Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1777
Revised: March 10, 2003
Accepted: March 29, 2003
Published online: August 15, 2003
AIM: To clone human 15ku selenoprotein gene.
METHODS: H9 human T cells were cultured in RPMI1640 medium supplemented with 100 mL/L fetal calf serum. mRNA was isolated from the cells. cDNA library was constructed by RT-PCR. The human 15ku selenoprotein gene was obtained by PCR and cloned into T vector and sequenced.
RESULTS: A unique cDNA fragment about 1244 bp was obtained. Sequence analysis identified an open reading frame within the cDNA. The gene had an in-frame TGA, which encoded selenocysteine (Sec), and a 3’-UTR SECIS element, which was required for synthesis of selenoprotein. The predicted protein molecular mass was about 15ku (162 residues). The result was identical with human liver 15ku selenoprotein gene published in Genbank.
CONCLUSION: Human 15ku selenoprotein gene can be successfully obtained from T cell line.
- Citation: Nan KJ, Li CL, Wei YC, Sui CG, Jing Z, Qin HX, Zhao LJ, Pan BR. Cloning of human 15ku selenoprotein gene from H9 T cells. World J Gastroenterol 2003; 9(8): 1777-1780
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1777
The trace element selenium (Se) is an essential human nutrient[1]. It has been shown to prevent cancers, especially liver and stomach cancers[2] in both epidemiological studies and clinical supplementation trials[3]. However, the mechanism by which Se suppresses tumor development remains unknown. Se is present in known human selenoproteins as amino acid selenocysteine (Sec)[4]. Sec is the active form of Se in selenoproteins and has important biological functions. Recently, a human 15ku selenoprotein (Sep15) containing Se in the form of Sec was identified. It was an acid protein with a PI value of 4.5. It was recently identified in human T cells[5,6] and was present in various human tissues, such as liver, kidney, testis and brain, but it was highly expressed in epithelial cells of the prostate gland[6] and thyroid[5]. The level of this selenoprotein was reduced substantially in hepatocellular carcinoma[7] and in a malignant prostate cell line[8]. Furthermore, epidemiological data have indicated a statistically significant inverse correlation between Se in the diet and occurrence of liver cancer. These facts have provoked our interest to study Sep15. The recent finding that the gene of Sep15 was located on human chromosome 1p31, often affected in human cancer[9], also supports the hypothesis that this protein might play a role in the development of cancers[5]. To get a better understanding of the relationship between Sep15 and tumor, and the mechanism by which it suppresses the tumor, we firstly cloned the gene of this selenoprotein.
H9 T cell line was purchased from ATCC. RPMI1640 was purchased from Gibco. Fetal bovine serum was from Hangzhou Sijiqing Company. Main biochemical reagents of T vector, T4 DNA ligase, Taq DNA polymerase and Trizol reagent were from Promega. Small amount plasmid extraction kit and PCR products purification kit were from Huashun Shengwu Engineering Company. Bacteria species JM109 was from the Department of Biochemistry of the Fourth Military Medical University. Agarose was from Huamei Shengwu Engineering Company. The restriction endonuclease Not I was from Takara. RNA extraction kit was from Promega. RT-PCR kit was from American Bior’s Company. The primers were synthesized by Georgia University of America. The forward primer was 5’-AGCGATGGCGGCTGGGCCGAG-3’. The backward primer was 5’-GATTTTTGAAACTTTTTATTTATA-3’.
mRNA extraction of H9 T cells H9 T cells were cultured in the RPMI1640 containing new born bovine serum under the condition of 50 mL/L CO2 at 37 °C in a CO2 incubator. About 107 H9 T cells were absolutely split with trizol reagent. Total RNA was extracted with chloroform, deposited with isopropanol, then dried at 37 °C. mRNA was isolated with a mRNA purification kit from Promega[10]. The procedure in details was performed according to the instructions of the kit.
RT-PCR[11] By using the reverse transcription system from Promega[12], cDNA synthesis was performed according to the following instructions. mRNA previously extracted and random hexamers were used to synthesize first strand of cDNA[13], which was used to synthesize the double-strand DNA in the latter PCR[14]. PCR was performed as follows[15,16]. The total volume of the PCR amplification system was 100 μL. First, 10 μL reverse transcription reaction liquid, 4 μL 20 pmol/ L primers (each 2 μL), 8 μL 10 mmol/L dNTP, 10 μL 10×PCR reaction liquid, and 10U TaqDNA were added consecutively, then water was added to a total volume of 100 μL. The PCR was performed by incubating at 94 °C for 2 min, at 94 °C for 1 min, at 55 °C for 1 min, at 72 °C for 1 min, totally 35 cycles. The reaction mixture was incubated at 72 °C for 7 min. Finally all the PCR products were used for 10 g/L agarose gel electrophoresis to purify the products. Purification was performed according to the instructions of the gel extraction kit from Huashun Company.
Linkage and conversion 5 μL T vector, 1 μL T4DNA ligase, 1 μL 10×buffer were added into previously purified PCR products, incubated at 16 °C overnight. 5 μL linkage products was picked up to infect competence bacteria of JM109. The conversion products were spread on a LB agarose plate containing ampicillin at 37 °C overnight.
Restriction endonuclease digestion and evaluation First, 3 monoclonal colonies were randomly chosen, and put into 10 g/L LB containing ampicillin, the culture was shaken at 37 °C overnight in air bath, then the plasmid of PGEM-T-15ku-selenoprotein was extracted. The procedure was performed according to the instructions of the low-dose plasmid extraction kit from Huashun Company. 5 μL previously extracted plasmid was digested with Not I. The reaction system containing 5 μL plasmid, 1 μL Not I, 2 μL 10×BSA buffer, 2 μL 10×H buffer, 2 μL 10×TritonX-100, and 8 μL sterilized water was performed at 37 °C for 3 hours. Finally 5 μL reaction mixture was used for 10 g/L agarose gel electrophoresis for 45 minutes. At the same time, the plasmid DNA was sent to the laboratory of Georgia University in America to sequence the DNA.
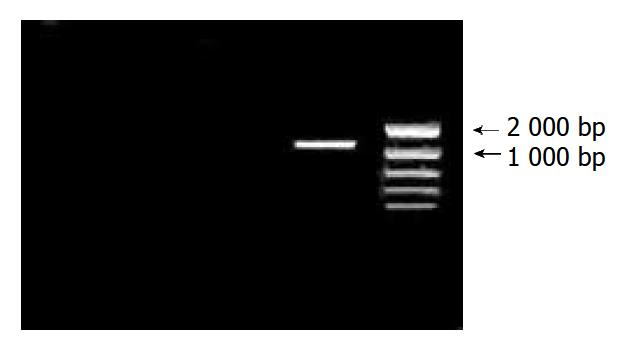
After 35 cycles of RT-PCR, 5 μL PCR products were used for 10 g/L agarose gel electrophoresis. When compared with the DNA marker, cDNA fragment was between 1000 bp and 2000 bp. It was coincident with the length of purposed gene (Figure 1).
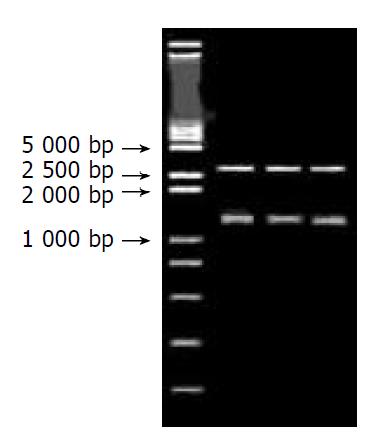
After 3 monoclonal colonies were randomly chosen, the plasmid was extracted and digested with Not I. Then, 5 μL was used for 10 g/L agarose gel electrophoresis, the straps were found to be coincident with the length of purposed gene (Figure 2).
The sequencing of PGEM-T-15ku-selenoprotein showed that the Sep15 gene we cloned was completely coincident with the sequence in GenBank. It was 100% homology with the human Sep15 gene. The sequences were as follows.
1 agcgatggcg gctgggccga gtgggtgtct ggtgccggcg tttgggctac ggttgttgtt ggcgactgtg
71 cttcaagcgg tgtctgcttt tggggcagag ttttcatcgg aggcatgcag agagttaggc ttttctagca
141 acttgctttg cagctcttgt gatcttctcg gacagttcaa cctgcttcag ctggatcctg attgcagagg
211 atgctgtcag gaggaagcac aatttgaaac caaaaagctg tatgcaggag ctattcttga agtttgtgga
281 tgaaaattgg gaaggttccc tcaagtccaa gcttttgtta ggagtgataa acccaaactg ttcagaggac
351 tgcaaatcaa gtatgtccgt ggttcagacc ctgtattaaa gcttttggac gacaatggga acattgctga
421 agaactgagc attctcaaat ggaacacaga cagtgtagaa gaattcctga gtgaaaagtt ggaacgcata
491 taaatcttgc ttaaattttg tcctatcctt ttgttacctt atcaaatgaa atattacagc acctagaaaa
561 taatttagtt ttgcttgctt ccattgatca gtcttttact tgaggcatta aatatctaat taaatcgtga
631 aatggcagta tagtccatga tatctaagga gttggcaagc ttaacaaaac ccatttttta taaatgtcca
701 tcctcctgca tttgttgata ccactaacaa aatgctttgt aacagacttg cggttaatta tgcaaatgat
771 agtttgtgat aattggtcca gttttacgaa caacagattt ctaaattaga gaggttaaca agacagatga
841 ttactatgcc tcatgtgctg tgtgctcttt gaaaggaatg acagcagact acaaagcaaa taagatatac
911 tgagcctcaa cagattgcct gctcctcaga gtctctccta tttttgtatt acccagcttt ctttttaata
981 caaatgttat ttatagttta caatgaatgc actgcataaa aactttgtag cttcattatt gtaaaacata
1050 ttcaagatcc tacagtaaga gtgaaacatt cacaaagatt tgcgttaatg aagactacac agaaaacctt
1121 tctagggatt tgtgtggatc agatacatac ttggcaaatt tttgagtttt acattcttac agaaaagtcc
1191 atttaaaagt gatcatttgt aagaccaaaa tataaataaa aagtttcaaa aatc
Se was recognized as an essential trace element in human and animal’s life by WHO in 1978. The relationship between Se and many kinds of disease including cancer is a hot point of medical research[17,18]. Usually, Se is incorporated into proteins in the forms of selenocysteine (Sec) and selenomethionine (SeMet). The term "selenoprotein" is restricted to the proteins which contain Se in the form of selenocysteine[19]. Selenoproteins are distinguished from proteins which nonspecifically incorporate selenomethionine not contributing to the biological function of the Se. About 21 specific selenoproteins have been identified in mammals and bacteria, and 18 of them could have biological functions attributed to them. Mammalian Se-containing proteins can be divided into three groups: proteins containing nonspecifically incorporated Se, specific Se-binding proteins, and specific selenocysteine-containing selenoproteins[5]. Selenoproteins with a known function identified so far include five glutathione peroxidases, two deiodinases, several thioredoxin reductases, and selenophosphate synthetase 2. Sep15, selenoprotein P, selenoprotein W, an 18-ku selenoprotein and several selenoproteins identified in silico from nucleotide sequence databases have been found to contain selenocysteine, but their functions are not known. Gel electrophoretic separation of tissue samples from rats labeled with 75Se showed the existence of further Se-containing proteins[5].
It has been shown that Se could prevent cancer in a variety of animal model systems[20,21]. Both epidemiological studies and supplementation trial supported its efficacy in humans[22]. However, the mechanism by which Se suppresses tumor development remains unknown[23]. Se is present in known human selenoproteins as selenocysteine. Selenocysteine represents the 21st amino acid and is encoded by UGA triplet in selenoprotein mRNA[24,25]. Although UGA most often functions as a stop codon, UGA-encoded incorporation of selenocysteine into the growing polypeptide is determined by the presence of a specific stem-loop secondary structure within the 3’-untranslated region of the selenoprotein mRNA[26]. Sep15 was firstly found in human T cells, and it contains a selenocysteine residue encoded by TGA. Its coding sequence has no homology to known protein-encoding genes. Computer analysis of transcript map databases indicated that this gene included five exons and four introns[27]. Recent findings indicate that the chromosome, in which the gene of Sep15 is located, is a genetic locus commonly mutated or deleted in human cancers. One in-frame TGA codon and two stem loop structures resembling selenocysteine insertion sequence (SECIS) elements were identified in the 3’-untranslated region of the gene, and only one of them was functional[9]. Examination of the available cDNA sequence of this protein revealed that two polymorphisms were located at position 811(C/T) and 1125 (G/A)[28] within the 3’-untranslated region. They were organized into two alleles, C811/G1125 and T811/A1125 in the 68%/32% frequency distribution. These 3’-untranslated region polymorphisms resulted in changes in selenocysteine incorporation into protein and different response. To Se supplementation[9]. Human epidemiological studies have revealed that Se has a negative correlation with the occurrence of prostate cancer[29,30] and lung cancer[31]. Moreover, recent investigations have shown that Se supplementation may be effective on the reduction of common human cancers, including prostate cancer[9], colon[32] cancer and lung cancer. Northern blot analysis of the human Sep15 mRNA demonstrated that the expression of Sep15 was significantly decreased in malignant prostate cancer cell line and in hepatocellular carcinoma cell line. The Sep15 protein levels in liver tumors, adjacent tissues, and normal hepatic tissue were significantly different. The Sep15 level was significantly decreased in tumors compared with that in the normal control. It was consistent with the observation that Sep15 protein was not detectable in mouse prostate adenocarcinoma cells, while normal mouse prostate showed a strong signal with Sep15 protein-specific antibodies.
Different expression patterns of the Sep15 protein in normal and malignant tissues, the occurrence of polymorphism associated with protein expression, the role of Se in differential regulation of polymorphisms, and loss of heterozygosity at the Sep15 locus in certain human tumor types make us suggest that Sep15 may be involved in cancer development[28]. We have cloned the gene of Sep15 in our country for the first time in order to study the expression of Sep15 protein in different tissue, its structure, function and the relationship with cancer due to the worldwide cancer[33-37] , and digestive in china[38-40].
Edited by Xu XQ and Wang XL
| 1. | Yu MW, Horng IS, Hsu KH, Chiang YC, Liaw YF, Chen CJ. Plasma selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection. Am J Epidemiol. 1999;150:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Tröbs M, Renner T, Scherer G, Heller WD, Geiss HC, Wolfram G, Haas GM, Schwandt P. Nutrition, antioxidants, and risk factor profile of nonsmokers, passive smokers and smokers of the Prevention Education Program (PEP) in Nuremberg, Germany. Prev Med. 2002;34:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Popova NV. Perinatal selenium exposure decreases spontaneous liver tumorogenesis in CBA mice. Cancer Lett. 2002;179:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Leblondel G, Mauras Y, Cailleux A, Allain P. Transport measurements across Caco-2 monolayers of different organic and inorganic selenium: influence of sulfur compounds. Biol Trace Elem Res. 2001;83:191-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Behne D, Kyriakopoulos A. Mammalian selenium-containing proteins. Annu Rev Nutr. 2001;21:453-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 303] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN. Association between the 15-kDa selenoprotein and UDP-glucose: glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2001;276:15330-15336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Chin-Thin W, Wei-Tun C, Tzu-Ming P, Ren-Tse W. Blood concentrations of selenium, zinc, iron, copper and calcium in patients with hepatocellular carcinoma. Clin Chem Lab Med. 2002;40:1118-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the Selenium and Vitamin E Cancer Prevention Trial: rationale and design. Prostate Cancer Prostatic Dis. 2000;3:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kumaraswamy E, Malykh A, Korotkov KV, Kozyavkin S, Hu Y, Kwon SY, Moustafa ME, Carlson BA, Berry MJ, Lee BJ. Structure-expression relationships of the 15-kDa selenoprotein gene. Possible role of the protein in cancer etiology. J Biol Chem. 2000;275:35540-35547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol. 2002;8:124-127. [PubMed] |
| 11. | Liu LX, Jiang HC, Liu ZH, Zhou J, Zhang WH, Zhu AL, Wang XQ, Wu M. Integrin gene expression profiles of human hepatocellular carcinoma. World J Gastroenterol. 2002;8:631-637. [PubMed] |
| 12. | Jiang RL, Lu QS, Luo KX. Cloning and expression of core gene cDNA of Chinese hepatitis C virus in cosmid pTM3. World J Gastroenterol. 2000;6:220-222. [PubMed] |
| 13. | Li Y, Yang L, Cui JT, Li WM, Guo RF, Lu YY. Construction of cDNA representational difference analysis based on two cDNA libraries and identification of garlic inducible expression genes in human gastric cancer cells. World J Gastroenterol. 2002;8:208-212. [PubMed] |
| 14. | Li Y, Lu YY. Applying a highly specific and reproducible cDNA RDA method to clone garlic up-regulated genes in human gastric cancer cells. World J Gastroenterol. 2002;8:213-216. [PubMed] |
| 15. | Zhao Y, Wu K, Xia W, Shan YJ, Wu LJ, Yu WP. The effects of vitamin E succinate on the expression of c-jun gene and protein in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:782-786. [PubMed] |
| 16. | Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:250-253. [PubMed] |
| 17. | Ujiie S, Kikuchi H. The relation between serum selenium value and cancer in Miyagi, Japan: 5-year follow up study. Tohoku J Exp Med. 2002;196:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Hara S, Shoji Y, Sakurai A, Yuasa K, Himeno S, Imura N. Effects of selenium deficiency on expression of selenoproteins in bovine arterial endothelial cells. Biol Pharm Bull. 2001;24:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Mostert V. Selenoprotein P: properties, functions, and regulation. Arch Biochem Biophys. 2000;376:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Riondel J, Wong HK, Glise D, Ducros V, Favier A. The effect of a water-dispersible beta-carotene formulation on the prevention of age-related lymphoid neoplasms in mice. Anticancer Res. 2002;22:883-888. [PubMed] |
| 21. | Popova NV. Perinatal selenium exposure decreases spontaneous liver tumorogenesis in CBA mice. Cancer Lett. 2002;179:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Nakaji S, Fukuda S, Sakamoto J, Sugawara K, Shimoyama T, Umeda T, Baxter D. Relationship between mineral and trace element concentrations in drinking water and gastric cancer mortality in Japan. Nutr Cancer. 2001;40:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Calvo A, Xiao N, Kang J, Best CJ, Leiva I, Emmert-Buck MR, Jorcyk C, Green JE. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325-5335. [PubMed] |
| 24. | Korotkov KV, Novoselov SV, Hatfield DL, Gladyshev VN. Mammalian selenoprotein in which selenocysteine (Sec) incorporation is supported by a new form of Sec insertion sequence element. Mol Cell Biol. 2002;22:1402-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Zhang W, Cox AG, Taylor EW. Hepatitis C virus encodes a selenium-dependent glutathione peroxidase gene. Implications for oxidative stress as a risk factor in progression to hepatocellular carcinoma. Med Klin (Munich). 1999;94 Suppl 3:2-6. [PubMed] |
| 26. | Mansur DB, Hao H, Gladyshev VN, Korotkov KV, Hu Y, Moustafa ME, El-Saadani MA, Carlson BA, Hatfield DL, Diamond AM. Multiple levels of regulation of selenoprotein biosynthesis revealed from the analysis of human glioma cell lines. Biochem Pharmacol. 2000;60:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565-3576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 470] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Hu YJ, Korotkov KV, Mehta R, Hatfield DL, Rotimi CN, Luke A, Prewitt TE, Cooper RS, Stock W, Vokes EE. Distribution and functional consequences of nucleotide polymorphisms in the 3'-untranslated region of the human Sep15 gene. Cancer Res. 2001;61:2307-2310. [PubMed] |
| 29. | Brooks JD, Metter EJ, Chan DW, Sokoll LJ, Landis P, Nelson WG, Muller D, Andres R, Carter HB. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urol. 2001;166:2034-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Gasparian AV, Yao YJ, Lü J, Yemelyanov AY, Lyakh LA, Slaga TJ, Budunova IV. Selenium compounds inhibit I kappa B kinase (IKK) and nuclear factor-kappa B (NF-kappa B) in prostate cancer cells. Mol Cancer Ther. 2002;1:1079-1087. [PubMed] |
| 31. | Tröbs M, Renner T, Scherer G, Heller WD, Geiss HC, Wolfram G, Haas GM, Schwandt P. Nutrition, antioxidants, and risk factor profile of nonsmokers, passive smokers and smokers of the Prevention Education Program (PEP) in Nuremberg, Germany. Prev Med. 2002;34:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Casimiro C. [Etiopathogenic factors in colorectal cancer. Nutritional and life-style aspects. 2]. Nutr Hosp. 2002;17:128-138. [PubMed] |
| 33. | Sun XW, Shen BZ, Shi MS, Dai XD. Relationship between CD44v6 expression and risk factors in gastric carcinoma patients. Shijie Huaren Xiaohua Zazhi. 2002;10:1129-1132. |
| 34. | Zhou HB, Zhang JM, Yan Y. Inactivation of DPC4 gene in colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:1140-1142. |
| 35. | Zhou XD, Yu JP, Ran ZX, Luo HS, Yu BP. Expression of cFLIP and p53 mutation in adenocarcinoma of colon. Shijie Huaren Xiaohua Zazhi. 2002;10:536-539. |
| 36. | Dong K, Li B, Qin Y, Li CZ, Lui JY, Sun ZL, Sun ZF. Methyla-tion pattern analysis in CpG islands of p15 and p16 tumor suppressor genes in pancreatic carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 2002;10:1264-1267. |
| 37. | Cui M, Zhang HJ, An LG. Tumor growth Inhibition by polysac-charide from Coprinus comatus. Shijie Huaren Xiaohua Zazhi. 2002;10:287-290. |
| 38. | Gong JQ, Fang CH. Relationship between the oval cells and de-velopment of hepatocellular carcinoma in rats. Shijie Huaren Xiaohua Zazhi. 2002;10:1133-1139. |
| 39. | Yang L, Wang YP, Wu DY, Zhang SM, Li JY, Zhang YC, Xin Y. Pathological behaviors and molecular mechanisms of signet-ring cell carcinoma and mucinous adenocarcinoma of stomach: a com-parative study. Shijie Huaren Xiaohua Zazhi. 2002;10:516-524. |
| 40. | Zhu JS, Zhu L, Wang L, Zhuang QX, Hu B, Da W, Chen WX, Chen GQ, Ma JQ. Autologous peripheral blood stem cell com-bined with high-dose arterial chemotherapy for advanced gas-tric cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:1408-1411. |