respectively, Figure 3C and D). In the four MS + MSCs subgroups, the mice in the 5-day subgroup found the platform within the shortest time, which was significantly improved compared to that of the 3-day subgroup (P = 0.041, Figure 3G). The mice of the MS + AS101/MSCs group took longer to find the platform at day 5 than the MS + MSCs group (P = 0.013, Figure 3D). The camera also recorded the movement path of the mice, and the trajectory at day 5 is shown in Figure 3H. Compared with the other MS model groups, the MS + MSCs group showed a clearer motion trajectory, whereas the mice in the MS group demonstrated obvious rotation and touching of the wall after entering the water. These phenomena also occurred in the MS + AS101/MSCs group, but were relatively rare.
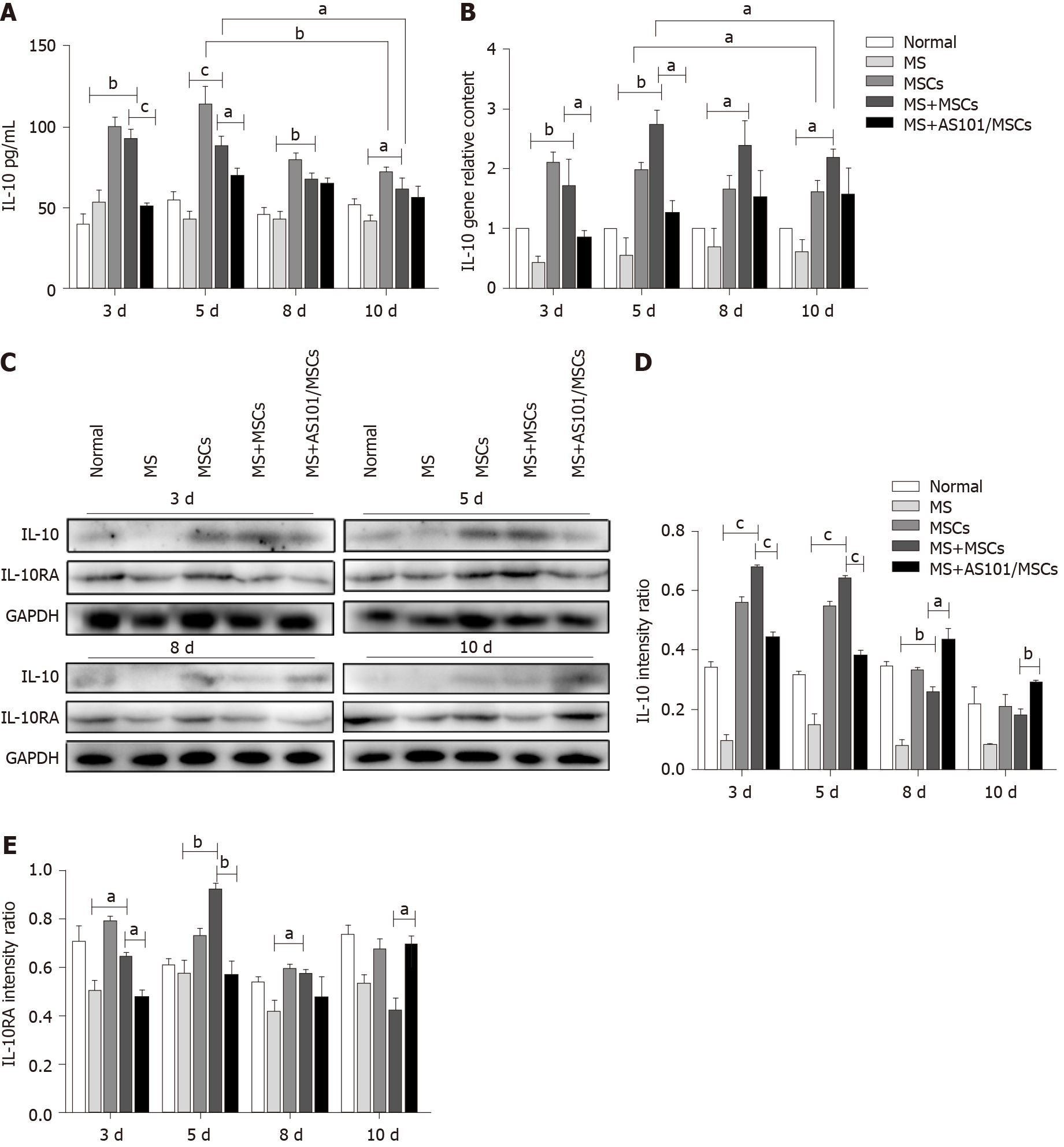
To explore the potential inflammatory response after the rotation, ELISA was used to measure the levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, and the anti-inflammatory cytokine IL-10 in the serum of UC-MSCs-treated mice. At the four time points, the levels of IL-1β, IL-6, and TNF-α in the MS groups were clearly higher than those of the Normal group (bP < 0.01), but there was no difference between the MS + MSCs and MS groups (Supplementary Figure 1A-C). RT-qPCR results showed that compared with the MS group, the mRNA levels of pro-inflammatory cytokines increased in the MS + MSCs group at certain time periods (aP < 0.05; Supplementary Figure 1D-F). AS101 could also inhibit the secretion of pro-inflammatory cytokines in UC-MSCs to a certain extent (aP < 0.05). These results indicated that the rotation led to an in vivo inflammatory response in the mouse model of MS. Specifically, there was an increase in the IL-10 levels in the MS + MSCs groups at 3, 5, 8, and 10 days compared to the MS group (P = 0.002, cP < 0.001, P = 0.002, and P = 0.040, respectively; Figure 4A), and the MS + AS101/MSCs group had lower levels of IL-10 expression compared to the MS + MSCs group at 3 and 5 d (cP < 0.001 and P = 0.031, respectively; Figure 4A). Furthermore, RT-qPCR results confirmed a significant increase in IL-10 levels when the mice were treated with UC-MSCs across the four time points (MS + MSCs groups vs MS groups, P = 0.009, P = 0.009, P = 0.048, and P = 0.049, respectively). Similarly, the levels of IL-10 in the MS + AS101/MSCs group were suppressed at 3 and 5 d (P = 0.035 and P = 0.011, respectively; Figure 4B). Compared to the IL-10 levels at day 5, those at day 10 were significantly decreased in the MSCs and MS + MSCs groups (P = 0.002 and P = 0.014, respectively) in both the serum and inner ear (Figure 4A and B).
Given that the MWM tests showed that the UC-MSCs therapy had a positive effect on MS symptoms at 3 and 5 d post-treatment, and ELISA and RT-qPCR showed that MS caused an inflammatory response, with an increase in IL-10 levels in the MS + MSCs group, we next explored the influence of the treatment on the signaling pathway downstream of IL-10. Western blot results showed significantly enhanced levels of IL-10, IL-10RA, JAK2, STAT3, and phosphorylated JAK2 and STAT3 in the MS + MSCs subgroups compared to the MS subgroups at the four time points post-transplantation (aP < 0.05). After IL-10 inhibition, the expression levels of the above proteins were reduced in the MS + AS101/MSCs group at 3, 5, and 8 days after transplantation (aP < 0.05; Figure 4 and 5). Thus, the secretion of IL-10 in UC-MSCs may be a factor contributing to the improvement of the symptoms of MS. These findings imply that the IL-10/STAT3 signaling pathway is associated with the benefits of UC-MSCs therapy for MS.
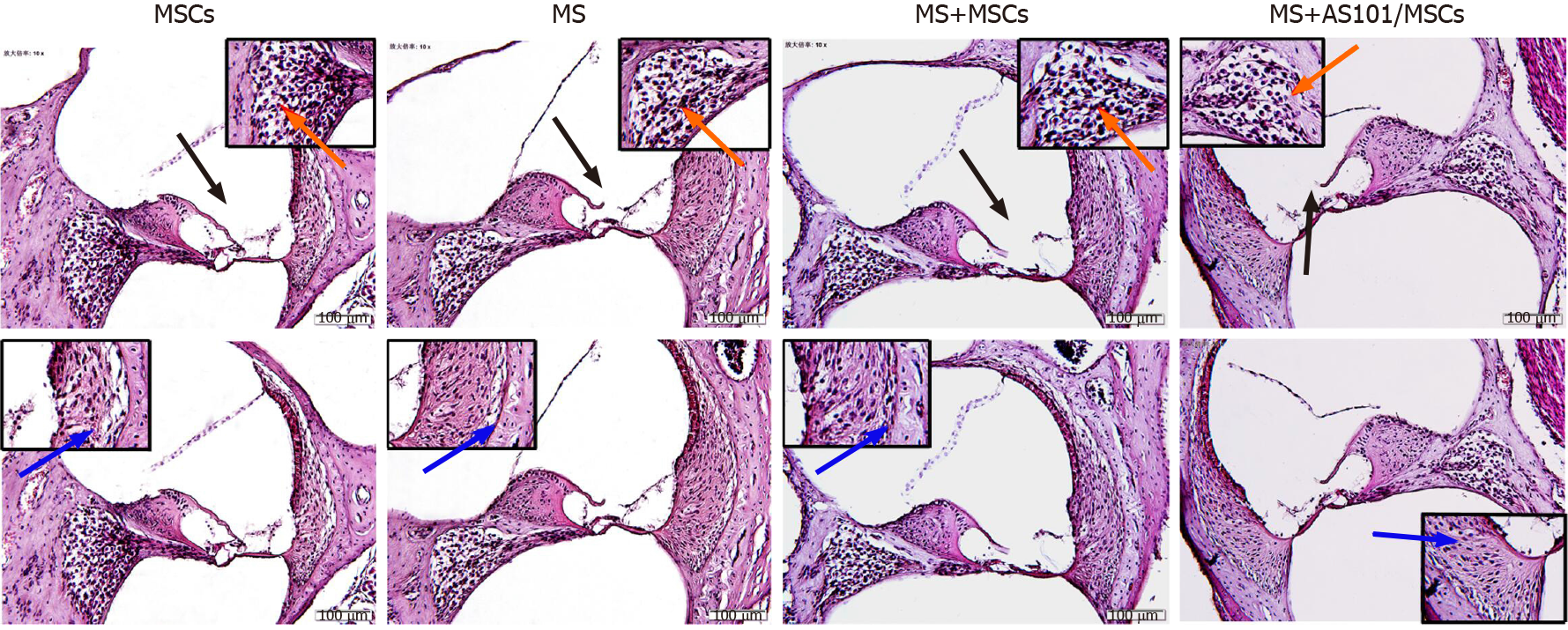
The vestibular cortex plays a major role in the pathogenesis of MS. Therefore, we histologically evaluated cochlea sections stained with HE. The morphological changes in the four groups showed no significant differences. The organ of Corti, vascular striate, and spiral ganglion cells also showed no difference among the groups. Further observations showed that the MS group had no significant cell edema or inflammatory cell infiltration (Figure 6).
MS refers to a series of physiological reactions that occur when the organs are subjected to imbalance, and may include symptoms such as nausea, vomiting, pallor, cold sweats, and headache[1,20]. Studies have shown that up to 90% of people have had at least one incidence of MS in their lifetime[21]. With a sufficiently long period of unbalanced motion, almost anyone can develop MS[22,23]. However, the pathogenesis of MS has not been fully revealed to date. In addition to the current sensory conflict hypothesis, the vestibular internal conflict theory is worth mentioning, which hypothesizes that clinical symptoms develop due to sensing of sudden changes in fluid transfer and gravity in the vestibular cortex by the otoliths and semicircular canals[24]. Miller et al[25] found that the levels of the pro-inflammatory cytokines IL-6 and TNF-α increased in clinical trials of acute stress. Thus, the occurrence of MS can be regarded as the acute stimulation of the vestibule undergoing repeated accelerated movement. Our research began with focus on the vestibular immune environment and explored new ways to treat MS.
Recent studies have shown that MSCs have immune-regulatory functions and can exert immunosuppressive effects both in vitro and in vivo[26]. Accordingly, MSCs have great value in clinical applications, including the induction of tolerance to allogeneic transplantation such as GvHD[27]. Moreover, many studies have shown that MSCs secrete and mediate the expression of immunosuppressive soluble cytokines such as transforming growth factor-β, hepatocyte growth factor, IL-2, and IL-10[28,29]. The present study showed that after prophylactic transplantation of UC-MSCs, the mice in the MS + MSCs groups performed significantly better in the MWM test than those in the MS groups. Moreover, after inhibiting the secretion of IL-10 in UC-MSCs, the MWM test performance of the mice decreased. The MWM is a commonly used laboratory behavioral test for assessing memory and cognition, and is most often used in models of Alzheimer disease[30]. Normal mice develop a memory of the platform position in the MWM test. After MS modeling, the body control ability of the mice decreases, and the swimming trajectories to find the platform will be significantly altered. Therefore, the MWM test can make up for the expression of subjective symptoms such as nausea and vomiting during MS to obtain more intuitive results, which was confirmed in the present study.
A stable vestibular immune microenvironment is necessary for its normal function. IL-10 plays an important role in the immune regulation. IL-10 is mainly produced by immune cells, including macrophages, T regulatory cells, and dendritic cells[31]. Woo et al[32] reported that IL-10 receptors are expressed in the cochlear lateral wall, particularly in the spiral ligament fibrocytes. Based on these previous findings, our results suggested that UC-MSCs enter and change the internal environment of the cochlea possibly by secreting IL-10 and activating IL-10 alpha receptors in the cochlear lateral wall fibrocytes, thereby reducing sensitivity of the vestibular cortex during unbalanced movement. However, we found that the effects of UC-MSCs treatment for MS were short-lived, and the effect of IL-10 on the vestibular microenvironment weakened over time.
We conducted further experiments to assess the internal mechanisms contributing to the changes of the vestibular microenvironment. Previous studies indicated that the changes in the levels of JAK2 and STAT3 or their phosphorylated forms in the cochleae are related to IL-10[33]. Notably, JAK2 and STAT3 proteins were expressed to some extent in the Normal group. Although these levels did not normalize after UC-MSCs treatment, they were significantly increased compared with those of the MS group. The short-term MS modeling process may explain the lack of difference in cochlear histopathology among the four groups.
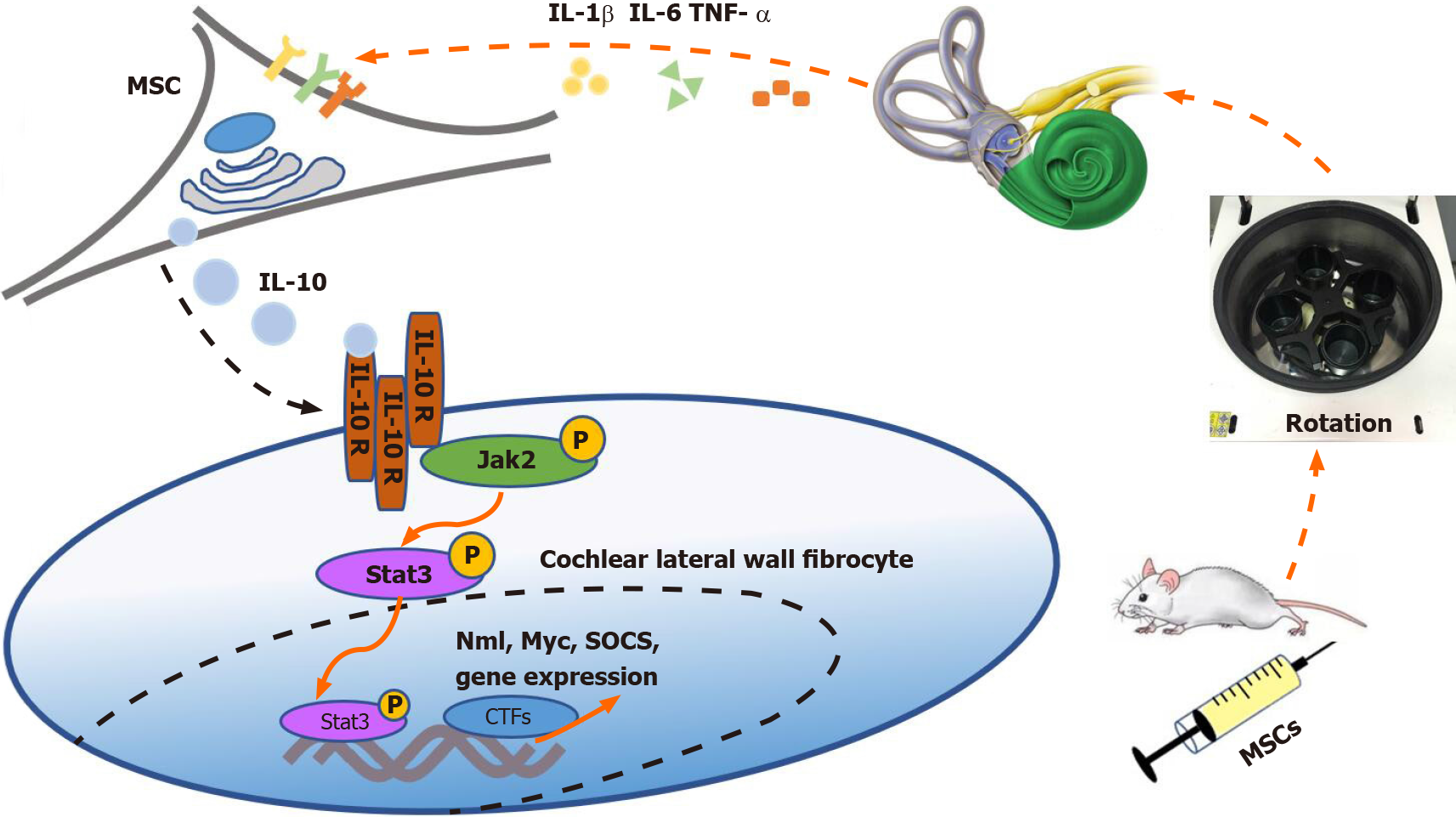
As mentioned above, the level of IL-10 increased after prophylactic transplantation of UC-MSCs, which was consistent with the results of the MWM test. Moreover, the therapeutic effect of the MSCs diminished with co-treatment of the IL-10 inhibitor. Based on our results, we speculate that MSCs entering the tail vein of mice can sense inflammatory cytokine stimulation in the vestibular microenvironment[34]. The MSCs were then stimulated to secrete large amounts of IL-10 in a short time. IL-10 binds to receptors on fibrocytes in the cochlear lateral wall to trigger the JAK2/STAT3 signaling pathway, eventually reducing the sensitivity of the vestibule to vertigo[32] (Figure 7). As mentioned above, disturbance of the vestibular immune micro-environment is presumably the cause of MS. However, IL-10 secreted by MSCs not only directly acts on the cochlear lateral wall but may also exert anti-inflammatory effects in other inflammation-related diseases by shifting the phenotype of the macrophages in tissues from M1 to M2[35]. Hu et al[36] have found that macrophages in the cochlear tissue are differentially localized in correlation with their functional characteristics. The relationship among MSCs, macrophages, and the vestibular immune microenvironment needs to be further evaluated.
There are also some limitations to the design of this study. Previous studies have shown that the IL-10 inhibitor AS101 can also interfere with the expression of other ILs[37]. Therefore, transgenic animals whose IL-10 signaling is inhibited may be a good choice, or gene editing techniques, such as sgRNA, may be used. Further studies will clearly be needed to more completely understand the molecular mechanisms modulated by MSCs in MS and other immune-related diseases. In addition, we will explore new ways to administer the treatment, such as, by collecting MSC-derived exosomes for oral or nasal mucosa administration to explore whether the same therapeutic effect is achieved as observed with intravenous infusion. Above all, MSCs are expected to become a new method for the clinical prevention and treatment of MS.
Prophylactic transplantation of UC-MSCs can alleviate the clinical symptoms of MS in mice, particularly at 3-5 d after preventive transplantation. The mechanism for UC-MSCs to reduce the sensitivity of vestibular cortex imbalance may be the secretion of IL-10. The next step is to demonstrate the possibility of curing MS in the vestibular environment by intermittent transplantation of MSCs. Above all, MSCs are expected to become a new method for the clinical prevention and treatment of MS.
Motion sickness (MS) is a disease that occurs during an unbalanced movement, and approximately 90% of people experience MS at least once in their lives. MS can present with gastrointestinal symptoms and activation of the autonomic nervous system. Additionally, it is accompanied by an increase in the levels of pro-inflammatory factors in the inner ear. However, the commonly used anti-inflammatory hormonal drugs have many side effects. Mesenchymal stem cells (MSCs) exert strong immunosuppressive effects and thus may serve as a therapeutic option for MS.
We can use the immunoregulatory properties of MSCs to suppress MS. Additionally, clarification of the molecular mechanisms underlying these properties may yield novel targets for the preventive treatment of MS.
In this study, we aimed to explore whether umbilical cord-derived MSCs (UC-MSCs) can suppress MS in a mouse model.
UC-MSCs were cultured and phenotypically characterized. A total of 144 (equal numbers of males and females) 5wkold BALB/c mice were randomly divided into five groups, and UC-MSCs were infused into the tail veins of the MSCs group and MS + MSCs group. AS101-treated UC-MSCs were infused into the MS + AS101/MSCs group for prophylaxis. The mice were subjected to the Morris water maze test to experience MS and monitored for any sign of dizziness. Enzyme-linked immunosorbent assay (ELISA) and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were used to assess the expression levels of inflammatory cytokines in the peripheral blood and petrous temporal bones of the mice. The petrous temporal bone samples were also histologically evaluated via hematoxylin-eosin (HE) staining. Additionally, Western blot analysis was performed to assess the cochlear levels of proteins involved in the JAK2/STAT3 signaling pathway.
Results of the Morris water maze test demonstrated that transplantation of UC-MSCs suppressed the symptoms of MS in mice. The UC-MSC-transplanted mice found the water maze platform faster than the MS group. The levels of interleukin-10 (IL-10) in the cochlear tissues were increased after transplantation with UC-MSCs, based on the ELISA and RT-qPCR results. Moreover, Western blot analysis showed that transplantation of UC-MSCs activated the JAK2/STAT3 signaling pathway in the cochlear tissues. These effects were abolished when the transplanted mice were treated with the IL-10 inhibitor AS101. Histologically, no obvious difference was observed among the petrous temporal bones of the mice in the five groups.
Prophylactic transplantation of UC-MSCs can suppress MS in mice, particularly at 3-5 d after transplantation. This reduced sensitivity of the vestibular cortex to imbalance presumably results from improvement of the immune microenvironment by IL-10 secreted by UC-MSCs.
In the future, we will explore additional MSC culture methods and cell-free administration of the MSC-derived active factors into mice. For example, we will culture MSCs in three dimensions (3D) to mimic their in vivo microenvironment, and administer MSC-derived exosomes to the oral or nasal mucosa. In this way, we hope to translate the anti-MS effect of MSCs to the clinic.
The authors are grateful to Dr. Ju XL for help with regard to experimental design and funding support. The authors also would like to thank the NHC Key Laboratory of Otorhinolaryngology of Shandong University for kindly providing MWM experiment platform. We also thank Li D for inspiring discussions.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soriano-Ursúa MA, Tawil B S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Koch A, Cascorbi I, Westhofen M, Dafotakis M, Klapa S, Kuhtz-Buschbeck JP. The Neurophysiology and Treatment of Motion Sickness. Dtsch Arztebl Int. 2018;115:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Farmer AD, Ban VF, Coen SJ, Sanger GJ, Barker GJ, Gresty MA, Giampietro VP, Williams SC, Webb DL, Hellström PM, Andrews PL, Aziz Q. Visually induced nausea causes characteristic changes in cerebral, autonomic and endocrine function in humans. J Physiol. 2015;593:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Romano F, Caramia N, Straumann D, Nalivaiko E, Bertolini G. Cross-coupling vestibular stimulation: motion sickness and the vestibulo-sympathetic reflex. J Neurol. 2017;264:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Brandt T, Bauer M, Benson J, Huppert D. Motion sickness in ancient China: Seasickness and cart-sickness. Neurology. 2016;87:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Zhang LL, Wang JQ, Qi RR, Pan LL, Li M, Cai YL. Motion Sickness: Current Knowledge and Recent Advance. CNS Neurosci Ther. 2016;22:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Russo FY, Ralli M, De Seta D, Mancini P, Lambiase A, Artico M, de Vincentiis M, Greco A. Autoimmune vertigo: an update on vestibular disorders associated with autoimmune mechanisms. Immunol Res. 2018;66:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Russo FY, De Seta D, Lahlou G, Borel S, Nguyen Y, Bouccara D, Sterkers O, Bernardeschi D, Mosnier I. Fluctuating Hearing Loss in the Only Hearing Ear: Cochlear Implantation in the Contralateral Deaf Side. Otolaryngol Head Neck Surg. 2018;158:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Huang M, Gao JY, Zhai ZG, Liang QL, Wang YM, Bai YQ, Luo GA. An HPLC-ESI-MS method for simultaneous determination of fourteen metabolites of promethazine and caffeine and its application to pharmacokinetic study of the combination therapy against motion sickness. J Pharm Biomed Anal. 2012;62:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Golding JF, Gresty MA. Pathophysiology and treatment of motion sickness. Curr Opin Neurol. 2015;28:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Murdin L, Golding J, Bronstein A. Managing motion sickness. BMJ. 2011;343:d7430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Mishra KP, Yadav AP, Shweta, Chanda S, Majumdar D, Ganju L. Serum levels of immunoglobulins (IgG, IgA, IgM) in Antarctic summer expeditioners and their relationship with seasickness. Cell Immunol. 2011;271:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Rohleder N, Otto B, Wolf JM, Klose J, Kirschbaum C, Enck P, Klosterhalfen S. Sex-specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinology. 2006;31:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Vandewalle J, Luypaert A, De Bosscher K, Libert C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol Metab. 2018;29:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 356] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 14. | Pistoia V, Raffaghello L. Mesenchymal stromal cells and autoimmunity. Int Immunol. 2017;29:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Chen C, Liang J, Yao G, Chen H, Shi B, Zhang Z, Zhao C, Zhang H, Sun L. Mesenchymal stem cells upregulate Treg cells via sHLA-G in SLE patients. Int Immunopharmacol. 2017;44:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 495] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 17. | Yan W, Li D, Chen T, Tian G, Zhou P, Ju X. Umbilical Cord MSCs Reverse D-Galactose-Induced Hepatic Mitochondrial Dysfunction via Activation of Nrf2/HO-1 Pathway. Biol Pharm Bull. 2017;40:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Yu XH, Cai GJ, Liu AJ, Chu ZX, Su DF. A novel animal model for motion sickness and its first application in rodents. Physiol Behav. 2007;92:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3541] [Cited by in RCA: 3374] [Article Influence: 177.6] [Reference Citation Analysis (0)] |
| 20. | Mo FF, Qin HH, Wang XL, Shen ZL, Xu Z, Wang KH, Cai YL, Li M. Acute hyperglycemia is related to gastrointestinal symptoms in motion sickness: an experimental study. Physiol Behav. 2012;105:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Herron DG. The ups and downs of motion sickness. Am J Nurs. 2010;110:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Burton MJ, Roland PS, Rosenfeld RM. Extracts from The Cochrane Library: Scopolamine (hyoscine) for preventing and treating motion sickness. Otolaryngol Head Neck Surg. 2010;142:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Arshad Q, Cerchiai N, Goga U, Nigmatullina Y, Roberts RE, Casani AP, Golding JF, Gresty MA, Bronstein AM. Electrocortical therapy for motion sickness. Neurology. 2015;85:1257-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Singh P, Yoon SS, Kuo B. Nausea: a review of pathophysiology and therapeutics. Therap Adv Gastroenterol. 2016;9:98-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1060] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 27. | Zhao L, Chen S, Yang P, Cao H, Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 28. | Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest. 2014;124:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 29. | Pedrosa M, Gomes J, Laranjeira P, Duarte C, Pedreiro S, Antunes B, Ribeiro T, Santos F, Martinho A, Fardilha M, Domingues MR, Abecasis M, P da Silva JA, Paiva A. Immunomodulatory effect of human bone marrow-derived mesenchymal stromal/stem cells on peripheral blood T cells from rheumatoid arthritis patients. J Tissue Eng Regen Med. 2020;14:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Huber CM, Yee C, May T, Dhanala A, Mitchell CS. Cognitive Decline in Preclinical Alzheimer's Disease: Amyloid-Beta versus Tauopathy. J Alzheimers Dis. 2018;61:265-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 31. | Bassi ÊJ, Moraes-Vieira PM, Moreira-Sá CS, Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva A, Câmara NO. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534-2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Woo JI, Kil SH, Oh S, Lee YJ, Park R, Lim DJ, Moon SK. IL-10/HMOX1 signaling modulates cochlear inflammation via negative regulation of MCP-1/CCL2 expression in cochlear fibrocytes. J Immunol. 2015;194:3953-3961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Li L, Zhang J, Chen J, Xu-Monette ZY, Miao Y, Xiao M, Young KH, Wang S, Medeiros LJ, Wang M, Ford RJ, Pham LV. B-cell receptor-mediated NFATc1 activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma. Blood. 2018;132:1805-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 810] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 35. | Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 398] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 36. | Hu BH, Zhang C, Frye MD. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res. 2018;362:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Ling D, Liu B, Jawad S, Thompson IA, Nagineni CN, Dailey J, Chien J, Sredni B, Nussenblatt RB. The tellurium redox immunomodulating compound AS101 inhibits IL-1β-activated inflammation in the human retinal pigment epithelium. Br J Ophthalmol. 2013;97:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |