Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1446
Peer-review started: April 28, 2021
First decision: June 23, 2021
Revised: July 14, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 26, 2021
Processing time: 180 Days and 13 Hours
Retinal degeneration is a major contributor to visual dysfunction worldwide. Although it comprises several eye diseases, loss of retinal pigment epithelial (RPE) and photoreceptor cells are the major contributors to their pathogenesis. Early therapies included diverse treatments, such as provision of anti-vascular endothelial growth factor and many survival and trophic factors that, in some cases, slow down the progression of the degeneration, but do not effectively prevent it. The finding of stem cells (SC) in the eye has led to the proposal of cell replacement strategies for retina degeneration. Therapies using different types of SC, such as retinal progenitor cells (RPCs), embryonic SC, pluripotent SCs (PSCs), induced PSCs (iPSCs), and mesenchymal stromal cells, capable of self-renewal and of differentiating into multiple cell types, have gained ample support. Numerous preclinical studies have assessed transplantation of SC in animal models, with encouraging results. The aim of this work is to revise the different preclinical and clinical approaches, analyzing the SC type used, their efficacy, safety, cell attachment and integration, absence of tumor formation and immunorejection, in order to establish which were the most relevant and successful. In addition, we examine the questions and concerns still open in the field. The data demonstrate the existence of two main approaches, aimed at replacing either RPE cells or photoreceptors. Emerging evidence suggests that RPCs and iPSC are the best candidates, presenting no ethical concerns and a low risk of immunorejection. Clinical trials have already supported the safety and efficacy of SC treatments. Serious concerns are pending, such as the risk of tumor formation, lack of attachment or integration of transplanted cells into host retinas, immunorejection, cell death, and also ethical. However, the amazing progress in the field in the last few years makes it possible to envisage safe and effective treatments to restore vision loss in a near future.
Core Tip: Retinal degeneration is a major cause of visual loss worldwide, having no cure or suitable treatments. Transplantation of stem cells into the eye to replace lost cells is being evaluated as a new therapeutic strategy. Establishing the most suitable source of stem cells and ethical and safety concerns still require to be solved. Preclinical studies and ongoing clinical trials support stem cells as a promising therapeutic approach to restore vision loss.
- Citation: German OL, Vallese-Maurizi H, Soto TB, Rotstein NP, Politi LE. Retina stem cells, hopes and obstacles. World J Stem Cells 2021; 13(10): 1446-1479
- URL: https://www.wjgnet.com/1948-0210/full/v13/i10/1446.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i10.1446
Stem cells (SC) are rather undifferentiated cells present in most tissues of nearly all multicellular organisms, from humans[1-3], to plants[4-6], having the amazing capacity to either activate self-renewal or differentiate into specific cell types[7]. Hence, they are potentially capable of regenerating whole tissues or organs subjected to ablations or damages. This capacity varies between species. It is particularly remarkable in Platyhelminthes, such as the planarian worms, which when beheaded can regenerate their entire bodies in just a few days[8-10]. Many cold-blooded species retain a considerable regenerative capacity; some teleosts, like zebrafish, can regenerate their limbs, spinal cord, retina, heart and brain. By contrary, regenerative capacity in mammals is quite restricted[11,12].
SC have attracted great attention due to their potential for regeneration and knowledge about them has increased enormously in the last few years. Most SC have a rather simple morphology, resembling undifferentiated cells, and essentially, they have the same organelles and molecular machinery present in most eukaryotic cells. However, embryonic pluripotent SCs (PSCs) are capable during development of an extraordinary feat, to give rise to nearly all the cell types present in the body. In addition, after ablations or injuries to a given tissue, they activate a complex response leading to their reentry into the cell cycle and, eventually, to the activation of a differentiation pathway, acquiring the morphology and functions of the cells of the damaged tissue, thus being able to replace lost cells.
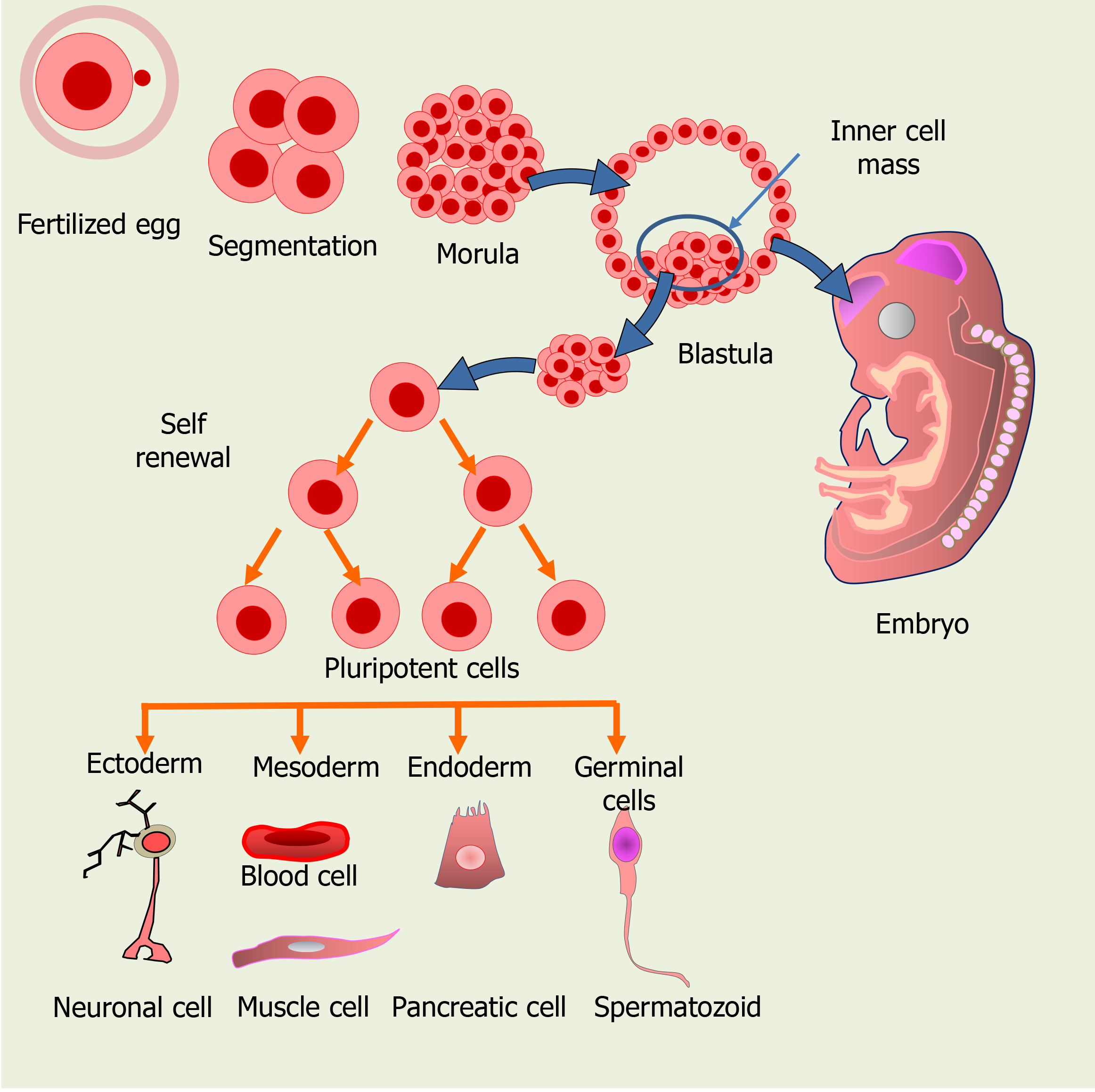
Based on their capacity for differentiation, SC can be classified either as unipotent, multipotent, pluripotent or totipotent. Whereas unipotent SC, such as epidermal or muscle SC, are able to generate only one type of cells upon differentiation[13,14], multipotent SC can generate a very limited amount of cell types, belonging to a closely related cell family[14], as usually is the case of adult SC. Müller glial SC in the retina belong to this group, as they potentially differentiate into just one or two retinal neuronal cell types and Müller glial cells (MGCs). PSCs, such as embryonic SC (ESCs) in the inner cell mass of the blastula, may differentiate into nearly all the cell types, while totipotent SC, like the zygote and the first few cells derived from it during zygote segmentation, have the highest capacity for differentiation and can give rise to all the cell types of an organism (Figure 1).
Based on their source, there are two main types of SC, ESC and adult (mature) SC. ESCs are included in a family of Stem/progenitor cells found in 3-5 d old embryos, which includes retinal progenitor cells (RPCs) and mesenchymal stromal cells. ESCs are pluripotent cells that can give rise to cells of the three embryonic germ layers: ectoderm, endoderm or mesoderm, and can generate all tissues in the body.
A major breakthrough in SC research was achieved less than a decade ago; the introduction of a few SC specific genes, such as Myc, Sox2, Oct3/4 and Klf4, induced somatic cells to acquire the morphology, characteristics, and markers of SC, thus being defined as induced PSCs (iPSCs). Their transplantation in nude mice generates tumors exhibiting tissues from the three germ layers[15], suggesting they may be used for cell replacement therapies.
Mature SC are present in small amounts in adult, differentiated tissues; these multipotent cells can give rise to a few types of specialized cells[2]. Mature SC are also found in the umbilical cord and placenta after birth. They also include hematopoietic SC (HSC) from bone marrow (BM), peripheral blood or umbilical cord blood, and are commonly used for transplantation, one of their advantages being that they rarely generate unwanted cell types.
The most relevant features of SC are their ability for self-renewal and for giving rise to multiple cell types. A very active SC self-renewal occurs during the first stages of development, both in the morula and in the inner mass of the blastula to generate the new cells required for the developing organism (Figure 1). Later, SC can activate in damaged tissues, proliferating and then differentiating to replace lost cells. In addition to the “ready to use” pool of undifferentiated SC, some differentiated cells may eventually undergo a dedifferentiation process, reentering the cell cycle and becoming “active” SC. To regain the proliferating capacity, they repress genes required for cell differentiation, activating those required for a proliferative, undifferentiated state[16].
Noteworthy, SC are similar to cancer SC in their ability to self-renew and generate large populations of more differentiated descendants; they also share the phenotypic plasticity that allows them to enter or exit the cell cycle, an ability closely associated with the stemness properties and invasiveness of cancer cells[17]. These morphological and functional similarities compromise current therapeutic efforts, as SC transplantation very frequently leads to deregulation of the mitotic cell cycle and tumor formation[18,19].
The ability of SC to repair damaged tissues and regenerate lost cells has encouraged scientists and clinicians to explore their use to treat or cure different diseases. However, many issues have still to be unraveled before using SC safely in humans. The aim of this review is to analyze the characteristics and potential advantages of using SC for treating retina neurodegenerative diseases, and the risks that remain to be addressed before using these cells in the clinic.
Contrary to early beliefs, nerve tissues in many animals, including mammals, have SC able to give rise to new neurons[20-23], sparking the hope for regenerative approaches to neurodegenerative diseases. However, at least presently, it seems nearly impossible to regenerate the brain as a whole. Humans have about 1011 neurons with different morphologies and multiple functions[24], which added to the complex interactions among the different neuronal types, and between neurons and other cell types (i.e., glial cells), imposes tremendous limitations for brain regeneration.
Retinal degeneration (RD) represents one of the most common causes of irreversible vision impairment leading to blindness worldwide. It comprises several eye diseases, including age-related macular degeneration (AMD), diabetic retinopathy, Stargardt’s disease and retinitis pigmentosa (RP). Among them, AMD is the leading cause of severe vision loss in people over 60 years old, with a global prevalence of 8.7 %, and it currently affects about 200 million persons in western world countries[25]. In spite of the numerous differences between these diseases, dysfunctions of RPE and photoreceptors (PHRs) are the major contributors to their pathogenesis[26].
The retina is part of the central nervous system (CNS); fortunately, its structure is simpler than that of the brain, making its regeneration more feasible. The retina is responsible for receiving light and transforming it into electrical signals that travel to the brain, where visual images are generated. It is a thin tissue of easy access, located in the back of the eye, with an ordered layered organization and a limited number of cells. These features make it possible to use SC to develop strategies for alleviating or, eventually curing, retinal neurodegenerative diseases.
Moreover, and in contrast to other organs of the body, the eye is a relatively immune privileged organ, which supports the likelihood of retina transplantation[27-29]. The ocular immune privilege, which is intended to limit local immune and inflammatory responses, can also contribute to avoid the rejection of grafts placed in the anterior chamber of the eye and protect the eye from inflammatory insults[30,31]. However, this protection is not absolute and this must be taken into account in cell replacement strategies. Treatments using iPSCs may activate immune rejection, as they can upregulate genes that induce a T cell response, and thus lead to the rejection of transplanted cells. Since the molecular mechanisms involved in these processes are incompletely understood[32], many studies resort to immunosuppression to reduce the risk of rejection[33]. Increasing our knowledge of the molecular processes that inhibit immune rejection of transplants would contribute to elaborate new strategies to facilitate the acceptance of tissue allografts during retinal transplantation.
The initial efforts to treat RD were oriented to halt degenerative processes of the retina. The proposed therapies included a variety of medical compounds and treatments, including several neurotrophic factors, aimed at controlling oxidative stress and cell death[34,35] or anti-vascular endothelial growth factor agents, to prevent the formation of leaky blood vessels, mainly used to treat wet AMD[36]. However, these strategies pose diverse difficulties. In wet AMD, treatments to avoid or minimize choroidal neovascularization, very frequently cause complications, such as uveitis and vitreous hemorrhages, which compromise their effectiveness. On the other hand, the clinical efficacy of the neuroprotective strategies has not been firmly established. Furthermore, surgical interventions like laser therapies may not prevent the progression of the disease in patients with AMD or other RD and can cause inflammatory-related damages[37-39].
The finding of SC in the retina has provided additional tools to develop strategies for repairing the damaged retinas. Therapies using different types of SC, such as stem/progenitor cells, RPCs, PSCs, ESCs, iPSCs, and mesenchymal SCs (MSCs), capable of self-renewal and of differentiating into multiple cell types, have now gained ample consensus. Numerous preclinical studies using SC in several animal models of RD have been performed and their results are encouraging. Emerging evidence suggests that RPCs are among the best candidates for RD treatment; they do not present ethical concerns and they have a relatively low risk of tumorigenesis and immunorejection[40]. Moreover, increasing evidence suggests that the combination of SC therapies with the provision of survival molecules might provide the best strategy to treat RD.
Regeneration following retina damages might require the recapitulation of developmental specification/differentiation programs. Following PHR damage, zebrafish retina evidences an increase in the expression of several developmental competence factors, required for generating ganglion, amacrine, and PHR cells[41]. Retinal injury might turn on cell specification programs in neuronal progenitor cells, which recapitulate the temporal expression sequence occurring during retina development and hence provide precursors suitable for replacing lost cells. A critical question is, which are the features that characterize and distinguish SC from other cells present in the organism? Cumulative evidence indicates that their extraordinary capacities depend, in part, on complex interactions between cell surface proteins and a variety of external and internal signals that activate signaling pathways to regulate pluripotency. These external signals include LIF/STAT3, Wnt/β-catenin, FGF/ERK, TGF/SMAD, bone morphogenetic protein (BMPs), Sonic Hedgehog, and the Wnts and Notch proteins[42].
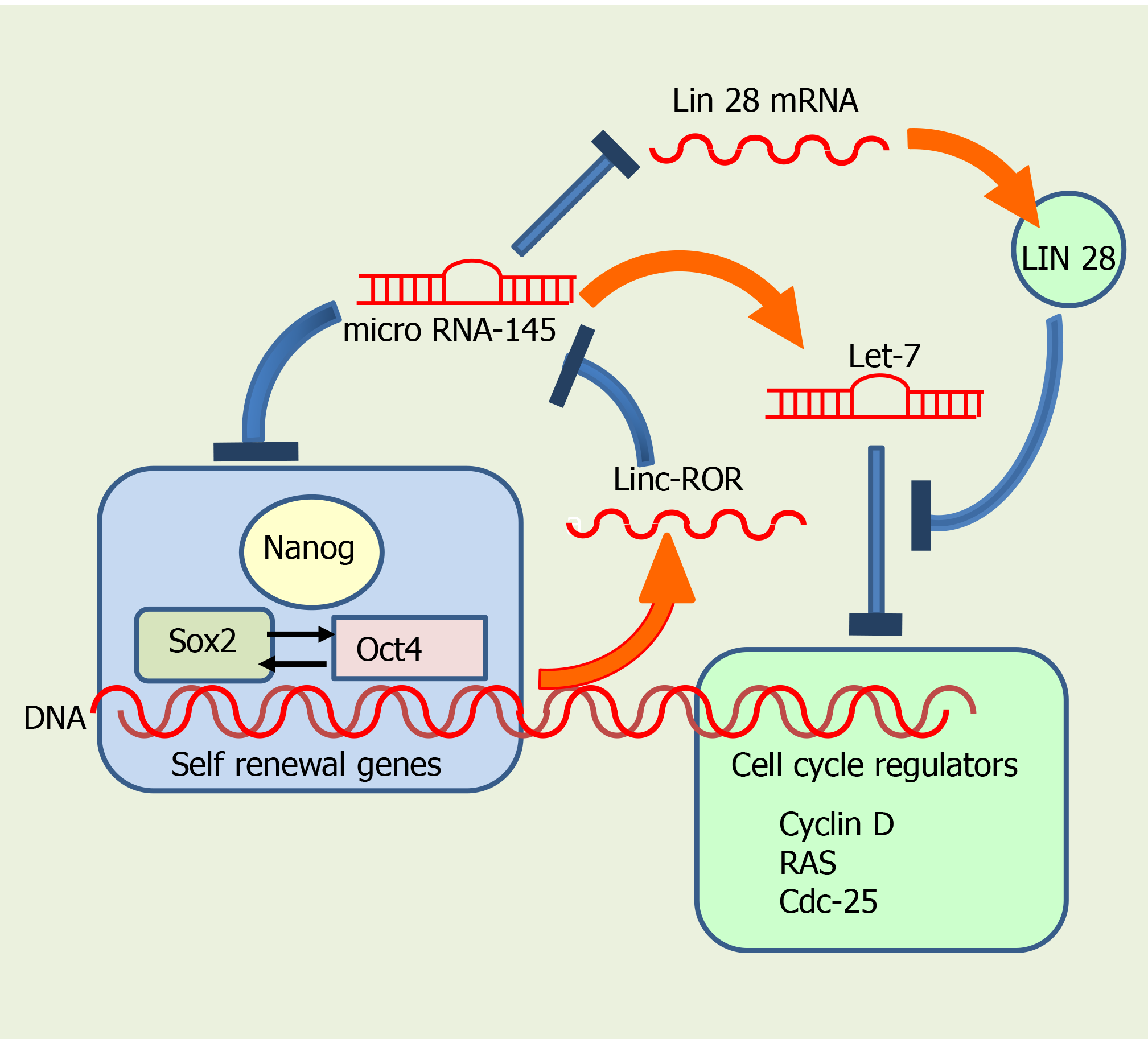
The internal regulatory system comprises many transcription factors, such as Oct4, Sox2 and Nanog, which interact with specific target genes that regulate self-renewal and pluripotency[43,44] (Figure 2). Oct4 (also known as Oct3 o Pou5f1) controls the Sox2 transcription factor, involved not only in self-renewal of SC, but also in embryo development. Sox2 maintains SC in an undifferentiated state, after concluding embryo development[45,46], being important also for regulating proliferation and differentiation of neuronal SC progenitors[47,48]. In addition, both Sox2 and Oct4 interact upstream with the promoter of Nanog, another transcription factor, activating different genes that inhibit differentiation[49,50] (Figure 2). Nanog is involved in the self-renewal of ESC and is critical for maintaining pluripotent cells in an undifferentiated state[51]. Interestingly, genetic deletion of Nanog in ESCs does not abolish pluripotency, although it reduces cell self-renewal activity. As a general rule, Nanog prevents SC differentiation, so it is considered a guardian of pluripotency[50].
In addition, epigenetical regulation of global changes in genetic expression control SC rate of mitosis, along with other morphological and physiological changes. Acetylation or methylation of H3 and H4 histones regulate the distribution of active and inactive forms of chromatin, and consequently genetic transcription in mouse pluripotent cells. Noteworthy, mouse ESCs have poised (bivalent) domains containing both active and inactive forms of chromatin; hence, poised chromatin has histone modifications associated with both gene activation and repression[52]. Most bivalent domains are associated with the so called, highly conserved noncoding elements, found in clusters around different genes, including many transcription factors implicated in the regulation of cell differentiation during development[53]. Some of these transcription factors are members of the Sox, Fox, Pax, Irx and Pou families. Bivalent domains in SC are thought to maintain stemness by equilibrating the expression of relevant genes involved in differentiation, whereas signals released during development give way to an irreversible differentiation process[54].
Sox2 binds to bivalently marked promoters of poised proneural genes in neural progenitor cells and a subset of other genes, to maintain the bivalent chromatin state, and prevent excessive polycomb repressive complex 2 activities. It decreases the tri-methylation of H3 on lysine 27 (H3K27me3) through histone methyl transferase activity. H3K27me3 often interacts in bivalent domains with H3K4me3, another epigenetic modification to H3, which plays a significant role in SC fate determination and early embryo development. Thus, Sox2 maintains a permissive epigenetic state, enabling proper activation of the neuronal differentiation program under suitable neurogenic cues[55]. Therefore, Sox2 plays an essential role in preserving pluripotency of SC and its interplay with Oct4 and Nanog generates a network that preserves the pluripotent state of SC.
MicroRNAs (miRNAs) are also major regulators of self-renewal and differentiation, modulating the expression of genes involved in cell cycle progression and pluripotential state. Several miRNAs have been proposed to target transcripts that, directly or indirectly, coordinate cell cycle progression in embryonic, somatic and cancer SC. Among these miRNAs, miRNA (MiR)-290-295, miR-302, miR-17-92, miR-106-b25 and miR-106a-363, together with members of the let-7 family, regulate ESC cell cycle, mostly by facilitating the G1/S transition[56,57].
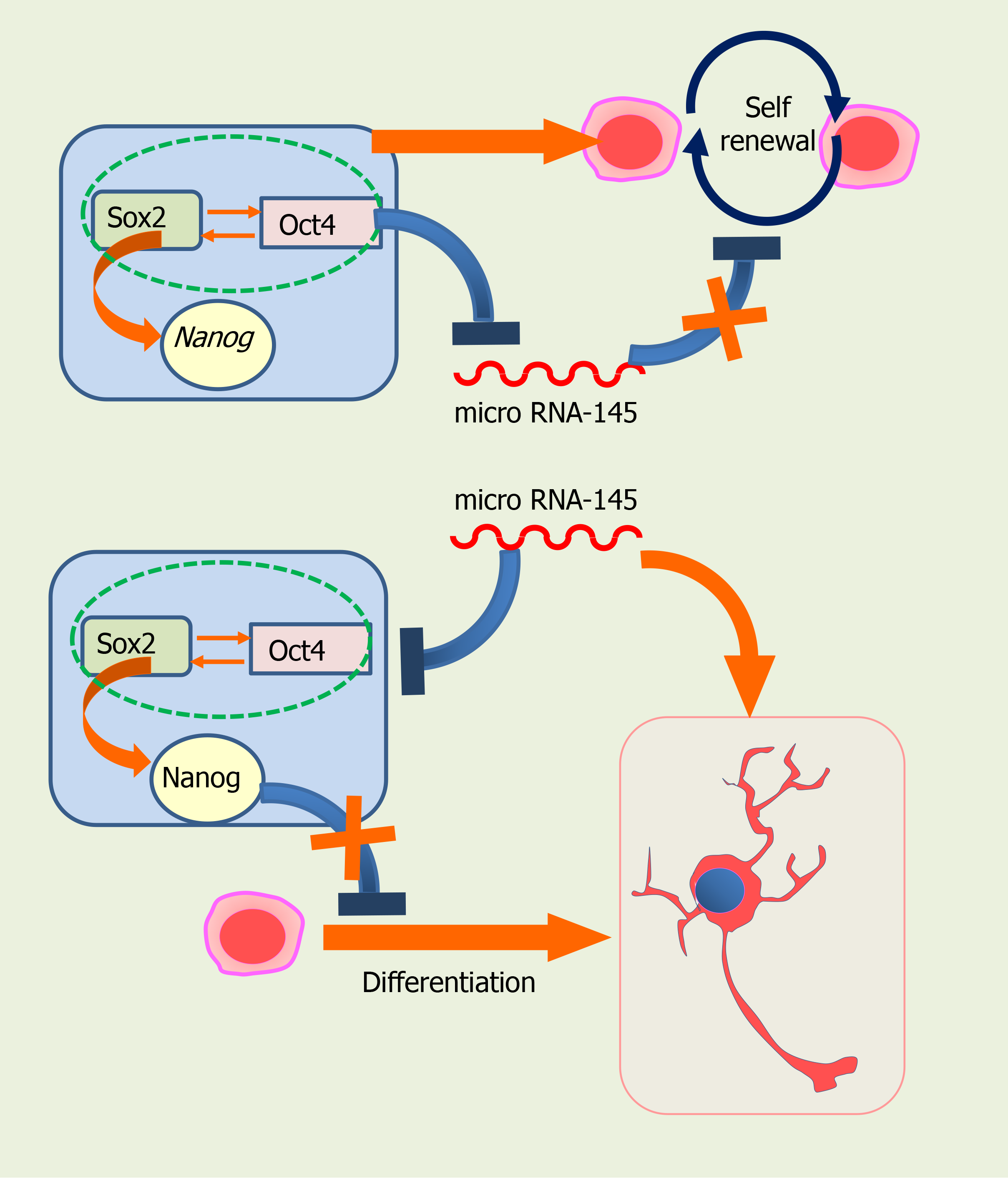
MiRNAs also participate in regulating and promoting SC differentiation (Figure 3). miR-145 is important during hESCs differentiation, having the pluripotency factors Oct4, Sox2, and Klf4 as direct targets (Figure 3). Overexpression of miR-145 Leads to repression of the 3' untranslated regions of Oct4, Sox2, and Klf4, thus inhibiting self-renewal and promoting differentiation. Interestingly, Oct4 binds to miR-145 promoter and inhibits its transcription in hESCs, acting as a negative loop[58].
MiR-145 promotes neuronal differentiation and regulates neural SC by repressing the expression of sex determining region Y-box2, and Sox2 (Figure 2), along with that of Lin28, a well-characterized RNA binding protein and a pluripotency promoter, which suppresses the biogenesis of let-7 miRNA. MiR-145 also upregulates let-7a and let-7b during neurogenesis[59]. In turn, the let-7 family inhibits proliferation by interfering with cell cycle regulators such as RAS, Cyclin D, and CDC25. Pos-transcriptional regulation of let-7 by Lin28 appears to be required for normal development. Moreover, let-7 might have a central role in the regulation of ‘stemness’, by repressing self-renewal and promoting differentiation, not only during normal development, but also in cancer cells[60,61].
Exogenous LIN28 expression has been shown to suppress Let-7 activity, thus reverting inhibition of cell proliferation in human neural progenitor cells[61-65] (Figure 2). Moreover, Sox2 is required for preserving the expression of physiological levels of Lin28 in the developing neural tube[61]. Sox2 binds to a promoter region of LIN28, and promotes acetylation, by interacting with the histone acetyltransferase complex[61]. Collectively, these data imply that miRNAs and several transcription factors regulate self-renewal in SC, interacting very precisely to decide whether they continue in the cell cycle or start their differentiation.
Wang et al[66] established an additional mechanism for regulation of pluripotency, involving a large intergenic noncoding RNA (lincRNA), the linc-regulator of reprogramming, or linc-ROR, which belongs to a larger group of non-coding RNA (ncRNA). While the vast majority of the mammalian genomic DNA is transcribed, the largest fraction are ncRNAs, as only a small fraction are protein-coding genes. In addition to the well-known transfer RNAs, ribosomal RNAs, and miRNAs, these ncRNAs include long ncRNAs (lncRNAs), which are longer than 200 nucleotides.
LincRNAs are transcribed from both strands of DNA in intergenic regions[67], and have both exons and introns. LncRNAs are shorter than lincRNAs, and they both have single-stranded sequences, able to form secondary structures[67]. LincRNA transcripts are generally found in the mammalian nucleus, while lncRNA transcripts are usually in the cytoplasmic region. LincRNAs regulate the transcription of neighboring genes by increasing or repressing transcriptional activation, and are believed to be involved in several pathologies. In contrast, the exact functions of lncRNAs are not fully established. Linc-ROR was the first identified linc-RNA; it promotes reprogramming of differentiated cells into iPSCs and maintains ESCs[68]. Noteworthy, linc-ROR interacts with several miRNAs and has been reported to be controlled by Oct4, Sox2 and Nanog in iPSCs. The presence of binding sites for these pluripotency transcription factors in linc-ROR indicates they regulate its expression, and hence that of human ESC (hESC)[68]. In turn, linc-ROR has been shown to maintain hESC self-renewal by functioning as a “sponge”, trapping miR-145 and preventing miRNA-mediated suppression of the pluripotency factors Oct4, Nanog, and Sox2[66] (Figure 2).
Linc-ROR has also been proposed to modulate the reprogramming of human iPSCs[69]. LINC-ROR may also be oncogenic, having as a target the EF-hand calcium binding protein tescalcin, an oncogene significantly upregulated in ocular melanoma cell lines and animal models[70].
Most of the above described regulatory signals and mechanisms are operative in the retina. Our knowledge on the mechanisms regulating SC function in this tissue has enormously increased during the last two decades. Growth factors and signaling pathways, such as fibroblast growth factor (FGF2), epidermal growth factor (EGF), insulin growth factor (IGF), ciliary neurotrophic factor (CNTF), Wnt/β-catenin, Notch–Delta, and others, regulate the regenerative response in the retina, supporting and maintaining the status of endogenous SC by activating proliferation and reprogramming cells to replace injured or dead retinal neurons. Some inhibitory extracellular matrix or cell adhesion molecules, along with Bmp4 and other signaling pathways, often have the opposite effect[71]. Other systemic factors such as hormones, growth factors, cells of the immune system and blood, are regulators of the regenerative behavior of endogenous retinal SC[72].
Substantial evidence underscores the role of trophic factors in regulating SC activity. Early work evidenced the relevance of trophic factors in activating SC after injuries; several trophic factors and other molecules, including FGF2, EGF, SC factor, erythropoietin, and brain derived trophic factor (BDNF) increase adult neurogenesis by stimulating generation of new neuronal cells or improving their survival[73]. Trophic factors usually contribute to improve the milieu in which SC proliferate; provision of an enriched environment to neural stem/progenitor cells promotes neurogenesis in the brain subventricular zone after inducing a cortical stroke[74]. Furthermore, SC appear to contribute to enrich their own environment by releasing trophic and survival molecules. MSC secrete many survival factors such as EGF, IGF-1, FGF2, BMP-7, TGF-b1, and interleukin-6 (IL-6), among other factors, which protect injured cells[75-77]. These factors might regulate SC proliferation, migration, differentiation, and interactions after injuries. Interestingly, enriched environment has a neuroprotective effect in the retina in diverse animal models of pathological situations, such as glaucoma and ischemia reperfusion, preserving or increasing BDNF levels[78,79]; whether it also impacts on SC regulation is a pending question that demands further research.
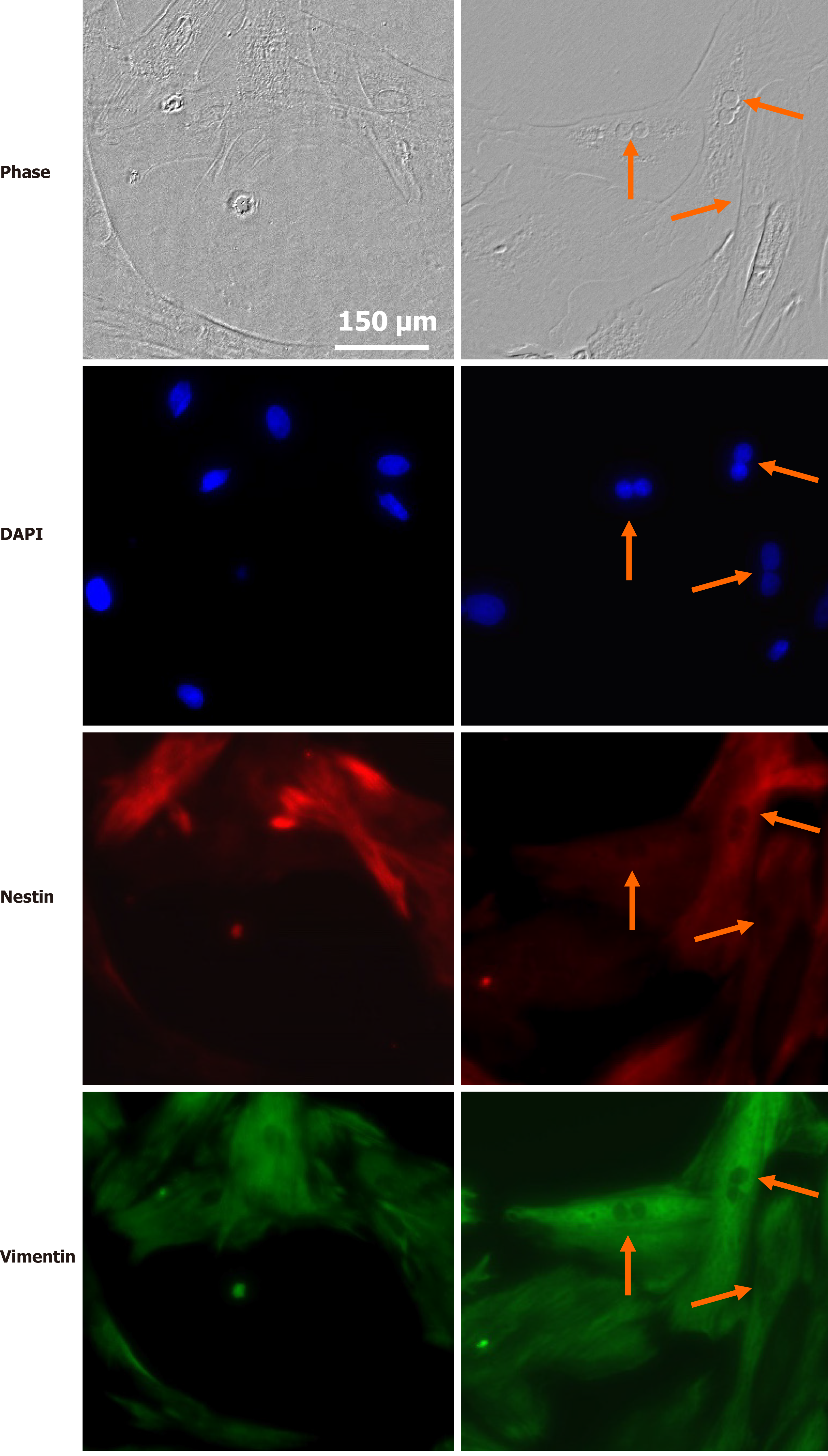
Retina SC have profiles similar to those of RPCs at early stages of eye development. They respond to many well-known intracellular factors, including the transcription factors Pax6, Chx, Rx, Six, Sox, Prox, Pitx, and others[80-82]. Their regenerative potential is epigenetically regulated[83-85], and they are also influenced by trophic and survival factors. MGCs have been proposed as retina SC and our research group has established that trophic factors as glial-derived neurotrophic factor (GDNF), FGF2, insulin and IGF-1 increase their proliferation and expression of SC markers such as Pax6 and nestin. This implies that the intrinsic, but dormant proliferative capacity of these cells can be regulated by trophic factors and other environmental molecular cues[86] (Figure 4).
Interestingly, dental pulp SC, which originate from the neural crest, are able to differentiate into neurons when supplemented with EGF, FGF2, and retinoic acid[87]. When transplanted intravitreally, they have been shown to secrete significant amounts of nerve growth factor, BDNF, neurotrophin-3, and GDNF, promoting neuroprotection and axon regeneration in retinal ganglion cells after axon injury[88-90]. Further research is required to identify the trophic factors required for preserving multipotentiality and proliferation in retina SC.
Transcription factors and miRNAs have been shown to control the formation of new retinal neurons derived from endogenous SC. Particularly, miRNAs are involved in controlling the ability of MGCs in non-mammalian and mammalian vertebrates to generate new RPCs[91]. In zebrafish, which, as mentioned before, effectively regenerates the retina after injury[92], miR-216a regulates reprogramming in MGCs, maintaining them in a quiescent state in undamaged retina. miR-216a suppression is necessary and sufficient for MGC dedifferentiation and proliferation, having the disruptor of telomeric silencing-1-like (Dot1 L) as a target; this miR-216a/Dot1 L regulatory axis mediates the initiation of retina regeneration through the Wnt/β-catenin pathway[93]. In addition, miR-9 has been recently identified in zebrafish MGC as a critical factor controlling retinal NSCs proliferation and fate[94], its depletion increasing the number of neural progenitors and neurons. miR-9 has different targets, including lin-28, which is necessary to promote the proliferation of retinal NSCs after injury[95], TLX and ONECUT mRNAs, which promote NSCs differentiation into neurons in both zebrafish and humans[94]. miR-9 might act as a negative regulator of the Sox2-Ascl1a/Atoh7-Lin-28 pathway to prevent MGC proliferation and as an activator of the TLX-ONECUT pathway for reprogramming endogenous MGC into functional retinal neurons[96]. Furthermore, miR-9 is involved in regulating mouse RSC differentiation through repression of polypyrimidine tract-binding protein 1 (PTBP1) expression, which is a repressor for polypyrimidine tract-binding protein 2 (PTBP2), during neuronal differentiation. Both proteins are highly expressed in the fetal stage and show lower transcript levels in the mature brain, retaining PTBT1 expression in glial cells and that of PTBP2 mostly in neurons[97]. miR-9 promotes the differentiation of neuronal cells from mouse RSCs, reducing the expression of PTBP1 and consequently increasing the expression of PTBP2[98]. Moreover, over-expression of miR-25 and miR-124, or let-7 antagonism induces the expression of proneural transcription factor Ascl1, a crucial regulator in retinal regeneration[99], and promotes the conversion of mature MGC into a neuronal/RPC phenotype[100].
In contrast to miRNA, most biological functions of lncRNAs are still poorly understood. They are believed to control key biological processes, including cell proliferation, apoptosis, differentiation, oxidative stress and inflammation, and have been shown to regulate SC maintenance and neural SCs proliferation[101,102]. In the eye, lncRNAs also appear to regulate SC maintenance, cell lineage commitment, and cellular phenotype differentiation[103] and have been involved in several ocular pathological conditions, such as glaucoma, proliferative vitreoretinopathy, diabetic retinopathy, and ocular tumors[104-107]. Interestingly, a retina-specific lncRNA, Vax2os, is involved in cell cycle progression in PHR progenitor cells during development of the mammalian retina[108,109]. Other lncRNAs, such as RNCR2[110], MIAT[111], and Gomafu have been found in the developing retina[112]. MIAT plays a critical role in regulating mammalian retinal cell specification and is involved in several diseases leading to visual impairment as well[113]. Expression levels of the lncRNA MALAT1 are significantly upregulated in diabetic retinas and it has been suggested to regulate retinal neurodegeneration in several rodent models[114]. MALAT1 expression is upregulated in cultured MGCs and retinal ganglion cells following stress, while its suppression decreases reactive gliosis, suggesting that MALAT1 dysregulation leads to neurodegenerative processes[115].
An advantage of SC therapies in the eye is that they may provide a tool for achieving cellular regeneration in diseases leading to the loss of particular cell types, as retinal ganglion cells are to be replaced in glaucoma, and PHRs or RPE cells in AMD or RP. Noteworthy, the recovery of a particular cell type might also have a trophic role, restoring the provision of trophic factors released by that cell type. As stated above, the fact that the vitreous cavity is a relatively immune-privileged site also contributes to the feasibility of SC transplantation.
These advantages have prompted researchers to establish the most suitable sources of SC and define which protocols to apply for each particular situation. Two main approaches can be described; one of them takes into account the cell type contributing to the degeneration; thus, many studies focus in RPE cells, when their damage plays a pivotal role in the pathogenesis of the disease, such as AMD; a second approach aims to directly replace the retinal neurons affected in each disease.
In the first approach, the efforts have been directed mainly to transplanting SC-derived RPE cells into the eye, either as a monolayer or as a cell suspension. Numerous protocols have been assayed, with different advantages and disadvantages; establishing the most suitable therapeutic strategy demands a careful analysis of all of them. A successful treatment would allow the replacement of the damaged RPE cells, thus preventing the progression of diseases such as AMD. A potential disadvantage is that in these diseases the initial degeneration of RPE cells is usually followed by the degeneration of neurons, mainly PHRs. Since most of the SC used are unipotent and will only generate new RPE cells, this approach has the limitation that it can only be useful at early stages of the disease, as no replacement of lost PHRs or other neuronal cells will be achieved when used at advanced stages.
Using SC to replace retina neurons faces several obstacles. The first is the difficulty to achieve their differentiation into the required neuronal cell type. Even if this is surpassed, the integration of the newly generated cells into the host retina confronts numerous critical complications such as their engrafting onto Bruch’s membrane, achievement of their adequate polarization, reestablishing an adequate circuitry, and finally avoiding immune rejection[116,117].
PHR death is the cause of most RDs, excluding glaucoma. Hence, a second approach to treat most RDs is to directly replace PHRs. With this aim, several strategies have been evaluated. Early work showed that transplants of embryonic murine retinas into the anterior chamber of adult eyes survive and develop, resulting in the differentiation of both neurons and MGC, with few cases of graft rejection[118,119]. Subsequent strategies involve the reactivation of the dormant potential for regeneration of endogenous populations of cells within the retina to generate new PHRs or also to attempt retinal repair by transplantation of healthy PHRs into the vitreous or the subretinal space[120,121].
Since the expectation of recovering visual function by using SC emerged, there has been an active search to establish the most adequate SC sources. As occurs with other tissues in the body, the retina has several cell types that display properties of SC. This is evident in fish and amphibians during development and regeneration. During embryogenesis, most of the retina originates from the ciliary marginal zone, a ring of cells found at the periphery of the retina. In contrast, different sources might provide new neurons during regenerative responses. In the amphibian retina of urodeles, RPE cells play a critical role during retina regeneration; these cells dedifferentiate into retinal progenitors that have to recapitulate the normal development of the retina, first proliferating, and then differentiating into the different retinal cell types[122]. In zebrafish, MGCs are recruited following damage of the retina, and transiently de-differentiate, express SC markers, and re-enter the cell cycle, to generate retinal progenitors that migrate to replace the lost neurons[92].
Consequently, cumulative evidence has shown that the ciliary margin zone, the RPE cells, the iris, and MGCs are the major sources of SC in the eye[71,120,123-125]. Some of these cells are represented by stem and low-differentiated cells and others by latent, differentiated progenitors[71,120,123-125]. In spite of their different origin, all of them share the essential requisites of SC, namely, the capacity for unlimited self-renewal and the ability to differentiate into different cell lineages. These traits turn them into ideal sources for recovering the different cell types lost in diverse retina degenerative diseases, thus repairing the retina and restoring vision. The feasibility of reprogramming resident, non-neuronal cell types into neurons in vivo[126] has led to include them as well among the SC types apt to contribute to neuronal regeneration in the retina.
We will now describe briefly the most explored candidates for cell replacement in the retina and the research, including clinical trials, that support their use.
RPCs are among the most promising SC type for treating RDs (Table 1). All cells in the retina derive from them, making these cells, obtained from human fetal retinas, an attractive source of SC for retina repairing. The evolutionary conserved, temporal organization of the genesis of each cell type, and even of particular subtypes, during development of the retina is well established[127]. RPCs proliferate actively, are generally multipotent and have been shown to produce a specific repertoire of cell types at defined developmental stages, suggesting they exhibit intrinsic changes in their state of competence along development[128]. Noteworthy, RPCs are very heterogeneous regarding their gene expression[129]. Although intrinsic cues play a central role in defining cell fate, this fate is not strictly determined and the context, i.e., regulatory and transcription factors, miRNAs, active/inactive signaling pathways, influences the cell response to a certain perturbation[127]. In addition, extrinsic cues may contribute to modulating the temporal progression of cell fate acquisition; soluble factors released by ganglion or amacrine cells can limit the generation of the respective cell type[129,130], implying their involvement in feedback inhibition.
| Retinal disease | Type of SC used | Type of intervention | Clinical trial status | Results: Advantages and disadvantages | Ethical concerns |
| RP, AMD | Fetal RPCs[33,147-150] | Subretinal or intravitreal transplantation | Yes[40,151] | Rescue of PHRs and low risk of tumorigenesis. Able to differentiate into MGCs and retinal neurons; improvement of retinal sensitivity. Adverse effects: limited amount of cells and low tissue integration. Promising therapeutic treatment. | Little |
| RP, AMD, most retinal degenerations | PSCs and iPSCs | Reprogramed to iPSC[40] | Yes[40,200,293] | Potential replacement of damaged retinal cells. Low immunogenicity. Adverse effects: risk of teratoma development[40,50,156,160-163]. | Little or none |
| Genomic instability in iPSC. Further research still needed. | |||||
| AMD, retinal degenerations | RPE | Transplantation of RPE sheets from human fetal eyes in AMD patients | Yes[204,283] | Substantial rescue of PHRs. Visual improvement[147,148]. | Concerns about using human tissues |
| Little or no evidences of draft rejection[33,203,204,281,285]. | |||||
| Adverse effects: unwanted cell aggregation; lack of attachment; cases of anoikis[162,163]. | |||||
| AMD, stargardt disease | Human ESC-RPE | Injection of differentiated ESC-RPE cells in subretinal space[238] | Yes phase 1/2a trial[175,176] | Cells form monolayers and display typical RPE features[171-173]. | Concerns about using human tissues |
| Improvement of visual acuity. No tumorigenicity or rejection after 4 years[187]. | |||||
| Cell sheets preserve RPE characteristics better than cell suspension[175-178]. Absence of serious adverse effects[131,188]. Effectiveness still uncertain. | |||||
| AMD | iPSC-RPE | Autologous transplantation into an AMD patient of cell-sheets of RPE, differentiated from iPSC obtained from the skin’s patient[195] | Yes[195,199,200] | Preservation of main RPE features. | Important concerns about safety |
| Long term survival of transplanted iPSC-RPE cells[196,199,200]. Visual acuity stable for 4 years[197,198]. | |||||
| Presence of mutations in iPSCs[199]. Little immune rejection[202]. Adverse effects: Risk of tumorigenesis. | |||||
| Still pending to establish the adequate iPSC-RPE cells and the effectiveness of transplantation. | |||||
| AMD, stargardt macular distrophy | human fetal SC | Transplantation of human fetal RPE cells into subretinal space | Yes[176,203] | Improvement of visual parameters.No immunosuppression; No restoring of retinal morphology; no expression of retinal markers[169,208]. Adverse effects: little graft rejection in patients with AMD.Further research still required to provide effective and safe treatments. | Concerns about using aborted human fetuses |
| Retinal degeneration | MGCs | Not established | No | Express most SC markers; potentially capable of retinal regeneration after reprogramming. Obstacles: gliosis, low regenerative potential. | Not determined |
| Retinal degeneration, retinal injuries and uveitis | Bone marrow (MSC) and hematopoietic SC | Intravitreal injection (in mouse models of RP) | No | Promote regeneration of different retinal cells[238,246-248]. Safety not determined. | Not determined |
SC-derived RPCs (SC-RPCs) obtained from embryos are a possible source of cells for retina cell replacement strategies[131]. These multipotent cells display markers indicative of retinal SC fate, such as Pax6, Vsx2, Lhx2, Six3, Rax, and can differentiate into MGCs and the six types of retinal neurons[132-136]. Establishing the strategies required for achieving the differentiation of SC-RPCs into the multiple cell types that constitute the retina and drawing a more precise roadmap of the cues involved is crucial for their successful use in regeneration schemes.
