Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1360
Peer-review started: March 1, 2021
First decision: June 23, 2021
Revised: July 2, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: October 26, 2021
Processing time: 238 Days and 14.7 Hours
With developments in the field of tissue engineering and regenerative medicine, the use of biological products for the treatment of various disorders has come into the limelight among researchers and clinicians. Among all the available biological tissues, research and exploration of adipose tissue have become more robust. Adipose tissue engineering aims to develop by-products and their substitutes for their regenerative and immunomodulatory potential. The use of biodegradable scaffolds along with adipose tissue products has a major role in cellular growth, proliferation, and differentiation. Adipose tissue, apart from being the powerhouse of energy storage, also functions as the largest endocrine organ, with the release of various adipokines. The progenitor cells among the heterogeneous population in the adipose tissue are of paramount importance as they determine the capacity of regeneration of these tissues. The results of adipose-derived stem-cell assisted fat grafting to provide numerous growth factors and adipokines that improve vasculogenesis, fat graft integration, and survival within the recipient tissue and promote the regeneration of tissue are promising. Adipose tissue gives rise to various by-products upon processing. This article highlights the significance and the usage of various adipose tissue by-products, their individual characteristics, and their clinical applications.
Core Tip: Promising evidence supports clinical application of derivatives of adipose-derived stem cells enriched with numerous growth factors and adipokines for improved vasculogenesis, graft integration, and survival within the recipient tissue. Analysis of its differential characteristics and practical applications became a necessity. In this review, we highlight the significance and usage of various adipose tissue by-products, their individual characteristics, and their clinical applications along with the evidence supporting its use.
- Citation: Sharma S, Muthu S, Jeyaraman M, Ranjan R, Jha SK. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World J Stem Cells 2021; 13(10): 1360-1381
- URL: https://www.wjgnet.com/1948-0210/full/v13/i10/1360.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i10.1360
With developments in the field of tissue engineering and regenerative medicine, the use of biological products for the treatment of various disorders has come into the limelight among researchers and clinicians. Among all the biological products, keen interest has been shown in adipose tissue and its by-products for translation from bench to clinical applications, with due consideration to its various unique properties. Because of the heterogeneous cellular population of adipose tissue, adipose tissue-derived products possess a greater advantage of regeneration compared with bone marrow-derived products[1,2].
Traditionally adipose tissue or fat graft transfer was practiced for elective cosmetic procedures and plastic and reconstructive surgery[3]. Because of the regeneration potential possessed by adipose tissue, use has become widespread in cosmetic-dermatological procedures, facial rejuvenation, breast and buttocks augmentation, and genital aesthetics[4-6]. Cellular viability and survival at the transplanted site depends on various factors involving the recipient site, donor site, laboratory processing, and manipulation[7,8].
Adipose tissue engineering aims to develop by-products and their substitutes for regeneration and restoring function[9]. That further eliminates the need for organ transplants and mechanical device placement. The use of biodegradable scaffolds along with adipose tissue products has a major role in cellular growth, proliferation, and differentiation[10,11]. Once implanted at an appropriate site, scaffolds degrade, progenitor cells proliferate with the help of growth factors and cytokines to form new tissue. Preclinical studies have investigated the ability of biomolecules and biodegradable three-dimensional scaffolds to interact with adipose tissue products to promote the adipogenesis of stem cells in vitro[12-15]. In this review, we discuss the differential characteristics of adipose tissue derivatives and elucidate their applications in clinical scenarios.
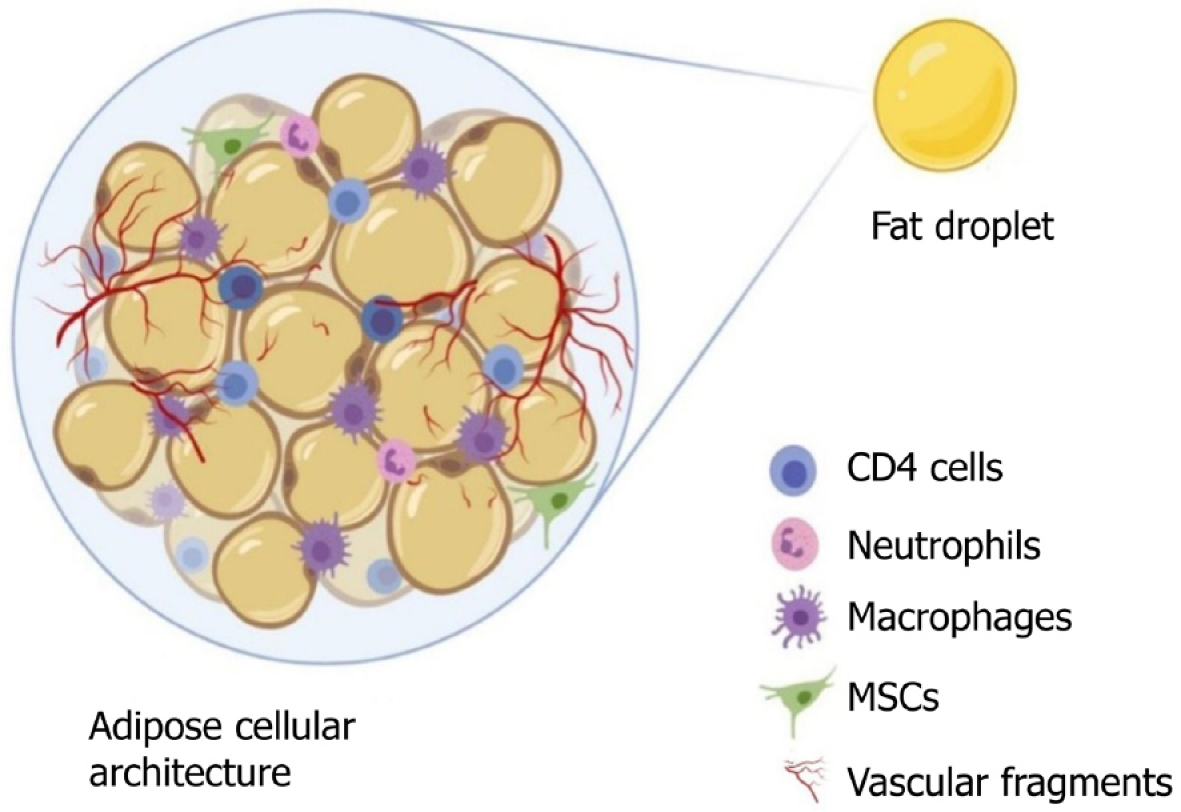
An adipocyte is usually 50-150 mm in diameter, it dies from ischemia if it grows larger, and its life expectancy varies from few months to 10 years in humans[16]. Adipose tissue, a specialized connective tissue with lipid-rich adipocytes, it contains a heterogeneous population of cells but adipocytes represents only 20% or less of the cellular mixture[17]. Based on adipocyte morphology, the two types of adipose tissue are white adipose tissue found in adults and brown adipose tissue found in newborns[18]. Adipose tissue, apart from being the powerhouse of energy storage, also functions as the largest endocrine organ, with the release of various adipokines. Adipose tissue-derived adipokines include leptin, adiponectin, apelin, chemerin, interleukin (IL)-6, 8, and 10; monocyte chemoattractant protein (MCP)-1, plasminogen activator inhibitor (PAI)-1, retinol binding protein (RBP)-4, tumor necrosis factor (TNF)-α, progranulin, complement C1q tumor necrosis factor-related protein (CTRP)-4, interferon (IFN)-γ, and interferon-γ-inducible protein (IP)-10, which are readily available sources to induce stem cells[19-22]. The adipokines work in a paracrine fashion when transplanted as a cellular therapeutic tool[23,24]. The components of adipose tissue are lipid-laden adipocytes, fibroblasts, neural and vascular progenitor cells, multipotent progenitor cells, pericytes, extracellular matrices, cytokines, growth factors, and immune cells such as CD4+ T cells, as shown in Figure 1. The progenitor cells in adipose tissue are of paramount importance, as they have the capacity for regeneration of these tissues. The progenitor cells differentiate into mesodermal, ectodermal, and endodermal lineages[25-27]. Tissue engineering experts focus on adipose tissue and its products for their plasticity, relative ease of harvest, and potential autologous usage. Stem cells and progenitor cells from freshly prepared SVFs constitute up to 3%, which represents 2500-fold more than the stem cells isolated from bone marrow source (0.002%)[28].
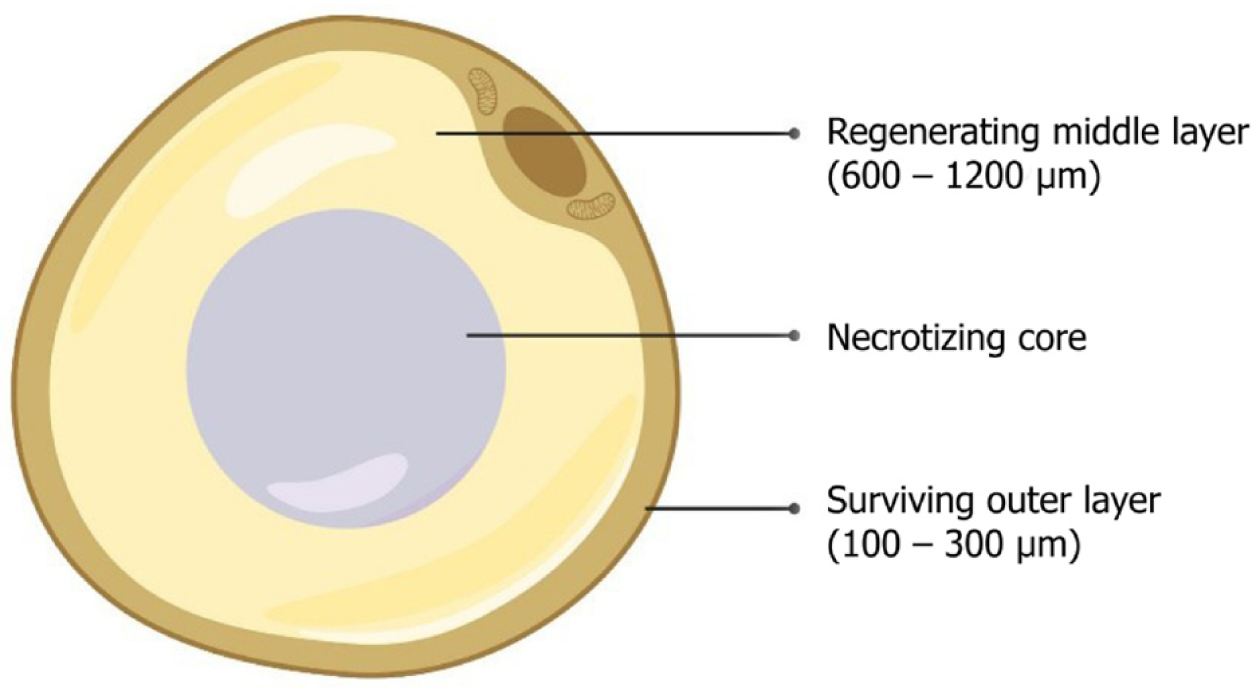
The literature on adipose tissue survival and regeneration depicts “cell survival theory” and “cell replacement theory”[29-32]. Various studies have proven the promising results of adipose-derived stem cell (ADSC)-assisted fat grafting, which provides numerous growth factors and adipokines for improved vasculogenesis, fat graft integration, and survival within the recipient tissue[8,33]. Adipocytes contain three zones namely, (1) the outer surviving layer (100–300 microns), (2) middle regenerating layer (600–1200 microns), and (3) inner necrotic layer, as shown in Figure 2[30-32,34].
