Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1853
Revised: January 6, 2003
Accepted: January 18, 2003
Published online: August 15, 2003
AIM: To demonstrate whether class I MHC molecule, transporter associated with antigen processing (TAP), and heat-shock proteion70 (HSP70) expressed in liver cancer cells before the design and construction of CTL vaccine against hepatocellular carcinoma (HCC).
METHODS: We studied 30 HCC specimens by labeled streptavidin biotin (LSAB) method of immunohistochemistry.
RESULTS: The results showed that the majority of HCC cells investigated naturally expressed class I MHC and TAP, which were different from other tumor cells. Furthermore, we found that HSP70 expressed not only in cellular cytoplasm, but also on the cell surface in HCC.
CONCLUSION: Our findings indicate that our understanding about immune escape mechanisms employed by HCC cells may be further improved. It is important to design and construct CTL vaccine against HCC.
- Citation: Deng XL, Chen W, Cai MY, Wei DP. Expression of class I MHC molecule, HSP70 and TAP in human hepatocellular carcinoma. World J Gastroenterol 2003; 9(8): 1853-1855
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1853.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1853
It is commonly accepted that tumor rejection is mediated by lymphocytes, and most notably, by cytotoxic T lymphocytes (CTL). The recognization of a tumor cell by CTL is regulated by interactions between T-cell receptor and antigenic peptide -MHC complex. TAP, HSP70 and class I MHC molecule are important components in endogenous processing of peptides through the MHC class I pathway. Among many proteins that contribute to MHC class I assembly, TAP complex is one of the most important components. It translocates endogenously processed peptides from cytosol into ER for binding to class I MHC, resulting in surface presentation of these complexes to CTL. Most of the studies described the defect of endogenous processing function in tumors such as melanomas, cervical carcinomas, and renal cell carcinomas[1-3]. These findings suggest that TAP down-regulation might represent an important mechanism for immune escape of malignant cells in tumors. Another importantly associated presentation protein is heat-shock proteion70 (HSP70). HSP70 has long been known to be located in the cytoplasm, where it performs chaperoning function. Recent studies have also indicated that conditional over-expression of HSP70 in target cells enhances the susceptibility to CTL[4]. Moreover, HSP70 may enhance TAP function[5]. Therefore, there is an increasing realization that expression of these molecules on tumor cell surface may potentially affect CTL-mediated recognition.
HCC is one of the most malignant cancers. However, to date most of the studies that described expressions of class I MHC, TAP, and HSP70 were carried out with cell lines[6]. Limited information is available about these molecules in HCC tumor tissues. Nevertheless, it is important to demonstrate whether these molecules can also be expressed in HCC in vivo before the design and construction of CTL vaccine against HCC.
Thirty pathological samples were obtained from surgically resected tissues of HCC patients in West China Medical Center of Sichuan University. The specimens were frozen immediately in liquid nitrogen. Cryostat sections (4 μm) were prepared and stored at -70 °C. Pathological diagnoses were made based on routinely processed HE sections.
Mouse anti-human HLA-ABC (DAKO Corp., working dilutions 1:100), rabbit anti-human TAP (Chemicon International, Inc., working dilutions 1:1000), mouse anti-human HSP70 (DAKO Corp., working dilutions 1:50), biotin labeled goat anti-mouse IgG and goat anti-rabbit IgG, HRP-labeled streptavidin and avidin biotin blocking system were all purchased from Beijing Zhongshan Corp.
The frozen sections were fixed in cold acetone for 10 min. 3% H2O2 containing methanol was added to block endogenous peroxidase. Fixed sections were incubated with normal goat serum for blocking and subsequently with mAb (HLA-ABC, TAP and HSP70 respectively) or PBS as control at 37 °C for 2 h, then washed with PBS. After incubation with biotin labeled goat anti-mouse IgG or goat anti-rabbit IgG for 30 min at 37 °C, and washed as before, HRP-labeled streptavidin was added. The following incubation and washing were exactly the same as above. Finally, freshly prepared substrate DAB was added for color development. The reaction was stopped with tap water rinse and then counterstained with hematoxylin and mounted for examination.
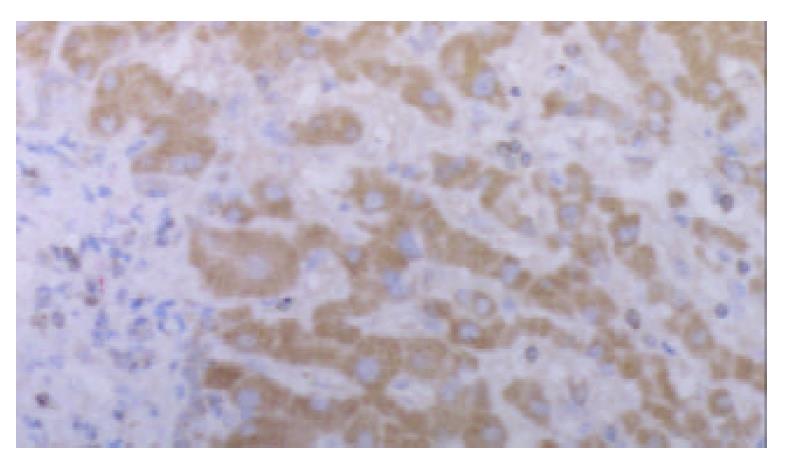
Strongly positive staining of class I MHC molecule was presented in all cases of HCC. The staining was mainly located on the membrane of the liver cancer cells, in the cytoplasm, and the perinuclear area. The staining showed granular pattern (Figure 1).
The positive staining was mainly located on the membrane of the liver cancer cells. Some of the cells showed positive staining in the cytoplasm and the perinuclear area.
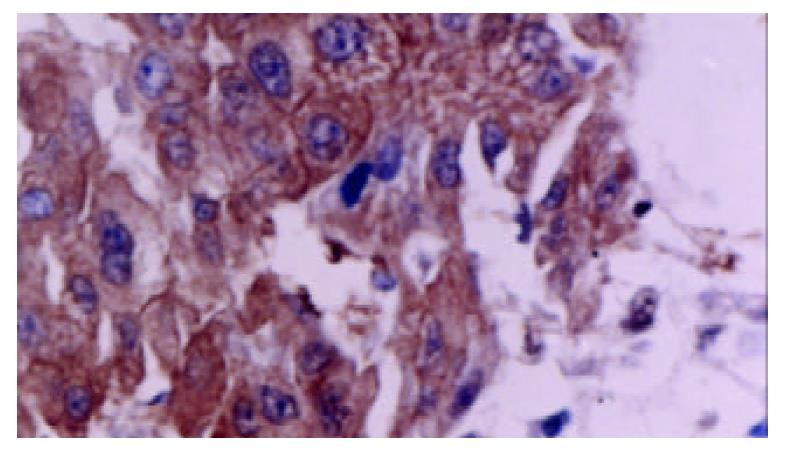
Tumor cells as well as adjacent non-neoplastic hepatocytes displayed intense and extensive positive staining. The staining was uniform throughout the specimen. The cytoplasmic staining showed granular pattern. The membranous staining displayed linear pattern, which made the interface between cells very clear (Figure 2).
Staining was typically uniform throughout the specimen. The cytoplasmic staining showed granular pattern. The membranous staining displayed linear pattern, which made the border between cells very clear.
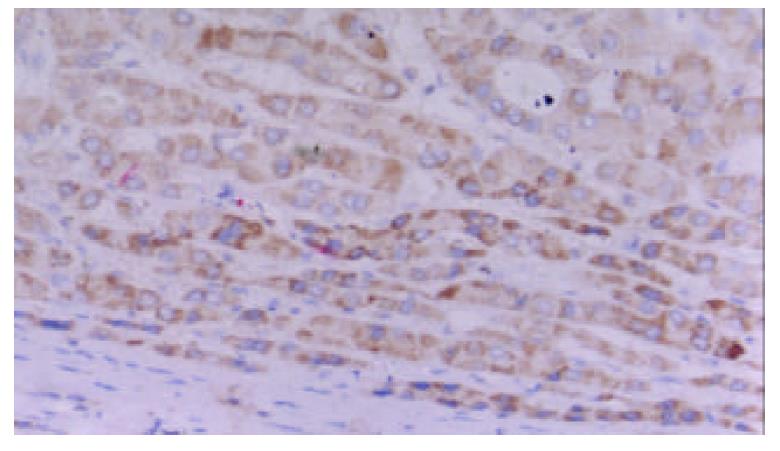
Positive staining for TAP was present in 29 of 30 cases. The staining was located in the cytoplasm and the perinuclear area. It showed granular intracytoplasmic pattern accompanied by variable staining intensity. Faint or negative staining was observed within sinusoids (Figure 3).
It showed granular intracytoplasmic pattern accompanied by variable staining intensity. Hepatocytes beside the sinusoid were more intensely stained than cells in the central tissues.
In this study we examined the expressions of class I MHC, TAP, and HSP70 in 30 HCC samples by immunohistochemical methods. The results showed that the majority of HCC investigated naturally expressed TAP, which was different from other tumors. This is consistent with the results of several other groups. Kurokohchi et al[6] examined TAP mRNA and HLA antigen in seven HCC cell lines. They found that only one HCC cell line (HuH-7) had low expression of HLA-B and C and TAP mRNA. All the other lines expressed high levels of both TAP mRNA and HLA. They speculated posttranscriptional events or failure to transport and load peptides for MHC might allow HCC cells to escape from CTL. Whereas our results suggested HCC cells with the expression of TAP might be more susceptible to host immunity. Recently, Alimonti et al[7] reported that TAP expression provided a general method for improving the recognition of malignant cells. They transfected TAP gene into the TAP- cell line and introduced it into tumor-burdened individuals. The result showed that TAP could improve tumor cell immunogenicity and host survival. This would be of a potential significance for the tumors with deficiency in components of antigen-processing molecules. However, Seliger et al[8,9] identified the structural alterations of TAP in the MHC class I antigen-processing pathway in melanoma after analyzing the sequence of TAP. The possibility of structural transformation of TAP in HCC cells could not be excluded and needs to be further studied.
As our understanding of the molecular aspects of the class I processing pathway has been improved, there is an increasing realization that expression of class I MHC antigen on cell surface is crucial for recognition by CTL. It is well established that many tumors escape T cell recognition by loss or down regulation of class I molecule expression on the surface of tumor cells. Previous reports described a correlation between HLA loss and TAP gene defects in some tumors[10]. So we also analyzed the expression of class I MHC on the surface of hepatocytes in HCC. Our investigation showed that most liver cancer cells in HCC tissues had strong class I MHC antigen expression. This is consistent with the results of several other groups[6,11]. The possible mechanism for the appearance of class I MHC antigen on hepatocytes remains controversial. One of the possibilities is that the expression of class I MHC antigen on HCC cells is related with viral infection. Sung et al[12] found that HCC patients were infected by HBV. Zhou et al[13] suggested cytokines such as γ-interferon released by T lymphocytes infiltrated in HCC tissues might be contributed to the expression of class I MHC molecules. The other possibility is that malignant transformation in HCC is characterized by expression of class I MHC molecules[11]. Although the underlying molecular mechanism is not well understood, the expression of class I MHC antigen on HCC cells may influence the behavior of tumor cells and has a significant effect on the reactivity of host immune system against HCC cells.
Earlier work suggested that HSP70 was shown to accumulate in the cytoplasm and the perinuclear area by cellular stress, such as non-lethal heat shock. In this study, we demonstrated that HSP70 expressed not only in cytoplasm, but also on the surface of cells in HCC. Most members of the HSP families do not possess signal peptides. So it is still unclear how these proteins are transported to the cell membrane. Morimoto et al[14] postulated that some HSP might be transported to the cell surface via autoregulatory mechanism like in normal cells. Our results indicate that HSP70 may be transported to the cell surface after binding to MHC molecules. The main reason of this speculation is that class I MHC is found on the membrane of HCC cells. HSP70 is known to have strong protein-binding capacities. Therefore, it is reasonable to suggest that MHC class I may be the best candidate. It is interesting that cell-surface localization of HSP70 may make it possible to increase the immunogenicity of HCC cells. Many evidences have shown that HSPs expressing on affected cell types is recognized by the immune system[15]. Binder et al[16] recently identified HSP-chaperoned peptides introduced into cytosol were quite efficient as compared with free peptides. They even suggested that HSP70 was involved not only in the afferent end of this process by chaperoning partially or fully unfolded polypeptide chains, but also in chaperoning the resulting antigenic peptides to the TAP complex. These evidences indicate that HSP70 expressing on the surface of cells may be potentially promising target molecules for the immunotherapy of HCC.
On the whole, our data suggest that HCC cells express TAP, HSP70, and class I MHC. The results indicate that the mechanism of HCC cellular escape should be re-evaluated. Especially when designing immunotherapeutic strategies for HCC, it is very important to consider the potential problems associated with the ability of tumor cells to present target epitopes for immune recognition. Moreover, it needs to stress that tumor progression is dependent on multiple factors. Several scenarios have been proposed to be responsible for tumor immune-escape mechanisms, including production of suppressive cytokines by tumor cells, and expression of Fas ligand on tumor cells[17]. A recent study from Chai et al[18] suggested that T cell-expressed CD80 had a regulatory function and played a key role in the induction of T cell unresponsiveness by co-stimulation-deficient antigen presentation. So elucidation of the immune deficiency against cancer progression has been a difficult task because no single mechanism can explain the complicated cancer-host immune interactions. It is important to determine a more appropriate approach of combining different strategies to control the outgrowth of HCC cells.
Edited by Xu JY and Wang XL
| 1. | Seliger B, Ritz U, Abele R, Bock M, Tampé R, Sutter G, Drexler I, Huber C, Ferrone S. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61:8647-8650. [PubMed] |
| 2. | Ritz U, Momburg F, Pilch H, Huber C, Maeurer MJ, Seliger B. Deficient expression of components of the MHC class I antigen processing machinery in human cervical carcinoma. Int J Oncol. 2001;19:1211-1220. [PubMed] |
| 3. | Dovhey SE, Ghosh NS, Wright KL. Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res. 2000;60:5789-5796. [PubMed] |
| 4. | Dressel R, Lübbers M, Walter L, Herr W, Günther E. Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J Immunol. 1999;29:3925-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Chen D, Androlewicz MJ. Heat shock protein 70 moderately enhances peptide binding and transport by the transporter associated with antigen processing. Immunol Lett. 2001;75:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kurokohchi K, Carrington M, Mann DL, Simonis TB, Alexander-Miller MA, Feinstone SM, Akatsuka T, Berzofsky JA. Expression of HLA class I molecules and the transporter associated with antigen processing in hepatocellular carcinoma. Hepatology. 1996;23:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Alimonti J, Zhang QJ, Gabathuler R, Reid G, Chen SS, Jefferies WA. TAP expression provides a general method for improving the recognition of malignant cells in vivo. Nat Biotechnol. 2000;18:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Seliger B, Ritz U, Abele R, Bock M, Tampé R, Sutter G, Drexler I, Huber C, Ferrone S. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61:8647-8650. [PubMed] |
| 9. | Seliger B, Bock M, Ritz U, Huber C. High frequency of a non-functional TAP1/LMP2 promoter polymorphism in human tumors. Int J Oncol. 2002;20:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Cromme FV, Airey J, Heemels MT, Ploegh HL, Keating PJ, Stern PL, Meijer CJ, Walboomers JM. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994;179:335-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Paterson AC, Sciot R, Kew MC, Callea F, Dusheiko GM, Desmet VJ. HLA expression in human hepatocellular carcinoma. Br J Cancer. 1988;57:369-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Sung CH, Hu CP, Hsu HC, Ng AK, Chou CK, Ting LP, Su TS, Han SH, Chang CM. Expression of class I and class II major histocompatibility antigens on human hepatocellular carcinoma. J Clin Invest. 1989;83:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zhou DX, Taraboulos A, Ou JH, Yen TS. Activation of class I major histocompatibility complex gene expression by hepatitis B virus. J Virol. 1990;64:4025-4028. [PubMed] |
| 14. | Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1017] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 15. | Schueller G, Paolini P, Friedl J, Stift A, Dubsky P, Bachleitner-Hofmann T, Jakesz R, Gnant M. Heat treatment of hepatocellular carcinoma cells: increased levels of heat shock proteins 70 and 90 correlate with cellular necrosis. Anticancer Res. 2001;21:295-300. [PubMed] |
| 16. | Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001;276:17163-17171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Restifo NP. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Chai JG, Vendetti S, Amofah E, Dyson J, Lechler R. CD152 ligation by CD80 on T cells is required for the induction of unresponsiveness by costimulation-deficient antigen presentation. J Immunol. 2000;165:3037-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |