Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1840
Revised: January 10, 2003
Accepted: February 17, 2003
Published online: August 15, 2003
AIM: To explore the inhibition of β-L-D4A on hepatitis B virus (HBV) in 2.2.15 cells derived from HepG2 cells transfected with HBV genome.
METHODS: 2.2.15 cells were plated at a density of 5 × 104 per well in 12-well tissue culture plates, and treated with various concentrations of β-L-D4A for 6 days. In the end, 5 μl of medium was used for the estimation of HBsAg and HBeAg, the other medium was processed to obtain virions by a polyethlene glycol precipitation method. At the same time, intracellular DNA was also extracted and digested with HindIII. Both DNAs were subjected to Southern blot, hybridized with a 32P-labeled HBV probe and autoradiographed. Intensity of the autoradiographic bands was quantitated by densitometric scans of computer and ED50 was calculated. Then Hybond-N membrane was washed and rehybridized with a 32P-labeled mtDNA-specific probe, and effect of β-L-D4A on mitochondrial DNA was studied. 2. 2.15 cells were also seeded in 24-well tissue culture plates, and cytotoxicity with different concentrations was examined by MTT method. ID50 was calculated. Structure-activity relationships between D2A and D4A were also studied as above.
RESULTS: Autoradiographic bands were similar between supernatant and intracellular HBV DNA. Episomal HBV DNA was inhibited in a dose-dependent manner. ED50 was 0.2 μM. HBsAg or HBeAg was not apparently decreased, and inhibition of mitochondrial DNA was not obvious. The experiment of cytotoxicity gained ID50 at 200 μM.
CONCLUSION: β-L-D4A possesses potent inhibitory effects on the replication of HBV in vitro with little cytotoxicity and mitochondrial toxicity, TI value is 1000. It is expected to be developed as a new clinically anti-HBV drug.
- Citation: Wu JM, Lin JS, Xie N, Liang KH. Inhibition of hepatitis B virus by a novel L-nucleoside, β-L-D4A and related analogues. World J Gastroenterol 2003; 9(8): 1840-1843
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1840.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1840
Like interferon, nucleoside analogues have become the focus of investigations of anti-HBV drugs[1]. However, most anti-HBV nucleoside analogues tested to date have at best only transient and limited effects in a small percentage of the general population of HBV-infected individuals and exist moderately to seriously side effects[2,3]. Vidarabine, ribavirin, acyclovir, ganciclovir, famciclovir, fialuridine, etc. have not been used widely. In recent years, considerable interest has been focused on the use of 2’, 3’-dideoxynucleosides (DDNs) for the treatment of chronic HBV infection[4]. DDNs are phosphorylated to triphosphate in cells, which in turn specifically inhibits the viral polymerase, terminates the elongation of HBV DNA, showing potent anti-HBV activities with relatively low cellular toxicities[5].
Lamivudine as one of DDNs has been used widely in clinic, and has a rapidly potent anti- HBV effect, but there is a rebound of HBV DNA after treatment, and drug resistance and viral mutants may appear after a long-term treatment with lamivudine[6]. Thus it is important to search for more effective agents against HBV, even with an improved therapeutic index. In this report, 2’, 3’-didehydro-2’, 3’-dideoxyadenine (β-L-D4A), a novel L-Nucleoside was demonstrated to effectively block the production of HBV in 2.2.15 cells in vitro. Mitochondrial effects and cytotoxicity were also investigated to evaluate the potential use of these compounds in treatment of HBV infection.