Yoshimura et al[35] demonstrated the sequence of changes that happen after grafting or transplantation of adipose tissue. In preclinical studies, it is shown that all adipocytes undergo apoptosis in the initial few days of grafting. Activation of adipogenesis was by adipose-derived progenitor cells, which were augmented by the adipokines at 3 mo after grafting. By the end of 9 mo, lipid droplets were absorbed by macrophages. The final fat graft retention at the recipient site was determined by the rate of successful replacement of the adipocytes[35]. ADSCs possess various advantages over bone marrow-derived mesenchymal stem cells (BM-MSCs)[36-38]. Harvesting adipose tissue by liposuction is less painful than bone marrow aspiration[39,40]. The quantity of stem/stromal cells obtained from adipose tissue than that obtained from bone marrow[41]. In long-term culture, ADSCs are more genetically stable[42,43].
Researchers demonstrated that ADSCs have a consistent phenotyp and reproductive capacity, based on cellular yield, cellular viability, adipocyte differentiation, and cell surface markers[44-46]. During initial culture, ADSCs are polygonal cells that adhere to the flask surface. They exhibit fibroblastic plastic morphology and expand in in vitro cultures. within 2 d of primary culture, 90% of cells become confluent when subcultured within 2 d of primary culture with a demonstrated ADSC yield of 87% and viability of 94%[47]. Luna et al[47] recovered 1 × 106 adipocytes, 1 × 106 ADSCs, 1 × 106 vascular endothelial cells, and 1 × 106 other cells from 1 g of adipose tissue.
During in vitro culture, the ADSCs immunophenotype changes from CD34+, major histocompatibility complex (MHC) I and II molecules, CD80+, CD86+, CD45+, CD11a+, CD14+, CD117+, human lymphocyte antigen (HLA)-DR+, NOG+, undifferentiated embryonic cell transcription factor (UTF)1+, WNT6+, and WNT8A+ to increased expre
MSCs from adipose tissue have strong positive expression of STRO-1+, CD29+, CD73+, CD90+, CD105+, CD166+ & CD44+ and weak positive expression for CD34+ and CD45+[56-57]. MSCs possess enhanced angiogenesis associated with the increased expression of CD105+ and CD34+. Proliferation and differentiation of MSCs is enhanced by CD9+, CD29+, CD44+, CD49d+, and CD106+[58-61]. Gronthos et al[62] demon
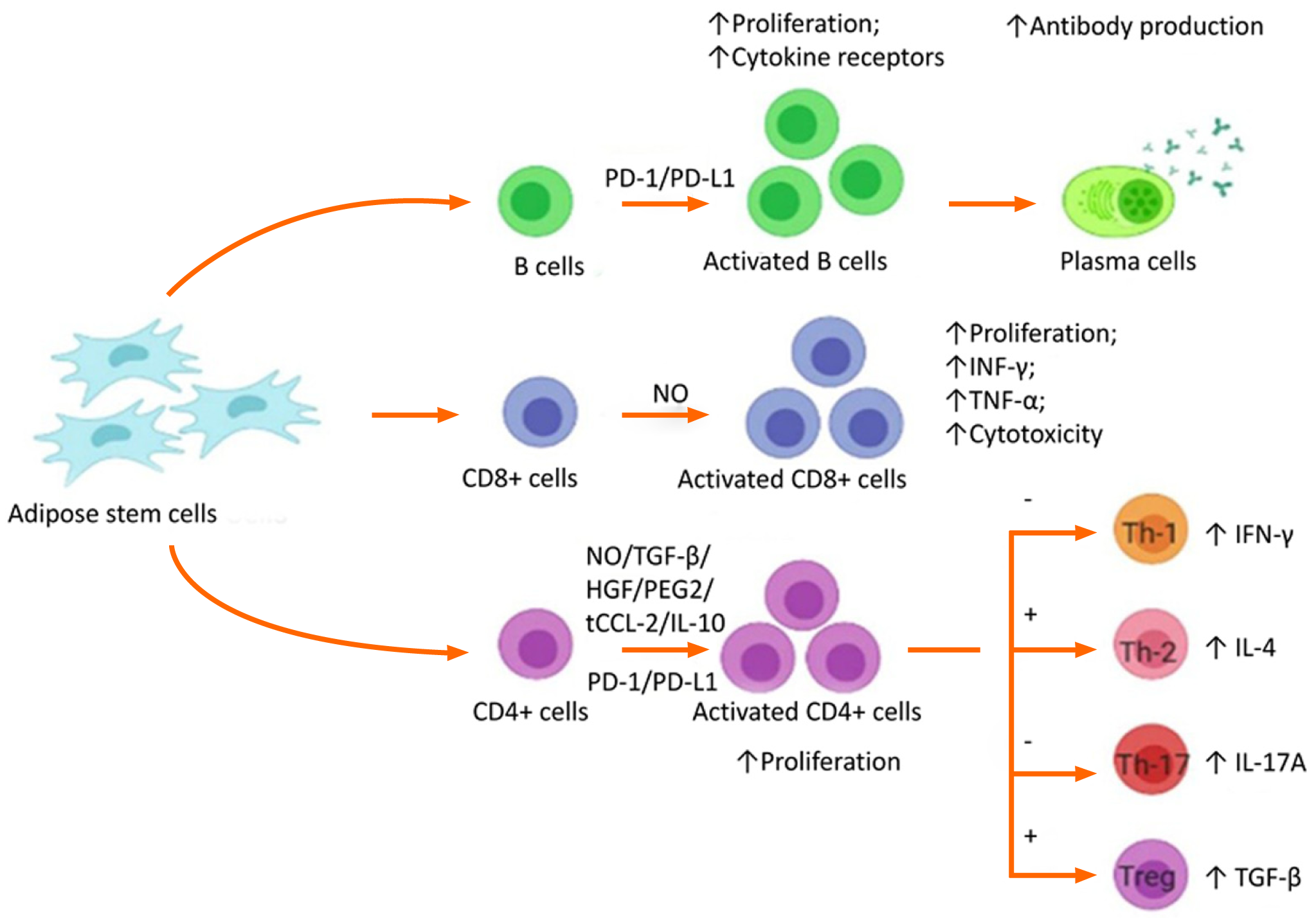
The cellular components of ADSC induce and activate quiescent native MSCs to secrete biological micromolecules at the site of injury to establish local homeostasis by increasing the permeability of cells at the injury site, downregulating inflammatory processes, and recognition of host-tissue progenitor cells for final differentiation into the cells of interest in the injured tissue[67]. Therefore, ADSCs activate adaptive cellular responses and secrete IL-1Ra, IL-1β, PGE2, IDO, IL-4 & -10, and TGF-β that modulate and prime the native immune cells. The micromolecular interactions lead to a cascade of events responsible for immunotolerance of engraftment to a foreign site.
The immunotolerant/immunosuppressive/immunomodulatory activity of ADSCs is caused by the interplay of regulatory T cells, cytotoxic T cells, and B cells as shown in Figure 3. Immunomodulatory activity includes the inhibition of INF-γ and TNF-α production by effector T lymphocytic cells, upregulation of IL-4 and IL-10 by native immune cells, and inhibition of proliferation, migration, and differentiation of B cells that produce immunoglobulins[68]. The activity of natural killer cells is suppressed by the production of indolamine 2,3-dioxygenase by MSC-like cells[69,70]. Dendritic cells enhance the expression of IL-4 IL-10 and suppressed INF-γ and TNF-α[71,72]. The cascade of events directly suppresses tissue inhibitors of matrix metalloproteinases (TIMPs) and matrix metalloproteinases (MMPs), resulting in the conversion of the proinflammatory environment to an anti-inflammatory environment. Adipose-derived MSCs secrete extracellular vesicles such as exosomes as a channel of communication with neighboring cells[73]. They act not only via direct cell-to-cell interaction but also via paracrine mechanisms[74] and are key mediators of signaling molecules such as TGF-β1[75], IL-10[76], PGE2[77], NO[78] and IDO[79,80]. The bioactive factors in-turn help to promote tissue regeneration and repair at the site of action.
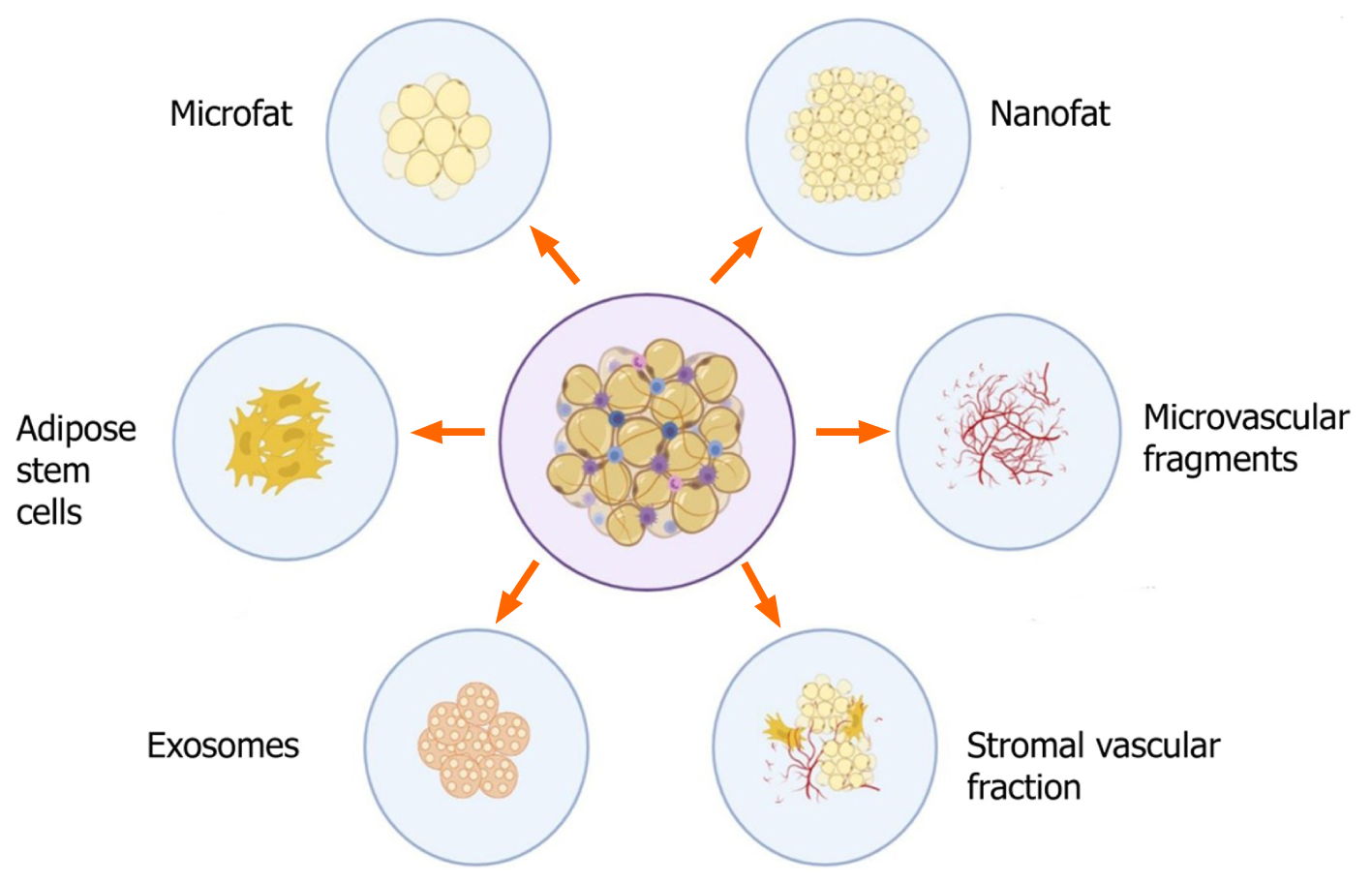
The various derivatives of adipose tissue that are of practical utility, with therapeutic potential are shown in Figure 4 and are discussed below:
Adipose stem cells: ADSCs are one of the forms of MSCs of adipose origin with an inherent property of self-renewal and multipotent differentiation[73,81]. Advantages of easy accessibility of the source, abundant availability, and fewer ethical concerns than embryonic stem cells, render ADSCs more suitable for use in regenerative medicine and tissue engineering. Tissue-engineered 3D scaffolds with ADSCs and biomolecules such as growth factors and extracellular matrix materials play a robust role in treating various disorders by cellular proliferation and differentiation[4]. Studies found that ADSCs have more grounded immunomodulatory impact than BM-MSCs[82-84]. Adipose tissue contains stem and progenitor cells in amounts of up to 3% of the uncultured SVF, which is 2500 times more than the stem cells obtained from bone marrow[85,86]. Depending on the donor and tissue harvesting site, lipoaspirates yield 1% to 10% stem cells, which is approximately 0.5 × 104 to 2 × 105 MSC/g adipose tissue[87]. ADSCs act in paracrine fashion by releasing adipocytokines, cytokines, and growth factors that form a secretome[88].
Following liposuction, adipose tissue is washed in phosphate-buffered saline containing 5% antibiotic, followed by tissue digestion by collagenase-1[89]. After the disintegration of adipose tissue, the resultant sample is transferred to tubes and centrifuged at 2000 rpm for 5 min to obtain a SVF that contains the ADSCs. The resultant cellular pellet is resuspended in 1 mL lysis buffer, recentrifuged at 2000 rpm for 5 min and the resulting cellular suspension is passed through a 70 μm cell strainer. The cellular mixture is transferred to lysine-coated culture plates and incubate at 37 °C with 5% CO2. To obtain ADSCs, about 500 mg of the adipose tissue cell suspension is inoculated in the wells[87,90]. According to International Fat Applied Technology Society, uncultured ADSCs express CD34+, CD45-, CD235a- and CD31-[63,91], whereas cultured ADSCs express CD73+, CD90+, CD105+, CD44+, CD45- and CD31-[91,93].
The short- and long-term storage of ADSCs have been investigated. The cellular proliferative capacity of ADSCs decreases with the length of storage[43]. Hence, they must be supplemented with 10% human serum or platelet-rich plasma (PRP) in 0.9% saline at 4 °C for the first 2 h and not more than 4 h[1,94]. For long-term storage, ADSCs can be stored at −80 °C in liquid nitrogen for up to 6 mo[95,96]. Certain strategies can be followed to enhance and optimize the potential of the ADSC, such as culturing them in hypoxic conditions, which not only enhances the immunomodulatory effect but also reduces the risk of chromosomal aberration and tumorigenesis[97]. Further, the cells can be cryopreserved in the early passages for future usage thereby making them available for future use[98].
ADSCs are used in cardiac tissue engineering where they enhance regeneration of myocardial tissues, improve left ventricular ejection fraction, and reduce the scar volume in ventricular wall in rodent models[99]. ADSCs are seeded as bioscaffold in the healing of cutaneous ulcers and soft tissue injuries[100,101]. Several studies have proven the beneficial role of ADSCs in multiple sclerosis, diabetes mellitus, and rheumatoid arthritis. The anti-inflammatory and immunomodulatory effects of ASCs have been demonstrated in various preclinical models of autoimmune diseases[102]. ADSCs have regenerative potential for bone and cartilage healing in addition to electrical stimulation of cells and tissues[103]. Various researchers across the globe have been working on the therapeutic efficacy of ADSCs in osteoarthritis knees and hips[104-106]. Agostini et al[107] developed a protocol for ADSC application in osteoarthritis in terms of isolation, dose, frequency, analysis, and follow-up. There is no consensus on the use of cultured vs uncultured cells in the management of osteoarthritis[106,108]. ADSCs have been used in sports injuries of ligaments and tendons[109,110], but evidence to support the safety and efficacy is lacking.
The potency of the stem cells to differentiate into various lineages including nerve cells and nervous tissue makes them a good candidate for use in neurological disorders. Cultured and uncultured cells have been used in the treatment of various neurological diseases such as Alzheimer’s disease, Parkinson’s disease, intervertebral disc, amyotrophic lateral sclerosis, multiple system atrophy, post-polio residual paralysis and traumatic brain injury[111-114]. The cells enhanced neurovasculogenesis, counterfeit fibrosis, oxidative stress, anti-inflammation, and neuromodulation. In neurological diseases, the research toward the use of ADSCs is ongoing in animal models.
Microfat: Compared with nanofat, microfat appears deep yellow, with a fine granular structure and intact three-dimensional adipose tissue architecture[115]. Microfat is composed of mature adipocytes, SVF cells, pericytes, capillary fragments, and fibrous scaffolds are well preserved[116-118]. Hence, they are a natural recipient site for survival of grafts, and provide a niche for SVF cellular mixtures for tissue regeneration and rejuvenation. Yang et al[117] harvested microfat from adipose tissue with cannulas that had side holes of less than 1 mm. Examination of the harvests revealed intact fat lobular structures without need of any inter-syringe passages of emulsification procedures. Side holes of < 0.8 mm allowed the processed microfat to easily pass through the needles without any blockage. Such harvests, used for skin and facial rejuvenation through 27 gauge needles, revealed the preservation of micro-functional units of fat tissue that retained the regenerative properties of ADSCs[117]. Caggiati et al[119] demonstrated a higher yield of ADSCs from lipoaspirates harvested by barbed compared with blunt cannulas, but they found that barbed cannulas cut the fibrous septum and produced higher quantities of coarse fibers that increased the probability of molecular blockage in the needle. The cellular components in the microfat induced tissue regeneration through the recruitment of monocyte/macrophage differentiation at the recipient site[35,120]. The cellular yield in microfat (2.28 ± 1.90) × 105 cells/mL is higher than the yield in nanofat (4.12 ± 1.37) × 104 cells/mL, indicating that nanofat involves mechanical emulsification[117]. Microfat components integrate into the local environment because of the intact microintegrity of cellular transplant, with inherent resistance to attack by host immune-mediated cells[121].
Microfat possess various advantages. (1) No mechanical emulsification or centrifugation is involved in preparation, other than the shearing forces during harvesting. (2) Intact vascular fragments with viable cellular components are present in the mixture. (3) There is less ischemic exposure time during adipose tissue harvest and while preparing the microfat graft. (4) Preservation of viable adipocytes that restore the degenerated and atrophied skin and subcutaneous tissues[117]. Compared with other adipose derivatives, microfat retains the three-dimensional architecture of the native tissue essential for the survival of mature adipocytes, thereby providing a natural niche for ADSC cells to ensure optimal tissue regeneration. Microfat is used in esthetic procedures. Because the three-dimensional architecture is retained in microfat, it is used as a lipofiller in breast reconstruction and facial and gluteal augmentation[122-125]. Microfat is also being tried in scar revision, burn injuries, lipodystrophies, rhinoplasty and wrinkles[3,40,117,126,127].
Nanofat: In 2013, Tonnard et al[128] developed nanofat, which is an ultrapurified adipose tissue-derived product that is devoid of mature adipocytes but contains CD34+ ADSCs, microvascular fragments, growth factors, biological peptides, and cytokines[93,94]. It is a liquefied, autologous injectable product with the property of biological integration with adjacent cells and tissues because of its homogenous consistency[129]. The size of nanofat components is approximately 400 to 600 μm[130]. Nanofat behaves much like adipose tissue-derived mesenchymal stromal cells. At the site of injury, stromal cells initiate a site-specific reparative response comprised of remodeling the extracellular matrix (ECM), enhanced and sustained angiogenesis, and immune system modulation. Because of the multi- and pluripotent nature of the cellular components in nanofat, it possesses the ability to differentiate into multiple lineages. Hence, nanofat can be used in preclinical and translational research in tissue engineering. Various preclinical and clinical studies have demonstrated antifibrotic, proangiogenic, neuroregenerative properties, and enhanced collagen deposition potential of adipose tissue-derived nanofat[131-134]. Apart from being an adipogenic derivative with ADSC, the proportion of ADSCs in nanofat is higher than that in microfat. The differences might be attributable to the method of preparation of the microfat, where the fibers and their accompanying capillaries that were the location of ADSCs are removed. In contrast, the ADSCs were mechanically separated from the native site and concentrated in nanofat, thereby making it more effective in terms of the number of ADSCs delivered to the target site. Sesé et al[135] estimated the total cellular load in mechanically prepared nanofat as 6.63 million cells/g of lipoaspirate whereas in enzymatically disintegrated SVF it was 0.68 million cells/g of lipoaspirate. The nucleated cellular count was 70% in nanofat and 7.3% in SVF. The cellular burden in nanofat contains predominantly the stromal cellular population[136].