Initially, the feasibility of expanding these cells in order to achieve a sufficient amount for transplantation, while simultaneously preserving their multipotency was a limiting factor for using RPCs isolated from the fetal neural retina. Promising in vitro and in vivo studies have shown that SC isolated from embryonic or fetal retina can be expanded in vitro[12], under particular conditions, such as low-oxygen culture conditions[137], paving the way for their use.
The ability of human or mouse fetal RPCs to repair retinal damages has been analyzed in multiple sub-retinal transplantation studies, which have evidenced they can rescue PHRs, preserving rhodopsin expression and visual function[18,138-141].
Human RPCs are able to differentiate into specific retinal cell types, including PHRs, preserving vision in rats[132,137,142-144]. RPCs isolated from retinas of postnatal day 1 mice, expanded in culture and grafted in the degenerating retina of RD or rho-/- mice differentiate and express PHR markers; in rho-/- mice, RPCs integrate in different retina layers, increasing outer nuclear layer thickness and improving light-mediated behavior[12]. Transplantation of RPCs in the subretinal space has been proved feasible; mice retinal progenitors obtained from postnatal day 1 retinas and injected into the subretinal space of different adult mouse models of retina degeneration can integrate, differentiate as PHRs and improve visual function[132,145]. Transplantation of human RPCs into Royal College of Surgeons (RCS) rats, an animal model in which a mutation in RPE cells leads to PHR degeneration, preserves the outer nuclear layer thickness and cell count and prevents visual loss[138]. Preclinical studies have also used RPCs obtained from human fetal retinas, between 14 wk and 20 wk of gestation, a time point at which PHR progenitors are exiting the cell cycle and initiating their differentiation[146]. Delivery of human RPCs by intravitreal transplantation has also been proved effective; injecting human RPCs, obtained from retinas of 16 wk to 18 wk’ gestational age, in the vitreal cavity of RCS rats has no adverse effects, and preserves PHR cell nuclei in the outer nuclear layer and visual function for 8 wk, decreasing afterwards[40]. However, the low efficiency of integration of RPCs in these studies, usually restricted to areas near the injection site, with few cells achieving differentiation are significant drawbacks still to be overcome.
Fetal RPCs have been used to repair atrophied retina areas in patients with RP or AMD[147-149] (Table 1). Intravitreal and subretinal delivery of allogeneic RPCs for RP treatment are still under evaluation in clinical trials. A sole intravitreal injection of human RPCs has been associated to an improvement in visual acuity in treated vs non-treated eyes, after a 12-month follow-up in phase I/IIa RP patients[151] and a phase IIa trial has been initiated[152] to evaluate safety.
Although RPCs emerge as a promising source of SC to achieve PHRs and retina regeneration, further knowledge is necessary to improve the efficiency of their integration, and control their differentiation into the required cell type, the extent of their reparative effect and, when allogeneic conditions are used, the magnitude of potential immune-derived damage.
PSCs are another potential source of cells to treat RDs. These cells were initially restricted to ESCs deriving from the inner cell mass of preimplantation embryos, and can be indefinitely maintained in the pluripotent state (Figure 1). This pluripotent state is preserved by a complex and coordinated gene network, along with several signaling pathways activated by environmental cues that start in blastocysts (in the blastula stage) and persist until gastrulation; at this time point, levels of Oct4 and Nanog decrease, and pluripotency can no longer be preserved[152-155]. However, a major step forward for SC-based therapies came from the in vitro technology developed by Takahashi and Yamanaka[15], and Takahashi et al[156], that allowed to obtain PSCs by inducing dedifferentiation of adult somatic cells through their reprogramming to a pluripotent state, thus generating iPSCs. As ESCs, iPSCs cells can be expanded indefinitely, preserving an undifferentiated state and, eventually, can differentiate into cells of the three germ layers: ectoderm, mesoderm and endoderm[157].
Numerous in vitro studies have now evaluated the capacity of IPSCs for retina cell replacement. Addition of low molecular-mass compounds to iPSC cultures leads to their differentiation into retinal progenitors, RPE cells and PHRs[158]. In a recent work, Fligor et al[159] demonstrated that three-dimensional retinal organoids derived from human iPSCs can recapitulate retina differentiation and are useful models to investigate guidance of developing neurites toward their targets.
The ability of human iPSCs to differentiate into a wide range of cell types turns them into a suitable and attractive alternative to ESCs, avoiding the ethical issues associated to the later. Hence, human iPSCs appear as likely candidates to be successfully used in the near future, providing better models for studying, treating, and eventually curing retinal degenerative diseases. Nonetheless, a crucial problem precluding their use is that they have been shown to give rise to teratomas when injected into immune-deficient mice[160, 156,161-163].
Further research on the mechanisms underlying self-renewal properties, and safety of human PSCs are still needed to solve the pending questions associated with their therapeutic possibilities[164] (Table 1).
Ciliary-derived cells and RPE cells have also been reported as a potential source of progenitor cells that can be mobilized to the injured retina[165]. RPE cells form a monolayer between the PHRs and the choroidal vasculature. They transport ions, water and metabolic end products from the subretinal space to the blood, they represent the only blood supply for the outer retina and have the critical function of constantly clearing shed PHR outer segments by phagocytosis. Their interactions with the Bruch’s membrane and the choriocapillaris constitute a barrier that regulates exchange of substances between the neural retina and the circulation. To perform these functions adequately, RPE cells must maintain a polarized structure, which is crucial for the homeostasis of the outer retina, the disruption of which leads to degenerative retinopathies[166].
Transplantation of SC-derived RPE cells has been in the spotlight for over 10 years as a cell replacement strategy, particularly for patients with macular disorders, in which the early loss of RPE cells leads to the subsequent death of PHRs. A huge amount of information has been accumulated regarding their efficacy and safety, and clinical trials are already on course.
RPE cells were among the first candidates evaluated for subretinal transplantation since they can be easily obtained from patients, for autologous replacement therapies, or even from corpses, and can be maintained in vitro for long periods. Autologous RPE cells have been used in patients with RP or AMD, transplanting patches of RPE cells into the damaged areas of the retina[147-149]. A weakness to the therapeutic possibilities of adult RPE cells is that the normal functions of RPE cells are not fully reestablished after these procedures, and they may retain some aging features[117].
Transplantation of RPE cells has many advantages, appearing as a suitable strategy to treat inherited diseases, such as AMD. The possibility of differentiating RPE cells from hESC (hESC-RPE) and from iPSC (iPSC-RPE) has paved the way for their use, since it provides a potentially unlimited source for the replacement of affected or dead RPE cells[167]. hESC-RPE cells can be obtained by culturing ESC colonies, in which cells spontaneously differentiate into RPE cells after removal of FGF. Sheets of RPE cells can be obtained from cultures of either ESCs, or iPSCs, and they can form confluent monolayers, reproducing many of the functions of RPE cells[168-170]. They not only form monolayers, but also display the typical RPE microvilli and pigment-containing melanosome granules, and express RPE markers, such as Na+K+ATPase, Pax6, and RPE65, together with proteins associated with tight junctions and involved in retinol cycling[163-171]. hESC-RPE cells have also been shown to express and release pigment epithelial derived factor from their apical surface[174]. Following their transplantation into RCS dystrophic rats, hESC-RPE cells survive in the subretinal space, expressing low levels of RPE65 and downregulating the cell cycle and Pax6 expression, while maintaining expression of other markers[172].
The efficacy of transplanting these cells as either a cell suspension or as sheets is a matter of extensive analysis. ESC-derived RPE cell suspensions were safely used in a phase I/IIa trial for treating AMD and Stargardt disease patients[175,176] (Table 1). However, the effectiveness of this strategy is still uncertain as these suspension are unable to form the typical RPE monolayers and to survive long periods of time[177,178]. Animal studies evidence the feasibility of subretinal transplantation of a hESC-RPE monolayer re-grown in a biocompatible membrane, which shows a normal implantation[179,180]. Current evidence suggests that cell sheets, rather than cell suspensions, might be more effective for preserving morphology, polarization, survival and physiology of RPE cells[177].
Different materials have been used to support RPE monolayers, with or without artificial scaffolds, as these materials may influence inflammation, adequate insertion and interaction of RPE cells with other cell types in the retina[181]. These patches allow the formation of tight junctions, required to acquire a fully polarized morphology, which, as stated above, is critical for RPE cells functions and for their interaction with PHR segments[182]. However, the survival of these patches after transplantation is a significant problem to deal with (Table 1).
The transplantation site is also still subject of considerable research. The subretinal space, a frequent transplantation site, is a relatively immune privileged site, and RPE cells located in this space have immunosuppressive functions. However, transplanted RPE grafts can be eventually rejected, as they can be attacked by immune cells due to their immunogenicity[181,183] (Table 1). Initial studies to evaluate the efficacy and safety of RPE cells for retina regeneration therapies were performed in animals, and most of them involved immunosuppression. Transplantation of RPE sheets prepared from human fetal eyes in RCS rat eyes indicates a substantial rescue of PHRs in the area of the RPE patches, with no evidence of draft rejection[33]. Later work evidenced that subretinal transplantation of hESC-RPE in RCS rat eyes leads to PHR rescue and improvement in visual performance; donor hESC-RPE localized adjacent to the RPE layer show no uncontrolled proliferation or evidence of tumor formation[171]. Similarly, long-term functional rescue is observed after subretinal injection of ESC-RPE in animal models of AMD and Stargardt disease, diseases in which RPE degeneration leads to PHR loss and visual deficiency; the retinas preserve PHR integrity and function, without evidence of teratomas or pathological changes[184].
Some problems still remain. Cell transplantations of RPE in the retina frequently causes unwanted cell aggregation or lack of attachment onto the Bruch´s membrane[185], with RPE cells tending to form aggregates (rosettes) instead of functional monolayers[172] or undergoing anoikis when dissociated from their usual extracellular matrix[186] (Table 1).
Further studies are required to solve these pending issues; nevertheless, cumulative information support ESC-RPE as a potentially useful and inexhaustible source of SC for treating retina degeneration.
The first clinical study evaluating the feasibility of transplantation of hESC-RPE cells to human patients was reported by Schwartz and colleagues in 2012[175]. These researchers injected differentiated hESC-RPE cells in the subretinal space of patients having macular degeneration. The cells integrated in the host RPE layer, forming monolayers, and improved visual acuity in over half of the patients, with no visible hyperproliferation, tumorigenicity or rejection-related inflammation even after 4 years[187]. A similar study evidenced an improvement in visual acuity and the absence of serious adverse effects[188]. In a Phase I trial, hESC-RPE cells on a coated, synthetic basement membrane transplanted in two patients with severe exudative AMD successfully survived for a year and increased visual acuity[189]. Therefore, retinal implants of ESC-RPE cells appear as a promising therapeutic tool to treat retinal diseases (Table 1).
iPSC-RPE are also a promising source of RPE cells for transplantation as they provide a virtually unlimited number of RPE cells, in a non-invasive manner (Table 1). iPSC-RPE cells have the same genetic background, and display morphological and functional characteristics of mature RPE cells, which they retain after their transplantation in the rodent retina[190-193]. iPSC-RPE cells have been established from mouse, monkey and human, using different methods to obtain the iPSCs and then induce their differentiation into RPE cells[194-196]. These cells preserve key RPE features; Carr et al[191] showed that following their transplantation into the RCS dystrophic rat, iPSC-RPE phagocyte PHR material, both in vitro and in vivo.
Several studies have reported that human iPSC-RPE cells also exhibit native RPE features, such as gene expression and cellular functions, and have immunosuppressive properties, suppressing T-cell activation in vitro[181].
Ensuring safety and efficacy are major concerns for their clinical applications. Tumorigenesis is a major risk, due to the reprogramming methods used and their possible contamination with iPSCs, which might contribute to formation of teratomas[181].
The potential use of iPSC-RPE cells has been tested in fewer clinical trials than ESC-RPE cells. The first human pilot trial was conducted by Mandai et al[197] and generated great public attention. iPSCs were induced from the patient´s skin cells by introducing reprogramming factors and then differentiated into RPE cells; an autologous RPE sheet was then prepared and transplanted into a patient with neovascular AMD[197]. The graft remained stable, with no signs of rejection or increased proliferation, and the patient´s visual acuity remained constant for four years[197,198]. A further trial was suspended due to the presence of mutations in iPSCs from another subject[199]. Takahashi´s group is currently evaluating the use of allogeneic iPSC-RPE cell grafts, which would be faster to prepare and easier to control genomic stability; their use requires to take into account the different factors leading to a higher risk of immune rejection.
In an early clinical study, Sugita et al[200] used iPSC-RPE cells derived from major histocompatibility complex (MHC) homozygous donors; after being transplanted into patients with exudative AMD having a matched MHC, the graft cells remained stable for one year, showing no abnormal growth. Although the patients experienced many moderate adverse events, such as corneal damages, retinal edema, elevated intraocular pressure, endophthalmitis, and mild immune rejection in the eye, this trial demonstrates long term survival and safety of transplanted iPSC-RPE cells[196,200,201]. Ongoing clinical studies in five patients with neovascular AMD have safety as their main concern and report the survival of the transplanted grafts and only one case of immune rejection[202]. Once the safety of their use is established, further clinical trials will be required to determine which iPSC-RPE cells are the most adequate and whether their transplantation is effective to improve visual function. Of note, treatments with iPSC-derived RPE cells are limited to early stages of RDs; they may be ineffective in patients with an irreversible PHR loss.
Fetal RPE cells provide another source of RPE cells for transplantation. In an early work, Algvere et al[203] transplanted human fetal RPE cells into the subretinal space in AMD patients, with no immunosuppression; although patients with exudative lesions showed graft rejection, patients with geographic atrophy of dry AMD presented little evidence of rejection (Table 1). Even when alerting about the risk of rejection, they concluded that it is feasible to transplant human RPE into the submacular space of nonexudative AMD patients without adversely affecting visual function. Further clinical trials show that subretinal human RPE allografts transplanted into eyes of AMD patients, without immunosuppression, exhibit high rejection rates after 2 years, probably through a disruption in the blood retinal barrier; on the other hand, small extrafoveal transplants remain essentially unchanged for over 2 years in non-exudative AMD without immunosuppression[204]. Interestingly, transplants of fetal retinal tissues as intact sheets, or even as aggregates, in the sub retinal space in rats have been shown to survive, adhered to the host RPE sheets[116,205,206]. In these cases, the occurrence of a pre-existing RPE sheet in the transplanted recipient provides paracrine trophic support for the grafted tissue, preventing cell death[205,206].
Human fetal neural SC are another possible source of SC. These cells, obtained from aborted fetuses, were identified by the expression of the cell-surface marker CD133, and cultured under conditions that induce rosette formation. After injection into the subretinal space in RCS rats, these cells survived away from the injection site and improved visual parameters, even when they neither restored retinal morphology, nor expressed retinal markers[207,208] (Table 1).
Taken as a whole, the above findings imply that transplantation of fetal cells is a promising approach, still requiring further research to provide an effective and safe treatment.
MGCs emerge as a further promising source of SC for retina regeneration. MGCs are the principal glial cells in the retina and play crucial roles in the preservation of retinal structure and function[209]. They provide the main trophic and metabolic support for retinal neurons, playing a major role in the preservation of homeostasis, the regulation of nerve signal transduction, and the formation of synaptic structures in the retina. Emerging evidences suggest that MGCs are dormant stem-like cells present throughout the retina that serve as a source of progenitor cells to regenerate retinal neurons after injury[209,210]. In teleost fish, MGCs are a major source of progenitors for retina regeneration after injury. In the damaged zebrafish retina, the activation of a reprogramming process in MGCs leads to their de-differentiation to generate neuronal progenitor cells, which proliferate and finally differentiate into all the cell types forming the retina[92]. This remarkable regeneration capacity is much diminished in vertebrates; although vertebrate MGCs have been established as retina SC, they have a very limited capacity to achieve retina regeneration upon damage. In spite of this limitation, MGCs would provide an intrinsic source of SC, in contrast to ESCs, iPSCs, or embryonic fetal RPCs, for regenerative purposes. A further complication is that injuries to the mammalian retina turn on a reactive process in MGCs, termed “gliosis”, through which MGCs initially orchestrate a neuroprotective response and then, if the injury persists, turn on a pro-inflammatory response that further impairs neuronal function and tissue repair (Figure 5). This gliotic process is common to other glial SC in the CNS; following injuries to nerve tissues, astrocyte-like cells with SC properties activate a reactive gliotic response that interferes with neurogenesis, turning gliosis into a considerable obstacle for regenerative processes[211] (Table 1).
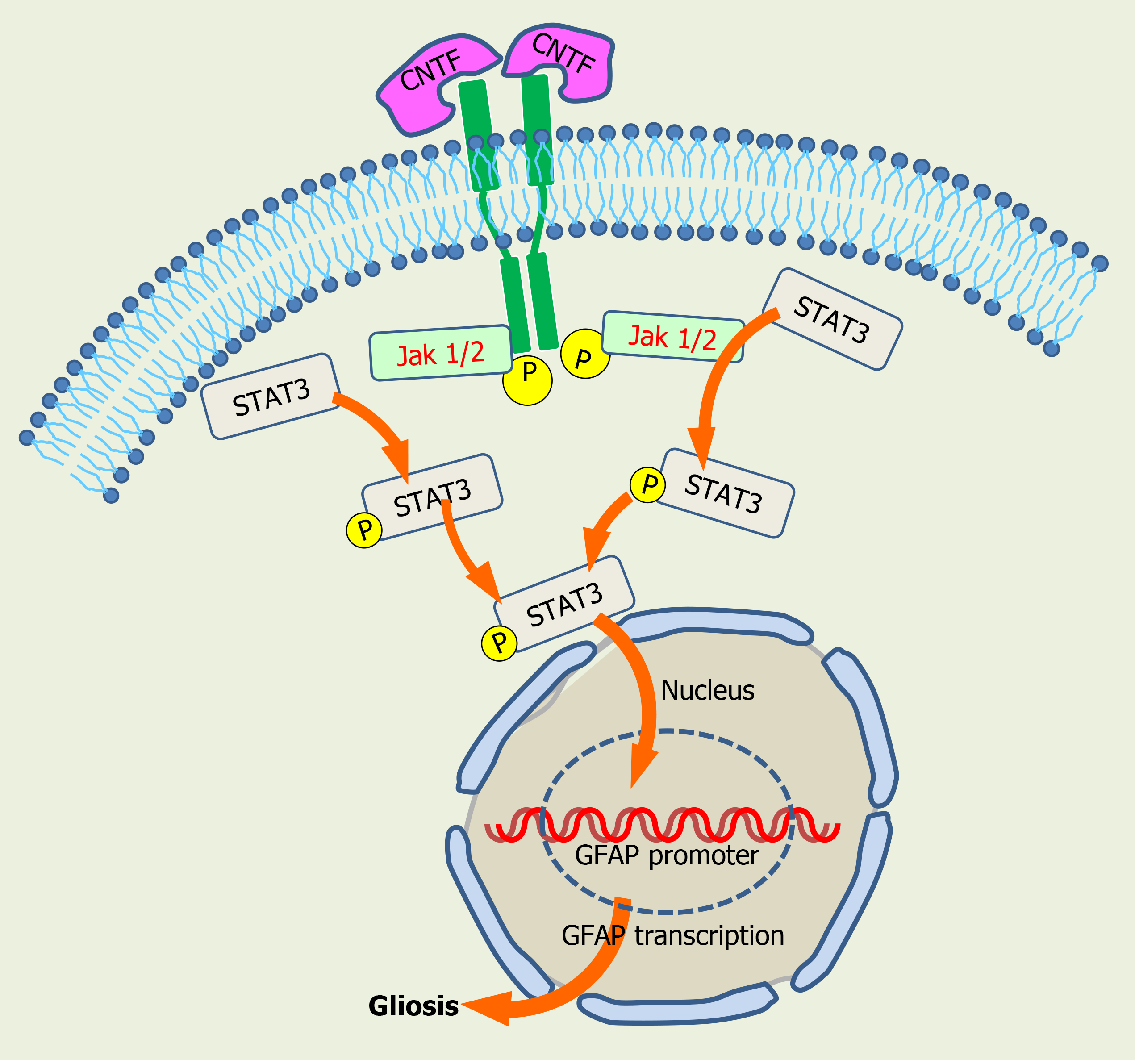
During this gliotic response MGCs up-regulate the expression of intermediate filaments and recruit macrophages. Reactive gliosis following transplantation also occurs in response to many other donor cell types, including neuronal cells, iPSCs, and PHR precursors, when transplanted either into the vitreous or in the subretinal space. This suggests gliosis is independent of the type and origin of transplanted SC. Intravitreally transplanted cells secrete CNTF and IL-6, among other factors, that activate the JAK2/STAT3 cascade, and STAT3 mediates glial fibrillary acidic protein (GFAP) upregulation (Figure 5). Noteworthy, pharmacological inhibition of STAT3 in BM MSC reduces GFAP expression and improves their retinal engraftment[212]. Moreover, activation of JAK/STAT signaling cascades is required for increasing proliferation of MGC-derived progenitors in NMDA-injured chicken retinas[213].
In spite of eliciting gliotic responses and scar formation in damaged retinas, that prevent neurite elongation and cell migration, MGCs still retain regenerative capabilities. Several studies have shown that a reduced amount of vertebrate MGC de-differentiates and re-enters the cell cycle, after different injuries, and eventually differentiate as retinal neurons[214].
MGCs have been shown to replace some or all retinal cell types in various species[215]. In response to damage, or when exposed to a combination of insulin and FGF2, MGCs in the chick retina can de-differentiate into proliferating progenitor cells, re-enter the cell cycle and express neuronal transcription factors such as CASH-1, PAX6 and CHX10[210,216]. However, their neurogenic competence is limited and they can only generate a few amacrine and bipolar cells[216-218].
In the mouse retina, neurotoxic injury activates proliferation and the expression of progenitor markers in MGCs[219], which can then differentiate into specific neuronal types[220,221]. Our work has shown that oxidative stress induces the de-differentiation and increases the proliferation of rat cultured MGCs[222]. Moreover, in mixed neuro-glial cultures, MGCs preserve the proliferative potential and SC characteristics of retina progenitor cells, even after successive reseedings, and also stimulate their differentiation as PHRs, increasing opsin expression and markers of PHR function, such as glutamate uptake and light-dependent cGMP degradation[223]. Interestingly, MGCs from the rd1 mice retina fail to preserve their proliferative capacity and the expression of SC markers, such as Sox2 and Nestin, in mixed rd1 neuron-glial cultures. Nestin expression is recovered when rd1 MGCs are co-cultured with wild type neurons and, conversely, it decreases in wild type MGCs co-cultured with rd1 neurons; this suggests that an active crosstalk between MGCs and PHRs is essential for the preservation of the regenerative potential of MGCs[224].
A recent work shows that culturing of human surgical retinal explants obtained from the equatorial retina reveals spontaneously migrating cells that express ESC markers, as Pax6, Sox2, Nestin and also MGC markers, such as GFAP and glutamine synthetase. This implies that following injury, this area of the retina might provide a source of RPCs, since it generates cells that possess the potential for regeneration, with markers consistent with Müller cell lineage[225].
As a whole, these findings imply that although with a restricted capacity, MGCs have the potential for neuronal regeneration. Understanding the mechanisms that limit this regenerative capacity and how to unleash it would allow reprogramming of MGCs as a source of progenitors for retina regeneration. Furthermore, given the undesired long-term effects of reactive gliosis, a better comprehension of the mechanisms of gliosis is essential before considering the use of MGCs for transplantation therapies.
A recent promising strategy has been successfully applied to unleash SC features in MGCs, through the transfer of cytoplasmic materials between transplanted and recipient cells. This transfer, by membrane fusion, exosome delivery or other methods of intercellular trafficking has been shown as an efficient tool for reprogramming cells[226,228]. Endogenous hematopoietic stem and progenitor cells transplanted into retinas of genetic and drug-induced mouse models of retina degeneration efficiently fuse with retinal MGCs in vivo, reprogramming them back to a neural progenitor-like state, to finally differentiate into PHRs, improving the electrophysiological response and the regeneration of the retina[228]. This supports transfer of intracellular material from SC as a new tool for turning on regenerative programs in MGCs.
Although this transfer appears as a promising strategy, understanding the molecular mechanisms involved is crucial for improving effectiveness of MGCs reprogramming. Extensive research has been devoted to uncover the diverse signaling pathways and the genetic network leading to MGCs reprogramming[229]. In zebrafish, this reprogramming involves the activation of different injury-induced genes that regulate neurogenic competence; among them, the proneural transcription factor ascl1a emerges as a crucial regulator in retinal regeneration[99]. Overexpressing Ascl1 and modifying MGCs epigenome, with a histone deacetylase inhibitor, promotes the generation of inner retina neurons from MGCs in adult mice after retinal damage[230]; this implies Ascl1 activation and epigenetic regulation are required for MGCs reprogramming. In contrast, the Hippo pathway repression of the transcription cofactor YAP blocks the ability of MGCs to adopt a proliferative, progenitor-like identity[231]. Exciting new data uncovers the genetic networks that control the regenerative capacity of MGCs in zebrafish and mice[232]. Retina injury triggers a reactive state in MGCs in zebrafish and mouse; however, while most zebrafish MGCs then adopt a progenitor fate, a dedicated gene regulatory network, which includes upregulation of nuclear factors I (NFI) factors, restore reactive mice MGCs to quiescence. Deletion of NFI factors in mice MGCs allows the generation of MGC-derived inner retina neurons, implying these factors are crucial to suppress neurogenesis from MGCs[232]. Although many questions remain to be answered, these findings suggest MGC reprogramming is a promising tool to unleash the SC potential of MGCs, which would contribute to human retina regeneration (Table 1).
As regardless of their source, most SC have the capacity to differentiate into nearly all of the cell types occurring in the human body, SC types like those from umbilical or placental tissues, have been evaluated in animal studies and in clinical trials for their therapeutic use in the eye. Umbilical tissues contain adult MSC, and other non-ocular cell types, such as placental cells and bone-marrow derived MSCs are also available[117,233-235]. An early study compared the efficacy of these three human-derived types of SC injected at early stages of RD in the subretinal space in RCS rats. Cells obtained from human umbilical cords were the most effective, rescuing larger areas of PHRs and preserving visual function, with no sign of tumor formation[236]. These cells were found to rescue phagocytic dysfunction in RCS-derived RPE cells in culture, by releasing trophic factors such as BDNF, GDNF, hepatocyte growth factor, and bridge molecules that bind to PHR outer segments and facilitate RPE phagocytosis[237] (Table 1). Adipose, BM and umbilical MSCs have been shown to secrete multifunctional exosomes with low risk of toxicity and immunological rejection[238]. In a clinical trial evaluating the safety and tolerability of the subretinal injection of human umbilical cord tissue-derived cells in patients with visual impairment due to geographic atrophy[239], patients showed a variable and consistent increase in visual acuity after 12 months, with no rejection or tumor formation[240]. Umbilical cord cells might thus provide a potential source of SC, able to contribute to PHR survival and function.
The use of BM SC has also gained relevance, given the evidences that these cells can rescue degenerating and ischemic retina[238]. Studies injecting BM SC subretinally in mouse models of RP show improvements in visual parameters and in the structure and function of RPE cells and PHRs[241,242] (Table 1).
BM SCs comprise MSC and HSC. MSC are easily accessible primary cells, with various biological functions and properties such as multi-lineage differentiation, anti-inflammation, immune suppression, and neuroprotection. They express specific cell surface markers including CD105, CD73, CD44, CD90, CD166, CD146 CD54, and CD49. HSC are capable of self-renewal, and can be identified by cell surface markers, like CD34+ in humans[243]. They have been used for transplantation treatments in retinal diseases, as in mice models of diabetic retinopathy or in ischemia-reperfusion injury[244].
BM SCs have generated great expectations for treating several RDs, including retinal injury, and autoimmune uveitis. Their intravenous injection reduces laser-induced damage in the retina, by inhibiting apoptosis and inflammatory responses, even when they do not migrate to the injured retina[245]. These cells release many soluble factors and exosomes; interestingly, exosome administration prevents the potential risks caused by MSC transplantation, mainly allogeneic and xenogeneic immunological rejection, and malignant transformation. MSCs can also be incorporated intravitreally into the damaged retinas, releasing molecules that activate the cell cycle, thus promoting regeneration of different retinal cells[238,246-248]. Although promising, the safety of these treatments remains to be established.
ESCs are pluripotent cells, with the ability to differentiate into any cell type in the body; hence, in addition to giving rise to ESC-RPE cells they represent an attractive source for replacement of retinal neurons. ESCs have been cultured with Wnt and nodal antagonists, involved in patterning the embryo and in the maintenance of pluripotency and carcinogenesis[249], and then with activin to induce retinal fate, thus generating cells with a PHR phenotype, expressing Rhodopsin and recoverin[250]. Using a combination of noggin, Dickkopf (dkk1), an inhibitor of Wnt signaling pathway, and IGF-1, Lamba et al[251] generated retinal progenitors from human ESCs, which integrate into degenerating retinas, increasing PHR differentiation.
ESCs emerge as a promising source of cells for retinal replacement. Nevertheless, extensive research is still necessary to identify the signals that promote retinal fate, allowing ESC differentiation into particular neuronal types, and to establish whether neurons derived from ESCs can be functionally integrated in human host retinas.
Moreover, taking advantage of the ability of ESCs for differentiating into multiple cell types is challenged by ethical questions, since human embryos have to be used as donors to obtain them. In contrast, as discussed for RPE cells, iPSCs represent a source to generate ESC-like cells that do not require a human embryonic cell donor, posing no ethical objections. However, transforming donor cells into iPSCs still has the potential risk of developing tumors or cancer cells[116,252] (Table 1).
iPSCs are similar to human ESCs in their morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity. The successful reprogramming of adult somatic cells (i.e., skin, or blood cells) into iPSCs by introducing the so called Yamanaka´s factors (Oct3/4, Sox2, Klf4 and c-Myc)[15], allows the generation of essentially all kind of tissues, including human dermal fibroblasts[15,156,253-255], as these iPSCs can differentiate into cell types from the three germinal layers in vitro[256]. One of the main difficulties of this approach is the requirement for c-Myc, a proto-oncogene capable of transforming iPSCs into cancer cells; to avoid it, a combination of Oct4, Sox2, Nanog and Lin28 has been used[257]. Human iPSCs have been shown to generate several types of neurons and even brain organoids, showing both excitatory and inhibitory synapses and exhibiting functional synaptic activity[258] and demonstrating an extraordinary capacity to emulate human fetal synapses[259]. An amazing finding was that cultured human iPSCs release intrinsic cues that allow them to recapitulate the main steps of retinal development, leading to the formation of three-dimensional retinal tissue exhibiting rather differentiated, photosensitive PHRs. Thus, iPSCs cells have proved to be very effective in developing organoids of three-dimensional tissues, containing mini optic vesicles, with characteristics similar to those of tissues and organs developed in vivo[18,260,261]. These organoids are useful to investigate the pathophysiology of various diseases and to evaluate therapeutic strategies[262].
While this approach is very promising, several safety concerns should be dealt with before using iPSCs for treating degenerative retina diseases. A troublesome finding is the report that iPSCs retain an epigenetic memory, since the pluripotent cells obtained by these reprogramming methods preserve residual DNA methylation signatures, characteristic of their original donor cell types[263]. Alteration of the genome due to in vitro manipulation, leading to oncogene mutations, is another major concern[18,262,264]. Due to the reprogramming process and their active cell division, iPSCs can accumulate mutations with a high risk of developing cancer cells[265]. This is a shared feature of many SC, which harbor malignant SC in their niches, able to maintain an active self-renewal while generating differentiated cells[266]. Although embryonic and adult iPSCs have the capacity of preventing the accumulation of genetic damages and avoid their propagation to daughter cells, this capacity is hampered by mutations occurred during their life span. Many critical functions of SC, like self-renewal, survival, proliferation, and differentiation are regulated by Jak/STAT kinase, phosphatidylinositol 3-kinase/phosphatase, NF-κB, and other signaling pathways, the dysregulation of which could lead to cancer development[267].
Cancer SC are particularly tolerant to DNA damages and fail to undergo senescence or regulated cell death. In spite of the accumulation of genetic lesions, they remain proliferating, contributing to form tumors and resisting chemo- and radio-therapy[266]. Since the risk of different cancer types correlates strongly with the amount of mitotic cycles of the normal self-renewing cells, SC are believed to be particularly prone to generating cancer cells and tumors[268], a feature that threatens their use in regenerative medicine.
The versatility of iPSC-derived organoids described above and their similarity to specific tissues and organs turn them into effective tools to evaluate the progression of multiple diseases and the effectiveness of new drugs. This has become apparent during the coronavirus disease 2019 (COVID-19) pandemic. This disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), identified in Wuhan, China, in December 2019. The respiratory system is the primary infected organ, but several other organs, including the CNS, the eyes and the retina may also be infected[269,270].
The angiotensin-converting enzyme 2 (ACE2) is the main host cell receptor for the entry of SARS-CoV-2. The eye expresses ACE2, and this expression is present in its inner part, including the retina[271-273]. Furthermore, SARS-CoV-2 is detected in post mortem retinas of COVID-19 patients[274]. As human iPSC-derived retinal organoids have been used to investigate disease progression, and for drug testing[275], the onset of the COVID-19 pandemic led researchers to use iPSC-derived organoids to investigate both SARS-CoV-2 pathogenesis and the effectiveness of antiviral drugs[276]. iPSC-derived human neuronal progenitor cells, neurospheres and brain organoids are permissive to SARS-CoV-2[277], suggesting SARS-CoV-2 can productively infect the brain and might thus be involved in the neurological symptoms observed in the disease. As ACE2 is expressed in human iPSC-derived retinal organoids and monolayer cultures derived from their dissociation and both platforms can be infected by a GFP-expressing lentivirus with a SARS-CoV-2 spike (S) protein[278], these platforms appear as suitable models to investigate SARS-CoV-2 pathogenesis and evaluate drug efficacy.
In addition, to using SC for cell replacement strategies, the feasibility of transplanting PHR cells into the damaged retinas has been a subject of extensive study. Using these cells presents particular advantages and difficulties. Their position as a layer in the retina makes their transplantation feasible; however, since PHRs do not divide, how to reach a critical mass of replacement cells, sufficient to lead to vision improvement is a pending, imperative question that remains to be answered[116, 169].
On the other hand, regeneration of PHRs faces less complications that of brain neurons. The short single axons of PHRs would allow an easier reestablishment of the appropriate contacts and connections, when compared to the complex neuronal circuitries found in other regions of the CNS. Axonal growth would be further facilitated since the myelin components that bind to the Nogo 66 receptor, inhibiting this growth in the brain, are relatively absent in the retina[116,279].
Early work evidenced that when PHR sheets or dissociated cells are transplanted into the subretinal space of rd (C3H rd/rd) mice, the transplanted PHRs survive for one month, developing outer segment-like processes, and synaptic terminals[141,280]. Transplantation of PHRs as a cell suspension in rd1 (C3H/HeNHsd rd1) mice regenerates a functional outer nuclear layer[281,282]. Even if no host rod cells are left in the degenerating retina, the transplanted PHRs reestablish the outer nuclear layer, preserve their appropriate polarization, and adequately reconnect their axons with the host neurons.
In an early clinical trial, subretinal transplantation of a sheet of PHR cells obtained from corpses to two advanced RP patients, without immunosuppression, showed no evidence of rejection. Although no improvement in visual acuity was observed, this trial demonstrated that PHRs can be harvested from human cadaveric eyes and safely transplanted to patients with RP[283]. Subretinal transplantation of human fetal retinal micro-aggregates in patients with RP and neovascular AMD evidences an apparent high tolerance for graft tissue, even when no positive effect on visual function has been observed. Interestingly, the transplantation of retinal micro-aggregate suspensions or retinal sheets from human fetuses in patients with RP and wet AMD leads to a transient improvement of light sensitivity in about 30% of the patients[284]. Similarly, subretinal transplantation of fetal retinal sheets in RP and AMD patients improves their visual acuity, supporting the efficacy of these therapies[281,285,286].
Other clinical trials are currently taking place. Many variables occurring during PHR transplants still require to be defined before successfully using these transplants in RD patients.
Regulation of SC death is a further critical problem that most regenerative processes still face. Thus, the initial exacerbation of the cell cycle in ESC, required for replenishing the cell loss occurring during degeneration, leads to a progressive accumulation of DNA damages, to which ESCs are very sensitive[287-289], and leads them to trigger apoptosis even after low damage doses[290]. The reason for this sensitivity remains unclear, as, in contrast, adult SC evidence a variable sensitivity to damage. Deregulated proliferation of SC increases the risk of mutations associated with cancer development. Thus, ESCs have to choose between cell death resistance, which may lead to the accumulation of mutations and cancer, or a high sensitivity to DNA damage, which may cause SC depletion, and regeneration failure[290], due to the activation of cell death response to preserve genetic stability[291].
In addition, PHR death is intrinsically generated in several retina degenerative diseases, such as RP, due to genetic causes. These causes will persist, even when allogenic therapies with human iPSCs allow a successful transplantation. Therefore, the combination of cell replacement therapies with new strategies aimed at inhibiting cell death will be essential to prevent the death of the newly generated PHRs.
Finally, it is important to mention the risks regarding the inadequate use of SC therapies. Attracted by the big economic benefits, a growing “stem cell” industry is developing[292]. The excitement regarding the therapeutic possibilities of SC and their potential capacity for regenerating most damaged tissues has led to the emergence of many unauthorized “Stem cell clinics” around the world, proposing treatments that lack carefully tested protocols, thus exposing desperate patients to high risks. Due to the relatively easy anatomic access and physiological monitoring of the retina, SC therapies for many ocular conditions are already being offered for improving or curing eye degenerative diseases without providing solid scientific data. Usually the efficacy of the treatments is exaggerated, and the potentially serious health risks of the untested “stem cell therapies” underestimated. It is important to remark that, up to now, only few SC therapies have been approved by the United States Food and Drug Administration[117]. Our knowledge on SC therapies develops much faster than the regulations supervising the ethical and legal concerns related to their clinical use. Hence, governmental offices around the world have neither approved nor disapproved numerous SC treatments offered in multiple websites. In spite of the warnings regarding the risks of undergoing non-approved stem-cell based, ocular treatments, such as that of the American Academy of Ophthalmology (2016), many vulnerable patients throughout the world have been deceived into believing that they are under safe, legal, which instead have repeatedly resulted in severe vision loss (American Academy of Ophthalmology, 2016. Intraocular Stem Cell Therapy).
Degeneration of RPE and PHR are the main pivotal causes of RD, a major cause of blindness around the world. The finding of SC in the retina, potentially able to restore the lost cells and repair this degeneration, has opened a new era toward more effective treatments or eventually cures for these diseases. Most SC types like the iPSCs, RPE, and RPCs, are potentially able to replenish the cell loss. Up to now, RPE and RPCs, derived from multiple sources, emerge as the best candidates for treating RD and several “proof of concept” studies support their use. However, none of the proposed SC fulfill yet all the requirements to successfully restore vision and significant problems remain to be addressed before using them safely in the clinic. The list of difficulties is significantly large; while some SC do not attach adequately to their substrata, others are immune-rejected, only survive for short periods of time, fail to integrate into the host retina or do not improve visual function[116]. How to avoid the risk of tumor development, and to address ethical issues are questions still unanswered and a careful balance of benefits and potential harms is required to choose an effective strategy for transplanting SC in the retina.
Additional pending difficulties are how to control the migration of the new cells in the host retina, their differentiation into the required cell types and their reestablishment of synaptic connectivity. Since our knowledge regarding the mechanisms underlying these processes are in their infancy, clinicians strongly rely in the capacity of the tissues surrounding the implanted grafts to provide the cues for the successful integration and function of the newly generated cells.
In spite of these enormous obstacles, outstanding progress has been made around the world to uncover the molecular mechanisms and understand the pathways that control SC potential. This progress allows us to hope that, in a near future these obstacles will be overcome, thus paving the road toward the treatment or eventual cure of RD by using SC.
We are greatly indebted to Victoria Simon PhD and Ms. Edgardo Buzzi from the Instituto de Investigaciones Bioquímicas of Bahía Blanca, for their generous advices and recommendations in the preparation of figures and pictures.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: the corresponding author is a member of the editorial board or peer reviewer of a BPG journal.
Specialty type: Ophthalmology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nussler AK, Zhang GL S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 963] [Article Influence: 160.5] [Reference Citation Analysis (35)] |
| 3. | Chagastelles PC, Nardi NB. Biology of stem cells: an overview. Kidney Int Suppl (2011). 2011;1:63-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Aichinger E, Kornet N, Friedrich T, Laux T. Plant stem cell niches. Annu Rev Plant Biol. 2012;63:615-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Heidstra R, Sabatini S. Plant and animal stem cells: similar yet different. Nat Rev Mol Cell Biol. 2014;15:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Pierre-Jerome E, Drapek C, Benfey PN. Regulation of Division and Differentiation of Plant Stem Cells. Annu Rev Cell Dev Biol. 2018;34:289-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1031] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 8. | Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 556] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 9. | Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science. 2011;332:811-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 477] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 10. | Reddien PW. The Cellular and Molecular Basis for Planarian Regeneration. Cell. 2018;175:327-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 11. | Zambusi A, Ninkovic J. Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish. World J Stem Cells. 2020;12:8-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 12. | Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004;23:149-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Kalra K, Tomar PC. Stem cell: basics, classification and applications. Am J Phytomed Clin Ther. 2014;2:919-930. |
| 14. | Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019;6:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 15. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18209] [Article Influence: 958.4] [Reference Citation Analysis (0)] |
| 16. | Cai S, Fu X, Sheng Z. Dedifferentiation: A New Approach in Stem Cell Research. BioScience. 2007;57:655-662. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129:2310-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Singh R, Cuzzani O, Binette F, Sternberg H, West MD, Nasonkin IO. Pluripotent Stem Cells for Retinal Tissue Engineering: Current Status and Future Prospects. Stem Cell Rev Rep. 2018;14:463-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 20. | Temple S. The development of neural stem cells. Nature. 2001;414:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1105] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 21. | McKay R. Stem cells in the central nervous system. Science. 1997;276:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1106] [Cited by in RCA: 1067] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 22. | Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kamelska-Sadowska AM, Wojtkiewicz J, Kowalski IM. Review of the Current Knowledge on the Role of Stem Cell Transplantation in Neurorehabilitation. Biomed Res Int. 2019;2019:3290894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | LaMantia SA. The changing brain. Early brain development. In: Purves D, Augustine G, Fitzpatrick D, Hall WC, LaMantia AS, McNamara JO, Williams SM. Neuroscience. 3rd ed. Sinauer Associates, Inc, 2004: 514. |
| 25. | Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3985] [Cited by in RCA: 3450] [Article Influence: 313.6] [Reference Citation Analysis (0)] |
| 26. | Ao J, Wood JP, Chidlow G, Gillies MC, Casson RJ. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin Exp Ophthalmol. 2018;46:670-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Streilein JW, Ma N, Wenkel H, Ng TF, Zamiri P. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002;42:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 545] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 29. | Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Vendomèle J, Khebizi Q, Fisson S. Cellular and Molecular Mechanisms of Anterior Chamber-Associated Immune Deviation (ACAID): What We Have Learned from Knockout Mice. Front Immunol. 2017;8:1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Niederkorn JY. Mechanisms of immune privilege in the eye and hair follicle. J Investig Dermatol Symp Proc. 2003;8:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep. 2010;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. | Little CW, Castillo B, DiLoreto DA, Cox C, Wyatt J, del Cerro C, del Cerro M. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci. 1996;37:204-211. [PubMed] |
| 34. | Önger ME, Delibaş B, Türkmen AP, Erener E, Altunkaynak BZ, Kaplan S. The role of growth factors in nerve regeneration. Drug Discov Ther. 2017;10:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018;65:50-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 36. | Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol. 2019;4:e000398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Han JW, Choi J, Kim YS, Kim J, Brinkmann R, Lyu J, Park TK. Comparison of the neuroinflammatory responses to selective retina therapy and continuous-wave laser photocoagulation in mouse eyes. Graefes Arch Clin Exp Ophthalmol. 2018;256:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Guymer RH, Wu Z, Hodgson LAB, Caruso E, Brassington KH, Tindill N, Aung KZ, McGuinness MB, Fletcher EL, Chen FK, Chakravarthy U, Arnold JJ, Heriot WJ, Durkin SR, Lek JJ, Harper CA, Wickremasinghe SS, Sandhu SS, Baglin EK, Sharangan P, Braat S, Luu CD; Laser Intervention in Early Stages of Age-Related Macular Degeneration Study Group. Subthreshold Nanosecond Laser Intervention in Age-Related Macular Degeneration: The LEAD Randomized Controlled Clinical Trial. Ophthalmology. 2019;126:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 39. | Lek JJ, Brassington KH, Luu CD, Chen FK, Arnold JJ, Heriot WJ, Durkin SR, Chakravarthy U, Guymer RH; Laser in Early Stages of Age-Related Macular Degeneration Study Writing Committee. Subthreshold Nanosecond Laser Intervention in Intermediate Age-Related Macular Degeneration: Study Design and Baseline Characteristics of the Laser in Early Stages of Age-Related Macular Degeneration Study (Report Number 1). Ophthalmol Retina. 2017;1:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Tang Z, Gu P. Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials. Cell Death Dis. 2020;11:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 41. | Lahne M, Brecker M, Jones SE, Hyde DR. The Regenerating Adult Zebrafish Retina Recapitulates Developmental Fate Specification Programs. Front Cell Dev Biol. 2020;8:617923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Huang G, Ye S, Zhou X, Liu D, Ying QL. Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network. Cell Mol Life Sci. 2015;72:1741-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 43. | Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3597] [Cited by in RCA: 3378] [Article Influence: 168.9] [Reference Citation Analysis (0)] |
| 44. | Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1833] [Cited by in RCA: 1847] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 45. | Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367-2382. [PubMed] |
| 46. | Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1031] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 47. | Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805-3819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 561] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 48. | Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol. 2005;25:2475-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 50. | Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T, Suemori H, Nakatsuji N, Tada T. Pluripotential competence of cells associated with Nanog activity. Mech Dev. 2005;122:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 51. | Gawlik-Rzemieniewska N, Bednarek I. The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells. Cancer Biol Ther. 2016;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 52. | Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4051] [Article Influence: 213.2] [Reference Citation Analysis (0)] |
| 53. | Woolfe A, Goodson M, Goode DK, Snell P, McEwen GK, Vavouri T, Smith SF, North P, Callaway H, Kelly K, Walter K, Abnizova I, Gilks W, Edwards YJ, Cooke JE, Elgar G. Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol. 2005;3:e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 686] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 54. | Li F, Wan M, Zhang B, Peng Y, Zhou Y, Pi C, Xu X, Ye L, Zhou X, Zheng L. Bivalent Histone Modifications and Development. Curr Stem Cell Res Ther. 2018;13:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, Terskikh AV. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2015;112:E1936-E1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Chakraborty M, Hu S, Visness E, Del Giudice M, De Martino A, Bosia C, Sharp PA, Garg S. MicroRNAs organize intrinsic variation into stem cell states. Proc Natl Acad Sci U S A. 2020;117:6942-6950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Mens MMJ, Ghanbari M. Cell Cycle Regulation of Stem Cells by MicroRNAs. Stem Cell Rev Rep. 2018;14:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 58. | Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 874] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 59. | Morgado AL, Rodrigues CM, Solá S. MicroRNA-145 Regulates Neural Stem Cell Differentiation Through the Sox2-Lin28/let-7 Signaling Pathway. Stem Cells. 2016;34:1386-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 60. | Büssing I, Slack FJ, Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 497] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 61. | Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013;110:E3017-E3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 62. | Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 63. | Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1215] [Cited by in RCA: 1147] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 64. | Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 777] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 65. | Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 643] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 66. | Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 642] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 67. | Zhang P, Wu W, Chen Q, Chen M. Non-Coding RNAs and their Integrated Networks. J Integr Bioinform. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 444] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 68. | Pan Y, Li C, Chen J, Zhang K, Chu X, Wang R, Chen L. The Emerging Roles of Long Noncoding RNA ROR (lincRNA-ROR) and its Possible Mechanisms in Human Cancers. Cell Physiol Biochem. 2016;40:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 69. | Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, Manos PD, Datta S, Lander ES, Schlaeger TM, Daley GQ, Rinn JL. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 828] [Cited by in RCA: 791] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 70. | Fan J, Xing Y, Wen X, Jia R, Ni H, He J, Ding X, Pan H, Qian G, Ge S, Hoffman AR, Zhang H, Fan X. Long non-coding RNA ROR decoys gene-specific histone methylation to promote tumorigenesis. Genome Biol. 2015;16:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 71. | Yu H, Vu TH, Cho KS, Guo C, Chen DF. Mobilizing endogenous stem cells for retinal repair. Transl Res. 2014;163:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Grigoryan EN. Molecular Factors of the Maintenance and Activation of the Juvenile Phenotype of Cellular Sources for Eye Tissue Regeneration. Biochemistry (Mosc). 2018;83:1318-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Uzun G, Subhani D, Amor S. Trophic factors and stem cells for promoting recovery in stroke. J Vasc Interv Neurol. 2010;3:3-12. [PubMed] |
| 74. | Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 75. | Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31-F42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 878] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 76. | Miller SB, Martin DR, Kissane J, Hammerman MR. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am J Physiol. 1994;266:F129-F134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Tsuji K, Kitamura S. Trophic Factors from Tissue Stem Cells for Renal Regeneration. Stem Cells Int. 2015;2015:537204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | González Fleitas MF, Devouassoux JD, Aranda ML, Calanni JS, Chianelli MS, Dorfman D, Rosenstein RE. Enriched environment provides neuroprotection against experimental glaucoma. J Neurochem. 2020;152:103-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Fleitas MFG, Aranda ML, Diéguez HH, Milne G, Langellotti L, Miranda M, Altschuler F, Dorfman D, Rosenstein RE. The "Use It or Lose It" Dogma in the Retina: Visual Stimulation Promotes Protection Against Retinal Ischemia. Mol Neurobiol. 2020;57:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155-5167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 378] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 81. | Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012;35:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 82. | Markitantova Y, Simirskii V. Inherited Eye Diseases with Retinal Manifestations through the Eyes of Homeobox Genes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration. Proc Natl Acad Sci U S A. 2013;110:19814-19819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Seritrakul P, Gross JM. Genetic and epigenetic control of retinal development in zebrafish. Curr Opin Neurobiol. 2019;59:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | VandenBosch LS, Reh TA. Epigenetics in neuronal regeneration. Semin Cell Dev Biol. 2020;97:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Insua MF, Simón MV, Garelli A, de Los Santos B, Rotstein NP, Politi LE. Trophic factors and neuronal interactions regulate the cell cycle and Pax6 expression in Müller stem cells. J Neurosci Res. 2008;86:1459-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells. 2017;35:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 88. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544-7556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 89. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 90. | Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | Konar GJ, Ferguson C, Flickinger Z, Kent MR, Patton JG. miRNAs and Müller Glia Reprogramming During Retina Regeneration. Front Cell Dev Biol. 2020;8:632632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 93. | Kara N, Kent MR, Didiano D, Rajaram K, Zhao A, Summerbell ER, Patton JG. The miR-216a-Dot1l Regulatory Axis Is Necessary and Sufficient for Müller Glia Reprogramming during Retina Regeneration. Cell Rep 2019; 28: 2037-2047. e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Madelaine R, Sloan SA, Huber N, Notwell JH, Leung LC, Skariah G, Halluin C, Paşca SP, Bejerano G, Krasnow MA, Barres BA, Mourrain P. MicroRNA-9 Couples Brain Neurogenesis and Angiogenesis. Cell Rep. 2017;20:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 95. | La Torre A, Georgi S, Reh TA. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci U S A. 2013;110:E2362-E2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 96. | Madelaine R, Mourrain P. Endogenous retinal neural stem cell reprogramming for neuronal regeneration. Neural Regen Res. 2017;12:1765-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 97. | Cheung HC, Hai T, Zhu W, Baggerly KA, Tsavachidis S, Krahe R, Cote GJ. Splicing factors PTBP1 and PTBP2 promote proliferation and migration of glioma cell lines. Brain. 2009;132:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 98. | Qi X. The role of miR-9 during neuron differentiation of mouse retinal stem cells. Artif Cells Nanomed Biotechnol. 2016;44:1883-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 99. | Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 100. | Wohl SG, Hooper MJ, Reh TA. MicroRNAs miR-25, let-7 and miR-124 regulate the neurogenic potential of Müller glia in mice. Development. 2019;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1477] [Cited by in RCA: 1574] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 102. | Ramos AD, Diaz A, Nellore A, Delgado RN, Park KY, Gonzales-Roybal G, Oldham MC, Song JS, Lim DA. Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell. 2013;12:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 103. | Rapicavoli NA, Poth EM, Blackshaw S. The long noncoding RNA RNCR2 directs mouse retinal cell specification. BMC Dev Biol. 2010;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 104. | Li F, Wen X, Zhang H, Fan X. Novel Insights into the Role of Long Noncoding RNA in Ocular Diseases. Int J Mol Sci. 2016;17:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Shen Y, Dong LF, Zhou RM, Yao J, Song YC, Yang H, Jiang Q, Yan B. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. J Cell Mol Med. 2016;20:537-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 106. | Xu XD, Li KR, Li XM, Yao J, Qin J, Yan B. Long non-coding RNAs: new players in ocular neovascularization. Mol Biol Rep. 2014;41:4493-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Yan B, Tao ZF, Li XM, Zhang H, Yao J, Jiang Q. Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 108. | Rapicavoli NA, Blackshaw S. New meaning in the message: noncoding RNAs and their role in retinal development. Dev Dyn. 2009;238:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Meola N, Pizzo M, Alfano G, Surace EM, Banfi S. The long noncoding RNA Vax2os1 controls the cell cycle progression of photoreceptor progenitors in the mouse retina. RNA. 2012;18:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 110. | Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 483] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 111. | Ishii N, Ozaki K, Sato H, Mizuno H, Susumu Saito, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Satoshi Saito, Nakamura Y, Tanaka T. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 516] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 112. | Sone M, Hayashi T, Tarui H, Agata K, Takeichi M, Nakagawa S. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J Cell Sci. 2007;120:2498-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 113. | Wan P, Su W, Zhuo Y. Precise long non-coding RNA modulation in visual maintenance and impairment. J Med Genet. 2017;54:450-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Yao J, Wang XQ, Li YJ, Shan K, Yang H, Wang YN, Yao MD, Liu C, Li XM, Shen Y, Liu JY, Cheng H, Yuan J, Zhang YY, Jiang Q, Yan B. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol Med. 2016;8:1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Yao J, Wang XQ, Li YJ, Shan K, Yang H, Wang YN, Yao MD, Liu C, Li XM, Shen Y, Liu JY, Cheng H, Yuan J, Zhang YY, Jiang Q, Yan B. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol Med. 2016;8:346-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 116. | MacLaren RE, Bennett J, Schwartz SD. Gene Therapy and Stem Cell Transplantation in Retinal Disease: The New Frontier. Ophthalmology. 2016;123:S98-S106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 117. | Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, Takahashi M, Nagiel A, Schwartz SD, Bharti K. Retinal stem cell transplantation: Balancing safety and potential. Prog Retin Eye Res. 2020;75:100779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 118. | ROYO PE, QUAY WB. Retinal transplantation from fetal to maternal mammalian eye. Growth. 1959;23:313-336. [PubMed] |
| 119. | del Cerro M, Gash DM, Rao GN, Notter MF, Wiegand SJ, Sathi S, del Cerro C. Retinal transplants into the anterior chamber of the rat eye. Neuroscience. 1987;21:707-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 120. | Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 121. | Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA. Cellular strategies for retinal repair by photoreceptor replacement. Prog Retin Eye Res. 2015;46:31-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 122. | Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48:1003-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 123. | Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 124. | Grigoryan EN. Endogenous Cell Sources for Eye Retina Regeneration in Vertebrate Animals and Humans. Russ J Dev Biol. 2018;49:, 314-326. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 125. | Aladdad AM, Kador KE. Adult Stem Cells, Tools for Repairing the Retina. Curr Ophthalmol Rep. 2019;7:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 126. | Varela A, Lanzetta D, Savino EA. The effects of 4-pentenoic and pentanoic acid on the hypoxic rat atria. Arch Int Physiol Biochim. 1989;97:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 127. | Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci. 2014;15:615-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 128. | Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 752] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 129. | Trimarchi JM, Stadler MB, Cepko CL. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One. 2008;3:e1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 130. | Poggio CE, Salvato A. When are implants needed? Am J Orthod Dentofacial Orthop. 2005;128:688-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 131. | Sowden JC. ESC-derived retinal pigmented epithelial cell transplants in patients: so far, so good. Cell Stem Cell. 2014;15:537-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45:4167-4173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 133. | Klassen H, Ziaeian B, Kirov II, Young MJ, Schwartz PH. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J Neurosci Res. 2004;77:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 134. | Schmitt S, Aftab U, Jiang C, Redenti S, Klassen H, Miljan E, Sinden J, Young M. Molecular characterization of human retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:5901-5908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Mayer EJ, Carter DA, Ren Y, Hughes EH, Rice CM, Halfpenny CA, Scolding NJ, Dick AD. Neural progenitor cells from postmortem adult human retina. Br J Ophthalmol. 2005;89:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 136. | Yang P, Seiler MJ, Aramant RB, Whittemore SR. In vitro isolation and expansion of human retinal progenitor cells. Exp Neurol. 2002;177:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 137. | Baranov PY, Tucker BA, Young MJ. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue Eng Part A. 2014;20:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, Qiu A, Luo H, Hicks C, Zeng J, Zhu J, Lu J, Sfeir N, Wen C, Zhang M, Reade V, Sinden J, Sun X, Shaw P, Young M, Zhang K. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289:6362-6371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 139. | Li SY, Yin ZQ, Chen SJ, Chen LF, Liu Y. Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs. Curr Eye Res. 2009;34:523-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 140. | Gouras P, Tanabe T. Survival and integration of neural retinal transplants in rd mice. Graefes Arch Clin Exp Ophthalmol. 2003;241:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 141. | Kwan AS, Wang S, Lund RD. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp Neurol. 1999;159:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 142. | Coles BL, Angénieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A. 2004;101:15772-15777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 143. | Zhang K, Ding S. Stem cells and eye development. N Engl J Med. 2011;365:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Tucker BA, Redenti SM, Jiang C, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010;31:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 145. | MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 764] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 146. | Hendrickson A, Bumsted-O'Brien K, Natoli R, Ramamurthy V, Possin D, Provis J. Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res. 2008;87:415-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 147. | Joussen AM, Heussen FM, Joeres S, Llacer H, Prinz B, Rohrschneider K, Maaijwee KJ, van Meurs J, Kirchhof B. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006;142:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 148. | Maaijwee K, Heimann H, Missotten T, Mulder P, Joussen A, van Meurs J. Retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: long-term results. Graefes Arch Clin Exp Ophthalmol. 2007;245:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 149. | Maaijwee K, Joussen AM, Kirchhof B, van Meurs JC. Retinal pigment epithelium (RPE)-choroid graft translocation in the treatment of an RPE tear: preliminary results. Br J Ophthalmol. 2008;92:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 150. | Kuppermann BD, Boyer DS, Mills B, Yang J, Klassen HJ. Safety and activity of a single, intravitreal injection of human retinal progenitor cells (jCell) for treatment of retinitis pigmentosa (RP). Investig Ophthalmol Vis Sci. 2018;59:2987-2987. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 151. | Safety and Efficacy of Intravitreal Injection of Human Retinal Progenitor Cells in Adults With Retinitis Pigmentosa. [accessed 2021 Jan 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03073733. gov Identifier: NCT03073733. |
| 152. | Romito A, Cobellis G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016;2016:9451492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 153. | Osorno R, Tsakiridis A, Wong F, Cambray N, Economou C, Wilkie R, Blin G, Scotting PJ, Chambers I, Wilson V. The developmental dismantling of pluripotency is reversed by ectopic Oct4 expression. Development. 2012;139:2288-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 154. | Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219-4231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 396] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 155. | Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell. 2010;18:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 156. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14326] [Article Influence: 842.7] [Reference Citation Analysis (0)] |
| 157. | Heymann MA, Hoffman JI. Problem of patent ductus arteriosus in premature infants. Paediatrician. 1978;7:3-17. [PubMed] |
| 158. | Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169-3179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 159. | Fligor CM, Langer KB, Sridhar A, Ren Y, Shields PK, Edler MC, Ohlemacher SK, Sluch VM, Zack DJ, Zhang C, Suter DM, Meyer JS. Three-Dimensional Retinal Organoids Facilitate the Investigation of Retinal Ganglion Cell Development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells. Sci Rep. 2018;8:14520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 160. | Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Mácia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 161. | Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 162. | Zhang WY, de Almeida PE, Wu JC. Teratoma formation: A tool for monitoring pluripotency in stem cell research. Stemcellbook. 2012;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 163. | Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Schrøder HD, Burns JS, Kassem M. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009;18:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 164. | Martin RM, Fowler JL, Cromer MK, Lesch BJ, Ponce E, Uchida N, Nishimura T, Porteus MH, Loh KM. Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nat Commun. 2020;11:2713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 165. | Jeon S, Oh IH. Regeneration of the retina: toward stem cell therapy for degenerative retinal diseases. BMB Rep. 2015;48:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 166. | Caceres PS, Rodriguez-Boulan E. Retinal pigment epithelium polarity in health and blinding diseases. Curr Opin Cell Biol. 2020;62:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 167. | Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, Humayun MS. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog Retin Eye Res. 2015;48:1-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 168. | Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 169. | Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576-2585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 170. | Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 439] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 171. | Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, Girman S, Bischoff N, Sauvé Y, Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 172. | Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A, Lundh P, Semo M, Ahmado A, Gias C, da Cruz L, Moore H, Andrews P, Walsh J, Coffey P. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214:347-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 173. | Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 282] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 174. | Zhu D, Deng X, Spee C, Sonoda S, Hsieh CL, Barron E, Pera M, Hinton DR. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest Ophthalmol Vis Sci. 2011;52:1573-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 175. | Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 971] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 176. | Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 886] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 177. | Diniz B, Thomas P, Thomas B, Ribeiro R, Hu Y, Brant R, Ahuja A, Zhu D, Liu L, Koss M, Maia M, Chader G, Hinton DR, Humayun MS. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087-5096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 178. | Hu Y, Liu L, Lu B, Zhu D, Ribeiro R, Diniz B, Thomas PB, Ahuja AK, Hinton DR, Tai YC, Hikita ST, Johnson LV, Clegg DO, Thomas BB, Humayun MS. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res. 2012;48:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 179. | Koss MJ, Falabella P, Stefanini FR, Pfister M, Thomas BB, Kashani AH, Brant R, Zhu D, Clegg DO, Hinton DR, Humayun MS. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatán minipigs. Graefes Arch Clin Exp Ophthalmol. 2016;254:1553-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 180. | Kashani AH, Martynova A, Koss M, Brant R, Zhu DH, Lebkowski J, Hinton D, Clegg D, Humayun MS. Subretinal Implantation of a Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium Monolayer in a Porcine Model. Adv Exp Med Biol. 2019;1185:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 181. | Sugita S, Mandai M, Kamao H, Takahashi M. Immunological aspects of RPE cell transplantation. Prog Retin Eye Res. 2021;100950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 182. | Bharti K, Miller SS, Arnheiter H. The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24:21-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 183. | Sugita S. Role of ocular pigment epithelial cells in immune privilege. Arch Immunol Ther Exp (Warsz). 2009;57:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 184. | Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 185. | Tsukahara I, Ninomiya S, Castellarin A, Yagi F, Sugino IK, Zarbin MA. Early attachment of uncultured retinal pigment epithelium from aged donors onto Bruch's membrane explants. Exp Eye Res. 2002;74:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 186. | Tezel TH, Del Priore LV, Kaplan HJ. Reengineering of aged Bruch's membrane to enhance retinal pigment epithelium repopulation. Invest Ophthalmol Vis Sci. 2004;45:3337-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 187. | Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Invest Ophthalmol Vis Sci. 2016;57:ORSFc1-ORSFc9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 188. | Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4:860-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 189. | da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, Feng G, Whitlock M, Robson AG, Holder GE, Sagoo MS, Loudon PT, Whiting P, Coffey PJ. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 190. | Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 191. | Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 192. | Zahabi A, Shahbazi E, Ahmadieh H, Hassani SN, Totonchi M, Taei A, Masoudi N, Ebrahimi M, Aghdami N, Seifinejad A, Mehrnejad F, Daftarian N, Salekdeh GH, Baharvand H. A new efficient protocol for directed differentiation of retinal pigmented epithelial cells from normal and retinal disease induced pluripotent stem cells. Stem Cells Dev. 2012;21:2262-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 193. | Maruotti J, Sripathi SR, Bharti K, Fuller J, Wahlin KJ, Ranganathan V, Sluch VM, Berlinicke CA, Davis J, Kim C, Zhao L, Wan J, Qian J, Corneo B, Temple S, Dubey R, Olenyuk BZ, Bhutto I, Lutty GA, Zack DJ. Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc Natl Acad Sci U S A. 2015;112:10950-10955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 194. | Iwasaki Y, Sugita S, Mandai M, Yonemura S, Onishi A, Ito S, Mochizuki M, Ohno-Matsui K, Takahashi M. Differentiation/Purification Protocol for Retinal Pigment Epithelium from Mouse Induced Pluripotent Stem Cells as a Research Tool. PLoS One. 2016;11:e0158282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 195. | Sugita S, Kamao H, Iwasaki Y, Okamoto S, Hashiguchi T, Iseki K, Hayashi N, Mandai M, Takahashi M. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Invest Ophthalmol Vis Sci. 2015;56:1051-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 196. | Sugita S, Iwasaki Y, Makabe K, Kamao H, Mandai M, Shiina T, Ogasawara K, Hirami Y, Kurimoto Y, Takahashi M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Reports. 2016;7:635-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 197. | Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata KI, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 2017;376:1038-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 988] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 198. | Takagi S, Mandai M, Gocho K, Hirami Y, Yamamoto M, Fujihara M, Sugita S, Kurimoto Y, Takahashi M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol Retina. 2019;3:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 199. | Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 200. | Sugita S, Iwasaki Y, Makabe K, Kimura T, Futagami T, Suegami S, Takahashi M. Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Reports. 2016;7:619-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 201. | Sugita S, Makabe K, Fujii S, Iwasaki Y, Kamao H, Shiina T, Ogasawara K, Takahashi M. Detection of Retinal Pigment Epithelium-Specific Antibody in iPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem Cell Reports. 2017;9:1501-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 202. | Sugita S, Mandai M, Hirami Y, Takagi S, Maeda T, Fujihara M, Matsuzaki M, Yamamoto M, Iseki K, Hayashi N, Hono A, Fujino S, Koide N, Sakai N, Shibata Y, Terada M, Nishida M, Dohi H, Nomura M, Amano N, Sakaguchi H, Hara C, Maruyama K, Daimon T, Igeta M, Oda T, Shirono U, Tozaki M, Totani K, Sugiyama S, Nishida K, Kurimoto Y, Takahashi M. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J Clin Med. 2020;9:2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 203. | Algvere PV, Berglin L, Gouras P, Sheng Y, Kopp ED. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 204. | Algvere PV, Gouras P, Dafgård Kopp E. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol. 1999;9:217-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 205. | Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest Ophthalmol Vis Sci. 1998;39:2121-2131. [PubMed] |
| 206. | Aramant R, Seiler M, Turner JE. Donor age influences on the success of retinal grafts to adult rat retina. Invest Ophthalmol Vis Sci. 1988;29:498-503. [PubMed] |
| 207. | Kim BS, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 208. | McGill TJ, Cottam B, Lu B, Wang S, Girman S, Tian C, Huhn SL, Lund RD, Capela A. Transplantation of human central nervous system stem cells - neuroprotection in retinal degeneration. Eur J Neurosci. 2012;35:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 209. | Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 502] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 210. | Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 211. | Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 396] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 212. | Tassoni A, Gutteridge A, Barber AC, Osborne A, Martin KR. Molecular Mechanisms Mediating Retinal Reactive Gliosis Following Bone Marrow Mesenchymal Stem Cell Transplantation. Stem Cells. 2015;33:3006-3016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 213. | Beach KM, Wang J, Otteson DC. Regulation of Stem Cell Properties of Müller Glia by JAK/STAT and MAPK Signaling in the Mammalian Retina. Stem Cells Int. 2017;2017:1610691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 214. | Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 447] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 215. | Belecky-Adams TL, Chernoff EC, Wilson JM, Dharmarajan S. Reactive Muller glia as potential retinal progenitors. Neural Stem Cells New Perspect. 2013;75. [DOI] [Full Text] |
| 216. | Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 397] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 217. | Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 218. | Lahne M, Nagashima M, Hyde DR, Hitchcock PF. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu Rev Vis Sci. 2020;6:171-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 219. | Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654-13659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 344] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 220. | Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 221. | Benesch RE, Edalji R, Benesch R. Reciprocal interaction of hemoglobin with oxygen and protons. The influence of allosteric polyanions. Biochemistry. 1977;16:2594-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 222. | Abrahan CE, Insua MF, Politi LE, German OL, Rotstein NP. Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro. J Neurosci Res. 2009;87:964-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 223. | Simón MV, De Genaro P, Abrahan CE, de los Santos B, Rotstein NP, Politi LE. Müller glial cells induce stem cell properties in retinal progenitors in vitro and promote their further differentiation into photoreceptors. J Neurosci Res. 2012;90:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 224. | Volonté YA, Vallese-Maurizi H, Dibo MJ, Ayala-Peña VB, Garelli A, Zanetti SR, Turpaud A, Craft CM, Rotstein NP, Politi LE, German OL. A Defective Crosstalk Between Neurons and Müller Glial Cells in the rd1 Retina Impairs the Regenerative Potential of Glial Stem Cells. Front Cell Neurosci. 2019;13:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 225. | Too LK, Shen W, Mammo Z, Osaadon P, Gillies MC, Simunovic MP. Surgical retinal explants as a source of retinal progenitor cells. Retina. 2021;41:1986-1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 226. | Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112:519-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1137] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 227. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8246] [Cited by in RCA: 9804] [Article Influence: 544.7] [Reference Citation Analysis (0)] |
| 228. | Sanges D, Simonte G, Di Vicino U, Romo N, Pinilla I, Nicolás M, Cosma MP. Reprogramming Müller glia via in vivo cell fusion regenerates murine photoreceptors. J Clin Invest. 2016;126:3104-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 229. | Gao H, A L, Huang X, Chen X, Xu H. Müller Glia-Mediated Retinal Regeneration. Mol Neurobiol. 2021;58:2342-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 230. | Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017;548:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 231. | Rueda EM, Hall BM, Hill MC, Swinton PG, Tong X, Martin JF, Poché RA. The Hippo Pathway Blocks Mammalian Retinal Müller Glial Cell Reprogramming. Cell Rep. 2019;27:1637-1649.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 232. | Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, Saez C, Keuthan C, Palazzo I, Squires N, Campbell WA, Rajaii F, Parayil T, Trinh V, Kim DW, Wang G, Campbell LJ, Ash J, Fischer AJ, Hyde DR, Qian J, Blackshaw S. Gene regulatory networks controlling vertebrate retinal regeneration. Science. 2020;370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 233. | Canto-Soler V, Flores-Bellver M, Vergara MN. Stem Cell Sources and Their Potential for the Treatment of Retinal Degenerations. Invest Ophthalmol Vis Sci. 2016;57:ORSFd1-ORSFd9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 234. | Singh MS, MacLaren RE. Stem cells as a therapeutic tool for the blind: biology and future prospects. Proc Biol Sci. 2011;278:3009-3016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 235. | Singh MS, MacLaren RE. Stem Cell Treatment for Age-Related Macular Degeneration: the Challenges. Invest Ophthalmol Vis Sci. 2018;59:AMD78-AMD82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 236. | Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauvé Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 237. | Cao J, Murat C, An W, Yao X, Lee J, Santulli-Marotto S, Harris IR, Inana G. Human umbilical tissue-derived cells rescue retinal pigment epithelium dysfunction in retinal degeneration. Stem Cells. 2016;34:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 238. | Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, Zhang Y, Li Q, Zhang X, Li X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep. 2016;6:34562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 239. | A Safety Study of CNTO 2476 in Patients With Age-Related Macular Degeneration. National Library of Medicine. [accessed 2021 Jan 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01226628 ClinicalTrials.gov Identifier: NCT01226628. |
| 240. | Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience With a Subretinal Cell-based Therapy in Patients With Geographic Atrophy Secondary to Age-related Macular Degeneration. Am J Ophthalmol. 2017;179:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 241. | Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 242. | Lu B, Wang S, Girman S, McGill T, Ragaglia V, Lund R. Human adult bone marrow-derived somatic cells rescue vision in a rodent model of retinal degeneration. Exp Eye Res. 2010;91:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 243. | Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 371] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 244. | Goodell MA, Nguyen H, Shroyer N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat Rev Mol Cell Biol. 2015;16:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 245. | Jiang Y, Zhang Y, Zhang L, Wang M, Zhang X, Li X. Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. Int J Mol Sci. 2014;15:9372-9385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 246. | Mead B, Tomarev S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl Med. 2017;6:1273-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 247. | Zhang X, Liu J, Yu B, Ma F, Ren X, Li X. Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch Clin Exp Ophthalmol. 2018;256:2041-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 248. | Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, Djalilian AR. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci. 2018;59:5194-5200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 249. | Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 250. | Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, Takahashi M, Sasai Y. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:11331-11336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 251. | Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769-12774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 252. | Cramer AO, MacLaren RE. Translating induced pluripotent stem cells from bench to bedside: application to retinal diseases. Curr Gene Ther. 2013;13:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 253. | Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3332] [Cited by in RCA: 3083] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 254. | Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2048] [Cited by in RCA: 1902] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 255. | Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1239] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 256. | Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 257. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7251] [Article Influence: 402.8] [Reference Citation Analysis (0)] |
| 258. | Wilson ES, Newell-Litwa K. Stem cell models of human synapse development and degeneration. Mol Biol Cell. 2018;29:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 259. | Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012;7:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 668] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 260. | Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 706] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 261. | Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, Wright LS, Shen W, Capowski EE, Percin EF, Perez ET, Zhong X, Canto-Soler MV, Gamm DM. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 262. | Scudellari M. How iPS cells changed the world. Nature. 2016;534:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 263. | Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1842] [Cited by in RCA: 1686] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 264. | Boyd AS, Rodrigues NP, Lui KO, Fu X, Xu Y. Concise review: Immune recognition of induced pluripotent stem cells. Stem Cells. 2012;30:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 265. | Hong SG, Dunbar CE, Winkler T. Assessing the risks of genotoxicity in the therapeutic development of induced pluripotent stem cells. Mol Ther. 2013;21:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 266. | Vitale I, Manic G, De Maria R, Kroemer G, Galluzzi L. DNA Damage in Stem Cells. Mol Cell. 2017;66:306-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 267. | Matsui WH. Cancer stem cell signaling pathways. Medicine (Baltimore). 2016;95:S8-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 268. | Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1274] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 269. | Allison SJ. SARS-CoV-2 infection of kidney organoids prevented with soluble human ACE2. Nat Rev Nephrol. 2020;16:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 270. | Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125-136.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 512] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 271. | Napoli PE, Nioi M, d'Aloja E, Fossarello M. The Ocular Surface and the Coronavirus Disease 2019: Does a Dual 'Ocular Route' Exist? J Clin Med. 2020;9:1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 272. | Hong N, Yu W, Xia J, Shen Y, Yap M, Han W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol 2020 epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 273. | Makovoz B, Moeller R, Zebitz Eriksen A, tenOever BR, Blenkinsop TA. SARS-CoV-2 Infection of Ocular Cells from Human Adult Donor Eyes and hESC-Derived Eye Organoids. 2020 Preprint. Available from: SSRN: 3650574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 274. | Casagrande M, Fitzek A, Püschel K, Aleshcheva G, Schultheiss HP, Berneking L, Spitzer MS, Schultheiss M. Detection of SARS-CoV-2 in Human Retinal Biopsies of Deceased COVID-19 Patients. Ocul Immunol Inflamm. 2020;28:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 275. | Ho BX, Pek NMQ, Soh BS. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 276. | Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, Korth C, Schaal H, Gopalakrishnan J. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39:e106230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 277. | Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, Gong HR, Lee AC, Zou Z, Yau T, Wu W, Hung IF, Chan JF, Yuen KY, Huang JD. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 278. | Ahmad Mulyadi Lai HI, Chou SJ, Chien Y, Tsai PH, Chien CS, Hsu CC, Jheng YC, Wang ML, Chiou SH, Chou YB, Hwang DK, Lin TC, Chen SJ, Yang YP. Expression of Endogenous Angiotensin-Converting Enzyme 2 in Human Induced Pluripotent Stem Cell-Derived Retinal Organoids. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 279. | Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 700] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 280. | Gouras P, Du J, Kjeldbye H, Kwun R, Lopez R, Zack DJ. Transplanted photoreceptors identified in dystrophic mouse retina by a transgenic reporter gene. Invest Ophthalmol Vis Sci. 1991;32:3167-3174. [PubMed] |
| 281. | Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 282. | Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, Barnard AR, MacLaren RE. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 283. | Kaplan HJ, Tezel TH, Berger AS, Wolf ML, Del Priore LV. Human photoreceptor transplantation in retinitis pigmentosa. A safety study. Arch Ophthalmol. 1997;115:1168-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 284. | Humayun MS, de Juan E Jr, del Cerro M, Dagnelie G, Radner W, Sadda SR, del Cerro C. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000;41:3100-3106. [PubMed] |
| 285. | Radtke ND, Aramant RB, Seiler M, Petry HM. Preliminary report: indications of improved visual function after retinal sheet transplantation in retinitis pigmentosa patients. Am J Ophthalmol. 1999;128:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 286. | Radtke ND, Seiler MJ, Aramant RB, Petry HM, Pidwell DJ. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am J Ophthalmol. 2002;133:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 287. | Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220:586-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 288. | Hong Y, Stambrook PJ. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci U S A. 2004;101:14443-14448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 289. | Solozobova V, Rolletschek A, Blattner C. Nuclear accumulation and activation of p53 in embryonic stem cells after DNA damage. BMC Cell Biol. 2009;10:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 290. | Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 291. | Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2741] [Cited by in RCA: 2732] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 292. | Berger I, Ahmad A, Bansal A, Kapoor T, Sipp D, Rasko JEJ. Global Distribution of Businesses Marketing Stem Cell-Based Interventions. Cell Stem Cell. 2016;19:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 293. | Klassen H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin Biol Ther. 2016;16:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |