Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1756
Revised: January 21, 2003
Accepted: March 10, 2003
Published online: August 15, 2003
AIM: To construct a recombinant vector which can express outer membrane protein (OMP) with Mr18000 and heat shock protein A (HspA) from Helicobacter pylori (H. pylori) in E. coli BL21, and to exploit the possibility for obtaining the vaccine conferring protection from H. pylori infection.
METHODS: The target gene of HspA was amplified from H. pylori chromosome by PCR, and then inserted into the prokaryotic expression vector pET32a (+) by restrictive endonuclease enzyme kpn I, BamH I simultaneously. The recombinant vector was used to sequence, and then together with pET32a (+)/Omp18, digested by restrictive endonuclease enzyme Hind III and BamH I simultaneously. pET32a(+)/ HspA and Omp18 were recovered from 1% agarose gel by gel kit, and ligated with T4 ligase by BamH I digested viscidity end. The recombinant plasmid of pET32a(+)/HspA/Omp18 was transformed and expressed in E. coli BL21 (DE3) under induction of IPTG. After purification, its antigenicity of the fusion protein was detected by Western blot.
RESULTS: Enzyme digestion analysis and sequencing showed that the target genes were inserted into the recombinant vector, composed of 891 base pairs, encoded objective polypeptides of 297 amino acid residues. Compared with GenBank reported by Tomb et al there were 1.3% and 1.4% differences in obtained H. pylori nucleotide sequence and amino acid residues, respectively. SDS-PAGE analysis showed that relative molecule mass (Mr) of the expressed product was Mr 51000, Mr of protein expressed by pET32a (+) was about Mr 20000, and soluble expression product accounted for 18.96% of total bacterial protein. After purification with Ni+2-NTA agarose resins, the purification of recombinant fusion protein was about 95%. Western blot showed that recombinant fusion protein could be recognized by the patients’ serum infected with H. pylori and anti-Omp18 monoclone, suggesting that this protein had good antigenicity.
CONCLUSION: The gene coding for H. pylori Mr18000 OMP and HspA was cloned and expressed successfully. The results obtained lay the foundation for development of H. pylori protein vaccine and a quick diagnostic kit.
-
Citation: Jiang Z, Huang AL, Tao XH, Wang PL. Construction and characterization of bivalent vaccine candidate expressing HspA and
M r18000 OMP fromHelicobacter pylori . World J Gastroenterol 2003; 9(8): 1756-1761 - URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1756
Helicobacter pylori (H.pylori) is a microaerophilic, spiral and gram-negative bacillus first isolated from human gastric antral epithelium in 1982. It has been recognized as a human-specific gastric pathogen that colonizes the stomaches of at least half the world’s population[1], and there are approximately thousands of newly infected people annually. Most infected individuals are asymptomatic. However, in some individuals, their infections are associated with the development of peptic ulcer, gastric adenocarcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma and primary gastric non-Hodgkin’s lymphoma[2-20], moreover with extradigestive diseases[21-37]. This organism was recently categorized as a class I carcinogenetic factor by the World Health Organization, and direct evidence of carcinogenesis was recently demonstrated in an animal model[38,39]. Although there are many methods for eradication of H. pylori infection, such as bi-, tri- drug therapy, the definitive curative effects were acquired by using a serial of antibiotics, which has led to resistant H. pylori. Meantime medical side effects, patients’ endurance and compliance were challenged. This has drawn increasing interests of scientists in developing H. pylori vaccine so as to reduce and prevent H. pylori infection, extinct diseases associated with H. pylori infection. Immunization against this bacterium represents a cost-effective strategy to reduce global H. pylori-gastric cancer and peptic ulcer rates[40]. To date, H. pylori vaccine candidate antigens identified include urease enzyme, VacA, and so on[41-50]. Mr18000 and HspA are outer membrane proteins of H. pylori, and the vaccines prepared with Mr18000 OMP and HspA respectively were used to inoculate Balb/c mice, 70%-80% of experimental mice were protected. In order to acquire a better immunocompetent effect, some investigators suggested that bi-valent antigen vaccine was possibly superior to single antigen. So in this study, the recombinant plasmid encoding H. pylori Mr18000 OMP and HspA genes was constructed and expressed in BL21 to explore the possibility for obtaining a vaccine conferring protection from H. pylori infection.
A well-characterized strain of H. pylori was afforded by the Department of Microbiology, Chongqing University of Medical Sciences. Top10, BL21 E.coli strains and pET32a (+), pET32a(+)/Omp18 plasmid, anti-Omp18 antibody were provided by the Institute of Viral Hepatitis of Chongqing University of Medical Sciences. Restriction endonuclease enzymes (Kpn I, Hind III, BamHI) and T4DNA ligase were purchased from Promega, TagDNA polymerase was produced by the Immunology Department of Beijing University of Medical Sciences. Isopropyl-β-D-thiogalactopyranoside (IPTG), dNTP and oligonucleotide primers were obtained from Sigma.
Oligonucleotide primers were designed to amplify H. pylori open reading frame (ORFs) of HspA based on GenBank. The primers had a KpnI site incorporated into the 5’ end and a BamHI site at the 3’ end and their sequences were as follows (5’-3’): CCGGTACCATGAAGTTTCAACCATTAGG (forward) and CCGGATCCGTGTTTTTTGTGATCATGAC (reverse). The reverse 5’ end stop codon TAA was banned. Genomic DNA prepared from Chinese H. pylori strains was used as the template in PCR. The PCR consisted of 30 cycles of denaturation at 94 °C for 60 s, annealing at 52 °C for 50 s, and an extension step at 72 °C for 50 s. The products were visualized on 10 g·L-1 agarose gel and purified using a PCR purification kit. After digestion with the restriction endonuclease enzymes BamHI and KpnI simultaneously, the purified products were cloned into the compatible sites of the expression vector pET32a (+) by using T4 DNA ligase at a molar ratio of 4:1 at 4 °C overnight.
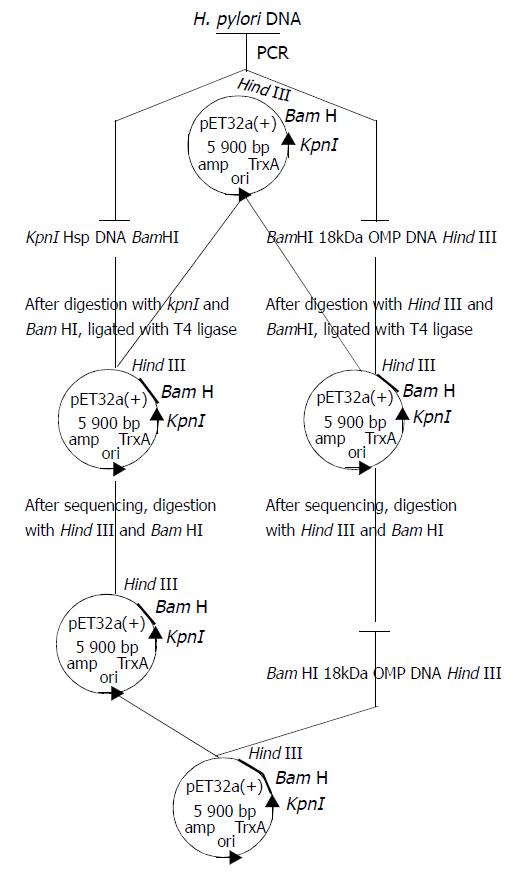
After the above connected products were transfected into Top10, pET32a(+)/HspA was selected and identified by the methods reported by Jiang et al[51]. After pET32a(+)/HspA and pET32a(+)/Omp18 were digested by restrictive endonuclease enzymes BamHI and Hind III simultaneously, the segments of Omp18 and pET32a(+)/HspA were recycled by gel extract kit, and ligated by using T4 DNA ligase at a molar ratio of 4:1 at 4 °C overnight. pET32a(+)/HspA /Omp18 was selected, appraised by PCR or enzyme digestion (Figure 1).
The single bacterial colony (Top10/pET32a (+)/HspA/Omp18) was picked, and cultivated in 2 ml LB broth containing 100 mg·L-1 of ampicillin, at 300 r·min-1 at 37 °C overnight, then recombinant plasmids were extracted according to the manufacturer’s instructions, in the meantime, identified by PCR and restriction endonuclease enzyme digestion. The recombinant plasmid was transfected into competent BL21 (DE3) E.coli strains by using standard procedures reported by Jiang et al[51] BL21 E.coli strains containing recombinant plasmid were grown until mid-log phase (optical density at 600 nm = 0.5 to 1.0), and then induced to express recombinant fusion protein by adding 1 mmol·L-1 IPTG for 4 h. Following induction, bacteria were harvested by centrifugation at 12000 r·min-1 for 2 min, resuspended in protein-buffer and seethed for 5 min. Total proteins were electrophoresed on 150 g·L-1 SDS-PAGE gel and stained with Coomassie. The rate of recombinant fusion protein to total protein was deduced by Image Master Totallab v1.11 software.
Due to C end of recombinant fusion antigen with six histidines, the recombinant fusion antigen was purified by using Ni2+-NTA agarose resin. Briefly, 500 ml of bacteria cultivated suspension was prepared, centrifugated, resuspended with the buffer liquid (50 mmol·L-1 phosphate, 300 mmol·L-1 NaCl, pH 7.0), and sonicated by ultrasonic wave with the energy of 600W × 35% for 40 min, and ultracentrifugated for 15 min at 10000 r·min-1 at 4 °C. The sonicated recombinant fusion antigen was purified by using Ni2+-NTA agarose resin with abluent (50 mmol·L- 1 phosphate, 300 mmol·L-1 NaCl, 20 mmol·L-1 imidazole, pH 7.80) and lavation (50 mmol·L-1 phosphate, 300 mmol·L-1 NaCl, 250 mmol·L-1 imidazole, pH 7.80) respectively, and quantified. The antigenicity of expressed recombinant fusion protein was determined by immunoblotting. Following electrophoretic transfer of SDS-PAGE-separated (150 g·L-1 acrylamide) recombinant fusion protein to 0.45 µm pore size PVDF membrane, and after a 30-min wash in tris-saline blotting buffer, antigen-impregated PVDF strips were incubated with the sera of patients infected with H. pylori and anti-Omp18 antibody for 2 h at RT. After a washing, the protein was detected by incubating the strips in alkaline phosphatase-conjugated goat anti-man IgG antibody for 1 h at RT.
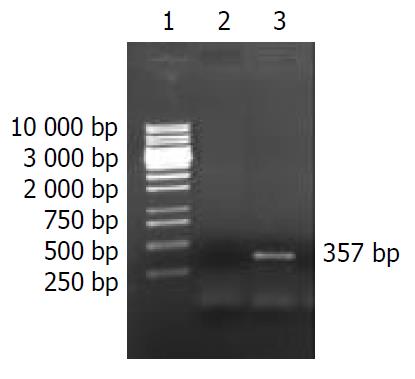
According to literature[55], H. pylori HspA ORF was amplified by PCR with Chinese H. pylori strain’s chromosomal DNA as the templates. The cloning products were electrophoresed and visualized by 10 g·L-1 agarose gel (Figure 2). It revealed that the size of HspA DNA fragment amplified by PCR was between 250-500 base pairs, and contained a gene of approximately 357 nucleotides, and was compatible with the previous reports[55].
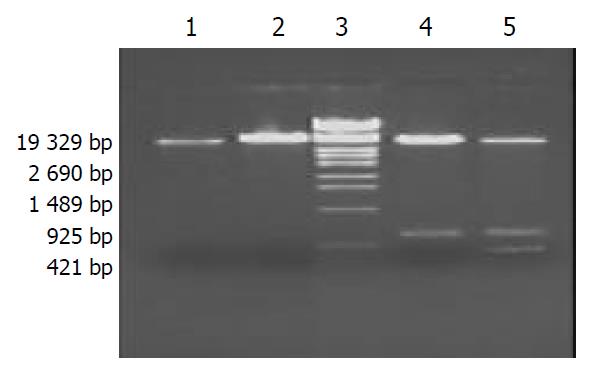
pET32a(+)/HspA identification by PCR After the single colony of Top10 E. coli /recombinant pET32a(+)/HspA was picked and incubated in 2 ml LB broth containing 100 mg·L-1 of ampicillin at 300 r·min-1 at 37 °C overnight, then 50 μl was incubated and seethed for 10 min, with the genomes of supernate and recombinant vector as templates respectively, products were amplified by PCR under the condition as mentioned above. The PCR products were visualized by 10 g·L-1 agarose gel electrophoresis (Figure 3). It indicated that recombinant plasmid contained the objective gene. At the same time, it was successful in transfecting recombinant plasmid into Top10 E. coli.
pET32a(+)/HspA-Omp18 identification by restriction enzyme digestion Recombinant plasmids pET32a(+)/HspA-Omp18 were digested by single, bi-, tri-enzyme digestion with HindIII, KpnI and BamHI, respectively, then digestive products were visualized on 10 g·L-1 agarose gel (Figure 4). It demonstrated that recombinant plasmids were digested to 357 bp, 528 bp DNA fragment, and contained the objective gene.
The nucleotide sequence of the cloned genes Mr18000 OMP and HspA inserted into pET32a (+) was analyzed by automated sequencing across the cloning junction, using the universal primer T7. The results showed that the cloned genes Mr18000 OMP and HspA were connected by BamH I enzyme digestion adhesion end. The cloned HspA genes contained 357 nucleotides with a promoter codon coding a putative protein of 119 amino acid residues with a calculated molecular mass of Mr13000, and provided a putative signal peptide. Compared with previous reports, 5 base pairs of the cloned gene and 2 amino acid residues (G→D, A→S) encoded were changed. The cloned gene Mr18000 OMP and its encoding protein sequences were published in GenBank (AF374387).
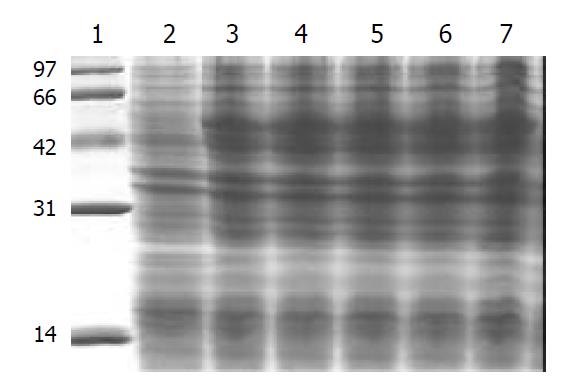
After pET32a(+)/HspA-Omp18 was transfected into BL21 E. coli strains, the strains with high expressions of fusion proteins were selected. BL21 (DE3) E.coli strains containing recombinant plasmid were grown until mid-log phase (optical density at 600 nm = 0.4 to 0.6), and then induced to express recombinant fusion protein by adding of 1 mmol·L-1 IPTG for 4 h. Following induction, bacteria were harvested by centrifugation at 12000 r·min-1 for 5 min, resuspended in protein-buffer and seethed for 5 min. Total protein was electrophoresed on 150 g·L-1 SDS-PAGE gel and stained with Coomassie. Its molecular mass was Mr 51000 by 150 g·L-1 SDS-PAGE gel analysis. After the recombinant bacteria were sonicated by ultrasonic wave and ultracentrifuged (10000 r·min-1, 15 min, 4 °C), the level of soluble fusion protein in the supernate was about 18.96% of total cellular protein. After purification by Ni2+-NTA agarose resin columniation, the purity of recombinant fusion protein was about 95% (Figure 5).
After bacteria BL21/pET32a(+)/HspA-Omp18, BL21/pET32a (+), BL21/pET32a(+)/HspA were induced to cultivate by adding of 1 mmol·L-1 IPTG for 4 h respectively, 1 ml of cultivated medium was respectively ultracentrifuged, resuspended in protein-buffer and seethed for 5 min. Total protein was electrophoresed on 150 g·L-1 SDS-PAGE gel, and then the proteins of SDS-PAGE-separated (150 g·L-1 acrylamide) were transferred to 0.45 µm pore size PVDF membrane at 14V, at 4 °C overnight. Following a 30-min wash in tris-saline blotting buffer, antigen-impregated PVDF strips were incubated with the sera of patients infected with H. pylori and anti-Omp18 antibody for 2 h at RT. After a washing, the proteins were detected by incubating the strips in alkaline phosphatase-conjugated goat anti-man IgG antibody for 1 h at RT. In this study, antigen-impregated PVDF strips of BL21/ pET32a(+)/HspA-Omp18 were recognized by anti-Omp18 antibody, showing brown strip corresponding to the site of the recombinant fusion protein. Antigen-impregated PVDF strips of BL21/pET32a(+)/HspA were recognized by patient’s sera infected with H. pylori, also showing brown strip corresponding to the site of the fusion protein, while antigen-impregated PVDF strips of BL21/pET32a(+) were not recognized by patient’s sera infected with H. pylori (Figure 6).
Heat shock phenomena were found by a geneticist Ritossa in studying on change of drosophila cell chromosome stimulated by heat in 1962. Heat shock protein, found by Tissiers et al in 1974, indwells in man, microbe, foliage, and animal. It belongs to secretory protein, and accounts for 5% of total cellular proteins. When cells are stimulated by environment, Hsps are induced to synthesize. A series of heat shock proteins, such as HspA, HspB, Hsp60, and Hsp70, synthesized by H. pylori, play a significant role in H. pylori pathogenesis, for example, taking part in regulation of cell immunization, initiating auto-immunoreaction of gastric epithelial cell, serving as a promotor in development of chronic gastric pathological changes, and mediating recognition and adhesion of pathogens with host. HspA and HspB genes encode 118, 545 amino acid residues respectively, corresponding to calculated molecular mass of Mr 13000, 58200. HspA is consisted of two domains: N domain is conservative sequence, associated with immune appearance; C domain is composed of 27 amino acid residues, including 8 histidines, and 4 cysteines. So the frame is a nickel combinative region, and plays a role in nickel ion translation and presentation. The experiments suggested that HspA, B were important factors in H. pylori conglutination and H. pylori active proteins stabilization under the extremely unfavorable conditions, meantime they increase urease activity. Others suggested that Hsp60 and HopZ (H. pylori outer membrane protein Z) of H. pylori were associated with H. pylori adhesion. HspA and HspB were homologous with E. coli GroEs and GroEI, associated with auto-immunoreaction, which might lead to the production of damaging auto-antibody. Animal experiments demonstrated that mice fed with HspA, B resulted in coronary artery sclerosis[52].
The outer membrane is a continuous structure on the surface of gram-negative bacteria and an asymmetric bilayer with phospholipids in the inner monolayer and the bulky glycolipid lipopolysaccharide (LPS). In the outer monolayer, H. pylori as bacterial pathogens, has particular significance as a potential target for inducing host protective immunity and escaping from the host’s immune system. Outer membrane vaccines have been used with considerable success to induce protection against a number of organisms, including the heat shock protein in H. pylori, urease A, and B. Mr18000 OMP is a lipoprotein (Lpp20) belonging to other outer membrane protein[53]. In an earlier study, immunoreactive species-specific Mr19500 H. pylori OMP actually was Lpp20-Mr18000 OMP. The Lpp20 antigen appears to be commonly expressed in all H. pylori strains examined so far. Furthermore, no cross-reaction was shown when antibodies (polyclonal and monoclonal) to H. pylori Lpp20 were used to immunoscreen closely related species of helicobacter, campylobacter, or a diverse range of other bacteria. It shows that the Lpp20 gene to be unique to H. pylori[54]. Keenan et al[53] demonstrated the protein was expressed on the surface of the bacteria by immunolabeling of H. pylori with gold-labeled anti-Lpp20 antibodies. Bacterial lipoproteins have been well described, not only as vaccine target candidates, but also as immunostimulatory molecules.
In order to overcome the weak antigenicity of a single antigen, H. pylori Mr18000 OMP and HspA gene were amplified by PCR, and inserted into pET32a(+) vector simultaneously in our study. The pET32a(+) vector was designed for cloning and high-level expression of peptide sequences with the 109aa Trx.·TagTM thioredoxin protein. Cloning sites were available to produce objective proteins also containing cleavable His·Tag and s·TagTM sequences for detection and purification. The expressed protein of pET32a(+) vector had a putative molecular mass of Mr 20000, so the expression of recombinant vector was a fusion protein with a calculated molecular mass of Mr 51000, consistent with our results. Compared with the reports, 1.8% of the cloned genes was mutated, and 1.7% of amino acid residues was changed. The reasons for the discrepancy might be as follows: (1) H. pylori chromosomal DNA as templates was different, (2) there were heterogeneity among H. pylori strains, and (3) H. pylori was provided with the ability of transformation, which could lead to H. pylori variation and genome reset[54]. But there was much homogeneity between them.
Todoroki et al[55] investigated the effect of DNA vaccines encoding H. pylori-heat shock proteins A and B (pcDNA3.1-hspA and -hspB) on inducing immune responses against H. pylori in mice. C57BL/six mice aged 5 weeks were immunized by a single injection of 10microg of pcDNA3.1-hspA and pcDNA3.1-hspB into intracutaneous tissue. Plasmid DNA lacking the inserted Hsp was injected as a control. The results demonstrated that DNA vaccines encoding H. pylori-Hsp induced significant immune response against H. pylori, decreased gastric mucosal inflammation, indicating that a pcDNA3.1-hspA or -hspB DNA vaccine can be a new approach against H. pylori in human. Jiang et al[51] reported that recombinant fusion OMP18 protein also had good antigenicity. While being an immunogenic marker, the patient sera infected with H. pylori Mr 18000 Omp antigen showed high sensitivity and specificity[56]. Moreover, a significant association was found between the serologic response to Mr 18000 Omp antigen and malignant outcome of H. pylori infection[57]. So the serum test for detecting antibody with lower-molecular-weight proteins of H. pylori could be used to identify H. pylori-infected patients at risk of peptic ulcer or malignancy. A recently published study also identified Mr 18000 Omp as a candidate following the successful immunization of mice with purified recombinant antigen. In our study, the purified Mr 51000 recombinant fusion HspA-Omp18 protein could be recognized by patients’ sera infected with H. pylori and anti-Omp18 monoclonal antibody, and the purified Mr 33000 recombinant fusion HspA protein could also be recognized by patients’ sera. The results demonstrated that recombinant fusion protein had good antigenicity. These showed that Mr 51000 recombinant fusion HspA-Omp18 protein would not only provide HspA characteristics, but possess Mr 18000 Omp specialty. Meantime, Mr 51000 recombinant fusion HspA-Omp18 protein was suggested to be a true vaccine candidate and not merely an immunogenic marker for H. pylori infection.
In addition to construction of the recombinant vector, looking for living carriers would be a key step. Immunization via the mucosal or intracutaneous-inoculated route offers the advantage that has the potential to stimulate both mucosal and systemic immunity. It is simple, safe and can be used for the immunization of a large population. Bacillus Calmette-Guerin (BCG), the attenuated strain of mycobacterium bovid and the current vaccine against tuberculosis, has been widely used as a living, innately immunogenic vehicle for multiple protective recombinant antigens for vaccines against pathogenic microorganisms. With the aim of developing a recombinant vaccine, vaccines against human immunodeficiency virus, diphtheria, pertussis, tetanus (DPT), and parasite[58-68] have been investigated. We are developing a living carrier-BCG to provide a mucosal or intracutaneous-inoculated vaccine vector to deliver Mr 51000 recombinant fusion HspA-Omp18 protein to antigen-presenting cells on mucosal surface. We believe that before long, H. pylori vaccine could be constructed successfully for eradicating H. pylori infection and H. pylori associated diseases.
Edited by Zhang JZ, Zhu LH and Wang XL
| 1. | Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthésy-Theulaz I, Losonsky G. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 5. | Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] |
| 7. | Xia HX, Fan XG, Talley NJ. Clarithromycin resistance in Helicobacter pylori and its clinical relevance. World J Gastroenterol. 1999;5:263-266. [PubMed] |
| 8. | Peng ZS, Liang ZC, Liu MC, Ouang NT. Studies on gastric epi-thelial cell proliferation and apoptosis in Hp associated gastric ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:218-219. |
| 9. | Xiao SD, Liu WZ. Current statue in treatment of Hp infection. Shijie Huaren Xiaohua Zazhi. 1999;7:3-4. |
| 10. | Meyer JM, Silliman NP, Dixon CA, Siepman NY, Sugg JE, Hopkins RJ. Helicobacter pylori and early duodenal ulcer status post-treatment: a review. Helicobacter. 2001;6:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Casella G, Buda CA, Maisano R, Schiavo M, Perego D, Baldini V. Complete regression of primary gastric MALT-lymphoma after double eradication Helicobacter pylori therapy: role and importance of endoscopic ultrasonography. Anticancer Res. 2001;21:1499-1502. [PubMed] |
| 12. | Hurenkamp GJ, Grundmeijer HG, Van Der Ende A, Tytgat GN, Assendelft WJ, Van Der Hulst RW. Arrest of chronic acid suppressant drug use after successful Helicobacter pylori eradication in patients with peptic ulcer disease: a six-month follow-up study. Aliment Pharmacol Ther. 2001;15:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Guo CQ, Wang YP, Liu GY, Ma SW, Ding GY, Li JC. Study on Helicobacter pylori infection and-p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:313-315. |
| 14. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Hu PJ. Hp and gastric cancer: challenge in the research. Shijie Huaren Xiaohua Zazhi. 1999;7:1-2. |
| 16. | Quan J, Fan XG. Progress in experimental research of Helicobacter pylori infection and gastric cancinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:1068-1069. |
| 17. | Delchier JC, Lamarque D, Levy M, Tkoub EM, Copie-Bergman C, Deforges L, Chaumette MT, Haioun C. Helicobacter pylori and gastric lymphoma: high seroprevalence of CagA in diffuse large B-cell lymphoma but not in low-grade lymphoma of mucosa-associated lymphoid tissue type. Am J Gastroenterol. 2001;96:2324-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041-2048. [PubMed] |
| 19. | Zhang XQ, Lin SR. Progress in research on the relationship be-tween Hp and stomach cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:206-207. |
| 20. | Hua JS. Effect of Hp: cell proliferation and apoptosis on stomach cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:647-648. |
| 21. | Armitage GC. Periodontal infections and cardiovascular disease--how strong is the association. Oral Dis. 2000;6:335-350. [PubMed] |
| 22. | Tsai CJ, Huang TY. Relation of Helicobacter pylori infection and angiographically demonstrated coronary artery disease. Dig Dis Sci. 2000;45:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Gocyk W, Nikliński T, Olechnowicz H, Duda A, Bielański W, Konturek PC, Konturek SJ. Helicobacter pylori, gastrin and cyclooxygenase-2 in lung cancer. Med Sci Monit. 2000;6:1085-1092. [PubMed] |
| 24. | Tsang KW, Lam WK, Kwok E, Chan KN, Hu WH, Ooi GC, Zheng L, Wong BC, Lam SK. Helicobacter pylori and upper gastrointestinal symptoms in bronchiectasis. Eur Respir J. 1999;14:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Caselli M, Zaffoni E, Ruina M, Sartori S, Trevisani L, Ciaccia A, Alvisi V, Fabbri L, Papi A. Helicobacter pylori and chronic bronchitis. Scand J Gastroenterol. 1999;34:828-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Daudén E, Jiménez-Alonso I, García-Díez A. Helicobacter pylori and idiopathic chronic urticaria. Int J Dermatol. 2000;39:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Ojetti V, Armuzzi A, De Luca A, Nucera E, Franceschi F, Candelli M, Zannoni GF, Danese S, Di Caro S, Vastola M. Helicobacter pylori infection affects eosinophilic cationic protein in the gastric juice of patients with idiopathic chronic urticaria. Int Arch Allergy Immunol. 2001;125:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Vainio E, Huovinen S, Liutu M, Uksila J, Leino R. Peptic ulcer and Helicobacter pylori in patients with lichen planus. Acta Derm Venereol. 2000;80:427-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Szlachcic A, Sliwowski Z, Karczewska E, Bielański W, Pytko-Polonczyk J, Konturek SJ. Helicobacter pylori and its eradication in rosacea. J Physiol Pharmacol. 1999;50:777-786. [PubMed] |
| 30. | Avci O, Ellidokuz E, Simşek I, Büyükgebiz B, Güneş AT. Helicobacter pylori and Behçet's disease. Dermatology. 1999;199:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Yazawa N, Fujimoto M, Kikuchi K, Kubo M, Ihn H, Sato S, Tamaki T, Tamaki K. High seroprevalence of Helicobacter pylori infection in patients with systemic sclerosis: association with esophageal involvement. J Rheumatol. 1998;25:650-653. [PubMed] |
| 32. | Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Parkinson AJ, Gold BD, Bulkow L, Wainwright RB, Swaminathan B, Khanna B, Petersen KM, Fitzgerald MA. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol. 2000;7:885-888. [PubMed] |
| 34. | Konno M, Muraoka S, Takahashi M, Imai T. Iron-deficiency anemia associated with Helicobacter pylori gastritis. J Pediatr Gastroenterol Nutr. 2000;31:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Annibale B, Lahner E, Bordi C, Martino G, Caruana P, Grossi C, Negrini R, Delle Fave G. Role of Helicobacter pylori infection in pernicious anaemia. Dig Liver Dis. 2000;32:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Choe YH, Kwon YS, Jung MK, Kang SK, Hwang TS, Hong YC. Helicobacter pylori-associated iron-deficiency anemia in adolescent female athletes. J Pediatr. 2001;139:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, Finci R, Yalçín A. Helicobacter pylori--is it a novel causative agent in Vitamin B12 deficiency. Arch Intern Med. 2000;160:1349-1353. [PubMed] |
| 38. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 39. | Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255-4259. [PubMed] |
| 40. | Hatzifoti C, Wren BW, Morrow WJ. Helicobacter pylori vaccine strategies--triggering a gut reaction. Immunol Today. 2000;21:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Dieterich C, Bouzourène H, Blum AL, Corthésy-Theulaz IE. Urease-based mucosal immunization against Helicobacter heilmannii infection induces corpus atrophy in mice. Infect Immun. 1999;67:6206-6209. [PubMed] |
| 42. | Liu X, Hu J, Zhang X, Fan D. Oral immunization of mice with attenuated Salmonella typhimurium expressing Helicobacter pylori urease B subunit. Chin Med J (Engl). 2002;115:1513-1516. [PubMed] |
| 43. | Lee CK, Soike K, Hill J, Georgakopoulos K, Tibbitts T, Ingrassia J, Gray H, Boden J, Kleanthous H, Giannasca P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Solnick JV, Canfield DR, Hansen LM, Torabian SZ. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta). Infect Immun. 2000;68:2560-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Chen Y, Zhang ZS, Wang JD, Zhou DY. Cloning and expression of adhesion gene hpaA of Helicobacter pylori. J First Mil Med Univ. 2000;20:210-213. |
| 47. | Chen Y, Wang J, Shi L. [In vitro study of the biological activities and immunogenicity of recombinant adhesin of Heliobacter pylori rHpaA]. Zhonghua Yixue Zazhi. 2001;81:276-279. [PubMed] |
| 48. | Kim BO, Shin SS, Yoo YH, Pyo S. Peroral immunization with Helicobacter pylori adhesin protein genetically linked to cholera toxin A2B subunits. Clin Sci (Lond). 2001;100:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Goto T, Nishizono A, Fujioka T, Ikewaki J, Mifune K, Nasu M. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun. 1999;67:2531-2539. [PubMed] |
| 51. | Jiang Z, Tao XH, Huang AL, Wang PL. A study of recombinant protective H.pylori antigens. World J Gastroenterol. 2002;8:308-311. [PubMed] |
| 52. | Metzler B, Mayr M, Dietrich H, Singh M, Wiebe E, Xu Q, Wick G. Inhibition of arteriosclerosis by T-cell depletion in normocholesterolemic rabbits immunized with heat shock protein 65. Arterioscler Thromb Vasc Biol. 1999;19:1905-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Keenan J, Oliaro J, Domigan N, Potter H, Aitken G, Allardyce R, Roake J. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect Immun. 2000;68:3337-3343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, Trust TJ. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect Immun. 2000;68:4155-4168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 55. | Todoroki I, Joh T, Watanabe K, Miyashita M, Seno K, Nomura T, Ohara H, Yokoyama Y, Tochikubo K, Itoh M. Suppressive effects of DNA vaccines encoding heat shock protein on Helicobacter pylori-induced gastritis in mice. Biochem Biophys Res Commun. 2000;277:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Shiesh SC, Sheu BS, Yang HB, Tsao HJ, Lin XZ. Serologic response to lower-molecular-weight proteins of H. pylori is related to clinical outcome of H. pylori infection in Taiwan. Dig Dis Sci. 2000;45:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Raymond J, Sauvestre C, Kalach N, Bergeret M, Dupont C. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr Infect Dis J. 2000;19:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Kawahara M, Hashimoto A, Toida I, Honda M. Oral recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing HIV-1 antigens as a freeze-dried vaccine induces long-term, HIV-specific mucosal and systemic immunity. Clin Immunol. 2002;105:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Kawahara M, Matsuo K, Nakasone T, Hiroi T, Kiyono H, Matsumoto S, Yamada T, Yamamoto N, Honda M. Combined intrarectal/intradermal inoculation of recombinant Mycobacterium bovis bacillus Calmette-Guérin (BCG) induces enhanced immune responses against the inserted HIV-1 V3 antigen. Vaccine. 2002;21:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Young SL, O'Donnell MA, Buchan GS. IL-2-secreting recombinant bacillus Calmette Guerin can overcome a Type 2 immune response and corticosteroid-induced immunosuppression to elicit a Type 1 immune response. Int Immunol. 2002;14:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Young S, O'Donnell M, Lockhart E, Buddle B, Slobbe L, Luo Y, De Lisle G, Buchan G. Manipulation of immune responses to Mycobacterium bovis by vaccination with IL-2- and IL-18-secreting recombinant bacillus Calmette Guerin. Immunol Cell Biol. 2002;80:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Zheng C, Xie P, Chen Y. Recombinant Mycobacterium bovis BCG producing the circumsporozoite protein of Plasmodium falciparum FCC-1/HN strain induces strong immune responses in BALB/c mice. Parasitol Int. 2002;51:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Méderlé I, Bourguin I, Ensergueix D, Badell E, Moniz-Peireira J, Gicquel B, Winter N. Plasmidic versus insertional cloning of heterologous genes in Mycobacterium bovis BCG: impact on in vivo antigen persistence and immune responses. Infect Immun. 2002;70:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Zheng C, Xie P, Chen Y. Immune response induced by recombinant BCG expressing merozoite surface antigen 2 from Plasmodium falciparum. Vaccine. 2001;20:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Chujoh Y, Matsuo K, Yoshizaki H, Nakasatomi T, Someya K, Okamoto Y, Naganawa S, Haga S, Yoshikura H, Yamazaki A. Cross-clade neutralizing antibody production against human immunodeficiency virus type 1 clade E and B' strains by recombinant Mycobacterium bovis BCG-based candidate vaccine. Vaccine. 2001;20:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Hiroi T, Goto H, Someya K, Yanagita M, Honda M, Yamanaka N, Kiyono H. HIV mucosal vaccine: nasal immunization with rBCG-V3J1 induces a long term V3J1 peptide-specific neutralizing immunity in Th1- and Th2-deficient conditions. J Immunol. 2001;167:5862-5867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Ohara N, Matsuoka M, Nomaguchi H, Naito M, Yamada T. Protective responses against experimental Mycobacterium leprae infection in mice induced by recombinant Bacillus Calmette-Guérin over-producing three putative protective antigen candidates. Vaccine. 2001;19:1906-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Luo Y, Chen X, Szilvasi A, O'Donnell MA. Co-expression of interleukin-2 and green fluorescent protein reporter in mycobacteria: in vivo application for monitoring antimycobacterial immunity. Mol Immunol. 2000;37:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |