Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1067
Peer-review started: July 6, 2020
First decision: July 30, 2020
Revised: August 13, 2020
Accepted: September 14, 2020
Article in press: September 14, 2020
Published online: October 26, 2020
Processing time: 111 Days and 17.2 Hours
Coronavirus disease 2019 (COVID-19), a pandemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), is growing at an exponential rate worldwide. Manifestations of this disease are heterogeneous; however, advanced cases often exhibit various acute respiratory distress syndrome-like symptoms, systemic inflammatory reactions, coagulopathy, and organ involvements. A common theme in advanced COVID-19 is unrestrained immune activation, classically referred to as a “cytokine storm”, as well as deficiencies in immune regulatory mechanisms such as T regulatory cells. While mesenchymal stem cells (MSCs) themselves are objects of cytokine regulation, they can secrete cytokines to modulate immune cells by inducing anti-inflammatory regulatory Treg cells, macrophages and neutrophils; and by reducing the activation of T and B cells, dendritic and nature killer cells. Consequently, they have therapeutic potential for treating severe cases of COVID-19. Here we discuss the unique ability of MSCs, to act as a “living anti-inflammatory”, which can “rebalance” the cytokine/immune responses to restore equilibrium. We also discuss current MSC trials and present different concepts for optimization of MSC therapy in patients with COVID-19 acute respiratory distress syndrome.
Core Tip: Coronavirus disease 2019 a disease caused by the severe acute respiratory syndrome coronavirus 2, is growing exponentially, with no treatments currently available. Preclinical and clinical studies have shown that mesenchymal stem cells (MSCs) work in reversing acute respiratory distress syndrome caused by other conditions such as influenza virus infection, or sepsis. In this review we discuss the unique ability of MSCs, to act as a “living anti-inflammatory”, which can “rebalance” the cytokine/immune responses to restore equilibrium.
- Citation: Lin F, Ichim TE, Pingle S, Jones LD, Kesari S, Ashili S. Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome. World J Stem Cells 2020; 12(10): 1067-1079
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1067.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1067
The severe respiratory consequences of the coronavirus disease 2019 (COVID-19) are caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with few effective treatments currently available. SARS-CoV-2 along with SARS-CoV and middle east respiratory syndrome coronavirus are coronaviruses that have caused significant human morbidity and mortality[1]. We are currently in the middle of a SARS-CoV-2 global pandemic. As of August 7, 2020, based on a Johns Hopkins University Coronavirus Resource Center Report, there have been at least 19.4 million confirmed cases worldwide, with at least 722706 deaths, and a mortality rate reaching approximately 3.7%. In the United States alone, approximately 5 million people are infected resulting in 161810 deaths and a mortality rate of 3.3%. COVID-19 infection of the lungs leads to extraordinary intensive care unit resource utilization and mortality.
One of the ways COVID-19 produces morbidity and mortality is by severely impairing lung function, causing a condition called acute respiratory distress syndrome (ARDS). This is characterized by a rapid onset of cytokine storm widespread lung inflammation, and sepsis-like conditions. Currently there is no effective anti-viral treatment for COVID-19. However, a few therapeutic strategies have been tested in the clinic or in trials for the treatment of patients with COVID-19. The anti-viral remdesivir can reduce death risk of severe COVID-19 patients as much as 62% when compared with standard care alone. Other anti-viral drugs such as but not limited to lopinavir-ritonavir, favipiravir, chloroquine and hydroxychloroquine have been proposed to treat COVID-19. Many of these anti-viral agents are currently being tested in clinical trials. Anti-inflammatory drugs such as dexamethasone were found to have beneficial effects in critically ill COVID-19 patients. Additionally, tocilizumab and siltuximab are interleukin-6 inhibitors being studied as therapeutics in critically ill patients with severe respiratory failure and elevated serum IL-6. The immunomodulatory functions of mesenchymal stem cells (MSCs) have been well documented in recent years. While MSCs themselves are objects of cytokine regulation, they can secrete cytokines to modulate immune cells by inducing anti-inflammatory regulatory Treg (T) cells, macrophages and neutrophils; and by reducing the activation of T and B cells, dendritic and nature killer (NK) cells. Consequently, they have therapeutic potential for treating severe cases of COVID-19.
Preclinical and clinical studies have shown that MSCs work in reversing ARDS caused by other conditions such as influenza virus infection, or sepsis. For example, MSCs derived from adipose[2-5], bone marrow[6-25], placental[26], amniotic membrane[27,28], umbilical cord[29-35], menstrual blood[36], and lung[37,38] origin, as well as conditioned media[39-46], have demonstrated reduction of pulmonary injury, and neutrophil accumulation. Early clinical trials[47,48] have shown safety of systemic infusions and bronchial instillations of MSCs for treating ARDS and other pulmonary complications. Most recently small studies[49,50] have also suggested that MSCs can be effective in COVID-19 ARDS; however, these approaches have not been well validated. In this review, we discuss the immunomodulatory effects of stem cells and the role of MSCs as potential therapeutic options for cytokine storm and/or ARDS in COVID-19.
SARS-CoV-2 enters host cells through the angiotensin converting enzyme 2 (ACE2) receptor. ACE2 expression was found to be high in the lung, heart, ileum, kidney and bladder[51]. ACE2 is highly expressed on the apical side of lung epithelial cells in the alveolar space, which is how SARS-CoV-2 virus can likely enter and destroy these cells. This is probably why early lung injury was often seen in the distal airway. The issue now becomes in what way does the immune system respond to viral infection in the lung. Innate immunity in the airway mainly includes epithelial cells, alveolar macrophages and dendritic cells. They fight against the virus until adaptive immunity is initiated. T cell mediated responses are initiated by antigen presentation via dendritic cells and macrophages. CD4+ T cells activate B cells to promote the production of virus-specific antibody CD8+ T cells which can kill virus infected cells. Approximately 80% percent of patients with COVID-19 are asymptomatic or experience only mild symptoms such as fever, dry cough and shortness of breath. However, some patients deteriorate quickly and develop ARDS[52]. Patients with severe diseases were reported to have increased plasma levels of proinflammatory cytokines, including IL-6, IL-8, IL-10, GM-CSF, macrophage inflammatory protein 1-alpha, and TNF-alpha[53].
Information regarding the pathological findings in COVID-19 is still limited, although several reports on this topic have been published recently. Xu et al[54] reported one case where the patient presented 15 d of symptoms. Biopsy samples from both lungs showed bilateral diffuse alveolar damage[55] with cellular fibromyxoid exudates and hyaline membrane formation, indicating ARDS in both lungs. Noteworthy is the observation that the pathological features of COVID-19 greatly resemble those seen in SARS and middle east respiratory syndrome coronavirus infections[56,57]. In addition, over-activation of T-cells was manifested by an increase of Th17 and high cytotoxicity of CD8 T cells, partially accounting for the severe immune injury in the patient.
Tian et al[58] described the early histopathological features in two patients who underwent postmortem for lung cancer but were later discovered to have had COVID-19 upon resection. The results of the lung evaluation from the two patients exhibited only nonspecific histologic changes, including edema, proteinaceous exudate, hyperplastic pneumocytes, patchy inflammation, and multinucleated giant cells with no hyaline membrane. Given that the two patients were asymptomatic from COVID-19 at the time of postmortem, they were likely only in the early stages of acute lung injury from the infection[58]. Tian et al[59] also conducted another postmortem study of four COVID-19 patients with a minimum of 15 days of symptoms, demonstrated ARDS in all biopsies.
Two COVID-19 autopsies on the lungs of a 77-year-old man revealing diffuse alveolar damage, the most common histopathologic correlation with ARDS, and on the lungs of a 42-year-old man presenting no evidence of diffuse alveolar damage/ARDS[60]. Magro et al[61] demonstrated in a report of five cases that diffuse alveolar damage was not prominent with the presentation of hyaline membranes, inflammation, and type II pneumocyte hyperplasia, all hallmarks of classic ARDS. These pulmonary findings were accompanied by significant deposits of terminal complement components C5b-9, C4d, and mannose binding lectin-associated serine protease 2, in the microvasculature, consistent with sustained, systemic activation of the alternative and lectin-based complement pathways. There was co-localization of COVID-19 spike glycoproteins with C4d and C5b-9 in the interalveolar septa and the cutaneous microvasculature of two cases examined. This indicated the pathophysiologic importance of complement in COVID-19. The results suggest that at least a subset of severe COVID-19 infection involves a catastrophic, complement-medicated thrombotic microvascular injury syndrome with sustained activation of the alternative and lectin-based cascades, possible pathways apart from virus spike protein engagement.
In general, ARDS is a common manifestation of cytokine storms and as well could be the cause of death in many COVID-19 patients, although other mechanisms may also be involved. A better understanding of COVID-19 patients’ underlying pathogenesis will pave the way for formulating a timely therapeutic strategy to reduce mortality.
MSCs are fibroblast-like and multipotent stromal cells. Human MSCs are positive for a number of cell surface markers including CD73, CD44, CD90, and CD105 and negative for the hematopoietic markers of CD34, CD45 and HLA-DR[62]. MSCs are traditionally isolated from bone marrow, and a variety of fetal, neonatal and adult tissues, including cord blood, peripheral blood, fetal liver and lung, adipose tissue, compact bone, dental pulp, dermis, endometrial, human islet, adult brain, skeletal muscle, amniotic fluid, synovium, and the circulatory system[63-65]. MSCs can differentiate into a variety of cell types of mesodermal origin, including osteoblasts, chondrocytes, cardiomyocytes, neural cells, smooth muscle cells and adipocytes[62,66-68]. MSCs are likely the only stem cell type that possesses both regenerative and immunomodulatory capabilities. Consequently, they have been used widely in the treatment of many degenerative and inflammatory diseases.
One property that greatly increases the value of MSCs in therapeutic applications is their ability to modulate immune responses. MSCs can exert their immunomodulatory function by producing many molecules having immunomodulatory effects, these include prostaglandin E2 (PGE2)[69], nitric oxide[70], indolamine 2,3-dioxigenase (IDO)[71], transforming growth factor beta[72,73], IL-6[74,75], hemoxygenase-1[76], leukocyte inhibitory factor[77], HLAG5 and chemokines[78], PDL1/2[79] and other surface markers-FasL[80]. MSCs can escape the immune system because bone marrow derived MSCs (BM-MSCs) are not recognized by NK cells as they lack expression of HLA Class I surface markers. They also lack expression of HLA Class II antigens, which is desirable for transplantation applications.
The immunosuppressive activity of MSCs is well described, with recent reports providing some mechanistic insights into key soluble factors and receptors. Programmed death-ligand 1/CD274 also known as B7 Homolog 1 (B7-H1) has been shown to be expressed in cultured MSCs and is strongly upregulated following IFN-γ stimulation. Combination therapy using rapamycin and MSCs induced immune tolerance to allografts, but monoclonal antibodies against B7-H1 were shown to abrogate this tolerance leading to allograft rejection[81]. The immunomodulatory effects of MSCs were mediated in part through upregulation of regulatory immune cells including CD4+CD25+FoxP3+ T cells[82,83] and tolerogenic dendritic cells[84] and a decrease in alloantibody levels. MSCs that expressed B7H1 may also induce the apoptosis of activated T-cells as a co-culture of CD4+CD25- T cells with MSCs resulting in significant upregulation of programmed cell death-1 receptor (PD-1) on activated T cells[85]. Similar results were reported by Chinnadurai et al[86] who further examined the role of IFN-γ in the “licensing” of MSCs to inhibit the proliferation of activated T cells[86]. Both MSCs and IFN-γ licensed MSCs inhibited T-cell proliferation; however, only IFN-γ licensed MSCs significantly inhibited Th1 cytokine (IFN-γ, TNFα and IL-2) production as well as T-cell degranulation. This IFN-γ licensed MSCs inhibitory effect on T-cells is thought to be dependent on IDO[71]; however, Chinnadurai showed that MSC IDO catalytic function is dispensable with regard to MSC driven T-cell inhibition. Chinnadurai et al[86] identified the B7-H1 PD1 pathways as essential effectors in blocking T-cell function. Further complexity was also suggested by a recent report that IFN-γ treatment of MSCs upregulated HLA-DR /Class II MHC after 48 h, and MSCs ability to inhibit T cells through B7-H1 was dependent upon the presence of HLA-DR[87].
MSCs express the adhesion molecules VCAM-1 and ICAM-1, which allow T-lymphocytes to adhere to their surface. Subsequently MSCs can affect them by molecules which have a short half-life. Their effect is in the immediate vicinity of the cell[70]. Examples of such molecules include nitric oxide, PGE2, HGF[88], and activation of receptor PD-1. MSCs reduce T cell proliferation between G0 and G1 cell cycle phases G[89], and decrease the expression of IFNγ of Th1 cells while increasing the expression of IL-4 of Th2 cells[90]. MSCs also inhibit the proliferation of B-lymphocytes between G0 and G1 cell cycle phases.
A novel mechanism for MSC-induced immunosuppression was recently proposed by Obermajer and colleagues who showed that cells of the Th17 type, predominantly associated with the rejection of allogeneic solid organ grafts, can be directly converted into a regulatory T cell type[91]. The induction of Tregs was preceded by development of a CD11b(hi)Gr1(int) myeloid-derived immunosuppressive cell-mediated Th17. They identified retinoic acid receptor-related orphan receptor γ as a common factor in the differentiation of T and Th17 cells. The identification of a specific subset of T cells IL-17A+Foxp3+ double-positive and ex-IL-17- producing IL-17A-Foxp3+ in this paper argues for direct conversion as the mechanism for MSC-mediated immuno-tolerance. This proposed mechanism where MSC-induced myeloid-derived immunosuppressive cells act as mediator for immune tolerance without complete immunosuppression may have significant implications for therapeutic applications.
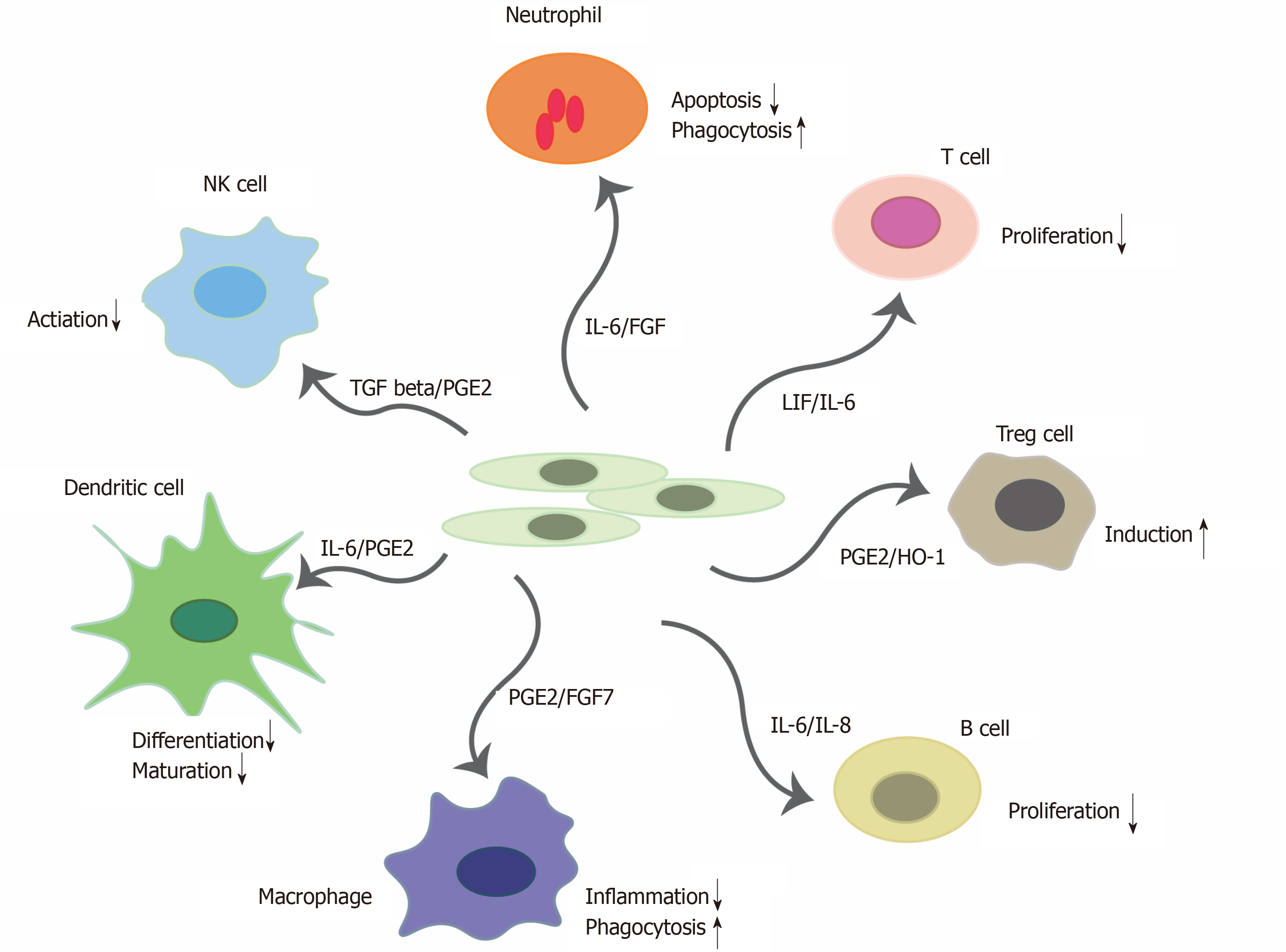
MSCs have an effect on macrophages, neutrophils, NK cells, mast cells and dendritic cells in innate immunity and effector T cells, regulatory T cells, and B cells in adaptive immunity illustrated in Figure 1. In severe COVID-19 patients, their immune responses to SARS-CoV-2 infection are usually over-activated. MSCs are able to exert their anti-inflammatory effect by regulating immune cells and balancing the immune responses. Furthermore, MSCs can migrate to the site of injury, where they polarize through GE2 macrophages into phenotype-2 which is characterized by an anti-inflammatory effect[92,93]. Further, PGE2 inhibits the ability of mast cells to degranulate and produce TNF-α. Proliferation and cytotoxic activity of NK cells are inhibited by PGE2 and IDO. MSCs also reduce the expression of NK cell receptors-NKG2D, NKp44 and NKp30[94], MSCs inhibit respiratory flare and apoptosis of neutrophils by production of cytokines IL-6 and IL-8[95]. Differentiation and expression of dendritic cell surface markers is inhibited by IL-6 and PGE2 of MSCs[96]. The immuno-suppressive effects of MSCs also depend on IL-10, but it is not certain whether they produce it alone, or only stimulate other cells to produce it[97,98].
MSCs have been shown to possess a comprehensive and powerful immuno-modulatory function to suppress excessive activation of the immune system, thus promoting endogenous repair by improving the microenvironment. There have been 13 MSCs therapies approved for treating a number of conditions (Table 1) outside of the United States, mainly in the EU, Japan, South Korea and India. Among the conditions, two adipose tissue derived MSC products, Alofisel® and Cupistem®, have been used for complex perianal fistulas in Crohn’s disease. The underlying mechanism of action is the MSC immunomodulatory and anti-inflammatory effects at the inflammation sites. Specifically MSCs impair proliferation of activated lymphocytes and reduce the inflammatory cytokines. Two BM-MSC products, Prochymal® and Temcell® HS, have been used for treating GvHD, due to MSCs immunomodulatory effects.
| Product name | Source | Autologous/Allogeneic | Indication | Company/Country | |
| 1 | Alofisel | Adipose tissue-derived stem cells | Allogeneic | Complex perianal fistuals in Crohn’s disease | TiGenix NV/Takeda PharmaceuticalEU |
| 2 | Chondrocytes-T-Ortho-ACI | Chondrocyte | Autologous | Cartilage damage, lesions and defects | Ortho Cell, Australia |
| 3 | Spherox | Chondrocyte | Autologous | Symptomatic articular cartilage defects | CO.DON AG, Germany and EU |
| 4 | Ossgrow | BM-MSCs | Autologous | Avascular necrosis | Regrow, India |
| 5 | Stempeucel | BM-MSCs | Allogeneic | CLI | Stempeutics, India |
| 6 | Porchymal | BM-MSCs | Allogeneic | GvHD in children | Osiris Therapeutics, Canada |
| 7 | Temcell HS | BM-MSCs | Allogeneic | GvHD | JCR Pharmaceuticals, Japan |
| 8 | NeuroNata-R | BM-MSCs | Autologous | Lou Gehrig’s disease, or ALS | Corestem, Korea |
| 9 | Cupistem | AT-MSCs | Autologous | Crohn’s fistula | Anterogen, Korea |
| 10 | Cartistem, | UC-blood-derived MSCs | Allogeneic | Damaged cartilage | Medipost Inc., Korea |
| 11 | Cellgram-AMI | BM-MSCs | Autologous | Acute myocardial infarction | Pharmicell, Korea |
| 12 | AstroStem | AT-MSCs | Autologous | Alzheimer’s disease | Nature cell, Korea |
| 13 | Stemilac | BM-MSCs | Autologous | Alzheimer’s disease | Nipro and Sapporo Medical University, Japan |
Preclinical study has demonstrated that MSCs can inhibit the progress of acute inflammation in the lungs and alleviate symptoms of respiratory distress[99]. The feasibility of utilizing MSCs for the treatment of ARDS has been demonstrated in animal models and extracorporeal lung models[100]. MSCs of adipose, bone marrow, placental, amniotic membrane, umbilical cord, menstrual blood, and lung, origin, as well as conditioned media with secreted exosomes, have demonstrated a reduction of pulmonary injury and neutrophil accumulation. In a recent study using a sheep model of ARDS[9], both endobronchial and intravenous administration of bone marrow-derived multipotent adult progenitor cells were effective for the treatment of ARDS.
Additionally, an analysis of 342 systemic infusions and 57 bronchial instillations (204 recipients) of cells of various origins for ARDS and other pulmonary issues demonstrated safety in early human clinical trials[47]. Recently, a study involving two patients with severe refractory ARDS, both showed improvement[99]. Both patients received 2 × 106 cells per kilogram of body weight. Subsequently, each of the patients improved with resolution of respiratory, hemodynamic, and multiorgan failure. In parallel, a decrease was seen in multiple pulmonary and systemic markers of inflammation, including epithelial apoptosis, alveolar-capillary fluid leakage, proinflammatory cytokines, microRNAs, and chemokines. In vitro studies of the MSCs demonstrated a broad anti-inflammatory capacity, including suppression of T-cell responses and induction of regulatory phenotypes in T cells, monocytes, and neutrophils. Some of these in vitro potency assessments correlated with, and were relevant to, the observed in vivo actions.
Currently, drugs alone or in combination with other therapeutic approaches have not afforded a cure; however a number of investigational drugs in clinical trials, including antivirals such as chloroquine/hydroxychloroquine, remdesivir, immune-based therapies and adjunctive therapies have shown promise, particularly in mitigating certain systemic markers according to NIH COVID-19 Guidelines. Potential antiviral drugs: remdesivir, chloroquine or hydroxychloroquine, hydroxychloroquine plus azithromycin, lopinavir/ritonavir and other HIV protease inhibitors. Immune-based therapy under evaluation: Convalescent plasma, Immunoglobulins: SAR-CoV-2-specific and non-SAR-CoV-2 specific, MSCs, Corticosteroids, Interferons alpha and beta, IL-1 and IL-6 inhibitors, Kinase inhibitors: Bruton’s tyrosine kinase inhibitors and Janus kinase inhibitors; Adjunctive therapy: antithrombotic therapy, vitamin C and vitamin D, zinc supplementation.
To date, there are over 50 clinical trials using MSCs to treat COVID-19 patients based on the registration in clinicaltrial.gov website, we listed the most relevant studies in Table 2. Umbilical cord MSCs (UC-MSCs), BM-MSCs, AT-MSCs and other MSCs, as well as exosomes from MSCs are used in clinical trials, among them UC-MSCs are the most desirable for treating severely compromised COVID-19 patients due to its rich and extensive source of stem cells, scalable expansion capability, and ability to be allogeneic as low MHC-I expression[49]. The dose and delivery times are also varied in different trials. Recent reviews have described the potential for and rationale of using different types of MSCs for treating severe COVID-19 patients to protect alveolar epithelial cells, to reclaim the pulmonary microenvironment, to induce anti-inflammatory macrophages, regulatory T and B cells, and regulatory dendritic cells. In addition, MSCs can inactivate T cells in order to prevent cytokine storm, prevent pulmonary fibrosis and cure lung dysfunction[101-104]. Details of MSCs clinical trials for COVID-19 have also been discussed in other reviews[101,104-106].
| NCT number | Cell type | Autologous | Phase | Sponsor |
| NCT04490486 | UC-MSCs | Allogeneic | I | Joshua M Hare, United States |
| NCT04456361 | UC-MSCs | Allogeneic | I | Instituto de Medicina Regenerativa, Mexico |
| NCT04313322 | UC -MSCs | Allogeneic | I | Stem Cells, Arabia |
| NCT04288102 | MSCs | NA | II | Beijing 302 Hospital |
| NCT04346368 | BM-MSCs | NA | I/II | Guangzhou Institute of Respiratory Disease |
| NCT04366323 | Adipose-derived MSCs | Allogeneic | I/II | Andalusian Network for Design and Translation of Advanced Therapies |
| NCT04273646 | UC-MSCs | Allogeneic | I | Wuhan Union Hospital, China |
| NCT04349631 | Adipose-derived MSCs | Autologous | II | Hope Biosciences |
| NCT04339660 | UC-MSCs | Allogeneic | I/II | Puren Hospital Affiliated to Wuhan University of Science and Technology |
| NCT04366063 | MSCs | NA | II/III | Royan Institute |
| NCT04352803 | Adipose-derived MSCs | Autologous | I | Regeneris Medical |
| NCT04355728 | UC-MSCs | Allogeneic | I/II | Camillo Ricordi |
| NCT04366271 | UC-MSCs | Allogeneic | II | Hospital Infantil Universitario Niño Jesús, Madrid, Spain |
| NCT04348461 | Adipose-derived MSCs | Allogeneic | I | Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz |
| NCT04345601 | BM-MSCs | Allogeneic | I | Baylor College of Medicine |
| NCT03042143 | UC-MSCs (CD362 enriched) | Allogeneic | I/II | Belfast Health and Social Care Trust |
| NCT04361942 | MSCs | Allogeneic | II | Red de Terapia Celular |
| NCT04269525 | UC-MSCs | Allogeneic | II | Zhi-Yong Peng, Hospital |
| NCT04333368 | UC-MSCs | Allogeneic | I | Assistance Publique - Hôpitaux de Paris |
| NCT04299152 | Cord blood stem cells (CB-SC) | Allogeneic | II | Tianhe Stem Cell Biotechnologies Inc. |
| NCT04341610 | Adipose-derived MSCs | Allogeneic | I/II | Rigshospitalet, Denmark |
| NCT04276987 | Adipose MSC-derived exosomes (inhalation) | Allogeneic | I | Ruijin Hospital |
| NCT03857841 | BM-MSC derived extracellular vesicles (UNEX-42) | Allogeneic | I | United Therapeutics |
MSCs have been used effectively to treat patients with COVID-19 in recent reports[50,107,108]. The underlying processes involve preventing the cytokine storm from occurring as well as reversing the cytokine storm in compromised patients. A total of seven patients with COVID-19 were enrolled in the study[50], the results have shown that MSCs significantly improved the functional outcome without observed adverse effects. The pulmonary function and symptoms of these patients were significantly improved in two days after MSC transplantation. Among them, two common and one severe patient recovered and were discharged within 10 d after the treatment. Compared to the placebo control group, the level of TNF-alpha was significantly decreased, and IL-10 increased in the MSCs treatment group. The gene profile showed MSCs were ACE2- and TMPRSS2- which indicate MSCs are free from COVID-19 infection. In another case report[107], UC-MSCs were infused into a severely compromised COVID-19 patient. The pulmonary function and symptoms of the patient were significantly improved in 2 d after UC-MSCs transplantation. The patient recovered and was discharged in 7 d after treatment. The percentage and counts of lymphocyte subsets (CD3, CD4, and CD8 T cell) were increased, and the level of IL-6, TNF-α, and C-reactive protein was shown to have significantly decreased after UC-MSCs treatment. Guo et al[108] reported a 31-patient trial with UC-MSCs infusion. After the first infusion of UC-MSCs, the SARS-CoV-2 PCR results of 30 patients (96.8%) became negative after a mean time of 10.7 d (SD, 4.2 d). Laboratory parameters tended to improve after UC-MSCs therapy compared to the status before treatment, including elevated lymphocyte count, decreased C-reactive protein and IL-6 levels. Thus far, the intravenous transplantation of MSCs has been shown to be effective for the treatment of patients with COVID-19 pneumonia, especially for the patients in critical condition.
Exosomes derived from MSCs have been studied in clinical trials for treating severely compromised COVID-19 patients[104,109,110]. Exosomes (ExoFloTM) derived from allogeneic bone marrow MSCs in a single 15 mL dose were evaluated in a 24-patient trial[110] for both safety and efficacy from days 1 to 14 post-treatment. No adverse events were observed; a survival rate of 83% was observed; 71 patents recovered, 13% remained critically ill though stable, and 16% patients expired for reasons unrelated to the treatment. Overall, after one treatment, patients’ clinical status and oxygenation improved with an average pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) increase of 192%. Laboratory values revealed significant improvements in absolute neutrophil count and lymphopenia with average CD3+, CD4+, and CD8+ lymphocyte counts increasing by 46%, 45%, and 46%, respectively. Likewise, acute phase reactants declined, with mean C-reactive protein, ferritin, and D-dimer reduction of 77%, 43%, and 42%, respectively. The study demonstrated the excellent safety profile and capacity to restore oxygenation, downregulate cytokine storm, and reconstitute immunity. Exosome derived from MSCs is a promising therapeutic candidate for severely compromised COVID-19 patients.
The social burden of COVID-19 is growing with the global pandemic. However, there is no effective or curative therapy for COVID-19, and preventive vaccines, other than the vaccine recently approved in Russia, but for which there is limited information, are still under development and will not be available until next year. The most recent clinical trials with MSCs may fulfill the unmet medical need of COVID-19, to reduce the related ARDS and cytokine storm. There are several issues that need to be addressed in order to move forward: dose, delivery times, type of MSCs, efficacy and cost-effectiveness. An understanding of all facets of MSCs and pathomechanism of COVID-19 is necessary to fully translate the MSCs therapy into a meaningful treatment for COVID-19. The next therapeutic strategies may focus on a combination approach using two or more types of MSCs, certain type of MSCs, and immune-based therapies or antiviral therapies to achieve maximal therapeutic efficacy.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Figueiredo CS, Li ZJ S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Guarner J. Three Emerging Coronaviruses in Two Decades. Am J Clin Pathol. 2020;153:420-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 2. | Jung YJ, Park YY, Huh JW, Hong SB. The effect of human adipose-derived stem cells on lipopolysaccharide-induced acute respiratory distress syndrome in mice. Ann Transl Med. 2019;7:674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Chen CH, Chen YL, Sung PH, Sun CK, Chen KH, Chen YL, Huang TH, Lu HI, Lee FY, Sheu JJ, Chung SY, Lee MS, Yip HK. Effective protection against acute respiratory distress syndrome/sepsis injury by combined adipose-derived mesenchymal stem cells and preactivated disaggregated platelets. Oncotarget. 2017;8:82415-82429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Lu H, Cook T, Poirier C, Merfeld-Clauss S, Petrache I, March KL, Bogatcheva NV. Pulmonary Retention of Adipose Stromal Cells Following Intravenous Delivery Is Markedly Altered in the Presence of ARDS. Cell Transplant. 2016;25:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 6. | Lu Z, Chang W, Meng S, Xu X, Xie J, Guo F, Yang Y, Qiu H, Liu L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 2019;10:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 7. | Silva JD, de Castro LL, Braga CL, Oliveira GP, Trivelin SA, Barbosa-Junior CM, Morales MM, Dos Santos CC, Weiss DJ, Lopes-Pacheco M, Cruz FF, Rocco PRM. Mesenchymal Stromal Cells Are More Effective Than Their Extracellular Vesicles at Reducing Lung Injury Regardless of Acute Respiratory Distress Syndrome Etiology. Stem Cells Int. 2019;2019:8262849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Xu AL, Rodriguez LA 2nd, Walker KP 3rd, Mohammadipoor A, Kamucheka RM, Cancio LC, Batchinsky AI, Antebi B. Mesenchymal Stem Cells Reconditioned in Their Own Serum Exhibit Augmented Therapeutic Properties in the Setting of Acute Respiratory Distress Syndrome. Stem Cells Transl Med. 2019;8:1092-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Cardenes N, Aranda-Valderrama P, Carney JP, Sellares Torres J, Alvarez D, Kocyildirim E, Wolfram Smith JA, Ting AE, Lagazzi L, Yu Z, Mason S, Santos E, Lopresti BJ, Rojas M. Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 2019;6:e000308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Li L, Dong L, Zhang J, Gao F, Hui J, Yan J. Mesenchymal stem cells with downregulated Hippo signaling attenuate lung injury in mice with lipopolysaccharideinduced acute respiratory distress syndrome. Int J Mol Med. 2019;43:1241-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Mokhber Dezfouli MR, Jabbari Fakhr M, Sadeghian Chaleshtori S, Dehghan MM, Vajhi A, Mokhtari R. Intrapulmonary autologous transplant of bone marrow-derived mesenchymal stromal cells improves lipopolysaccharide-induced acute respiratory distress syndrome in rabbit. Crit Care. 2018;22:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Schwede M, Wilfong EM, Zemans RL, Lee PJ, Dos Santos C, Fang X, Matthay MA. Effects of bone marrow-derived mesenchymal stromal cells on gene expression in human alveolar type II cells exposed to TNF-α, IL-1β, and IFN-γ. Physiol Rep. 2018;6:e13831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Masterson C, Devaney J, Horie S, O'Flynn L, Deedigan L, Elliman S, Barry F, O'Brien T, O'Toole D, Laffey JG. Syndecan-2-positive, Bone Marrow-derived Human Mesenchymal Stromal Cells Attenuate Bacterial-induced Acute Lung Injury and Enhance Resolution of Ventilator-induced Lung Injury in Rats. Anesthesiology. 2018;129:502-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Park J, Jeong S, Park K, Yang K, Shin S. Expression profile of microRNAs following bone marrow-derived mesenchymal stem cell treatment in lipopolysaccharide-induced acute lung injury. Exp Ther Med. 2018;15:5495-5502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Pedrazza L, Cunha AA, Luft C, Nunes NK, Schimitz F, Gassen RB, Breda RV, Donadio MV, de Souza Wyse AT, Pitrez PMC, Rosa JL, de Oliveira JR. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol. 2017;232:3552-3564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Hu S, Xu X, Li J, Liu A, Han J, Liu S, Liu L, Qiu H. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediators Inflamm. 2016;2016:2347938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Moodley Y, Sturm M, Shaw K, Shimbori C, Tan DB, Kolb M, Graham R. Human mesenchymal stem cells attenuate early damage in a ventilated pig model of acute lung injury. Stem Cell Res. 2016;17:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Hayes M, Curley GF, Masterson C, Devaney J, O'Toole D, Laffey JG. Mesenchymal stromal cells are more effective than the MSCs secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Med Exp. 2015;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015;192:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 20. | Hao Q, Zhu YG, Monsel A, Gennai S, Lee T, Xu F, Lee JW. Study of Bone Marrow and Embryonic Stem Cell-Derived Human Mesenchymal Stem Cells for Treatment of Escherichia coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells Transl Med. 2015;4:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Devaney J, Horie S, Masterson C, Elliman S, Barry F, O'Brien T, Curley GF, O'Toole D, Laffey JG. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Asmussen S, Ito H, Traber DL, Lee JW, Cox RA, Hawkins HK, McAuley DF, McKenna DH, Traber LD, Zhuo H, Wilson J, Herndon DN, Prough DS, Liu KD, Matthay MA, Enkhbaatar P. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Shalaby SM, El-Shal AS, Abd-Allah SH, Selim AO, Selim SA, Gouda ZA, Abd El Motteleb DM, Zanfaly HE, El-Assar HM, Abdelazim S. Mesenchymal stromal cell injection protects against oxidative stress in Escherichia coli-induced acute lung injury in mice. Cytotherapy. 2014;16:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Bustos ML, Huleihel L, Meyer EM, Donnenberg AD, Donnenberg VS, Sciurba JD, Mroz L, McVerry BJ, Ellis BM, Kaminski N, Rojas M. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl Med. 2013;2:884-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Rojas M, Parker RE, Thorn N, Corredor C, Iyer SS, Bueno M, Mroz L, Cardenes N, Mora AL, Stecenko AA, Brigham KL. Infusion of freshly isolated autologous bone marrow derived mononuclear cells prevents endotoxin-induced lung injury in an ex-vivo perfused swine model. Stem Cell Res Ther. 2013;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yan X, Fu X, Jia Y, Ma X, Tao J, Yang T, Ma H, Liang X, Liu X, Yang J, Wei J. Nrf2/Keap1/ARE Signaling Mediated an Antioxidative Protection of Human Placental Mesenchymal Stem Cells of Fetal Origin in Alveolar Epithelial Cells. Oxid Med Cell Longev. 2019;2019:2654910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Cui P, Xin H, Yao Y, Xiao S, Zhu F, Gong Z, Tang Z, Zhan Q, Qin W, Lai Y, Li X, Tong Y, Xia Z. Human amnion-derived mesenchymal stem cells alleviate lung injury induced by white smoke inhalation in rats. Stem Cell Res Ther. 2018;9:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Zhang S, Jiang W, Ma L, Liu Y, Zhang X, Wang S. Nrf2 transfection enhances the efficacy of human amniotic mesenchymal stem cells to repair lung injury induced by lipopolysaccharide. J Cell Biochem. 2018;119:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Huang Z, Liu H, Zhang X, Wen G, Zhu C, Zhao Y, Niu W, Qin Y, Chen H, Bai C, Liu G. Transcriptomic analysis of lung tissues after hUC-MSCs and FTY720 treatment of lipopolysaccharide-induced acute lung injury in mouse models. Int Immunopharmacol. 2018;63:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Xuan YY, Wu YY, Xie YL, Chu JG, Li GX, Wang LP. Human Mesenchymal Stem/Stromal Cells From Human Umbilical Cord Ameliorate Acute Respiratory Distress Syndrome in Rats: Factors to Consider. Crit Care Med. 2017;45:e736-e737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Lee FY, Chen KH, Wallace CG, Sung PH, Sheu JJ, Chung SY, Chen YL, Lu HI, Ko SF, Sun CK, Chiang HJ, Chang HW, Lee MS, Yip HK. Xenogeneic human umbilical cord-derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget. 2017;8:45626-45642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Zhu H, Xiong Y, Xia Y, Zhang R, Tian D, Wang T, Dai J, Wang L, Yao H, Jiang H, Yang K, Liu E, Shi Y, Fu Z, Gao L, Zou L. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice. Sci Rep. 2017;7:39889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Curley GF, Jerkic M, Dixon S, Hogan G, Masterson C, O'Toole D, Devaney J, Laffey JG. Cryopreserved, Xeno-Free Human Umbilical Cord Mesenchymal Stromal Cells Reduce Lung Injury Severity and Bacterial Burden in Rodent Escherichia coli-Induced Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45:e202-e212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Chang Y, Park SH, Huh JW, Lim CM, Koh Y, Hong SB. Intratracheal administration of umbilical cord blood-derived mesenchymal stem cells in a patient with acute respiratory distress syndrome. J Korean Med Sci. 2014;29:438-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 36. | Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Wang L, Shi M, Tong L, Wang J, Ji S, Bi J, Chen C, Jiang J, Bai C, Zhou J, Song Y. Lung-Resident Mesenchymal Stem Cells Promote Repair of LPS-Induced Acute Lung Injury via Regulating the Balance of Regulatory T cells and Th17 cells. Inflammation. 2019;42:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Silva JD, Lopes-Pacheco M, Paz AHR, Cruz FF, Melo EB, de Oliveira MV, Xisto DG, Capelozzi VL, Morales MM, Pelosi P, Cirne-Lima E, Rocco PRM. Mesenchymal Stem Cells From Bone Marrow, Adipose Tissue, and Lung Tissue Differentially Mitigate Lung and Distal Organ Damage in Experimental Acute Respiratory Distress Syndrome. Crit Care Med. 2018;46:e132-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Su VY, Lin CS, Hung SC, Yang KY. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Mohammadipoor A, Antebi B, Batchinsky AI, Cancio LC. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir Res. 2018;19:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 41. | Lee JH, Park J, Lee JW. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Abreu SC, Weiss DJ, Rocco PR. Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases? Stem Cell Res Ther. 2016;7:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 43. | Monsel A, Zhu YG, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther. 2016;16:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 44. | Liu FB, Lin Q, Liu ZW. A study on the role of apoptotic human umbilical cord mesenchymal stem cells in bleomycin-induced acute lung injury in rat models. Eur Rev Med Pharmacol Sci. 2016;20:969-982. [PubMed] |
| 45. | Chen J, Li Y, Hao H, Li C, Du Y, Hu Y, Li J, Liang Z, Li C, Liu J, Chen L. Mesenchymal Stem Cell Conditioned Medium Promotes Proliferation and Migration of Alveolar Epithelial Cells under Septic Conditions In Vitro via the JNK-P38 Signaling Pathway. Cell Physiol Biochem. 2015;37:1830-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967-L977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 47. | Zhao R, Su Z, Wu J, Ji HL. Serious adverse events of cell therapy for respiratory diseases: a systematic review and meta-analysis. Oncotarget. 2017;8:30511-30523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Cheng SL, Lin CH, Yao CL. Mesenchymal Stem Cell Administration in Patients with Chronic Obstructive Pulmonary Disease: State of the Science. Stem Cells Int. 2017;2017:8916570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020;23:E71-E83. [PubMed] |
| 50. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 51. | Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1933] [Cited by in RCA: 2216] [Article Influence: 443.2] [Reference Citation Analysis (0)] |
| 52. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30121] [Article Influence: 6024.2] [Reference Citation Analysis (3)] |
| 53. | Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1187] [Article Influence: 237.4] [Reference Citation Analysis (0)] |
| 54. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5785] [Article Influence: 1157.0] [Reference Citation Analysis (2)] |
| 55. | Colamartino ABL, Lemieux W, Bifsha P, Nicoletti S, Chakravarti N, Sanz J, Roméro H, Selleri S, Béland K, Guiot M, Tremblay-Laganière C, Dicaire R, Barreiro L, Lee DA, Verhoeyen E, Haddad E. Efficient and Robust NK-Cell Transduction With Baboon Envelope Pseudotyped Lentivector. Front Immunol. 2019;10:2873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 56. | Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, Lu Y, Wu D, He L, Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 576] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 57. | Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 58. | Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15:700-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 927] [Cited by in RCA: 1000] [Article Influence: 200.0] [Reference Citation Analysis (0)] |
| 59. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 60. | Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, United States. Am J Clin Pathol. 2020;153:725-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 607] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 61. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1635] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |
| 62. | Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 749] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 63. | Jiang B, Yan L, Wang X, Li E, Murphy K, Vaccaro K, Li Y, Xu RH. Concise Review: Mesenchymal Stem Cells Derived from Human Pluripotent Cells, an Unlimited and Quality-Controllable Source for Therapeutic Applications. Stem Cells. 2019;37:572-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 913] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 65. | Carlotti F, Zaldumbide A, Loomans CJ, van Rossenberg E, Engelse M, de Koning EJ, Hoeben RC. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets. 2010;2:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Nandy SB, Mohanty S, Singh M, Behari M, Airan B. Fibroblast Growth Factor-2 alone as an efficient inducer for differentiation of human bone marrow mesenchymal stem cells into dopaminergic neurons. J Biomed Sci. 2014;21:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Armiñán A, Gandía C, Bartual M, García-Verdugo JM, Lledó E, Mirabet V, Llop M, Barea J, Montero JA, Sepúlveda P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 68. | Liu J, Wang Y, Wu Y, Ni B, Liang Z. Sodium butyrate promotes the differentiation of rat bone marrow mesenchymal stem cells to smooth muscle cells through histone acetylation. PLoS One. 2014;9:e116183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSCs-derived prostaglandin E2. Blood. 2009;113:6576-6583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 502] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 70. | Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 496] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 71. | Kriegsmann K, Kriegsmann M, Cremer M, Schmitt M, Dreger P, Goldschmidt H, Müller-Tidow C, Hundemer M. Cell-based immunotherapy approaches for multiple myeloma. Br J Cancer. 2019;120:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885-5894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 73. | Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 683] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 74. | Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 474] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 75. | Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, Bron D, Toungouz M, Martiat P, Lagneaux L. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11:570-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 76. | Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691-3694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 77. | Nasef A, Mazurier C, Bouchet S, François S, Chapel A, Thierry D, Gorin NC, Fouillard L. Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Rizzo R, Campioni D, Stignani M, Melchiorri L, Bagnara GP, Bonsi L, Alviano F, Lanzoni G, Moretti S, Cuneo A, Lanza F, Baricordi OR. A functional role for soluble HLA-G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy. 2008;10:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Tyndall A, van Laar JM. Stem cell transplantation and mesenchymal cells to treat autoimmune diseases. Presse Med. 2016;45:e159-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 81. | Wang H, Qi F, Dai X, Tian W, Liu T, Han H, Zhang B, Li H, Zhang Z, Du C. Requirement of B7-H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transpl Immunol. 2014;31:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Gazdic M, Markovic BS, Arsenijevic A, Jovicic N, Acovic A, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N, Lukic ML, Volarevic V. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl. 2018;24:687-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 84. | Mohammadpour H, Pourfathollah AA, Zarif MN, Tahoori MT. TNF-α modulates the immunosuppressive effects of MSCs on dendritic cells and T cells. Int Immunopharmacol. 2015;28:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Yan Z, Zhuansun Y, Liu G, Chen R, Li J, Ran P. Mesenchymal stem cells suppress T cells by inducing apoptosis and through PD-1/B7-H1 interactions. Immunol Lett. 2014;162:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 86. | Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 87. | Jang IK, Yoon HH, Yang MS, Lee JE, Lee DH, Lee MW, Kim DS, Park JE. B7-H1 inhibits T cell proliferation through MHC class II in human mesenchymal stem cells. Transplant Proc. 2014;46:1638-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2363] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 89. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 835] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 90. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3279] [Article Influence: 156.1] [Reference Citation Analysis (0)] |
| 91. | Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, Dahlke MH. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J Immunol. 2014;193:4988-4999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 92. | Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med. 2017;6:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 93. | Takizawa N, Okubo N, Kamo M, Chosa N, Mikami T, Suzuki K, Yokota S, Ibi M, Ohtsuka M, Taira M, Yaegashi T, Ishisaki A, Kyakumoto S. Bone marrow-derived mesenchymal stem cells propagate immunosuppressive/anti-inflammatory macrophages in cell-to-cell contact-independent and -dependent manners under hypoxic culture. Exp Cell Res. 2017;358:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 94. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 95. | Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 96. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 985] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 97. | Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, Rosdiana I, Munir D. The Role of TNF-α induced MSCs on Suppressive Inflammation by Increasing TGF-β and IL-10. Open Access Maced J Med Sci. 2018;6:1779-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 98. | Xiao S, Huang G, Wei Z, Nie K, Liu Z, Deng C, Wang D. IL-10 Gene-Modified Human Amniotic Mesenchymal Stem Cells Augment Regenerative Wound Healing by Multiple Synergistic Effects. Stem Cells Int. 2019;2019:9158016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, Jitschin R, Rodin S, Corbascio M, El Andaloussi S, Wiklander OP, Nordin JZ, Skog J, Romain C, Koestler T, Hellgren-Johansson L, Schiller P, Joachimsson PO, Hägglund H, Mattsson M, Lehtiö J, Faridani OR, Sandberg R, Korsgren O, Krampera M, Weiss DJ, Grinnemo KH, Le Blanc K. In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med. 2015;4:1199-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 100. | Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2636] [Cited by in RCA: 2797] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 101. | Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep. 2020;16:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 102. | Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 103. | Rogers CJ, Harman RJ, Bunnell BA, Schreiber MA, Xiang C, Wang FS, Santidrian AF, Minev BR. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 104. | Tsuchiya A, Takeuchi S, Iwasawa T, Kumagai M, Sato T, Motegi S, Ishii Y, Koseki Y, Tomiyoshi K, Natsui K, Takeda N, Yoshida Y, Yamazaki F, Kojima Y, Watanabe Y, Kimura N, Tominaga K, Kamimura H, Takamura M, Terai S. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020;40:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 105. | Raza SS, Seth P, Khan MA. 'Primed' Mesenchymal Stem Cells: a Potential Novel Therapeutic for COVID19 Patients. Stem Cell Rev Rep. 2020;:. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Qin H, Zhao A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 107. | Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M, Wu T, Liu D, Tian S, Tian H, Chen S, Zhou C. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 108. | Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 109. | O'Driscoll L. Extracellular vesicles from mesenchymal stem cells as a Covid-19 treatment. Drug Discov Today. 2020;25:1124-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 515] [Article Influence: 103.0] [Reference Citation Analysis (0)] |