Copyright
©The Author(s) 2015.
World J Stem Cells. May 26, 2015; 7(4): 745-756
Published online May 26, 2015. doi: 10.4252/wjsc.v7.i4.745
Published online May 26, 2015. doi: 10.4252/wjsc.v7.i4.745
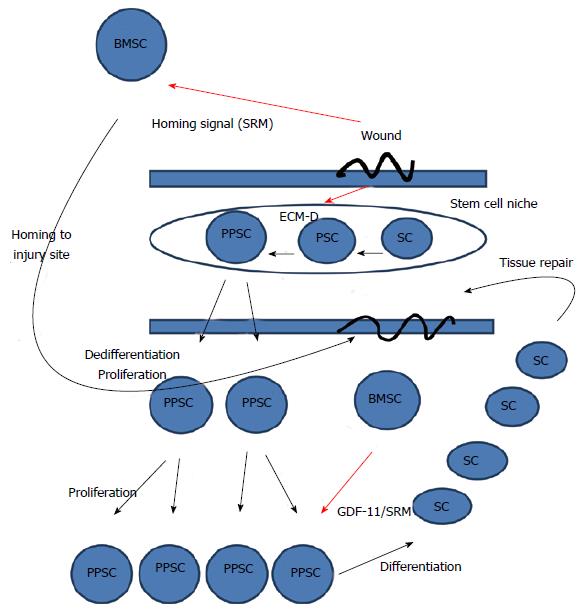
Figure 1 General model of wound healing.
The wounded state sends a homing signal to bone marrow stem cells and disrupts the ECM. Disruption of the ECM will shift the dynamic transition of potency towards dedifferentiation and the more pluripotent state. The more pluripotent state will cause the cells to proliferate. After proliferation, the migration of bone marrow stem cells to the wound site will release stem cell released molecules, including GDF-11, that stops proliferation and induces differentiation allowing newly differentiated somatic cells to repair the tissue. Thus, in our model, GDF11 is released from BMSCs and is a master regulator of stem cell transcription that inhibits cell proliferation and migration by down-regulating the expression of numerous genes involved in both these processes[93]. ECM-D: Extracellular matrix disruption; SC: Somatic cell; PPSC: Pluripotent stem cell; BMSC: Bone marrow stem cell; PSC: Potent stem cell.
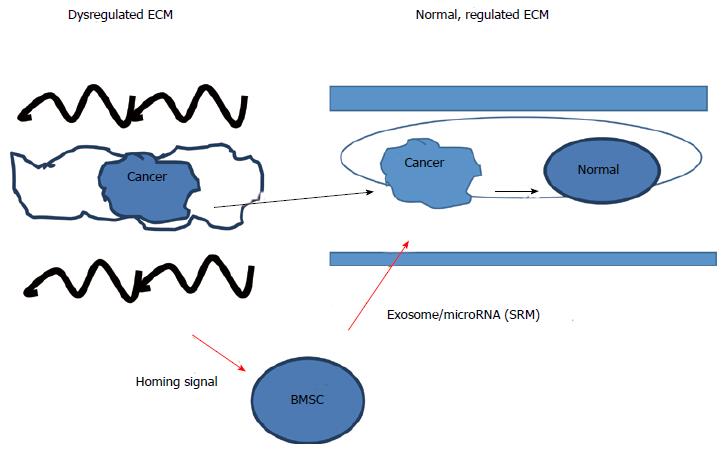
Figure 2 Regulation of the cancer/pluripotent phenotype by stem cells and extracellular matrix.
The cancer/pluripotent cell phenotype can be regulated by the extracellular matrix (ECM) and stem cells, where cancer cells can be removed from a dysregulated ECM and placed into a normal ECM and the cancer/pluripotent phenotype will revert to a normal, somatic cell phenotype. Likewise, if a dysregulated ECM is reconstructed into a normal state, the cancer/pluripotent phenotype will revert to the normal somatic cell phenotype. Further regulation of the cancer/pluripotent phenotype can be regulated by a number of factors, including microRNA contained within exosomes that were released from mesenchymal stem cells serving to change the state of the cancer cell into one of quiescence. MBSC: BM-stroma cell.
- Citation: Maguire G, Friedman P. Systems biology approach to developing S2RM-based “systems therapeutics” and naturally induced pluripotent stem cells. World J Stem Cells 2015; 7(4): 745-756
- URL: https://www.wjgnet.com/1948-0210/full/v7/i4/745.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i4.745