Nanofat grafting enhances neoangiogenesis without producing any visible scars, and provides a favorable outcome in esthetic medicine for breast, buttocks, and genital augmentation, facial rejuvenation, and facial volume augmentation[137,138]. Nanofat injections retract the atrophic scars because of the presence of adipose tissue-derived stromal cells, and avoids the need for surgical procedures. Nanofat components can regenerate dermis and subcutaneous fatty tissues and enhance the dermo-epidermal junction[139]. They regenerate by laying down adipose tissue-derived ECM, collagen deposition, and neoangiogenesis. Klinger et al[140] reported that autologous fat grafting allowed the regeneration of skin that was soft, flexible, and matched the color of neighboring skin. This concept of skin rejuvenation can be extrapolated to scars present in joints, eyelids, the face, and mouth.
Nanofat grafting beneath and within the substance of the scar improves the quality, integrity, and texture of burn scars[141]. Improved skin texture, elasticity, skin moisture, facial rejuvenation, and anti-aging properties can be achieved by combining nanofat (autocrine and paracrine effects) with platelet-rich fibrin (PRF)[142]. In a pre-clinical trial, nanofat injection improved the thickness of the dermal layer and promoted angiogenesis in the photoaged skin of nude mice[143]. A wide range of improvements in wrinkles, discolorations, and burn scars have been seen with nanofat application[141]. Esthetically, nanofat grafting is used for the correction of dark circles[144,145], malar bags[3], hollow eyes[145], and blepharoplasty[146]. Because of fat atrophy in the aging process, nanofat has emerged as a plausible technique for facial rejuvenation[126,147,148]. Nanofat is also being increasingly used in primary rhinoplasty procedures[149]. Nanofat is being used to correct slight skin irregularities that do not require cartilage grafting[127,150]. Segreto et al[151] evaluated the role of a combination of nanofat grafting with autologous PRP in chronic nonhealing infected wounds, where it enhanced the regeneration of soft tissue. The multi-differentiation potential of adipose tissue, which is a component in nanofat grafting, allows evaluation in avascular necrosis of femoral head, mild to moderate grades of osteoarthritis of the knees, tendinopathies, and nonunion of fractures. Preclinical and clinical studies of nanofat use have proven its regenerative capacity in various clinical settings.
Microvascular fragments: Microvascular fragments (MVFs), the byproduct of adipose tissue, range from 40–180 mm, and are composed of arteriolar, capillary, and vein segments[152-154]. MVFs release VEGF and basic fibroblast growth factor (bFGF) under culture conditions[155]. They are the richest source of proangiogenic factors that induce vasculogenesis in a paracrine fashion[154,156,157]. MVF contains Sca 1/VEG
It was initially speculated that the high vascularization potential of MVFs is mainly derived from stem cell populations. McDaniel et al[160] compared the regenerative properties of conventionally isolated adipose-derived stem cells and multipotent cells derived from an explant culture of microvascular fragments. They found that the latter source exhibited a higher proliferation rate, an increased expression of genes involved in differentiation, and an improved ability to form capillary-like structures. In line with the concept of the “stem cell niche,” the findings indicated that compared with single-cell isolates, microvascular fragments including stem cell components provides a more physiological environment that maximizes the regenerative activity. Transplanted or injected MVFs rapidly integrate with native tissues to promote neoangiogenesis in the physiological environment[161]. The three phases of MVF-induced neoangiogenesis are (1) immature vascular segments with high proliferation capacity; (2) vascular remodeling with a high rate of apoptosis; and (3) vascular maturation with microvascular network organization[162,163]. The complex pattern of neoangiogenesis is associated with the upregulation of angiogenic genes.
Researchers have observed the regenerative potential in cartilage defects and skeletal muscle injury[164-167], myocardial infarction[152,163,168], partial- or complete-thickness skin defects[169,170] and diabetes mellitus[171]. MVF loaded scaffolds can reverse lymphatic network disorders by reducing edema formation and promoting vasculogenesis in the area of repair[154,172-174]. With the available, diverse potentials of MVF, the application of MVF in the clinical setting is plausible with the imperative question of technical and regulatory compliance from bench to bedside.
SVF: SVF is an ultra-byproduct of adipose tissue through various processing methods[175-177]. The development of biocellular regenerative medicine and cellular biology, has increased the use of SVF by regenerative surgeons and researchers. The components of SVF are MSCs, HSCs, T-regulatory cells, pericyte endothelial cells, mast cells, complex microvascular beds with fibroblasts, WBC, dendritic cells, intra-adventitial smooth muscular-like cells, and others, and extracellular matrix[44,177-179]. A sufficient number of SVF cells can be obtained without culture. Current restrictions on the use of cultured cells in humans cite the possibilities of contamination, tumorigenesis, unexpected cell differentiation, and limited cell sources. SVFs behave much like BM-MSCs. SVF mixtures contain 30% MSCs, 3% endothelial cells, and 14% endothelial precursor cells[180]. BM-MSCs contain 0.001% MSCs, 0.1% endothelial cells, and 2% endothelial precursor cells[181]. About 2% of isolated SVF express the hematopoietic markers CD34+ and CD 45+ and 7% express the MSC markers CD105+, and CD146+[92,182,183]. SVF cells express cell surface markers similar to those of BM-MSCs, such as CD105+/SH2+, CD90+, CD29+, CD44+, CD71+, and SH3+, along with low expression of CD31+, CD45+, and CD24+[184-186].
Various methods of isolation of SVF have been described in the literature[90,187-190]. SVF isolation needs fat obtained by liposuction and transportation to a cGMP certified laboratory for further processing[191-193]. The global researchers and clinicians widely use the enzymatic method for isolating and harvesting SVF by collagenase enzymes. After the addition of collagenase enzyme to the lipoaspirate, digestion and disintegration of adipose cells take place and result in the formation of an aqueous mixture including two phases, a floating adipocyte fraction and precipitated cellular components[194,195]. The separation can be enhanced by density gradient centrifugation and filtration[191]. The lower portion of the tube contains a yellowish red pellet which is the SVF mixture that can be used for in vitro expansion[196]. Because of the ethical issues associated with SVF isolation by the enzymatic method, researchers opt for alternative methods for SVF isolation[197,198]. Researchers proposed mechanical agitation and disruption of adipose tissue, which releases a mixture of cellular components and stromal cells. The proportion of cells in the SVF obtained by mechanical disruption is lower that obtained by enzymatic degradation, but the regenerative potential of SVF mixtures obtained by mechanical disruption and enzymatic degradation are the same[198]. The anti-inflammatory effects of SVF result from the presence of higher amounts of IL-1 and 10 receptor antagonists, which are expressed by TNF-α and leptin released by monocytes and macrophages in the SVF mixture[199]. SVF mixtures provoke adaptive immunity by producing T-reg cells by suppressing the activation of dendritic cells[200,201].
Krawiec et al[202] demonstrated that SVF components seeded with biodegradable porous scaffolds in vivo for 8 wk resulted in the generation of tissue-engineered vascular grafts when populated with primary vascular components such as smooth muscle cells, endothelial cells, collagen, and elastin. ADSCs or SVF combined with PRP enhanced the regenerative potential in the form of neoangiogenesis, cellular proliferation, and differentiation[203]. The combination of SVF with PRP enhanced the stemness of MSCs, and the growth factors present in PRP induced locally available stem cells and prolonged the survival time and survival rates of cells present in the PRP[204]. Such combination treatments have been evaluated in preclinical and clinical trials of wound healing, osteoarthritis of the knees, bone and tendon regeneration, periodontal engineering, fat grafting procedures, and vascular diseases[203,205-208]. Osteochondral defects of the knees in goats were managed by the application of acellular type 1 and 3 collagen scaffolds along with SVF cells[209]. In rat models of acute kidney injury, the transplantation of autologous SVF cells induced antiapoptotic effects and the release of growth factors like VEGF and HGF to regenerate kidney cells[210]. In preclinical studies, the therapeutic effect, safety and functional outcome of autologous SVF uncultured or culture-expanded cells were observed in acute myocardial infarction, skin flap necrosis, and erectile dysfunction with cavernous nerve injury[211-213]. In human clinical studies, the use of SVF cells was extensively studied in conditions like breast reconstruction surgery, traumatic calvaria defects, types 1 and 2 diabetes mellitus, Crohn’s disease with enterocutaneous fistula, and burn injuries[214-218]. Zhao et al[219] evaluated the efficacy of SVF in diabetic feet in terms of cellular survival, proliferation rate and differentiation and they determined the characteristics of transplanted cells. Various researchers demonstrated endo
ADSC exosomes: Exosomes are the cell-free regenerative tool in the field of tissue engineering. Exosomes are extracellular vehicles that are endosome-derived lipid bilayer spherical vesicles of 40 to 150 nm in size. They are found in all cell types and body fluids and are comprised of cytokines, proteins, lipids, DNA, and RNA from the parent cell. Exosomes are an integral part of both the diagnostic and therapeutic methods used in various diseases. ADSC exosomes (EXOs) differ from other MSC EXOs in proliferation and differentiation abilities and immunosuppressive pathways. Compared with ADSCs, ADSC-derived exosomes possess have a high biosafety profile with low immunogenicity[227]. They protect cargoes from degradation, have tissue and target specificity, good tissue permeability, intercellular signaling and communication, immune function, tissue homeostasis, and development of cell fate[227].
Ogawa et al[228] observed that mRNAs of adipose tissue-derived exosomes have a significant role in metabolic, immunological, and cellular responses. Various studies demonstrated that adipose tissue-derived exosomes possess the capability of regeneration of muscles and bones, promotion of wound healing, and enhancement of cellular proliferation, and neoangiogenesis. ADSCs-EXOs enhance the proliferation and migration of vascular endothelial cells and promote vasculogenesis. They retain the fat graft volume by the stimulation of angiogenesis and regulating inflammatory responses[229,230]. ADSC EXOs were tested by Wang et al[231] for the promotion of wound healing in diabetic mice by enhancing angiogenesis, proliferation of fibroblasts, and collagen synthesis in the later stages. The evidence also supports the use of ADSC EXOs for treating diabetic foot patients[232]. To stabilize the ADSC EXOs concentration when used for local application, bioscaffolds like hydrogels or fibrin are augmented with exosomes to delay release at the therapeutic site[233,234]. ADSC EXOs are used for scarless cutaneous repair by retracting the size of scars, increasing the collagen 3 to collagen 1 ratio, and by regulating the migration, proliferation, differentiation, and gene expression of fibroblasts[235]. Kim et al[236] demonstrated that key cytokines and growth factors in ADSC EXOs facilitated tissue regeneration and repair through anti-oxidation, anti-wrinkle and skin-whitening activity. ADSC EXOs with multiple bioactive molecules for the management of aging warrants further extensive research. Zhao et al[237] demonstrated crosstalk that facilitated immune and metabolic homeostasis, providing a vital therapy for obesity and diabetes mellitus. ADSC EXOs combined with poly(lactide-co-glycolide) scaffolds improved the osteoinductive effects, MSC migration, and homing abilities in bone regeneration[238]. Chen et al[239] demonstrated that exosomes derived from miR-375-overexpressing ADSCs incorporated with hydrogel enhanced bone regeneration in a rat model of calvarial defect. Direct stem cell transplantation with ADSC EXOs primed by TNF-α, promoted the proliferation and differentiation of human osteoblasts promoted through the Wnt signaling pathway, which further widened the application of exosomes in bone regeneration[240]. Pers et al[241,242] reported that ADSC EXOs were a safe, effective, and inexpensive therapy for osteoarthritic knees. ADSC EXOs downregulated inflammation and oxidative stress in osteoarthritic knees[243]. Zhang et al[244] confirmed that intra-articular injection of ADSC EXOs inhibited cartilage and subchondral bone degradation and osteophyte formation and slowed the progression of the disease process in osteoarthritis. ADSC EXOs participated cellular communications and applications in plastic and cosmetic surgery. Although applications in clinical practice are lacking, ADSC EXOs have an increasing role in maximizing the therapeutic effectiveness for dermopathies and for tissue reconstruction[245]. There is a future for exosomes as a diagnostic tool for early identification of pathological processes involved in disease and as a therapeutic, targeted acellular conduit to optimize the pathological milieu at the site of interest.
An early therapy and drug development are dependent on translational phases, the best strategy is to standardize the process, and regularly acquire data to validate and certify the technologies being developed. The safety and efficacy of the developed therapy must be proven from various perspectives, including the donor, recipient, product, manufacturing, clinical application, and biovigilance. The procedures involved in making adipose-derived products involve the collection and preparation of fat tissue. The procedures require that the cells are obtained by safe methods without any adverse events. Clinicians who use the cells in their practice are obliged to abide by existing laws that control the use in clinical practice. The European Medicines Agency (EMA), United States Food and Drug Administration (FDA), and others consider adult human cells as biological products of two classes. The first involves cells processed with minimal manipulation techniques such as filtration, centrifugation, and mechanical disruption done in a single surgical window within a sterile operating environment. The second class of products undergoes significant manipulation such as expansion in cell culture, characterization, cultivation, and other manipulation techniques used outside of a single surgical window or the operating room. As per FDA and EMA regulations, adipose products are considered medicinal products that have to be harvested using validated procedures adhering to stringent regulatory protocols. The clinical quality attributes of therapeutic products ensure safety along with the maintenance of identity, purity, and potency as per the FDA Guidance for industry [Pharmaceutical development. Q8(R2). Current Step 4 version dated August 2009.Q8 (R2)]. In agreement with the reflection paper EMA/CAT/
Having discussed the practical constraints of the use of ADSCs for practical applications, the use of ADSC-derived exosomes as a therapeutic conduit is being explored[247]. Exosomes do not carry the risk of genetic instability and immune activation following their administration in the host environment. The differential advantage of using them vs their parent cell of origin include immune privilege in the host environment, which helps them to evade the native phagocytosis, their size in nanoscale enabling them to move in and out of cells with ease, their homing molecules on their surface enabling them to migrate to their site of interest[248]. Hence, with the above-conferred advantages in using ADSC-derived exosomes, they are the principal focus of research in advancing regenerative therapy in the future.
Of late, interest in adipose derivatives for regenerative therapies and constructive tissue engineering is on the rise. Having elaborated the biology, characteristics, immunology, and clinical applications of adipose-derived products, it is imperative that evidence to strengthen the clinical applicability of these therapeutic products is needed to warrant an official recommendation from regulatory authorities. Unraveling the utility of various adipose derivatives helps in the improvisation of the existing regenerative therapies and their associated biomedical applications.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan M, Yong KW S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH, Thi Nga V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 725] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 3. | Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond). 2017;20:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 4. | Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 653] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 5. | Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8352] [Cited by in RCA: 6638] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 6. | Feinberg AW. Engineered tissue grafts: opportunities and challenges in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2012;4:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 7. | Hsu VM, Stransky CA, Bucky LP, Percec I. Fat grafting's past, present, and future: why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet Surg J. 2012;32:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Tan SS, Ng ZY, Zhan W, Rozen W. Role of Adipose-derived Stem Cells in Fat Grafting and Reconstructive Surgery. J Cutan Aesthet Surg. 2016;9:152-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Flynn L, Woodhouse KA. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: A review. Bioact Mater. 2019;4:271-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 469] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 11. | Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Devel Ther. 2018;12:3117-3145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 12. | Bahmad HF, Daouk R, Azar J, Sapudom J, Teo JCM, Abou-Kheir W, Al-Sayegh M. Modeling Adipogenesis: Current and Future Perspective. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Young DA, Christman KL. Injectable biomaterials for adipose tissue engineering. Biomed Mater. 2012;7:024104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Klingelhutz AJ, Gourronc FA, Chaly A, Wadkins DA, Burand AJ, Markan KR, Idiga SO, Wu M, Potthoff MJ, Ankrum JA. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci Rep. 2018;8:523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Turner PA, Gurumurthy B, Bailey JL, Elks CM, Janorkar AV. Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids. Process Biochem. 2017;59:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Stenkula KG, Erlanson-Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. 2018;315:R284-R295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Church C, Horowitz M, Rodeheffer M. WAT is a functional adipocyte? Adipocyte. 2012;1:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939-3949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 747] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 20. | Żelechowska P, Kozłowska E, Pastwińska J, Agier J, Brzezińska-Błaszczyk E. Adipocytokine Involvement in Innate Immune Mechanisms. J Interferon Cytokine Res. 2018;38:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 758] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 22. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3330] [Article Influence: 158.6] [Reference Citation Analysis (0)] |
| 23. | Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 599] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911-9; quiz 920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1754] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 25. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 26. | Moon SH, Kim JM, Hong KS, Shin JM, Kim J, Chung HM. Differentiation of hESCs into Mesodermal Subtypes: Vascular-, Hematopoietic- and Mesenchymal-lineage Cells. Int J Stem Cells. 2011;4:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ballini A, Scacco S, Coletti D, Pluchino S, Tatullo M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine. Stem Cells Int. 2017;2017:3292810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Fraser JK, Zhu M, Wulur I, Alfonso Z. Adipose-derived stem cells. Methods Mol Biol. 2008;449:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | PEER LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946). 1955;16:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Pu LL. Mechanisms of Fat Graft Survival. Ann Plast Surg. 2016;77 Suppl 1:S84-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Bellini E, Grieco MP, Raposio E. The science behind autologous fat grafting. Ann Med Surg (Lond). 2017;24:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Harrison BL, Malafa M, Davis K, Rohrich RJ. The discordant histology of grafted fat: a systematic review of the literature. Plast Reconstr Surg. 2015;135:542e-555e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Hong KY. Fat grafts enriched with adipose-derived stem cells. Arch Craniofac Surg. 2020;21:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, Yoshimura K. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 491] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 35. | Yoshimura K, Eto H, Kato H, Doi K, Aoi N. In vivo manipulation of stem cells for adipose tissue repair/reconstruction. Regen Med. 2011;6:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Stem Cells Dev. 2012;21:2724-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 37. | Lee SH. The advantages and limitations of mesenchymal stem cells in clinical application for treating human diseases. Osteoporos Sarcopenia. 2018;4:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 39. | Schneider S, Unger M, van Griensven M, Balmayor ER. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017;22:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Simonacci F, Bertozzi N, Grieco MP, Raposio E. From liposuction to adipose-derived stem cells: indications and technique. Acta Biomed. 2019;90:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 41. | Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 42. | Meza-Zepeda LA, Noer A, Dahl JA, Micci F, Myklebost O, Collas P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12:553-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep. 2011;7:269-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 44. | Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1660] [Cited by in RCA: 1919] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 45. | Alonso-Goulart V, Ferreira LB, Duarte CA, Isabela Lemos de Lima, Enza Rafaela Ferreira, Bárbara Candido de Oliveira, Luna Nascimento Vargas, Dayane Dotto de Moraes, Isaura Beatriz Borges Silva, Rafael de Oliveira Faria, Aline Gomes de Souza, Leticia de Souza Castro-Filice. Mesenchymal stem cells from human adipose tissue and bone repair: a literature review. Biotechnol Res Innov. 2018;2:74-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 47. | Luna AC, Madeira ME, Conceição TO, Moreira JA, Laiso RA, Maria DA. Characterization of adipose-derived stem cells of anatomical region from mice. BMC Res Notes. 2014;7:552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 1050] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 49. | Screven R, Kenyon E, Myers MJ, Yancy HF, Skasko M, Boxer L, Bigley EC 3rd, Borjesson DL, Zhu M. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet Immunol Immunopathol. 2014;161:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Rady D, Abbass MMS, El-Rashidy AA, El Moshy S, Radwan IA, Dörfer CE, Fawzy El-Sayed KM. Mesenchymal Stem/Progenitor Cells: The Prospect of Human Clinical Translation. Stem Cells Int. 2020;2020:8837654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells. 2014;9:135-148. [PubMed] |
| 52. | Cheng KH, Kuo TL, Kuo KK, Hsiao CC. Human adipose-derived stem cells: Isolation, characterization and current application in regeneration medicine. Genomic Med Biomark Health Sci. 2011;3:53-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Hoogduijn MJ, Roemeling-van Rhijn M, Korevaar SS, Engela AU, Weimar W, Baan CC. Immunological aspects of allogeneic and autologous mesenchymal stem cell therapies. Hum Gene Ther. 2011;22:1587-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Mazini L, Rochette L, Amine M, Malka G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 56. | Zhong YC, Wang SC, Han YH, Wen Y. Recent Advance in Source, Property, Differentiation, and Applications of Infrapatellar Fat Pad Adipose-Derived Stem Cells. Stem Cells Int. 2020;2020:2560174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Zhao Y, Waldman SD, Flynn LE. The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells Tissues Organs. 2012;195:414-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Walenda T, Bork S, Horn P, Wein F, Saffrich R, Diehlmann A, Eckstein V, Ho AD, Wagner W. Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J Cell Mol Med. 2010;14:337-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Wei W, Xu C, Ye ZY, Huang XJ, Yuan JE, Ma TB, Lin HB, Chen XQ. [Biological characteristics of mesenchymal stem cell and hematopoietic stem cell in the co-culture system]. Sheng Li Xue Bao. 2016;68:691-698. [PubMed] |
| 60. | Perucca S, Di Palma A, Piccaluga PP, Gemelli C, Zoratti E, Bassi G, Giacopuzzi E, Lojacono A, Borsani G, Tagliafico E, Scupoli MT, Bernardi S, Zanaglio C, Cattina F, Cancelli V, Malagola M, Krampera M, Marini M, Almici C, Ferrari S, Russo D. Mesenchymal stromal cells (MSCs) induce ex vivo proliferation and erythroid commitment of cord blood haematopoietic stem cells (CB-CD34+ cells). PLoS One. 2017;12:e0172430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Ocansey DKW, Pei B, Yan Y, Qian H, Zhang X, Xu W, Mao F. Improved therapeutics of modified mesenchymal stem cells: an update. J Transl Med. 2020;18:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 62. | Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 997] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 63. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1366] [Article Influence: 113.8] [Reference Citation Analysis (2)] |
| 64. | Szöke K, Brinchmann JE. Concise review: therapeutic potential of adipose tissue-derived angiogenic cells. Stem Cells Transl Med. 2012;1:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 633] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 66. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2900] [Cited by in RCA: 2864] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 67. | Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol. 2016;231:1413-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 68. | Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 69. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 388] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 70. | Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018;2018:3057624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 71. | Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Yanagawa Y, Iwabuchi K, Onoé K. Co-operative action of interleukin-10 and interferon-gamma to regulate dendritic cell functions. Immunology. 2009;127:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Seo Y, Shin TH, Kim HS. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 74. | Muthu S, Bapat A, Jain R, Jeyaraman N, Jeyaraman M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021;8:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 75. | Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol Lett. 2015;168:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 76. | Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596-3599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 577] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 77. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 779] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 78. | Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 699] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 79. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3275] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 80. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 871] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 81. | Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012;2012:461718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 82. | Leto Barone AA, Khalifian S, Lee WP, Brandacher G. Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int. 2013;2013:383685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5011] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 84. | Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, Sung KW, Kim CW, Koo HH. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 85. | Baptista LS. Adipose stromal/stem cells in regenerative medicine: Potentials and limitations. World J Stem Cells. 2020;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 87. | Palumbo P, Lombardi F, Siragusa G, Cifone MG, Cinque B, Giuliani M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 88. | Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC, Deng WP. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 89. | Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 90. | Mailey B, Hosseini A, Baker J, Young A, Alfonso Z, Hicok K, Wallace AM, Cohen SR. Adipose-derived stem cells: methods for isolation and applications for clinical use. Methods Mol Biol. 2014;1210:161-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6:256-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 92. | Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013;83:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 93. | Brooks AES, Iminitoff M, Williams E, Damani T, Jackson-Patel V, Fan V, James J, Dunbar PR, Feisst V, Sheppard HM. Ex Vivo Human Adipose Tissue Derived Mesenchymal Stromal Cells (ASC) Are a Heterogeneous Population That Demonstrate Rapid Culture-Induced Changes. Front Pharmacol. 2019;10:1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Wu YD, Li M, Liao X, Li SH, Yan JX, Fan L, She WL, Song JX, Liu HW. Effects of storage culture media, temperature and duration on human adiposederived stem cell viability for clinical use. Mol Med Rep. 2019;19:2189-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Shaik S, Wu X, Gimble J, Devireddy R. Effects of Decade Long Freezing Storage on Adipose Derived Stem Cells Functionality. Sci Rep. 2018;8:8162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | De Rosa A, De Francesco F, Tirino V, Ferraro GA, Desiderio V, Paino F, Pirozzi G, D'Andrea F, Papaccio G. A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology. Tissue Eng Part C Methods. 2009;15:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Choi JR, Yong KW, Wan Safwani WKZ. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell Mol Life Sci. 2017;74:2587-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 98. | Yong KW, Choi JR, Dolbashid AS, Wan Safwani WKZ. Biosafety and bioefficacy assessment of human mesenchymal stem cells: what do we know so far? Regen Med. 2018;13:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 100. | Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 101. | Shingyochi Y, Orbay H, Mizuno H. Adipose-derived stem cells for wound repair and regeneration. Expert Opin Biol Ther. 2015;15:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 102. | Sabol RA, Bowles AC, Côté A, Wise R, Pashos N, Bunnell BA. Therapeutic Potential of Adipose Stem Cells. Adv Exp Med Biol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | Björninen M, Gilmore K, Pelto J, Seppänen-Kaijansinkko R, Kellomäki M, Miettinen S, Wallace G, Grijpma D, Haimi S. Electrically Stimulated Adipose Stem Cells on Polypyrrole-Coated Scaffolds for Smooth Muscle Tissue Engineering. Ann Biomed Eng. 2017;45:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Dall'Oca C, Breda S, Elena N, Valentini R, Samaila EM, Magnan B. Mesenchymal Stem Cells injection in hip osteoarthritis: preliminary results. Acta Biomed. 2019;90:75-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 105. | Jones IA, Wilson M, Togashi R, Han B, Mircheff AK, Thomas Vangsness C Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord. 2018;19:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 106. | Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019;8:504-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 324] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 107. | Agostini F, Rossi FM, Aldinucci D, Battiston M, Lombardi E, Zanolin S, Massarut S, Parodi PC, Da Ponte A, Tessitori G, Pivetta B, Durante C, Mazzucato M. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res Ther. 2018;9:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Roato I, Belisario DC, Compagno M, Lena A, Bistolfi A, Maccari L, Mussano F, Genova T, Godio L, Perale G, Formica M, Cambieri I, Castagnoli C, Robba T, Felli L, Ferracini R. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations. Int Orthop. 2019;43:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 109. | Eagan MJ, Zuk PA, Zhao KW, Bluth BE, Brinkmann EJ, Wu BM, McAllister DR. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J Tissue Eng Regen Med. 2012;6:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | de Aro AA, Carneiro GD, Teodoro LFR, da Veiga FC, Ferrucci DL, Simões GF, Simões PW, Alvares LE, de Oliveira ALR, Vicente CP, Gomes CP, Pesquero JB, Esquisatto MAM, de Campos Vidal B, Pimentel ER. Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Choi HS, Kim HJ, Oh JH, Park HG, Ra JC, Chang KA, Suh YH. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson's disease. Neurobiol Aging. 2015;36:2885-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 112. | Gugliandolo A, Bramanti P, Mazzon E. Mesenchymal Stem Cells: A Potential Therapeutic Approach for Amyotrophic Lateral Sclerosis? Stem Cells Int. 2019;2019:3675627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 113. | Chen BK, Staff NP, Knight AM, Nesbitt JJ, Butler GW, Padley DJ, Parisi JE, Dietz AB, Windebank AJ. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting Phase I human trials. Transfusion. 2015;55:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Uccelli A, Milanese M, Principato MC, Morando S, Bonifacino T, Vergani L, Giunti D, Voci A, Carminati E, Giribaldi F, Caponnetto C, Bonanno G. Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Mol Med. 2012;18:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 115. | Cohen SR, Womack H. Injectable Tissue Replacement and Regeneration: Anatomic Fat Grafting to Restore Decayed Facial Tissues. Plast Reconstr Surg Glob Open. 2019;7:e2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Tremolada C, Colombo V, Ventura C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr Stem Cell Rep. 2016;2:304-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 117. | Yang Z, Jin S, He Y, Zhang X, Han X, Li F. Comparison of Microfat, Nanofat and Extracellular Matrix/Stromal Vascular Fraction Gel for Skin Rejuvenation: Basic Research and Clinical Applications. Aesthet Surg J. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 118. | Trivisonno A, Alexander RW, Baldari S, Cohen SR, Di Rocco G, Gentile P, Magalon G, Magalon J, Miller RB, Womack H, Toietta G. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl Med. 2019;8:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 119. | Caggiati A, Germani A, Di Carlo A, Borsellino G, Capogrossi MC, Picozza M. Naturally Adipose Stromal Cell-Enriched Fat Graft: Comparative Polychromatic Flow Cytometry Study of Fat Harvested by Barbed or Blunt Multihole Cannula. Aesthet Surg J. 2017;37:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Borrelli MR, Hu MS, Longaker MT, Lorenz HP. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J Craniofac Surg. 2020;31:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Christ B, Franquesa M, Najimi M, van der Laan LJW, Dahlke MH. Cellular and Molecular Mechanisms of Mesenchymal Stem Cell Actions. Stem Cells Int. 2017;2017:2489041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 122. | Kim HY, Jung BK, Lew DH, Lee DW. Autologous Fat Graft in the Reconstructed Breast: Fat Absorption Rate and Safety based on Sonographic Identification. Arch Plast Surg. 2014;41:740-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Goldman BE. Micro Fat Grafting for Post Lumpectomy and Breast Reconstruction Deformities: Pearls for Success. Plast Reconstr Surg. 2015;136:101-102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 124. | Lindenblatt N, van Hulle A, Verpaele AM, Tonnard PL. The Role of Microfat Grafting in Facial Contouring. Aesthet Surg J. 2015;35:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 125. | Roberts TL 3rd, Toledo LS, Badin AZ. Augmentation of the buttocks by micro fat grafting. Aesthet Surg J. 2001;21:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Rihani J. Microfat and Nanofat: When and Where These Treatments Work. Facial Plast Surg Clin North Am. 2019;27:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 127. | Erol OO. Microfat Grafting in Nasal Surgery. Aesthet Surg J. 2014;34:671-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 129. | Zheng H, Qiu L, Su Y, Yi C. Conventional Nanofat and SVF/ADSC-Concentrated Nanofat: A Comparative Study on Improving Photoaging of Nude Mice Skin. Aesthet Surg J. 2019;39:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 130. | Cohen SR, Tiryaki T, Womack HA, Canikyan S, Schlaudraff KU, Scheflan M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthet Surg J Open Forum. 2019;1:ojz028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 131. | Serratrice N, Bruzzese L, Magalon J, Véran J, Giraudo L, Aboudou H, Ould-Ali D, Nguyen PS, Bausset O, Daumas A, Casanova D, Granel B, Andrac-Meyer L, Sabatier F, Magalon G. New fat-derived products for treating skin-induced lesions of scleroderma in nude mice. Stem Cell Res Ther. 2014;5:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Memar O, Nezamabadi A, Milani BY, Milani FY, Djalilian A. Nanofat grafting: basic research and clinical application. Plast Reconstr Surg. 2014;133:728e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 133. | Friji MT. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2014;134:333e-334e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Comparing different nanofat procedures on scars: role of the stromal vascular fraction and its clinical implications. Regen Med. 2017;12:939-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 135. | Sesé B, Sanmartín JM, Ortega B, Matas-Palau A, Llull R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast Reconstr Surg. 2019;144:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 136. | Copcu HE, Oztan S. New Mechanical Fat Separation Technique: Adjustable Regenerative Adipose-tissue Transfer (ARAT) and Mechanical Stromal Cell Transfer (MEST). Aesthet Surg J Open Forum. 2020;2:ojaa035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Menkes S, Luca M, Soldati G, Polla L. Subcutaneous Injections of Nanofat Adipose-derived Stem Cell Grafting in Facial Rejuvenation. Plast Reconstr Surg Glob Open. 2020;8:e2550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 138. | Cohen SR, Hewett S, Ross L, Delaunay F, Goodacre A, Ramos C, Leong T, Saad A. Regenerative Cells For Facial Surgery: Biofilling and Biocontouring. Aesthet Surg J. 2017;37:S16-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 139. | Fakih-Gomez N, Steward E, Orte Aldea M del C. Nanofat in Facial Rejuvenation: Step-by-Step Procedure, Patient Evaluation and Recovery Process. The American Journal of Cosmetic Surgery. 2021;38:27-35. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 140. | Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci V. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 141. | Jan SN, Bashir MM, Khan FA, Hidayat Z, Ansari HH, Sohail M, Bajwa AB, Shami HB, Hanif A, Aziz F, Choudhery MS. Unfiltered Nanofat Injections Rejuvenate Postburn Scars of Face. Ann Plast Surg. 2019;82:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 142. | Wei H, Gu SX, Liang YD, Liang ZJ, Chen H, Zhu MG, Xu FT, He N, Wei XJ, Li HM. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget. 2017;8:68542-68556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 143. | Xu P, Yu Q, Huang H, Zhang WJ, Li W. Nanofat Increases Dermis Thickness and Neovascularization in Photoaged Nude Mouse Skin. Aesthetic Plast Surg. 2018;42:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 144. | Ziade G, Karam D. Emulsified fat and nanofat for the treatment of dark circles. Dermatol Ther. 2020;33:e14100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 145. | Oh DS, Kim DH, Roh TS, Yun IS, Kim YS. Correction of Dark Coloration of the Lower Eyelid Skin with Nanofat Grafting. Arch Aesthetic Plast Surg. 2014;20:92-96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 146. | Tonnard P, Verpaele A, Carvas M. Fat Grafting for Facial Rejuvenation with Nanofat Grafts. Clin Plast Surg. 2020;47:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 147. | Boureaux E, Chaput B, Bannani S, Herlin C, De Runz A, Carloni R, Mortemousque B, Mouriaux F, Watier E, Bertheuil N. Eyelid fat grafting: Indications, operative technique and complications; a systematic review. J Craniomaxillofac Surg. 2016;44:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 148. | Le TP, Peckinpaugh J, Naficy S, Amadi AJ. Effect of autologous fat injection on lower eyelid position. Ophthalmic Plast Reconstr Surg. 2014;30:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Yuksel E, Spira M, Yazgan H. Role of Fat Grafting in Primary Rhinoplasty. Plast Reconstr Surg. 2012;130:44-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 150. | Kao WP, Lin YN, Lin TY, Huang YH, Chou CK, Takahashi H, Shieh TY, Chang KP, Lee SS, Lai CS, Lin SD, Lin TM. Microautologous Fat Transplantation for Primary Augmentation Rhinoplasty: Long-Term Monitoring of 198 Asian Patients. Aesthet Surg J. 2016;36:648-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 151. | Segreto F, Marangi GF, Nobile C, Alessandri-Bonetti M, Gregorj C, Cerbone V, Gratteri M, Caldaria E, Tirindelli MC, Persichetti P. Use of platelet-rich plasma and modified nanofat grafting in infected ulcers: Technical refinements to improve regenerative and antimicrobial potential. Arch Plast Surg. 2020;47:217-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 152. | Frueh FS, Später T, Scheuer C, Menger MD, Laschke MW. Isolation of Murine Adipose Tissue-derived Microvascular Fragments as Vascularization Units for Tissue Engineering. J Vis Exp. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 153. | Hoying JB, Boswell CA, Williams SK. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev Biol Anim. 1996;32:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 154. | Kamat P, Frueh FS, McLuckie M, Sanchez-Macedo N, Wolint P, Lindenblatt N, Plock JA, Calcagni M, Buschmann J. Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat-a review. Cytotherapy. 2020;22:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 155. | Sun XT, Ding YT, Yan XG, Wu LY, Li Q, Cheng N, Qiu YD, Zhang MY. Angiogenic synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in an in vitro quantitative microcarrier-based three-dimensional fibrin angiogenesis system. World J Gastroenterol. 2004;10:2524-2528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 156. | Frueh FS, Später T, Lindenblatt N, Calcagni M, Giovanoli P, Scheuer C, Menger MD, Laschke MW. Adipose Tissue-Derived Microvascular Fragments Improve Vascularization, Lymphangiogenesis, and Integration of Dermal Skin Substitutes. J Invest Dermatol. 2017;137:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 157. | Laschke MW, Menger MD. Adipose tissue-derived microvascular fragments: natural vascularization units for regenerative medicine. Trends Biotechnol. 2015;33:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 158. | Klein D, Weisshardt P, Kleff V, Jastrow H, Jakob HG, Ergün S. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One. 2011;6:e20540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 159. | Laschke MW, Später T, Menger MD. Microvascular Fragments: More Than Just Natural Vascularization Units. Trends Biotechnol. 2021;39:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 160. | McDaniel JS, Pilia M, Ward CL, Pollot BE, Rathbone CR. Characterization and multilineage potential of cells derived from isolated microvascular fragments. J Surg Res. 2014;192:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 161. | Shepherd BR, Chen HY, Smith CM, Gruionu G, Williams SK, Hoying JB. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 162. | Karschnia P, Scheuer C, Heß A, Später T, Menger MD, Laschke MW. Erythropoietin promotes network formation of transplanted adipose tissue-derived microvascular fragments. Eur Cell Mater. 2018;35:268-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 163. | Nunes SS, Krishnan L, Gerard CS, Dale JR, Maddie MA, Benton RL, Hoying JB. Angiogenic potential of microvessel fragments is independent of the tissue of origin and can be influenced by the cellular composition of the implants. Microcirculation. 2010;17:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 164. | Pilia M, McDaniel JS, Guda T, Chen XK, Rhoads RP, Allen RE, Corona BT, Rathbone CR. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur Cell Mater. 2014;28:11-23; discussion 23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 165. | Li MT, Ruehle MA, Stevens HY, Servies N, Willett NJ, Karthikeyakannan S, Warren GL, Guldberg RE, Krishnan L. * Skeletal Myoblast-Seeded Vascularized Tissue Scaffolds in the Treatment of a Large Volumetric Muscle Defect in the Rat Biceps Femoris Muscle. Tissue Eng Part A. 2017;23:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 166. | Orth M, Altmeyer MAB, Scheuer C, Braun BJ, Holstein JH, Eglin D, D'Este M, Histing T, Laschke MW, Pohlemann T, Menger MD. Effects of locally applied adipose tissue-derived microvascular fragments by thermoresponsive hydrogel on bone healing. Acta Biomater. 2018;77:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 167. | Ruehle MA, Li MA, Cheng A, Krishnan L, Willett NJ, Guldberg RE. Decorin-supplemented collagen hydrogels for the co-delivery of bone morphogenetic protein-2 and microvascular fragments to a composite bone-muscle injury model with impaired vascularization. Acta Biomater. 2019;93:210-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 168. | Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng. 2007;13:2871-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 169. | Frueh FS, Später T, Körbel C, Scheuer C, Simson AC, Lindenblatt N, Giovanoli P, Menger MD, Laschke MW. Prevascularization of dermal substitutes with adipose tissue-derived microvascular fragments enhances early skin grafting. Sci Rep. 2018;8:10977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 170. | Später T, Frueh FS, Menger MD, Laschke MW. Potentials and limitations of Integra® flowable wound matrix seeded with adipose tissue-derived microvascular fragments. Eur Cell Mater. 2017;33:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 171. | Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 172. | Saberianpour S, Heidarzadeh M, Geranmayeh MH, Hosseinkhani H, Rahbarghazi R, Nouri M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J Biol Eng. 2018;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 173. | Rouwkema J, Khademhosseini A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016;34:733-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 435] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 174. | Später T, Ampofo E, Menger MD, Laschke MW. Combining Vascularization Strategies in Tissue Engineering: The Faster Road to Success? Front Bioeng Biotechnol. 2020;8:592095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 175. | Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 176. | Muller S, Ader I, Creff J, Leménager H, Achard P, Casteilla L, Sensebé L, Carrière A, Deschaseaux F. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep. 2019;9:7250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 177. | Vezzani B, Shaw I, Lesme H, Yong L, Khan N, Tremolada C, Péault B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl Med. 2018;7:876-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 178. | Alexander RW. Understanding Adipose-derived Stromal Vascular Fraction (AD-SVF) Cell Biology and Use on the Basis of Cellular, Chemical, Structural and Paracrine Components: A Concise Review. Journal of Prolotherapy. 2012;4:e855-e869. |
| 179. | Ramakrishnan VM, Boyd NL. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng Part B Rev. 2018;24:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 180. | Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 181. | Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669-4674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 182. | Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 561] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 183. | Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 398] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 184. | Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 185. | Rojewski MT, Weber BM, Schrezenmeier H. Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfus Med Hemother. 2008;35:168-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 186. | Thitilertdecha P, Lohsiriwat V, Poungpairoj P, Tantithavorn V, Onlamoon N. Extensive Characterization of Mesenchymal Stem Cell Marker Expression on Freshly Isolated and In Vitro Expanded Human Adipose-Derived Stem Cells from Breast Cancer Patients. Stem Cells Int. 2020;2020:8237197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 187. | Aronowitz JA, Lockhart RA, Hakakian CS. A Method for Isolation of Stromal Vascular Fraction Cells in a Clinically Relevant Time Frame. Methods Mol Biol. 2018;1773:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 188. | van Dongen JA, Harmsen MC, Stevens HP. Isolation of Stromal Vascular Fraction by Fractionation of Adipose Tissue. Methods Mol Biol. 2019;1993:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Senesi L, De Francesco F, Farinelli L, Manzotti S, Gagliardi G, Papalia GF, Riccio M, Gigante A. Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction From Adipose Tissue: Preliminary Results. Front Cell Dev Biol. 2019;7:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 190. | Condé-Green A, Kotamarti VS, Sherman LS, Keith JD, Lee ES, Granick MS, Rameshwar P. Shift toward Mechanical Isolation of Adipose-derived Stromal Vascular Fraction: Review of Upcoming Techniques. Plast Reconstr Surg Glob Open. 2016;4:e1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 191. | SundarRaj S, Deshmukh A, Priya N, Krishnan VS, Cherat M, Majumdar AS. Development of a System and Method for Automated Isolation of Stromal Vascular Fraction from Adipose Tissue Lipoaspirate. Stem Cells Int. 2015;2015:109353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 192. | Rodriguez J, Pratta AS, Abbassi N, Fabre H, Rodriguez F, Debard C, Adobati J, Boucher F, Mallein-Gerin F, Auxenfans C, Damour O, Mojallal A. Evaluation of Three Devices for the Isolation of the Stromal Vascular Fraction from Adipose Tissue and for ASC Culture: A Comparative Study. Stem Cells Int. 2017;2017:9289213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 193. | Doi K, Tanaka S, Iida H, Eto H, Kato H, Aoi N, Kuno S, Hirohi T, Yoshimura K. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med. 2013;7:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 194. | Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 195. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5756] [Article Influence: 239.8] [Reference Citation Analysis (0)] |
| 196. | Riis S, Zachar V, Boucher S, Vemuri MC, Pennisi CP, Fink T. Critical steps in the isolation and expansion of adipose-derived stem cells for translational therapy. Expert Rev Mol Med. 2015;17:e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 197. | Shah FS, Wu X, Dietrich M, Rood J, Gimble JM. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 198. | Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 199. | Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 1135] [Article Influence: 189.2] [Reference Citation Analysis (0)] |
| 200. | Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Febbo I, Bunnell BA. Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells. 2017;35:2198-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 201. | Dong Z, Peng Z, Chang Q, Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One. 2013;8:e80364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 202. | Krawiec JT, Liao HT, Kwan LL, D'Amore A, Weinbaum JS, Rubin JP, Wagner WR, Vorp DA. Evaluation of the stromal vascular fraction of adipose tissue as the basis for a stem cell-based tissue-engineered vascular graft. J Vasc Surg. 2017;66:883-890.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 203. | Karina, Samudra MF, Rosadi I, Afini I, Widyastuti T, Sobariah S, Remelia M, Puspitasari RL, Rosliana I, Tunggadewi TI. Combination of the stromal vascular fraction and platelet-rich plasma accelerates the wound healing process: pre-clinical study in a Sprague-Dawley rat model. Stem Cell Investig. 2019;6:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 204. | Tobita M, Tajima S, Mizuno H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness. Stem Cell Res Ther. 2015;6:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 205. | Rigotti G, Charles-de-Sá L, Gontijo-de-Amorim NF, Takiya CM, Amable PR, Borojevic R, Benati D, Bernardi P, Sbarbati A. Expanded Stem Cells, Stromal-Vascular Fraction, and Platelet-Rich Plasma Enriched Fat: Comparing Results of Different Facial Rejuvenation Approaches in a Clinical Trial. Aesthet Surg J. 2016;36:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 206. | Butt G, Hussain I, Ahmad FJ, Choudhery MS. Stromal vascular fraction-enriched platelet-rich plasma therapy reverses the effects of androgenetic alopecia. J Cosmet Dermatol. 2020;19:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 207. | Van Pham P, Hong-Thien Bui K, Quoc Ngo D, Tan Khuat L, Kim Phan N. Transplantation of Nonexpanded Adipose Stromal Vascular Fraction and Platelet-Rich Plasma for Articular Cartilage Injury Treatment in Mice Model. J Med Eng. 2013;2013:832396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 208. | Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 209. | Jurgens WJ, Kroeze RJ, Zandieh-Doulabi B, van Dijk A, Renders GA, Smit TH, van Milligen FJ, Ritt MJ, Helder MN. One-step surgical procedure for the treatment of osteochondral defects with adipose-derived stem cells in a caprine knee defect: a pilot study. Biores Open Access. 2013;2:315-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 210. | Zhou L, Xu L, Shen J, Song Q, Wu R, Ge Y, Xin H, Zhu J, Wu J, Jia R. Preischemic Administration of Nonexpanded Adipose Stromal Vascular Fraction Attenuates Acute Renal Ischemia/Reperfusion Injury and Fibrosis. Stem Cells Transl Med. 2016;5:1277-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 211. | van Dijk A, Naaijkens BA, Jurgens WJ, Nalliah K, Sairras S, van der Pijl RJ, Vo K, Vonk AB, van Rossum AC, Paulus WJ, van Milligen FJ, Niessen HW. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011;7:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 212. | Sheng L, Yang M, Li H, Du Z, Yang Y, Li Q. Transplantation of adipose stromal cells promotes neovascularization of random skin flaps. Tohoku J Exp Med. 2011;224:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 213. | Qiu X, Fandel TM, Ferretti L, Albersen M, Orabi H, Zhang H, Lin G, Lin CS, Schroeder T, Lue TF. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 214. | Wu AY, Morrow DM. Autologous fat transfer with in-situ mediation (AIM): a novel and compliant method of adult mesenchymal stem cell therapy. J Transl Med. 2013;11:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 215. | Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, Floris M, Fiaschetti V, Floris R, Cervell V. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 216. | Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 409] [Article Influence: 29.2] [Reference Citation Analysis (1)] |
| 217. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 571] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 218. | Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 2014;40:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 219. | Zhao X, Guo J, Zhang F, Zhang J, Liu D, Hu W, Yin H, Jin L. Therapeutic application of adipose-derived stromal vascular fraction in diabetic foot. Stem Cell Res Ther. 2020;11:394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 220. | Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N, Dardzinski B, Gritzalis D, Antoniadis A, Froudarakis M, Kolios G, Bouros D. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 221. | Gentile P, Sterodimas A. Adipose Stem Cells (ASCs) and Stromal Vascular Fraction (SVF) as a Potential Therapy in Combating (COVID-19)-Disease. Aging Dis. 2020;11:465-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 222. | Mazini L, Ezzoubi M, Malka G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: flow chart and regulation updates before and after COVID-19. Stem Cell Res Ther. 2021;12:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 223. | Jeyaraman M, Ranjan R, Kumar R, Arora A, Chaudhary D, Ajay SS, Jain R. Cellular Therapy: Shafts of Light Emerging for COVID-19. Stem Cell Investig. 2020;7:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 224. | D'Esposito V, Passaretti F, Perruolo G, Ambrosio MR, Valentino R, Oriente F, Raciti GA, Nigro C, Miele C, Sammartino G, Beguinot F, Formisano P. Platelet-Rich Plasma Increases Growth and Motility of Adipose Tissue-Derived Mesenchymal Stem Cells and Controls Adipocyte Secretory Function. J Cell Biochem. 2015;116:2408-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 225. | Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C, Dickmeiss E, Drzewiecki KT. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 426] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 226. | Lapuente JP, Dos-Anjos S, Blázquez-Martínez A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res. 2020;15:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 227. | Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y, Wu W, Zhu N. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng Regen Med. 2021;18:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 228. | Ogawa R, Tanaka C, Sato M, Nagasaki H, Sugimura K, Okumura K, Nakagawa Y, Aoki N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010;398:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 229. | Mou S, Zhou M, Li Y, Wang J, Yuan Q, Xiao P, Sun J, Wang Z. Extracellular Vesicles from Human Adipose-Derived Stem Cells for the Improvement of Angiogenesis and Fat-Grafting Application. Plast Reconstr Surg. 2019;144:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 230. | Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 231. | Wang J, Yi Y, Zhu Y, Wang Z, Wu S, Zhang J, Hu X, Nie J. [Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 232. | Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 233. | Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, Akbariqomi M, Sanikhani NS, Absalan M, Tavoosidana G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J Biomed Mater Res A. 2020;108:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 234. | Taverna S, Pucci M, Alessandro R. Extracellular vesicles: small bricks for tissue repair/regeneration. Ann Transl Med. 2017;5:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 235. | Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, Hu B, Song J, Chen L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 236. | Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 237. | Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 238. | Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W, Zhang X, Wu G, Zhou Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces. 2018;10:5240-5254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 239. | Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, Jia L, Zhou Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52:e12669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 240. | Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng Part A. 2017;23:1212-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 241. | Pers YM, Quentin J, Feirreira R, Espinoza F, Abdellaoui N, Erkilic N, Cren M, Dufourcq-Lopez E, Pullig O, Nöth U, Jorgensen C, Louis-Plence P. Injection of Adipose-Derived Stromal Cells in the Knee of Patients with Severe Osteoarthritis has a Systemic Effect and Promotes an Anti-Inflammatory Phenotype of Circulating Immune Cells. Theranostics. 2018;8:5519-5528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 242. | Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23:2027-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 243. | Wang J, Guo X, Kang Z, Qi L, Yang Y, Wang J, Xu J, Gao S. Roles of Exosomes from Mesenchymal Stem Cells in Treating Osteoarthritis. Cell Reprogram. 2020;22:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 244. | Zhang R, Ma J, Han J, Zhang W. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11:6275-6289. [PubMed] |
| 245. | Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y, Wu Y, Wu M. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol. 2020;8:574223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 246. | Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, Liu Q, Zhang Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020;2020:8810813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 247. | Malekpour K, Hazrati A, Zahar M, Markov A, Zekiy AO, Navashenaq JG, Roshangar L, Ahmadi M. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes for Orthopedic Diseases Treatment. Stem Cell Rev Rep. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 248. | Riau AK, Ong HS, Yam GHF, Mehta JS. Sustained Delivery System for Stem Cell-Derived Exosomes. Front Pharmacol. 2019;10:1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |