Copyright
©The Author(s) 2020.
World J Stem Cells. Aug 26, 2020; 12(8): 857-878
Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.857
Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.857
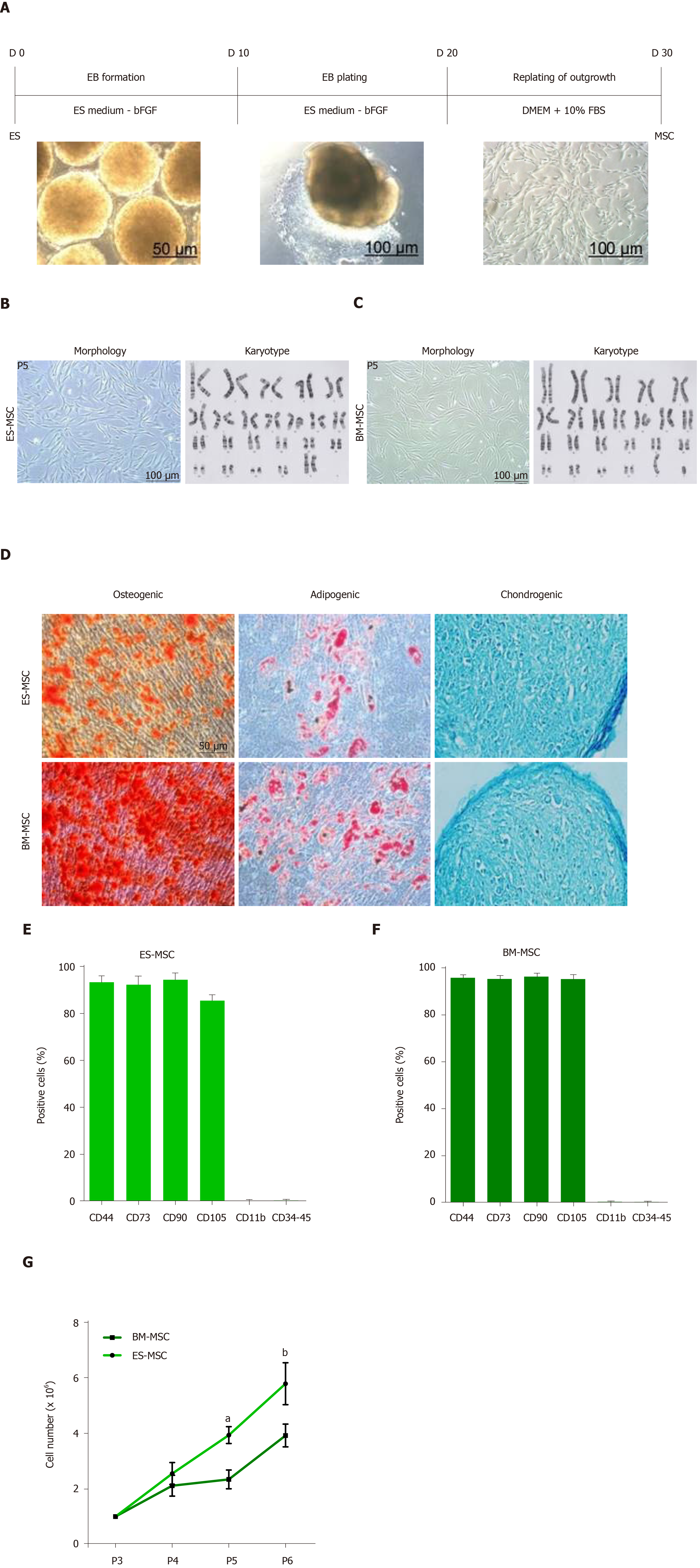
Figure 1 Derivation and identification of human embryonic stem cell-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells.
A: Schematic presentation of the procedure used to derive human mesenchymal stem cells (MSCs) from embryonic stem (ES) cells. Colonies of ES cells were enzymatically detached and cultured for 10 d in suspension to form embryoid bodies, which were then plated onto gelatin-coated tissue culture plates. After 10 d, outgrowths of the cells that sprouted from embryoid bodies were mechanically isolated by a cell scraper and subsequently expanded in mesenchymal stem cell culture medium; B and C: Morphology and karyotype of ES-MSCs and BM-MSCs. Passage-5 ES-MSCs and BM-MSCs showed a fibroblastic morphology and normal karyotype; D: Alizarin red staining after 14 d of culture in osteogenic medium indicated the osteogenic differentiation potential of ES-MSCs and BM-MSCs (P4). Oil red staining after 21 d of culture in adipogenic medium showed the adipogenic differentiation potential of ES-MSCs and BM-MSCs (P4). Alcian blue staining after 21 d of culture in chondrogenic medium showed chondrogenic differentiation potential of ES-MSCs and BM-MSCs; E, F: Flow cytometric analysis indicated that cultured ES-MSCs and BM-MSCs expressed CD44, CD90, CD73 and endoglin (CD105), but not hematopoietic lineage markers CD11b, CD34 and protein tyrosine phosphatase receptor type C (CD45); G: ES-MSCs proliferated more rapidly than BM-MSCs. Results are expressed as mean ± standard error,
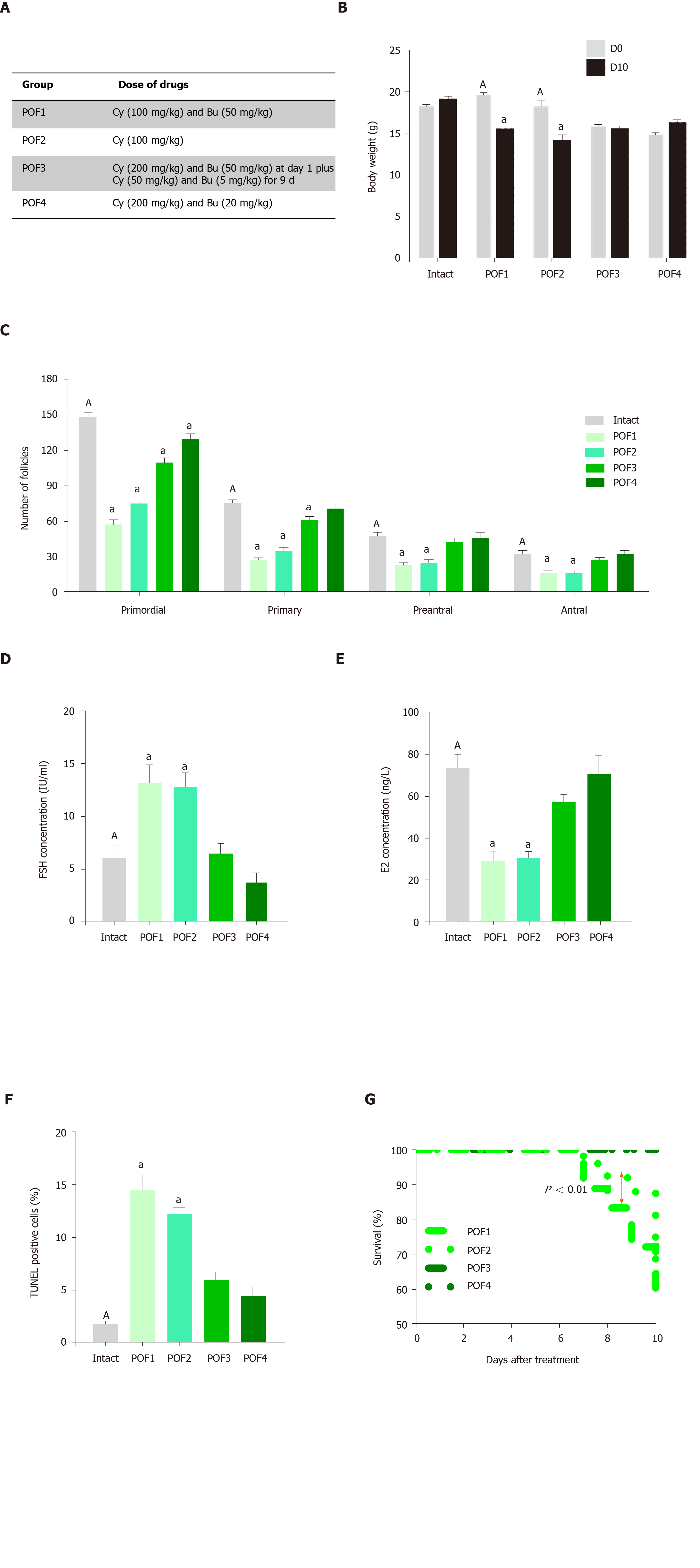
Figure 2 Establishment of a mouse model of premature ovarian failure.
A: Premature ovarian failure (POF) groups were treated with different dosages of cyclophosphamide and busulfan; B: Body weight changes in the intact and POF groups after 10 d showed that the POF1 and POF2 groups had significant decreases in body weights; C: Ovarian pathology of the intact and POF groups 10 d after injection of cyclophosphamide and busulfan . Follicle count revealed that there were fewer normal follicles in the POF groups than in the intact mice. The ovaries of the intact group contained large numbers of follicles at all developmental stages, whereas the atrophic ovaries of the POF groups had fewer follicles at each stage; D, E: Serum levels of follicle stimulating hormone and estradiol 10 d after injection of cyclophosphamide and busulfan. Serum levels of follicle stimulating hormone were significantly increased in the POF1 and POF2 groups compared to those of the intact group. Serum levels of estradiol were significantly decreased in the POF1 and POF2 groups compared with the intact group; F: Apoptosis rate in the ovary. Green fluorescence indicated the presence of apoptotic cells in the POF1, POF2, POF3 and POF4 groups; G: Survival rate in the POF groups after 10 d. The survival percent showed a significant decrease in the POF1 group compared with the POF2 group. All data are presented as mean ± standard error. Small letters (a) indicate the significance (P < 0.05) compared to groups labeled by similar capital letters (A); aP < 0.05 significance of experimental groups vs the intact group; n = 3-5. POF: Premature ovarian failure; Cy: Cyclophosphamide; Bu: Busulfan; FSH: Follicle stimulating hormone; E2: Estradiol.
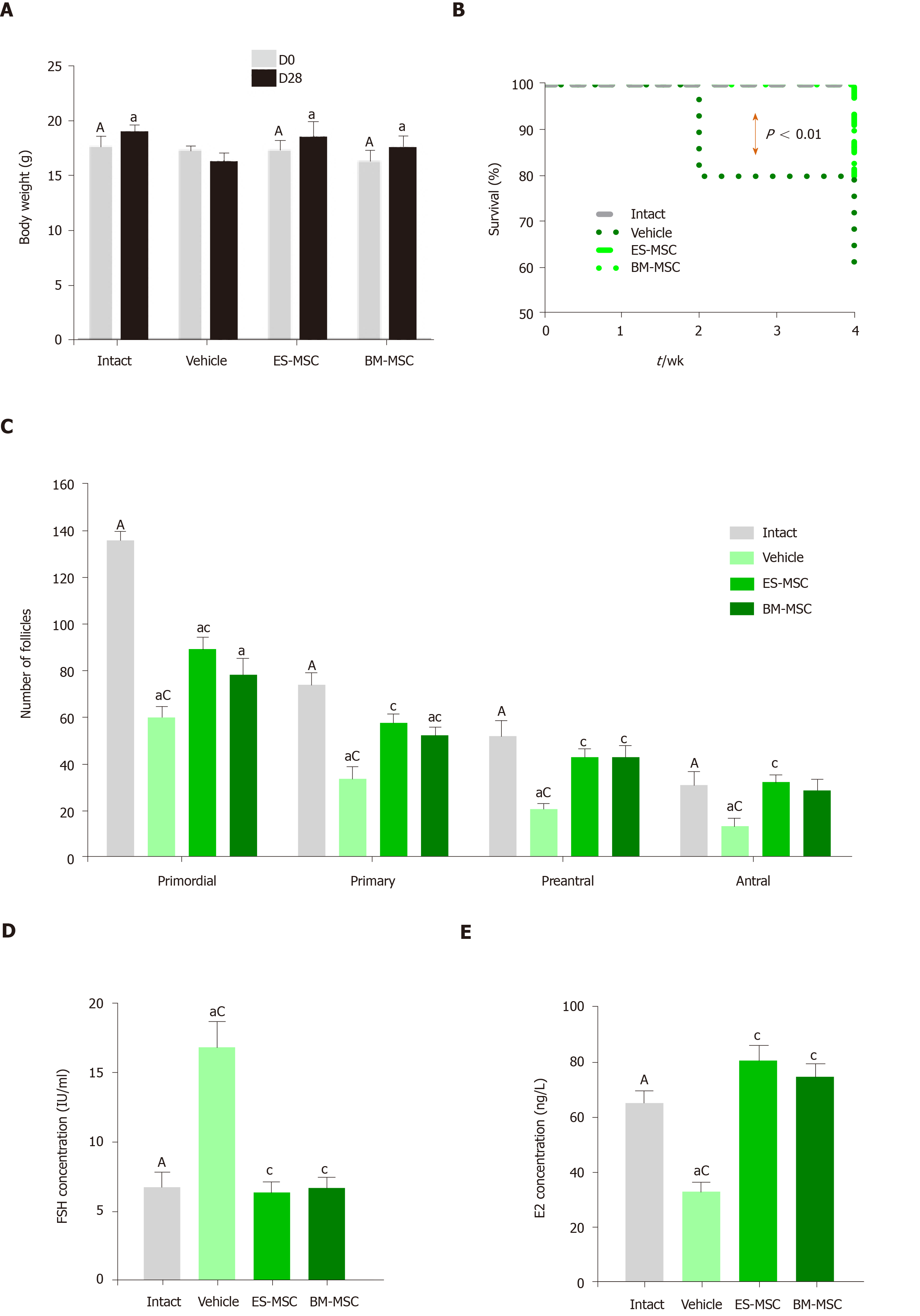
Figure 3 Effects of human embryonic stem cell-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells transplantation in mice with premature ovarian failure.
A: Transplantation of embryonic stem cell-derived mesenchymal stem cells (ES-MSCs) and/or bone marrow-derived mesenchymal stem cells (BM-MSCs) improved body weights in mice with premature ovarian failure after 4 wk; B: Survival rate 4 wk after ES-MSCs and/or BM-MSCs transplantation. Survival rate significantly increased in both the ES-MSCs and/or BM-MSCs transplanted mice (more than 60%) compared with the vehicle group (20%); C: The follicle number increased after transplantation. The number of follicles at all stages of development in both cell transplanted groups was significantly higher than that of the vehicle mice, while it was lower than the intact mice; D, E: Both cell transplantations rescued hormone secretion in premature ovarian failure mice. Serum follicle stimulating hormone levels decreased significantly in both cell transplanted groups compared to the vehicle group. The serum estradiol level significantly recovered after both cell transplantations compared to the vehicle group. All data are presented as mean ± standard error. Small letters (a, c) indicate the significance (P < 0.05) compared to groups labeled by similar capital letters (A, C); aP < 0.05 significance of experimental groups vs the intact group; cP < 0.05 significance of ES-MSC and BM-MSC groups vs the vehicle group; n= 3-5. ES-MSCs: Embryonic stem cell-derived mesenchymal stem cells; BM-MSCs: Bone marrow-derived mesenchymal stem cells; POF: Premature ovarian failure; FSH: Follicle stimulating hormone; E2: Estradiol.
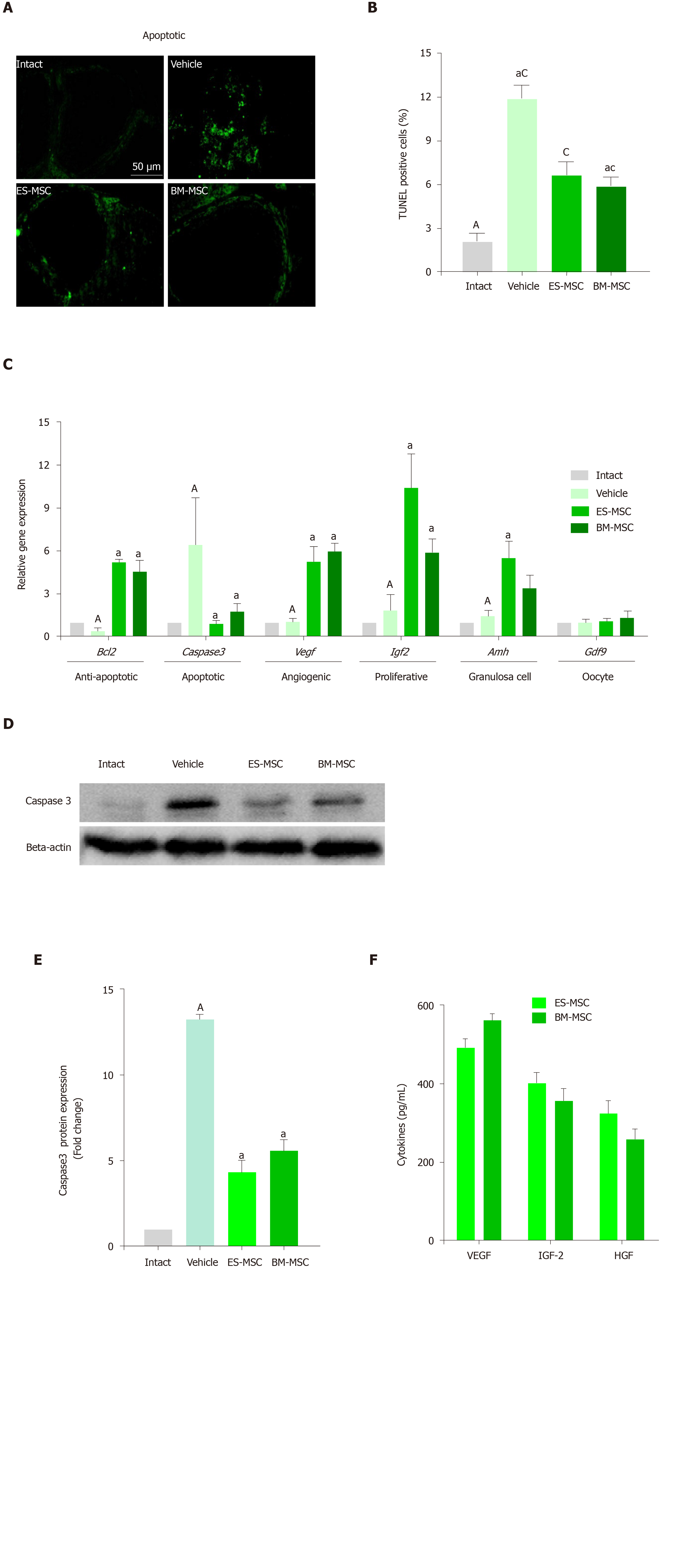
Figure 4 Human embryonic stem cell-derived mesenchymal stem cells and/or bone marrow-derived mesenchymal stem cells transplantation improved premature ovarian failure conditions.
A and B: Apoptosis was reduced after both cell transplantations. The green stain color indicates terminal deoxynucleotidyl transferase mediated 2-deoxyuridine 5-triphosphate nick end labelling-positive cells. Data at 4 wk showed decreased levels of apoptosis following transplantation of embryonic stem cell-derived mesenchymal stem cells (ES-MSCs) and/or bone marrow-derived mesenchymal stem cells (BM-MSCs); C: Gene expression analysis showed that the expressions of B-cell lymphoma 2, vascular endothelial growth factor, insulin-like growth factor 2, anti-Müllerian hormone and growth/differentiation factor 9 significantly increased in both cell transplanted groups compared with the vehicle group, whereas cysteine-aspartic proteases 3 (caspase 3) significantly decreased in both cell transplanted groups compared to the vehicle group; D, E: Western blot analysis for cleaved-caspase 3 expression in the ovarian tissue. The results showed that cleaved-caspase 3 in ovarian tissue of the vehicle group significantly increased compared to the intact group. Cleaved-caspase 3 protein expression levels decreased in the ovaries from the ES-MSCs and BM-MSCs transplantation groups compared with the vehicle group; F: ELISA assessment of conditioned media of ES-MSCs and BM-MSCs for vascular endothelial growth factor, insulin-like growth factor 2 and hepatocyte growth factor. The results showed that ES-MSCs and BM-MSCs secreted vascular endothelial growth factor, insulin-like growth factor 2,and hepatocyte growth factor in vitro. All data are presented as mean ± standard error. Small letters (a, c) indicate the significance (P < 0.05) compared to groups labeled by similar capital letters (A, C), aP < 0.05, significance of experimental groups vs the intact group. cP < 0.05, significance of ES-MSC and BM-MSC groups vs the vehicle group. For Figure 4C:aP < 0.05 significance of ES-MSC and BM-MSC groups vs the vehicle group, n = 3. ES-MSCs: Embryonic stem cell-derived mesenchymal stem cells; BM-MSCs: Bone marrow-derived mesenchymal stem cells; POF: Premature ovarian failure; Bcl-2: B-cell lymphoma 2; Caspase 3: Cysteine-aspartic proteases 3; Vegf: Vascular endothelial growth factor; Igf-2: Insulin-like growth factor 2; Amh: Anti-Müllerian hormone; Gdf9: Growth/differentiation factor 9.
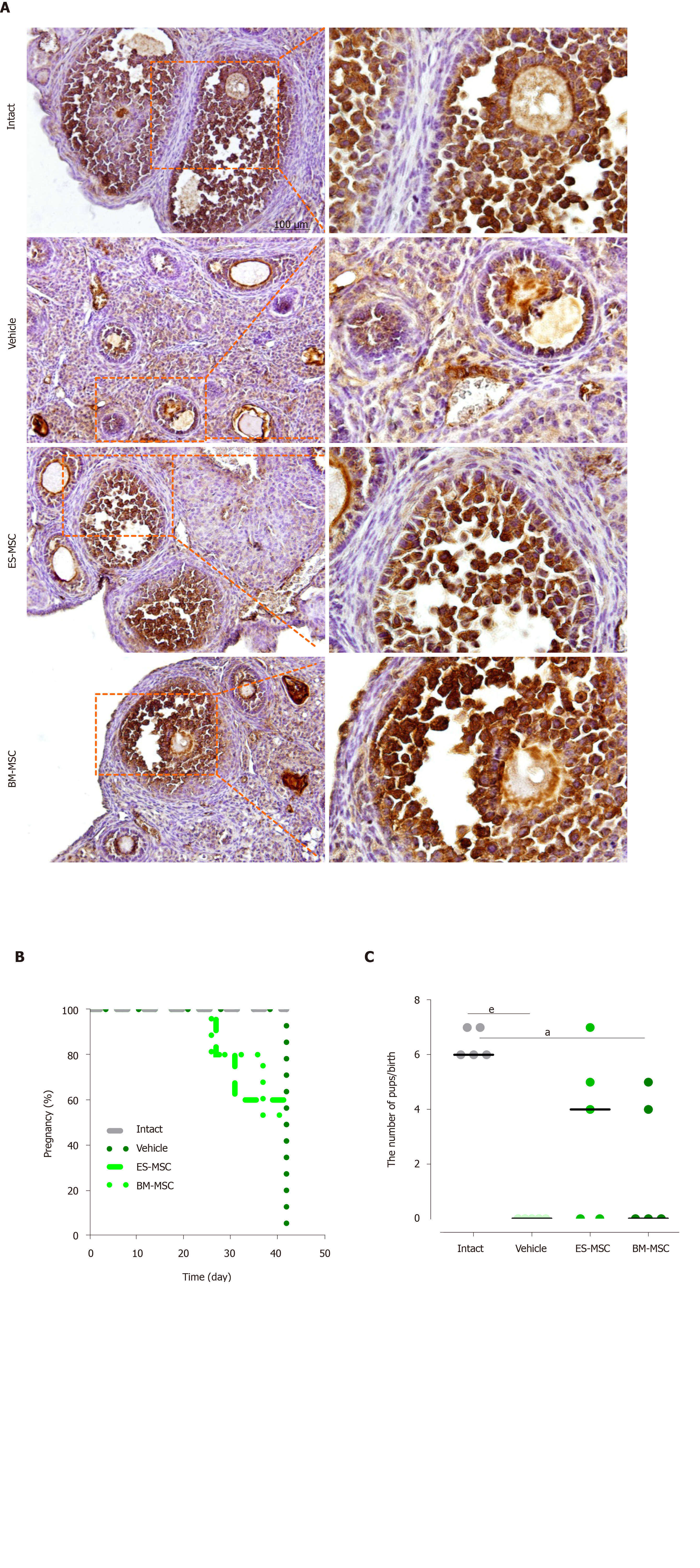
Figure 5 Human embryonic stem cell-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells transplantation prevented follicular atresia and restored fertility in premature ovarian failure mice.
A: Immunohistochemical staining showed increased anti-Müllerian hormone expression in the ovaries from the embryonic stem cell-derived mesenchymal stem cells (ES-MSCs) and bone marrow-derived mesenchymal stem cells (BM-MSCs) transplantation groups compared with the vehicle group; B, C: The percentage of pregnancies and the numbers of pups six weeks after both cell transplantations. The intact mice had a 100% (5 of 5) pregnancy rate, producing 32 live offspring. The embryonic stem cell-derived MSCs transplanted mice had a 60% (3 of 5) pregnancy rate, producing 16 live offspring. The bone marrow-derived MSCs transplanted mice had a 40% (2 of 5) pregnancy rate, producing nine live offspring. None of the premature ovarian failure mice became pregnant. Results are expressed as mean ± standard error, aP < 0.05, eP < 0.001, n = 3-5. ES-MSCs: Embryonic stem cell-derived mesenchymal stem cells; BM-MSCs: Bone marrow-derived mesenchymal stem cells.
- Citation: Bahrehbar K, Rezazadeh Valojerdi M, Esfandiari F, Fathi R, Hassani SN, Baharvand H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J Stem Cells 2020; 12(8): 857-878
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/857.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.857