Copyright
©The Author(s) 2015.
World J Stem Cells. Mar 26, 2015; 7(2): 281-299
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.281
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.281
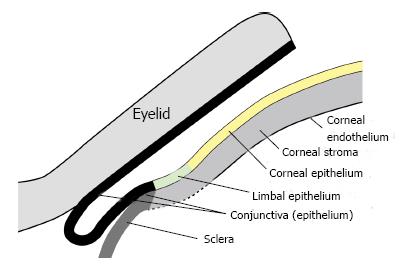
Figure 1 Diagrammatic representation of the tissues of the mouse ocular surface and eyelid.
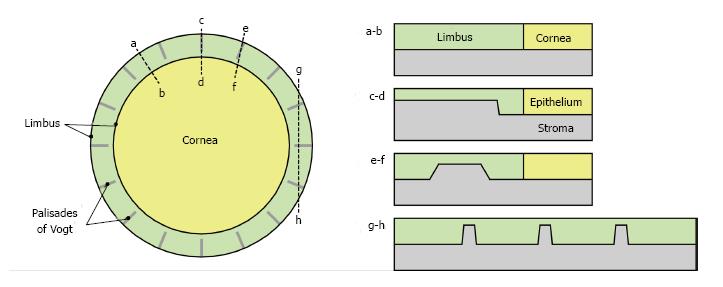
Figure 2 Palisades of Vogt.
Diagram showing the arrangement of the palisades of Vogt (upward projections of limbal stroma into the limbal epithelium) in the human limbus in plan view (left) and how they might appear in differently orientated sections through the limbus (right). Only 16 palisades are shown but in reality there are many more. For anatomy see references[114,115].
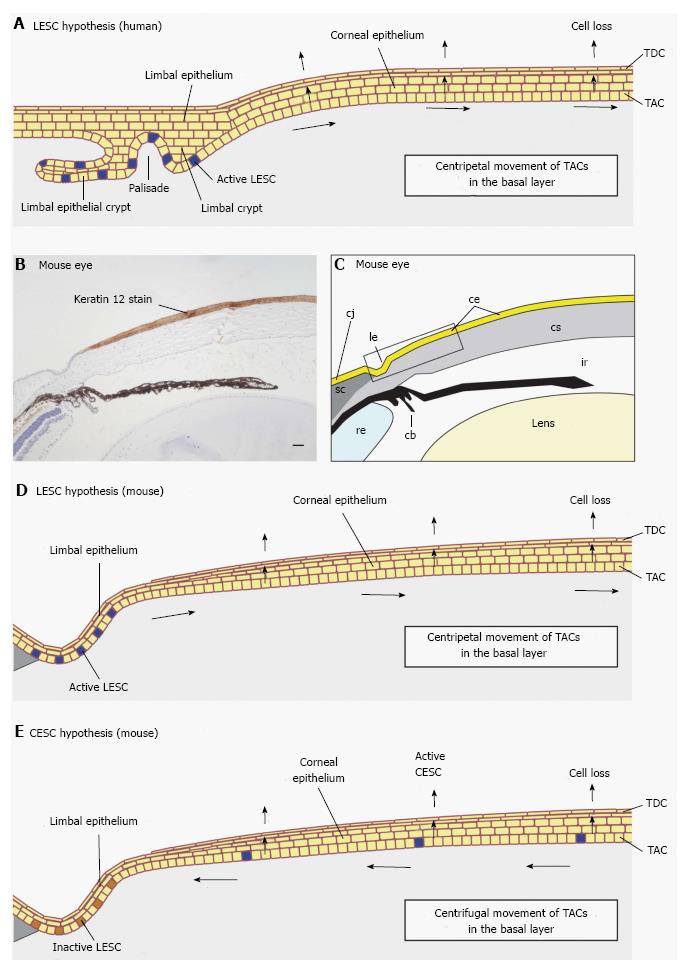
Figure 3 Limbal epithelial stem cell vs corneal epithelial stem cell hypotheses.
A: Diagram of human corneal epithelial maintenance according to the limbal epithelial stem cell (LESC) hypothesis showing active LESCs in the limbal epithelium in both a limbal crypt and a limbal epithelial crypt. The LESCs divide slowly replacing themselves and producing daughter transient (or transit) amplifying cells (TACs), which divide more quickly and move centripetally from the basal layer of the limbal epithelium to the basal layer of the corneal epithelium. After a final cell division TACs leave the basal layer, move through the suprabasal layers and are shed from the surface as terminally differentiated cells (TDCs); B: Histological section showing mouse cornea, limbus and part of the conjunctiva immunohistochemically stained for keratin 12 (K12; dark brown staining) to show the border between the corneal epithelium (K12 positive) and limbal epithelium (K12 negative); C: Drawing of photograph shown in (B) with different tissues labelled. The boxed area shows part of the limbal and corneal epithelia, equivalent to that represented in (D) and (E); D: Diagram of mouse corneal epithelial maintenance according to the LESC hypothesis. The principles are the same as described for (A); E: Diagram of mouse corneal epithelial maintenance according to the corneal epithelial stem cell (CESC) hypothesis. The CESCs divide slowly replacing themselves and producing daughter TACs, which divide more quickly and move centrifugally as originally proposed[1]. After a final cell division TACs leave the basal layer, move through the suprabasal layers and are shed from the surface. cb: Ciliary body; ce: Corneal epithelium; cj: Conjunctiva; cs: Corneal stroma; ir: Iris; le: Limbal epithelium; re: Retina; sc: Sclera. Photograph (B) is reproduced from Mort et al[18] with kind permission of Springer Science + Business Media.
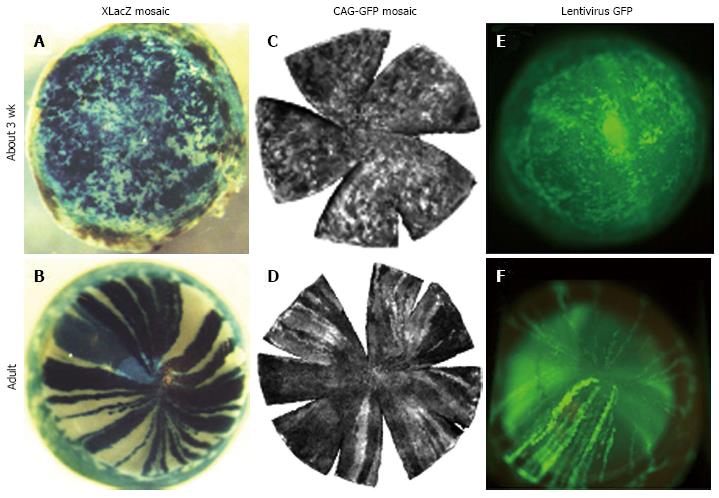
Figure 4 Transition from randomly orientated patches to radial stripes in corneal epithelia of different types of mosaic mice between 3 wk and adulthood.
A and B: β-gal staining in XLacZ X-inactivation mosaics[27]; C and D: Green fluorescent protein (GFP) fluorescence in CAG-GFP transgenic mosaics[77]; E and F: GFP fluorescence in corneal epithelium after transfecting conceptuses with lentiviral vectors encoding GFP at embryonic day 9 or 10[79]. Photographs (A and D) are reproduced from Developmental Dynamics[27] with kind permission of John Wiley and Sons, (C and D) are reproduced from Molecular Vision[77] with kind permission of the authors and editors, and photographs (E and F) are reproduced from Molecular Therapy[79] with kind permission of the authors and the Nature Publishing Group. This combination of photographs was first published by Mort et al[18].
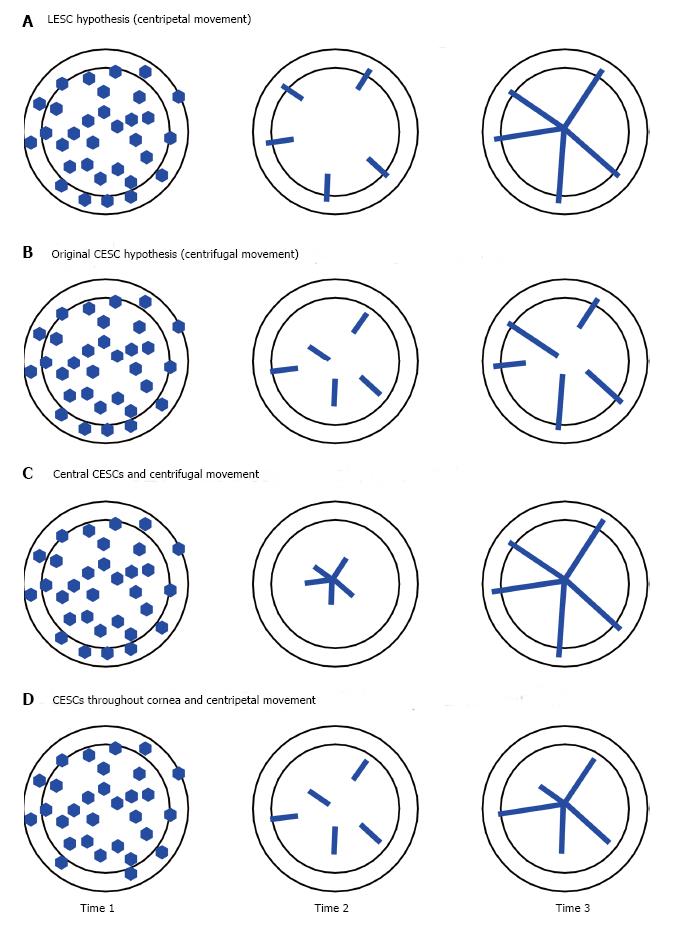
Figure 5 Hypothetical results from a lineage tracing experiment to distinguish between the limbal epithelial stem cell and corneal epithelial stem cell hypotheses.
In each figure the inner disc represents the corneal epithelium and the outer ring represents the limbal epithelium. If a reporter transgene is driven by a tamoxifen-inducible, ubiquitous promoter, a proportion of all the cell types in the ocular surface (and other tissues) will be labelled shortly after tamoxifen treatment. The frequency of labelled cells will depend partly on the dose of tamoxifen, which could be titrated to ensure only a few stem cells are labelled per eye. Time 1 is shortly after tamoxifen-treatment and the labelled cells may have divided to produce a small clone of labelled cells. By Time 2, the short-lived labelled clones produced by labelling transient (or transit) amplifying cells should have been shed from the corneal epithelium but long-lived labelled clones produced by long-lived labelled stem cells will remain. Expectations for distributions of labelled cells at Times 2 and 3 vary for the different hypotheses. A: The limbal epithelial stem cell hypothesis predicts clones of labelled cells will extend radially from the limbus and, by Time 3, clones of labelled cells will span the full radius; B: The original corneal epithelial stem cell (CESC) hypothesis predicts clones of labelled cells produced by labelled stem cells may also extend radially but will extend centrifugally from stem cells located throughout the corneal epithelium itself. Clones of labelled cells that do not arise from the centre of the cornea will not span the full radius; C: If the CESC hypothesis is modified so that all the CESCs are at the very centre of the cornea, centrifugal movement will produce clones of labelled cells that span the full radius by Time 3 but at Time 2 there should be no labelled peripheral cells produced by stem cells; D: If the CESC hypothesis is modified so that the CESCs are located throughout the corneal epithelium but movement is centripetal, clones of labelled cells that do not arise from the periphery of the cornea will not span the full radius. To distinguish between the various hypotheses it will be necessary to compare patterns of labelled clones at different times after tamoxifen treatment.
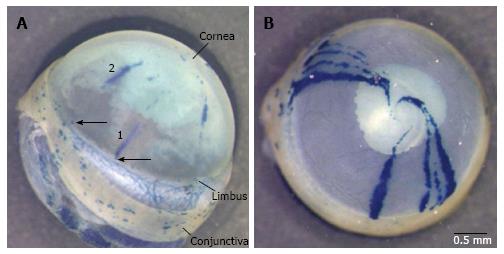
Figure 6 Preliminary results from a lineage tracing experiment to distinguish between the limbal epithelial stem cell and corneal epithelial stem cell hypotheses.
Eyes from CAGG-CreER; R26R-LacZ mice that were injected with tamoxifen to induce LacZ reporter gene expression and stained for β-galactosidase (β-gal) activity after different chase periods. The pigmented iris is visible through the cornea and appears grey, whereas the β-gal staining is blue. A: Side view of a β-gal-stained eye, after a chase period of 9 wk and 4 d, with several radial β-gal-positive stripes and small patches in the cornea and numerous β-gal-positive patches in the conjunctiva. (The conjunctiva is torn near the limbus and hangs down at the bottom right of the photograph, so the sclera is visible between the limbus and conjunctiva.) Stripe 1 is a limbus-cornea (LC) stripe, with its more peripheral end in the limbus (arrow), consistent with expectations of the limbal epithelial stem cell (LESC) hypothesis (see Figure 5). It also appears to be aligned with other β-gal-positive patches towards the centre of the cornea so it may be part of a longer discontinuous stripe. Stripe 2 is a cornea-cornea stripe with both ends in the cornea, consistent with the corneal epithelial stem cell hypothesis. However, it is radially aligned with a small β-gal-positive patch in the limbus (arrow), which could be the location of a β-gal-positive LESC. If so, stripe 2 might be a discontinuous stripe, which extended from a LESC that was not continuously active (consistent with the LESC hypothesis); B: Anterior (frontal) view of a β-gal-stained eye, after a 14-wk chase period, with eight radial β-gal-positive stripes. All eight stripes are LC stripes with one end at the limbus and many extend the full radius and have a curved end, consistent with a central spiral pattern, as reported for other chimaeric and mosaic eyes[27,72,73]. CAGG-CreER; R26R-LacZ mice were produced by crossing CAGG-CreER and R26R-LacZ mice [full names Tg(CAG-cre/Esr1*)5Amc and B6.129S4-Gt(ROSA)26Sortm1Sor/J; references[116,117]. LacZ reporter gene expression was induced at 12 wk by 3 injections of tamoxifen (100 μg/g body weight per injection).
- Citation: West JD, Dorà NJ, Collinson JM. Evaluating alternative stem cell hypotheses for adult corneal epithelial maintenance. World J Stem Cells 2015; 7(2): 281-299
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/281.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.281