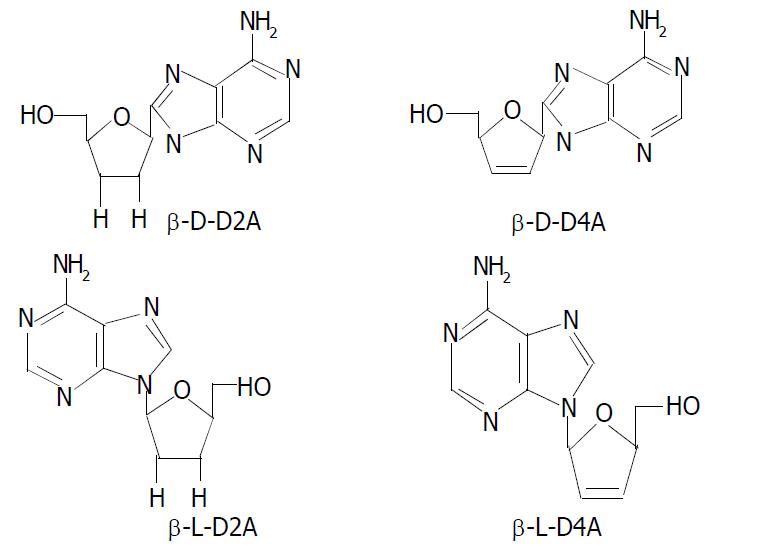
β-L-D4A was synthesized by ourselves with the help of Pharmaceutic College of Wuhan University and identified by infrared, mass spectra, nuclear-magnetic resonance. Lamivudine, ddC’ D-D4A, L-D2A, D-D2A were provided by Professor Cheng YC (School of Medicine, Yale University, New Haven, CT). All compounds were dissolved in phosphate-buffered saline (pH 7.4).
2.2.15 cells (clonal cells derived from hepG2 cells that were transfected with a plasmid containing HBV DNA) that could secrete heptitis B virions, kindly provided by Prof. Cheng, were incubated in DMEM medium with 10% (vol/vol) fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin at 37 °C in a moist atomosphere containing 5% CO2/95% air. The cells were inoculated at a density of 5 × 104/ml per well in 12-well tissue culture plates. The compounds studied were added to the medium 3 days after the inoculation. The cells were grown in the presence of drugs for 9 days with changes of medium every 3 days. On the 12th day, the culture medium was havested. An aliquot of the culture medium (5 μl) was used for estimation of HBV surface antigen (HBsAg) and HBV e antigen (HBeAg). The remaining medium was processed to obtain virions by a polyethylene glycol precipitation method[7]. The viral DNA recovered from the secreted particles was subjected to Southern blot analysis. Cellular DNA was isolated according to the standard protocols. Inhibition of viral DNA replication was determined by comparison of the viral DNA from drug-treated and nontreated cultures. The level of inhibition was determined by hybridization of the blots to an HBV-specific probe followed by autoradiography. Quantitation of the autoradiographs was performed by density scanning with a computer software.
Since some of the nucleosides used in the treatment of HBV, such as FIAU[8], could affect liver function, especially mitochondrial function, a detailed analysis of the effect of β-L-D4A on mitochondrial DNA synthesis was carried out. 2.2.15 cells were cultured as above and after 24 h in culture, treatment with the compounds was initiated. β-L-D4A was added at concentrations of 0.4 μM and 10 μM in DMEM. Cultures treated with 0.4 μM 2’3’-dideoxycytidine (ddC) were maintained in parallel as positive controls for damage of mitochondrial DNA. Blank control was also set. Cellular DNA was isolated according to the standard protocols and digested with restriction enzyme BamH I. Hybridizations and detection of the mitochodrial DNA were done according to the laboratory manual of molecular cloning. The probe was cytochrome oxidase III DNA labeled by 32pdCTP.
Cells were inoculated at a density of 5 × 103/ml per well in 24-well tissue culture plates. After 24 h in culture, the cells were treated with various concentrations of β-L-D4A in DMEM for 3 days. Then 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays were performed using the cell titer kitTM (Promega) following the standard procedure with the following exceptions. At the time points described as illustrations, 15 μl MTT reagent was added per well, allowed to incubate for 30 minutes, after which 130 μl stop/lysis buffer was added. Plates were sealed with ParafilmTM and left overnight at room temperature to allow solubilization of the formazan salt product. Absorbance was measured at 750 nm and 570 nm using a Thermomax (Molecular Devices, San Jose, CA), or a cytoFluor microplate reader (PE Biosystems, Foster City, CA). The data were normalized (A570-A750 nm) and the mean absorbance (5 wells/concentration) was plotted against drug concentration. The ID50 values were calculated as described above.
HBsAg and HBeAg in the culture medium were determined according to the protocols supplied by the manufacturer. Essentially, the culture medium was appropriately diluted with phosphate-buffered saline and absorbed on the surface of plates coated by antibody to HBsAg or HBeAg. After an incubation period, the plates were washed and incubated in orthophenylene diamine. After a 30-min incubation, the reaction was terminated by adding 1N sufuric acid. The A490 of the final reaction was read. Appropriate positivity and negativity were assayed along with the samples.
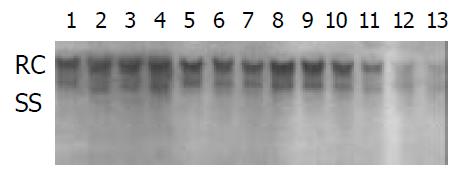
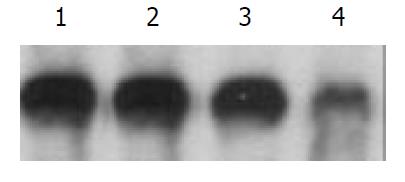
The 2.2.15 cell line was used to evaluate the antiviral activities of ddA analogues: D-D4A, L-D2A, D-D2A (Structures are shown in Figure 1). The antiviral effects were measured by an analysis of extracellular HBV DNA. The experiment revealed that the inhibition of HBV DNA was more strong by L-isomer than by D-isomer and much more notable by L-D4A than by L-D2A (Figure 2).
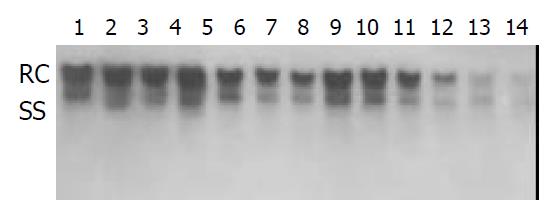
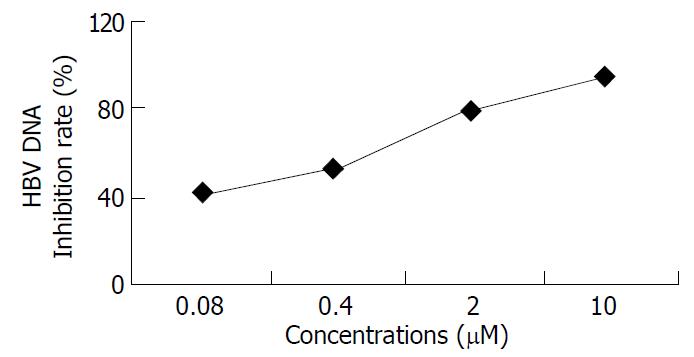
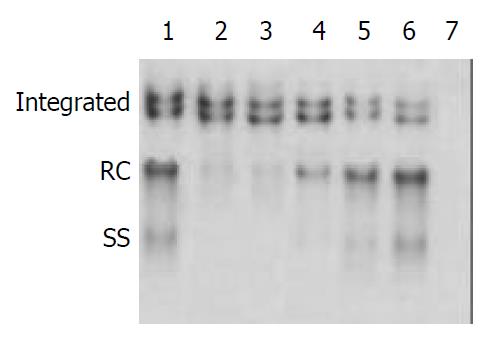
Inhibition of HBV DNA replication by β-L-D4A was evident as demonstrated by the amount of DNA obtained from the secreted viral particles as well as from the intracellular episomal particles. Concentrations of β-L-D4A ranging from 0.08 μM to 10 μM produced a dose-dependent inhibition (Figure 3). The intensity of autoradiographic bands for extracellular HBV DNA was quantitated by densitometric scans of computer and ED50 was calculated at 0.2 μM(Figure 4). Analysis of the intracellular episomal HBV DNA reflected similar trends in inhibition (Figure 5).
Hybridization of intracellular DNA to a cytochrome oxidase III was done to evaluate the effect of β-L-D4A on mitochondrial DNA. Mitochondrial DNA levels treated with 0.4 μM and 10 μM β-L-D4A were nearly similar to the blank control, but were obviously lower for ddC at 0.4 μM (Figure 6).
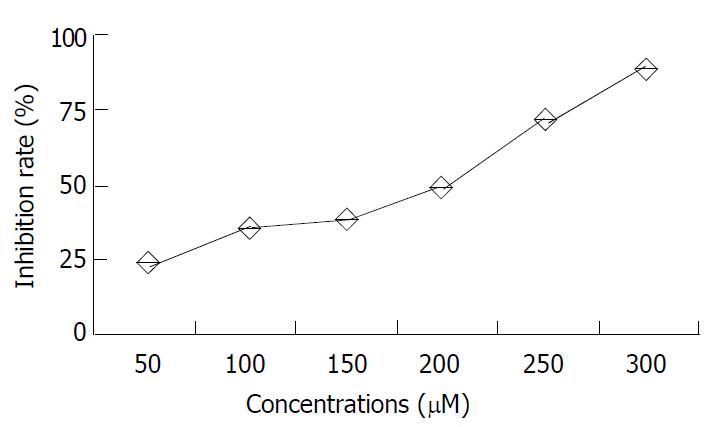
β-L-D4A did not show evident toxicity to 2.2.15 cells even at a concentration of 10 μM, but at high concentrations it had cytotoxicity, with 50% inhibitory concentration (ID50) of 200 μM, The data are shown in Table 1 and Figure 7.
| Dosage (μM) | n | A value (-x±s) | Inhibition rate (%) |
| 50 | 3 | 0.84 ± 0.22 | 22.3 |
| 100 | 3 | 0.75 ± 0.16 | 36.0 |
| 150 | 3 | 0.73 ± 0.12 | 37.2 |
| 200 | 3 | 0.67 ± 0.21 | 50.4 |
| 250 | 3 | 0.53 ± 0.15 | 73.5 |
| 300 | 3 | 0.43 ± 0.25 | 89.2 |
| 0 | 3 | 2.01 ± 0.23 | 0 |
Measurements of the levels of viral surface antigen and e antigen from the media of cultures treated with β-L-D4A revealed that β-L-D4A had no significant inhibitory effect on HBsAg and HBeAg at low concentrations, but had marked effect of reducing HBsAg and HBeAg at 2 μM and 10 μM, significantly different from the blank group (P < 0.05 or P < 0.01, respectively) (Shown in Table 2).
One rational approach to the development of drugs for the treatment of HBV infection in patients is to identify those compounds that specifically inhibit HBV DNA replication[9,10]. Since deoxynucleoside analogues were found to be effective on a variety of viruses, several compounds have been tested in vitro and in vivo against hepadnaviruses[9-12]. The selectivity of these compounds against HBV in general is assessed by their relative potency against HBV versus cellular toxicity[13-16]. Cytotoxicity studies are usually conducted by growth retardation assays that measure cell growth in the presence of compounds tested for three or four generations[17]. This growth retardation assay could not, however, detect delayed cytotoxicity of deoxynucleoside analogues such as ddC that have an effect on cellular mtDNA synthesis. Since mitochondria play an important role in organ function, it was hypothesized that the delayed toxicity as shown by peripheral neuropathy (e.g., observed in patients treated with ddC analogs) could be due to decreases in mtDNA[18]. In this study, in addition to assessing their anti-HBV activities, compounds were examined for their effects on 4-day cell growth and on mtDNA.
Deoxynucleosides such as D2A and D4A can exist as (+)-or (-)- enatiomers. From the results we got, we could see that (-)-isomers were superior to (+)-isomers, and β-L-D4A was the most potent inhibitor of HBV replication in 2.2.15 cells, which could almost completely block HBV DNA replication at a concentration of 4 μM. However, (+) -isomers were almost inactive against HBV replication when tested up to 8 μM, mechanism of which might be in that the compounds could be deaminated intracellularly to the inactive analogues as reported before[19].
β-L-D4A was the most potent inhibitor of HBV replication in 2.2.15 cells from our results. No effects on mtDNA were observed. Cell growth retardation with the administration of β-L-D4A at low concentrations was not evident, but at high concentrations, β-L-D4A began to show cytotoxicity in a dose-dependent manner with 50% inhibitory concentration (ID50) of 200 μM. Since β-L-D4A does not inhibit mtDNA synthesis at concentrations that inhibit virus production, the delayed toxicity, such as peripheral neuropathy, associated with the treatment of ddC[20] may not occur. In addition, β-L-D4A is not toxic to proliferting cells at concentrations that completely block the synthesis of HBV virion, suggesting that acute bone marrow toxicity may not be a concern. The study on dosage-activity relationships showed that β-L-D4A could inhibit HBV DNA replication in a markedly dose-dependent manner with 50% inhibitory concentration (ED50) of 0.2 μM. As the therapeutic index (TI value) was equal to ID50/ ED50, we got the TI value of β-L-D4A at 1 000. TI of lamivudine was 750 as reported in another article[21]. Therefore β-L-D4A like lamivudine possesses potent anti-HBV replication effect and has a higher TI value. In addition to that, β-L-D4A shows inhibition of expression of HBV antigens at high concentrations, indicating that β-L-D4A may be able to decrease the levels of HBsAg and HBeAg with a long term use.
About the mechanism of action of β-L-D4A, we think it is likely the inhibition of viral DNA polymerase, chain-termination resulted from incorporation into elongated DNA strand, or both. The mechanism needs to be further explored.
On the basis of the study of nucleoside analogs against HIV, human immunodeficiency virus resistant to ddC is not cross resistant to the thymidine analog zidovudine[22]. This permits the possibility of HBV treatment with β-L-D4A in the event of resistance to cytosine analogs as lamivudine. Furthermore, possibilities of a combined therapy of β-L-D4A and lamivudine for HBV can be explored.
Edited by Xu XQ and Wang XL
| 1. | Bridges EG, Cheng YC. Use of novel beta-L(-)-nucleoside analogues for treatment and prevention of chronic hepatitis B virus infection and hepatocellular carcinoma. Prog Liver Dis. 1995;13:231-245. [PubMed] |
| 2. | Freiman JS, McCaughan GW. Current limitations to nucleoside analogue therapy for chronic hepatitis B virus infection in the liver transplant and non-transplant settings. J Gastroenterol Hepatol. 2000;15:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Pan-Zhou XR, Cui L, Zhou XJ, Sommadossi JP, Darley-Usmar VM. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrob Agents Chemother. 2000;44:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Van Draanen NA, Tisdale M, Parry NR, Jansen R, Dornsife RE, Tuttle JV, Averett DR, Koszalka GW. Influence of stereochemistry on antiviral activities and resistance profiles of dideoxycytidine nucleosides. Antimicrob Agents Chemother. 1994;38:868-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Zoulim F. Therapy of chronic hepatitis B virus infection: inhibition of the viral polymerase and other antiviral strategies. Antiviral Res. 1999;44:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Lai CL, Yuen MF. Profound suppression of hepatitis B virus replication with lamivudine. J Med Virol. 2000;61:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Hawkins AE, Zuckerman MA, Briggs M, Gilson RJ, Goldstone AH, Brink NS, Tedder RS. Hepatitis B nucleotide sequence analysis: linking an outbreak of acute hepatitis B to contamination of a cryopreservation tank. J Virol Methods. 1996;60:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Tennant BC, Baldwin BH, Graham LA, Ascenzi MA, Hornbuckle WE, Rowland PH, Tochkov IA, Yeager AE, Erb HN, Colacino JM. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;28:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Chu CK, Boudinot FD, Peek SF, Hong JH, Choi Y, Korba BE, Gerin JL, Cote PJ, Tennant BC, Cheng YC. Preclinical investigation of L-FMAU as an anti-hepatitis B virus agent. Antivir Ther. 1998;3:113-121. [PubMed] |
| 10. | Colacino JM. Mechanisms for the anti-hepatitis B virus activity and mitochondrial toxicity of fialuridine (FIAU). Antiviral Res. 1996;29:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Lin JS, Kira T, Gullen E, Choi Y, Qu F, Chu CK, Cheng YC. Structure-activity relationships of L-dioxolane uracil nucleosides as anti-Epstein Barr virus agents. J Med Chem. 1999;42:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ma T, Pai SB, Zhu YL, Lin JS, Shanmuganathan K, Du J, Wang C, Kim H, Newton MG, Cheng YC. Structure--activity relationships of 1-(2-Deoxy-2-fluoro-beta-L-arabinofuranosyl)pyrimidine nucleosides as anti-hepatitis B virus agents. J Med Chem. 1996;39:2835-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Kotra LP, Xiang Y, Newton MG, Schinazi RF, Cheng YC, Chu CK. Structure-activity relationships of 2'-deoxy-2',2'-difluoro-L-erythro-pentofuranosyl nucleosides. J Med Chem. 1997;40:3635-3644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Qiu YL, Ptak RG, Breitenbach JM, Lin JS, Cheng YC, Kern ER, Drach JC, Zemlicka J. (Z)- and (E)-2-(hydroxymethylcyclopropylidene)-methylpurines and pyrimidines as antiviral agents. Antivir Chem Chemother. 1998;9:341-352. [PubMed] |
| 15. | Qiu YL, Ksebati MB, Ptak RG, Fan BY, Breitenbach JM, Lin JS, Cheng YC, Kern ER, Drach JC, Zemlicka J. (Z)- and (E)-2-((hydroxymethyl)cyclopropylidene)methyladenine and -guanine. New nucleoside analogues with a broad-spectrum antiviral activity. J Med Chem. 1998;41:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Du J, Surzhykov S, Lin JS, Newton MG, Cheng YC, Schinazi RF, Chu CK. Synthesis, anti-human immunodeficiency virus and anti-hepatitis B virus activities of novel oxaselenolane nucleosides. J Med Chem. 1997;40:2991-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Lu X, Gong S, Monks A, Zaharevitz D, Moscow JA. Correlation of nucleoside and nucleobase transporter gene expression with antimetabolite drug cytotoxicity. J Exp Ther Oncol. 2002;2:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Hostetler KY, Korba BE, Sridhar CN, Gardner MF. Antiviral activity of phosphatidyl-dideoxycytidine in hepatitis B-infected cells and enhanced hepatic uptake in mice. Antiviral Res. 1994;24:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Gudmundsson KS, Tidwell J, Lippa N, Koszalka GW, van Draanen N, Ptak RG, Drach JC, Townsend LB. Synthesis and antiviral evaluation of halogenated beta-D- and -L-erythrofuranosylbenzimidazoles. J Med Chem. 2000;43:2464-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2'3'-dideoxycytidine (ddC). Lab Invest. 2001;81:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Leung N. Liver disease-significant improvement with lamivudine. J Med Virol. 2000;61:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Descamps D, Flandre P, Joly V, Meiffrédy V, Peytavin G, Izopet J, Tamalet C, Zeng AF, Harel M, Lastère S. Effect of zidovudine resistance mutations on virologic response to treatment with zidovudine or stavudine, each in combination with lamivudine and indinavir. J Acquir Immune Defic Syndr. 2002;31:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |